Abstract
Background
Sinus venosus defects (SVD) of the inferior vena cava (IVC) type, or inferior SVDs, are an uncommon form of atrial communication located outside the confines of the fossa ovalis and involve override of the IVC. Despite numerous studies describing the anatomical and echocardiographic features of the inferior SVD, distinguishing this defect from a large secundum atrial septal defect (ASD) by echocardiography is often challenging. Accurate diagnosis of an inferior SVD and correct differentiation from a secundum ASD is essential for appropriate presurgical planning. Absence of the posterior rim in the parasternal short axis views may serve as a useful clue in diagnosing inferior SVDs. We sought to determine the utility of using the presence or absence of a posterior atrial rim in the parasternal short axis view to help distinguish an inferior SVD from a secundum ASD. This sign may help clinch the diagnosis when subcostal imaging is suboptimal.
Methods
We retrospectively reviewed transthoracic echocardiograms (TTE) from 15 patients with a known surgical diagnosis of an inferior SVD between 2004 and 2015. The presence or absence of a posterior rim in the parasternal short axis view was determined by two primary investigators. The posterior rim was also evaluated in 14 patients with a secundum ASD repair as controls. Echocardiograms were then reviewed blindly by attending level echocardiographers and cardiology fellows in training. Diagnostic accuracy was assessed both with and without the use of the posterior rim criterion. Statistical analysis was used to determine the effect of using the rim criterion on inferior SVD diagnosis. We also reviewed all surgically diagnosed secundum ASDs that were incorrectly diagnosed as inferior SVD by preoperative imaging and determined whether use of the posterior rim criterion would have resulted in the correct diagnosis.
Results
The posterior rim was absent in all 15 patients with a surgical diagnosis of inferior SVD and present in all 14 patients with a secundum ASD. For all observers, there was a statistically significant increase in diagnostic accuracy of inferior SVDs with the use of the rim criterion (p<0.0001). We noted that secundum ASDs with inferior extension also have persistent posterior rims. The rim criterion correctly classified all large secundum ASDs with inferior extension that were previously misdiagnosed by echocardiogram preoperatively.
Conclusions
Absence of the posterior rim (“bald” posterior wall) is a consistent finding in patients with an inferior SVD and distinguishes an inferior SVD from a large secundum ASD with inferior extension. Parasternal short axis evaluation of the posterior atrial rim is a helpful tool for all levels of physician training in improving diagnostic accuracy for detecting inferior SVDs and in distinguishing them from secundum ASDs.
Keywords: Inferior sinus venosus defect, atrial septal defect, echocardiogram
Introduction
Sinus venosus defects (SVD) are a rare form of congenital heart disease involving an interatrial communication outside the confines of the fossa ovalis. Although SVDs do not involve the atrial septum proper (i.e. the fossa ovalis and its surrounding muscular rim), they are placed in the same physiological category as atrial septal defects (ASD) and account for up to 11% of interatrial communications (1–4). SVDs of the inferior vena cava (IVC) type, or inferior SVDs, involve a deficiency in postero-inferior aspects of the atrial septum, resulting in a communication between the right and left atrial chambers through the mouth of the inferior vena cava (IVC) (2, 5, 6). An intact fossa ovalis and the IVC straddling over the defect are considered as additional prerequisites for diagnosing inferior SVDs. Anomalous pulmonary venous drainage to the IVC is a common finding, and has been regarded by some as an anatomical criterion for the diagnosis of inferior SVDs (2, 5, 6), whereas, others have not included this feature as a diagnostic criterion (1) because it is not present consistently.
In contrast to the secundum type of ASD, inferior SVDs are not amenable to transcatheter device closure, and thus require surgical repair. The presence of an inferior SVD may also influence the specific placement of the IVC cannula prior to cardiopulmonary bypass (1). Previous studies have reported challenges with accurate preoperative diagnosis of inferior SVDs, with the defects often being mistaken for large secundum ASDs with inferior extension (5–7). While combination defects within the atria may be present, defining the posteroinferior rim anatomy itself has profound implications upon treatment strategy and overall hospital course. For example, Banka and colleagues found that misdiagnosis of inferior SVDs was associated with longer hospitalizations, a more complicated hospital course, and poorer technical outcomes(6).
The importance of pre-surgical diagnostic precision has resulted in the use of multiple imaging modalities including transesophageal echocardiography and MRI (8, 9). Additionally, many studies have described transthoracic echocardiographic (TTE) findings to help facilitate diagnosis of inferior SVDs and their distinction from secundum ASDs. However, in these studies, the focus was upon the absence of an IVC rim in the subcostal sagittal views (1–3, 5, 7). Frequently in children, the subcostal coronal and sagittal views and many “in-between” views are obtained, along with methodical sweeping along a stack of planes in each view. This allows the diagnosis of SVDs by creating a mental three-dimensional picture of the position of the defect with respect to the IVC. However, this method also requires optimal images, which may be lacking in some older children and adults. Moreover, in adult echocardiography laboratories, such multiplanar subcostal imaging of the atrial septum is performed much less frequently. Previous studies have suggested that the parasternal short-axis view may demonstrate a deficiency in the posterior atrial rim (1–3).
Our aim was to determine if an absent posterior atrial rim in the parasternal short axis view was a consistent and reliable finding in all patients with inferior SVDs. We hypothesize that the identification of a “bald atrial wall”, seen in the absence of a posterior atrial rim in a parasternal short axis view, is a consistent feature found in inferior SVDs which will improve diagnostic accuracy.
Methods
We retrospectively reviewed the clinical data, echocardiography, and surgical notes from our surgical database for all patients between ages 0 and 18 years of age with a post-operative diagnosis of an inferior SVD at our institution over an 11 year time-period, between January 2004 and February 2015. Subjects diagnosed with a secundum ASD that subsequently underwent surgical closure from the same time period were used as controls.
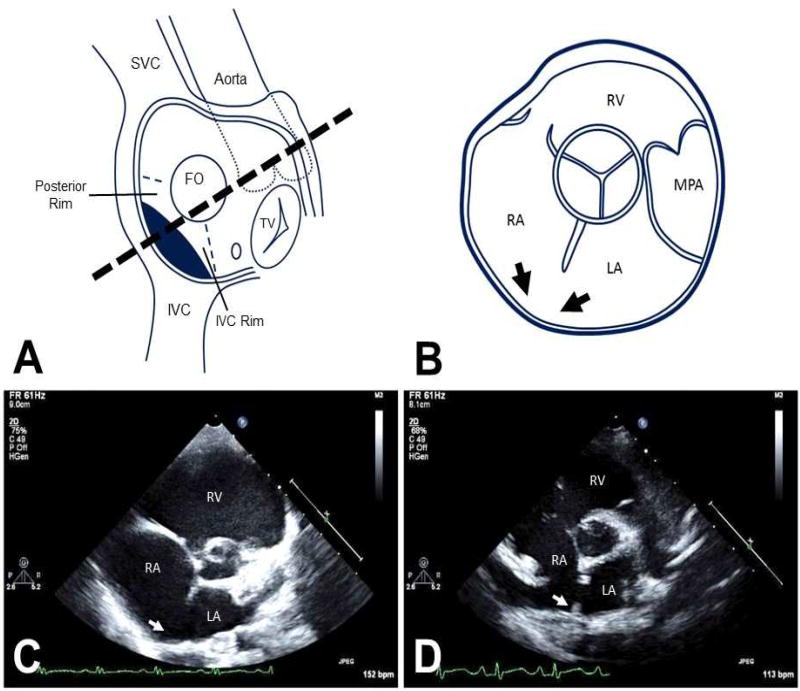
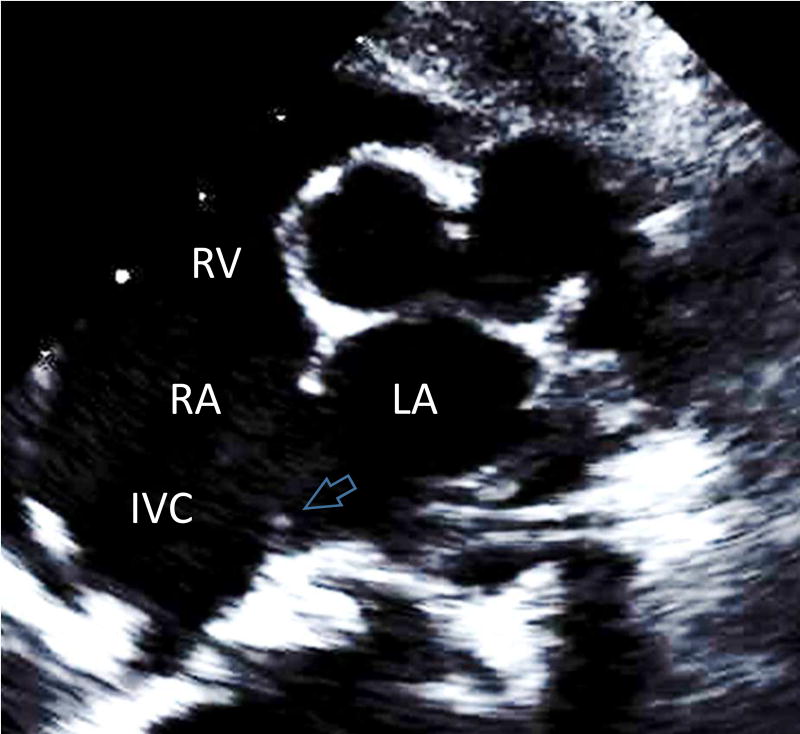
To be included in the study, patients must have had a full echocardiogram performed prior to surgical intervention. The minimal criterion for an inferior SVD defined for this study, was an atrial communication originating at the mouth of the IVC with the IVC straddling the defect. The presence of anomalous pulmonary venous drainage was noted when present, but not required to establish a diagnosis of inferior SVD. Our posterior rim criterion was defined as absence of a posterior atrial rim in the standard parasternal short axis view (giving a “bald” appearance to the posterior atrial wall). This criterion was used to support the diagnosis of inferior SVD, while the presence of a posterior rim, even a rudimentary one, went against this diagnosis (Fig 1A–D). In some patients, a slightly modified parasternal short axis view obtained by 20 degree counterclockwise rotation of the transducer was also available, as this view is frequently used in our institution to clarify the position of an ASD with respect to the IVC.
Figure 1.
Anatomy and corresponding parasternal short axis views of the atrial structures. A: The components of the atrial septum are depicted in an en-face view, highlighting the posterior and IVC rims. FO=fossa ovalis part of atrial septum is depicted by a circle. The position of the inferior SVD is shown by the dark shading. B: Cartoon of the corresponding parasternal short-axis view of TTE. The absent posterior rim of the atrial septum creating the "bald" posterior wall of the left atrium, is depicted by the two black arrows. C: Parasternal short axis view demonstrating absence of the posterior rim (white arrow) in a typical inferior SVD. D: Similar parasternal short axis view showing a secundum ASD with the presence of a tiny posterior rim (white arrow). IVC=inferior vena cava, LA=left atrium, MPA=main pulmonary artery, RA=right atrium. RV=right ventricle, SVC=superior vena cava, TV=tricuspid valve.
Echocardiograms were reviewed by two investigators (B.S., A.B.) to determine the presence or absence of a posterior rim. We then sought to determine whether this particular echocardiographic finding would facilitate the accurate diagnosis of inferior SVDs. Echocardiograms were reviewed blindly by three separate attending physicians who were more than 8 years past their fellowship training and were dedicated echocardiographers. Echocardiograms were also reviewed by two first-year cardiology fellows who were at the end of their first year of training (Fellows 1 and 2) and three second-year fellows (Fellows 3, 4 and 5). Lastly, we performed a reverse analysis and all the patients with a surgical diagnosis of secundum ASD who were incorrectly diagnosed as having an inferior SVD in preoperative echocardiographic report were identified and the presence of the posterior rim was determined by the investigators of this study. The study was approved by the Institutional Review Board of the Children’s Hospital of Philadelphia (IRB # 14-011648).
Statistics
Descriptive statistics of the demographic factors were reported for the two groups of patients separately. Percentage of accurate diagnosis was calculated and reported. Determination of diagnostic accuracy was done by performing a logistic regression analysis and generating receiver operating characteristic (ROC) curve and the area under the curve (AUC) was calculated as a measure of discriminatory power of the test. The values used for the predictor were either inferior SVD or secundum ASD. McNemars test was used to determine statistical significance of using the posterior rim criterion for inferior SVD diagnosis. A p value of <0.05 was considered significant.
Results
Using the aforementioned criteria, 15 patients were identified with surgically confirmed diagnoses of inferior SVDs. We found anomalous pulmonary venous return from either the right lower or right middle and lower pulmonary veins, in 11 patients (73%). The average age at the time of surgery was 4.8 years (range:1–14 years). Fourteen patients with secundum ASDs were used as controls. The demographics of the inferior SVD and secundum ASD population are illustrated in table 1.
Table 1.
Demographic data of patients with two different types of interatrial communications, inferior sinus venosus defects and secundum atrial septal defects.
| Inferior SVD | Secundum ASD | P value | |
|---|---|---|---|
| Total | 15 | 14 | |
| Male | 40% (n=6) | 29% (n=4) | 0.70 |
| Age at Surgery (years) | 4.8 ± 3.8 | 7.4 ± 5.6 | 0.16 |
| Anomalous Pulmonary Venous Return | 73% (n=11) | 0 | <0.0001 |
Inferior SVD= Inferior sinus venosus type ASD
Parasternal short axis views of all 15 patients with inferior SVDs were reviewed. All of the echocardiograms demonstrated a deficient posterior atrial rim, giving rise to a bald appearance of the posterior atrial wall (Fig 1B,C and Fig 5). In contrast, all 14 patients with secundum ASDs exhibited at least some remnant of posterior rim along the posterior atrial wall (Fig 1D).
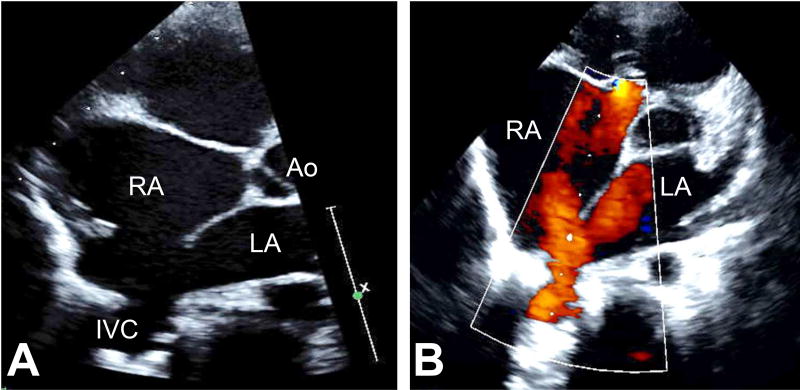
Figure 5.
A: A slightly modified parasternal short axis view obtained by rotating the transducer in counterclockwise direction by 20 degrees. This figure depicts two cardinal features of the inferior sinus venosus defect by echocardiogram: 1) absent posterior rim and 2) straddling of the IVC over the defect. B: By color Doppler interrogation, the straddling of the IVC is confirmed. The flow returning from the IVC splits into two components, one entering the LA and the other entering the RA.
All 29 patients were first reviewed by 3 blinded senior echocardiography attending physicians. Attending physicians #1 and #2 were already familiar with our proposed rim criterion and each correctly classified both defects 90% of the time. Inferior SVDs were correctly identified 80–87% of the time and secundum ASDs 93–100%.
The echocardiograms were then reviewed by a third attending who had not previously used the posterior rim criterion. Attending physician #3 correctly identified 60% (9/15) of inferior SVDs and 100% (14/14) of secundum ASDs. Attending #3 was then asked to again blindly classify the echocardiograms utilizing the posterior rim criterion for the diagnosis of inferior SVDs (Fig 1C). Attending #3 correctly diagnosed 87% (13/15) of inferior SVDs, but misclassified 3 secundum ASDs as inferior SVDs.
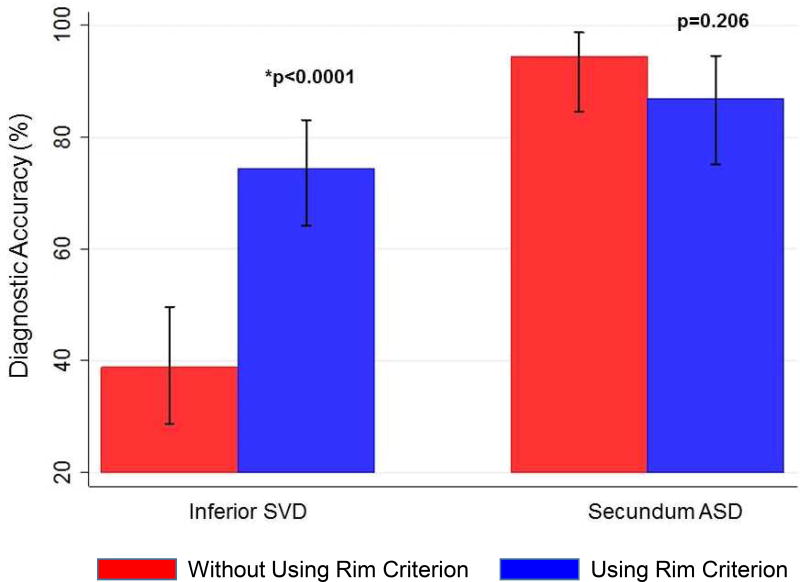
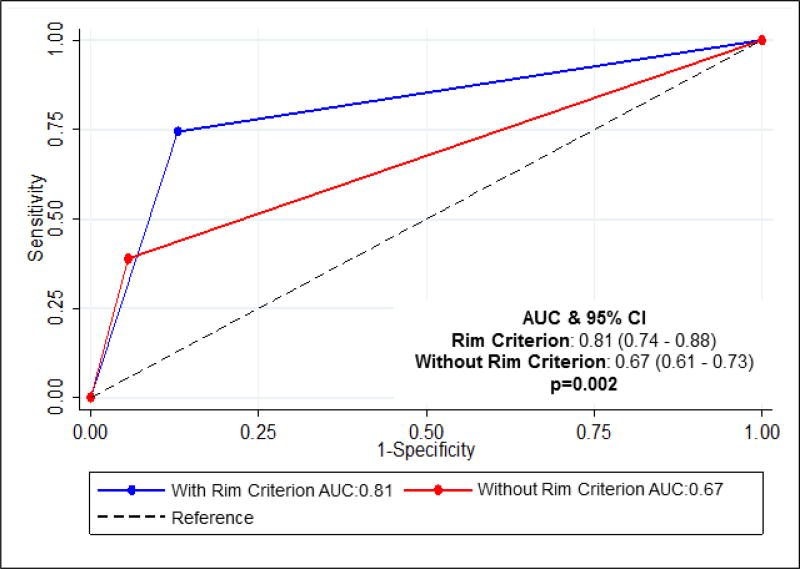
We then sought to determine whether the bald posterior atrial wall would facilitate ASD classification for cardiology fellows in-training. Two first-year fellows (Fellows 1,2) and three second-year fellows (Fellows 3–5) blindly reviewed a smaller subset of the echocardiograms (n=23) both with and without the posterior rim criterion. Prior to being introduced to the rim criterion, the fellows correctly classified 34.7% of inferior SVDs and 92.5% of secundum ASDs. After introduction of the rim criterion, the diagnostic accuracy of inferior SVD dramatically improved to 72% (p<0.0001), while there was no significant difference in secundum ASD accuracy (Fig 2a). Including all fellows and attendings, use of the rim criterion resulted in a statistically significant improvement in diagnoses of inferior SVDs, with no significant difference in diagnoses of secundum ASDs (Fig 2). Use of the rim criterion for inferior SVD diagnosis resulted in a significantly higher area under the curve (AUC) compared to that not using the criteron (0.81 vs 0.67) (Fig 3).
Figure 2.
Diagnostic accuracy of using the posterior rim criterion for all observers: attending echocardiographers and fellows. There is a significant improvement in inferior SVD diagnosis when using the rim criterion (p<0.0001).
Figure 3.
Receiver operating characteristic (ROC) curves were constructed for evaluating diagnostic accuracy using binary predictors, absence of posterior rim for predicting inferior SVD and presence of posterior rim for predicting a secundum ASD. The area under the curve (AUC) 17 for the rim criterion is 0.81 and is significantly higher than that not using the rim criterion (p=0.002).
As a secondary aim, we performed a reverse analysis of all 237 patients with a surgical diagnosis of a secundum ASD and identified 6 patients that were incorrectly diagnosed with inferior SVDs in their preoperative echocardiographic reports. All 6 of these patients had large secundum ASDs with inferior extension. Despite the presence of a large secundum defect with inferior extension, they consistently showed at least a rudimentary posterior rim (Fig 4). Had the posterior rim criterion been used during the initial echocardiographic diagnosis it would have correctly identified all 6 as secundum ASDs. Along with the rudimentary posterior rim in these 6 patients, we also noted that the anterior rims were qualitatively smaller than the anterior rims of inferior SVDs. All 6 patients were correctly assigned to the secundum ASD group in this study.
Figure 4.
Standard parasternal short axis view in a patient with a large secundum ASD that extends into the postero-inferior part of the atrial septum. Note the persistence of the rudimentary posterior rim (white arrow) even in this ASD. This feature is helpful in differentiating a large inferior secundum ASD from an inferior sinus venosus ASD. Here the IVC does not straddle the defect (open arrow). It enters the RA normally, to the right of the atrial septum.
Discussion
Precise delineation of the atrial anatomy remains an important step for pre-operative planning in ASD repair (1, 6). In the largest published series to date of 45 inferior SVDs, two-thirds of the patients were misdiagnosed by pre-operative imaging (6). Significantly, misdiagnosis was associated with poorer technical surgical outcomes and hospital course compared with patients undergoing secundum ASD repair(6). This underscores not only the importance of preoperative imaging, but also some of the challenges in differentiating an inferior SVD from a secundum ASD by echocardiography. Our study shows that the absence of a posterior atrial rim in the parasternal short axis view is a consistent and reliable finding in patients with inferior SVD and improves diagnostic accuracy of this lesion.
Anatomically, a secundum ASD is defined by a deficiency in septum primum and the defect invades the fossa ovalis and its muscular rim (3,10). Although there has been some debate over the specific criterion that define an inferior SVD (1, 5–7), it is generally accepted that this defect is characterized by an inferior vena cava straddling the defect with the atrial septal defect outside of the boundaries of the fossa ovalis and its muscular rim (Fig 5) (1, 3, 6, 10, 11). Inferior SVDs are often accompanied by anomalous pulmonary venous drainage, although there is disagreement as to whether that is actually required for the diagnosis (1, 6, 7). While our results showed a statistically significant difference in the presence of anomalous pulmonary venous drainage in inferior SVDs, we do not feel that the presence this finding is mandatory in diagnosing inferior SVDs. The inferior SVD is sometimes difficult to distinguish from large secundum defects with inferior extension due to the close proximity of the two lesions (7, 10). Additionally, mixed-type lesions with both secundum and inferior SVD defects have been described (12). This has led to numerous helpful studies describing echocardiographic features of the inferior SVD (1–3, 5, 7, 11). Notwithstanding, this lesion continues to remain a challenge to diagnose, particularly in comparison to a secundum ASD, as demonstrated by all of our observers.
We have noted the utility of using the parasternal short axis view, to evaluate the presence or absence of the posterior atrial rim in diagnosing inferior SVDs. We found that the observers who used the rim criterion had better diagnostic accuracy for diagnosing inferior SVDs than those who did not. Our study shows that there is a statistically significant improvement in diagnostic accuracy when using the parasternal short axis view and focusing upon the posterior rim. Notably this improvement was seen both at the attending and fellow levels of expertise. An unexpected finding was the misclassification of three secundum defects by Attending #3 after introduction of the rim criterion. Perhaps the reason for this was that these patients had a rudimentary posterior rim. Notwithstanding this misclassification, there was no statistically significant difference in secundum ASD diagnosis when using the rim criterion for all observers (Fig 2). These results suggest that the rim criterion may be useful for assisting with diagnosis of the inferior SVD for cardiologists at multiple levels of experience.
Differentiating a large secundum ASD from an inferior SVD may prove to be difficult at times. For the large secundum ASDs with postero-inferior extensions, we noted three interesting qualitative signs that were manifested in the short axis views: 1) a rudimentary posterior rim was present (atrial wall was not bald), 2) anterior, retroaortic rim was invariably smaller than that noted in inferior SVDs and 3) In slightly modified views the IVC can be seen entering to the right of the atrial septum (no straddle) (Fig 4). The deficient retroaortic rim may result from the large secundum ASD encroaching well into the fossa ovalis and its muscular rims. This truncates the retroaortic rim and makes it appear shorter in the short axis view. In inferior SVD, the fossa ovalis is spared, therefore, the anterior rim appears longer. In large secundum ASD with a rudimentary posterior rim, it is also helpful to assess the entrance of the IVC into the right atrium demonstrated by the modified short-axis view described by us. During sweeping maneuvers performed in the short axis view, the mouth of the IVC may often be detected.
One of the important implications of improved inferior SVD diagnosis, is the effect it may have upon pre-procedure planning of interatrial defect closure. Sinus venosus defects are currently a contraindication for catheter based device closure (3). Our study demonstrates that the parasternal short axis profile of the posterior rim facilitates identification of inferior SVD. We speculate this may help reduce the number of cases referred to the cardiac catheterization laboratory that are not amenable to device closure.
We also propose that this simple view will also be greatly useful for adult cardiologists, interested in the fields of imaging and adults with congenital heart disease. In teenagers and adults, multiplane subcostal views can be technically difficult, due to body habitus and the significant distance of the heart from the transducer. Therefore, in this group of patients subcostal images of the atrial septum may be quite poor and cannot diagnose inferior SVDs with confidence. Despite being extremely useful in evaluating other parts of the atrial septum, transesophageal echocardiography (TEE) faces difficulty in evaluating the IVC rim of ASDs, including secundum ASDs. Due to the close proximity of the IVC to the lower esophagus, it can often be challenging to obtain quality images of a thin IVC rim from standard TEE planes and highly modified planes may be necessary(13). Less experienced physicians may not be familiar with these modified TEE planes. Considering these potential limitations of TEE, we consider a set of TTE views may be more reliable than TEE in diagnosing inferior SVDs.
Three-dimensional echocardiography (3DE) using the transesophageal (3D-TEE) and transthoracic (3D-TTE) approaches have been used with some success in evaluating interatrial communications in children(3, 14). However, our study was a retrospective study, which analyzed standard TTEs performed for clinical purposes and 3DE images of the atrial septum were not available in these patients. Moreover, unlike 3D-TEE technique, 3D-TTE has not been as successful in identifying the ASD rims in children, due to lack of resolution and movement artifacts. At present, the successful use of 3D-TTE with inferior SVD diagnosis is limited to a few case reports(14). We feel that at present level of transducer technology, 3DE cannot be completely relied upon in making this diagnosis. It is possible that with improving transducer technology and with more wide-spread clinical use of this modality, 3DE may become a useful tool in diagnosing inferior SVDs. This also applies to the present state of 3D printing. Although a tool that has tremendous future potential, 3D printing is still in its infancy and depends on high quality 3D TTE or TEE data. At present, the 3D TTE data has not yet reached widespread use and high quality datasets needed for 3D printing are still suboptimal.
Limitations
In this study, direct surgical observation was used as the gold-standard for differentiating a secundum ASD from an inferior SVD. However, in an empty, flaccid heart on cardiopulmonary bypass a thin, sliver-like posterior rim may not be appreciated by direct observation, in which case the ASD would be labeled as inferior SVD, rather than a secundum ASD. In a blood-filled, beating heart TTE may pick up the sliver-like posterior rim, not detected by direct observation and this may be a potential source of discrepancy.
In recent years, an increasing number of imaging modalities have become more available for cardiovascular imaging including, computerized tomography (CT) and magnetic resonance imaging (MRI). While the use of multiple modalities such as CT, MRI, TEE, and 3D echocardiography could help clarify some of the anatomical distinctions between different defects, and may be informative in comparing diagnostic utility, our retrospective study was limited to the imaging tools that were used at the time of diagnosis. Additionally, at our center it is not routine practice to obtain pre-operative TEEs. Moreover, despite the availability of different imaging modalities, two-dimensional TTE remains the cornerstone for making this diagnosis. When TTE is the only modality being used, the view proposed by us can help clinch the diagnosis in doubtful cases.
The inferior SVD is a relatively uncommon defect. Similar to previous studies on inferior SVD, our study is limited by a small cohort (1, 5–7). While we have demonstrated illustrative differences in the parasternal short axis views of large secundum ASD with inferior extension and the inferior SVD, our study is limited by the low number of secundum ASDs with inferior extension. This also precluded meaningful statistical anlaysis of the length of the anterior rim in secundum ASDs versus inferior SVDs and observations about the anterior rim were more qualitative.
Conclusions
In summary, absent posterior atrial rim in the parasternal short axis view (“bald” posterior wall) by TTE is indeed a consistent and reliable finding in patients with surgically diagnosed inferior SVDs and shows the best correlation with direct surgical observation. It is, therefore, an important supportive criterion that helps distinguish between the inferior SVD and secundum ASD. This simple and well-known view may be particularly helpful in teenagers and adults who have limited subcostal views. The standard parasternal short axis view may improve diagnostic accuracy of inferior SVDs, in physicians at multiple levels of training and in those who have less experience with subcostal, multiplanar imaging.
Acknowledgments
We would like to thank Rachel Rogers and Xuemei Zhang from the CHOP Biostatistics and Data Management Core for assistance with statistical analyses.
Funding Sources: This work received support by the National Institutes of Health T-32 HL007915 (B.S.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomar M, Radhakrishnan S, Kaushal SK, Dagar KS, Iyer KS, Shrivastava S. Inferior-type caval vein defect--echocardiographic and surgical description of a large series of patients. Cardiol Young. 2012;22(3):270–8. doi: 10.1017/S104795111100120X. [DOI] [PubMed] [Google Scholar]
- 2.Van Praagh S, Carrera ME, Sanders SP, Mayer JE, Van Praagh R. Sinus venosus defects: unroofing of the right pulmonary veins--anatomic and echocardiographic findings and surgical treatment. Am Heart J. 1994;128(2):365–79. doi: 10.1016/0002-8703(94)90491-x. [DOI] [PubMed] [Google Scholar]
- 3.Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, Hijazi ZM, et al. Guidelines for the Echocardiographic Assessment of Atrial Septal Defect and Patent Foramen Ovale: From the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr. 2015;28(8):910–58. doi: 10.1016/j.echo.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Ettedgui JA, Siewers RD, Anderson RH, Park SC, Pahl E, Zuberbuhler JR. Diagnostic echocardiographic features of the sinus venosus defect. Br Heart J. 1990;64(5):329–31. doi: 10.1136/hrt.64.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crystal MA, Al Najashi K, Williams WG, Redington AN, Anderson RH. Inferior sinus venosus defect: echocardiographic diagnosis and surgical approach. J Thorac Cardiovasc Surg. 2009;137(6):1349–55. doi: 10.1016/j.jtcvs.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Banka P, Bacha E, Powell AJ, Benavidez OJ, Geva T. Outcomes of inferior sinus venosus defect repair. J Thorac Cardiovasc Surg. 2011;142(3):517–22. doi: 10.1016/j.jtcvs.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Plymale J, Kolinski K, Frommelt P, Bartz P, Tweddell J, Earing MG. Inferior sinus venosus defects: anatomic features and echocardiographic correlates. Pediatr Cardiol. 2013;34(2):322–6. doi: 10.1007/s00246-012-0449-7. [DOI] [PubMed] [Google Scholar]
- 8.Schantz D, Thompson D, Warren A. Magnetic resonance imaging-aided diagnosis of an inferior sinus venosus defect: a case report. Pediatr Cardiol. 2010;31(4):564–6. doi: 10.1007/s00246-010-9643-7. [DOI] [PubMed] [Google Scholar]
- 9.Wong DR, Hancock Friesen CL, Warren AE, O'Blenes SB. Echocardiographic diagnosis and repair of an inferior sinus venosus defect. Can J Cardiol. 2008;24(1):67. doi: 10.1016/s0828-282x(08)70554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy K, Ho S, Anderson R. Defining the morphologic phenotypes of atrial septal defects and interatrial communications. Images Paediatr Cardiol. 2003;5(2):1–24. [PMC free article] [PubMed] [Google Scholar]
- 11.al Zaghal AM, Li J, Anderson RH, Lincoln C, Shore D, Rigby ML. Anatomical criteria for the diagnosis of sinus venosus defects. Heart. 1997;78(3):298–304. doi: 10.1136/hrt.78.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz-Castellanos L, Espinola-Zavaleta N, Kuri-Nivon M, Ruiz JF, Keirns C. Atrial septal defect: anatomoechocardiographic correlation. J Am Soc Echocardiogr. 2006;19(9):1182–9. doi: 10.1016/j.echo.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 13.Remadevi KS, Francis E, Kumar RK. Catheter closure of atrial septal defects with deficient inferior vena cava rim under transesophageal echo guidance. Catheter Cardiovasc Interv. 2009;73(1):90–6. doi: 10.1002/ccd.21756. [DOI] [PubMed] [Google Scholar]
- 14.Chen CA, Wang JK, Hsu JY, Hsu HH, Chen SJ, Wu MH. Diagnosis of inferior sinus venosus atrial septal defects using transthoracic three-dimensional echocardiography. J Am Soc Echocardiogr. 2010;23(4):457 e4–6. doi: 10.1016/j.echo.2009.09.008. [DOI] [PubMed] [Google Scholar]