Abstract
Background
Uncertainty surrounds why hepatitis C virus (HCV) is concentrated among HIV-positive men who have sex with men (MSM). We used mathematical modelling to explore reasons for these infection patterns, and implications for HCV treatment-as-prevention.
Methods
Using a joint MSM HIV/HCV transmission model parameterized with UK behavioural data, we considered how biological (heightened HCV infectivity and reduced spontaneous clearance among HIV-positive MSM) and/or behavioural factors (preferential sexual mixing by HIV status and risk heterogeneity) could concentrate HCV infection in HIV-positive MSM as commonly observed (5-20 times the HCV prevalence in HIV-negative MSM; defined as the HCV ratio). We explored how HCV treatment-as-prevention impact varies under differing HCV ratios.
Results
Biological factors produced low HCV ratios (< 3), not explaining the skewed epidemic. However, combining preferential mixing by HIV status with sexual risk behaviour heterogeneity produced high HCV ratios (> 10) that were highly sensitive to both factors. Irrespective of the HCV ratio or behavioural/biological factors, HCV treatment of HIV-diagnosed MSM markedly reduced the HCV prevalence among HIV-positive MSM, but less impact was achieved among all MSM for lower HCV ratios.
Conclusions
Sexual behaviour patterns likely drive observed HCV infection patterns among HIV-positive MSM. Changes in these patterns could disseminate HCV amongst HIV-negative MSM, limiting the impact of targeting HCV treatment to HIV-diagnosed MSM.
Keywords: Hepatitis C virus, HIV, men who have sex with men, antiviral treatment, prevention
Introduction
An epidemic of hepatitis C virus (HCV) continues unfolding among HIV-positive men-who-have-sex-with-men (MSM) in the UK, Europe, the USA and Australia.1 HCV is a leading non-AIDS cause of death among MSM with HIV.2 The incidence of HCV among HIV-positive MSM is generally 5-20 times higher than in HIV-negative MSM.1,3 In the UK, the HCV seroprevalence among HIV-negative MSM was estimated to be 1.2% (0.6–2.1%) in 20094 but was 9.9% among HIV-positive MSM in 2012.5
Behavioural and biological factors have been proposed to account for the large discrepancy in HCV burden between HIV-positive and HIV-negative MSM.6,7 Biological factors include reduced chances of spontaneously clearing the HCV virus8,9 and higher HCV viral loads, potentially leading to greater infectivity in HIV-positive MSM.10,11
Behavioural factors include sexual partner selection based on HIV status and heterogeneity in sexual risk.6,12 Heterogeneities in risk and precautionary behaviours could incorporate differences in numbers of sexual partners and use of condoms, different preferences for ano-brachial insertion (fisting), and injecting illicit drugs.6,13–15 Additionally, partner selection and risk behaviours may interact, such as reduced condom use occurring between couples that assume they are sero-concordant.12 These heterogeneities in sexual risk and mixing could lead to groups with higher risk and thus greater sexually transmitted infection (STI) and HIV prevalences.
We developed a dynamic joint HIV/HCV transmission model among MSM to explore the contribution of behavioural and biological factors to why HCV is concentrated among HIV-positive MSM. We assessed how variations in these biological and behavioural factors may affect the HCV distribution, and evaluated the resulting implications for HCV treatment-as-prevention.5
Methods
Model derivation
A dynamic, deterministic model of HIV and HCV transmission among MSM was developed. We divided MSM into compartments (model schematic in Supplementary Figure S1, available as Supplementary data at IJE online) defined by HIV status (susceptible, undiagnosed acute infection, undiagnosed chronic infection and diagnosed HIV infection), HCV status (susceptible, chronic HCV, chronic HCV treatment failure) and low and high sexual risk groups, based on annual numbers of partners that MSM have anal sex with. A simplified model was designed, that was not intended to rigorously simulate the historical HIV epidemic as done in other studies.16
MSM enter the model when they reach sexual maturity and exit though death or ageing out of the model at 65 years of age. The model is dynamic, such that the risk of an individual acquiring HIV or HCV is related to the background prevalence of that infection, which can change over time. We assume that both diseases are transmitted through sexual episodes, which may involve unprotected anal intercourse, injecting drug use and fisting.17–20 Because individuals with more anal intercourse partners (whom we deem ‘high-risk’) also have a higher frequency of injecting drugs, fisting and other high-risk behaviours, high-risk MSM were assumed to have higher chances of HIV and HCV exposure.6,13–15
Once HIV infected, individuals enter undiagnosed acute HIV infection, with heightened HIV infectivity,21 subsequently transitioning to undiagnosed chronic HIV infection. Individuals are assumed not to become HIV diagnosed during the short (2.6 months) acute phase of HIV infection.21 On diagnosis, MSM transition to diagnosed chronic HIV, where a proportion receive HIV antiretroviral treatment (ART) which we assume reduces HIV infectivity22,23 and increases survival.24,25
Newly HCV-infected MSM that do not spontaneously clear HCV transition to chronic HCV. The HCV acute phase was not included due to its likely small contribution to HCV transmission.26 Those clearing HCV remain susceptible. When included in our analysis, HCV treatment is assumed to cure infection for a proportion of individuals but not to confer immunity. Successfully treated MSM remain at risk of reinfection. Unsuccessfully treated MSM move into the treatment failure class and remain infected with HCV.
To explore the implications of HIV infection for HCV infection/transmission patterns, we consider scenarios where HIV has no effect on HCV, and the alternative: where HIV infection increases HCV liver-related progression and mortality,27 reduces spontaneous clearance8,9 and increases HCV infectivity.10,11 We assume HCV does not impact on HIV disease progression or ART response.
Model parameterization
We modelled HIV and HCV transmission among sexually active MSM aged 15–65, parameterizing sexual behaviour with the UK component of the European MSM Internet Survey (EMIS-UK).28 EMIS was an online survey undertaken during June-August 2010, recruiting online and promoted offline through print media. Over 18 000 MSM living in the UK participated. From EMIS-UK data, we calculated the proportion of HIV-diagnosed MSM’s sexual partners they assumed were HIV-positive (36.2%) and condom use in these pairings (13.0% in latest sex act) compared with (68.0% in latest sex act) other MSM partnerships. EMIS-UK data also determined the heterogeneity in frequencies of sexual partnerships. When risk heterogeneity was explored, we divided the MSM population into categories of low and high risk by the annual number of casual sexual partnerships, 14 or less and 15 or greater, respectively, with 82.2% and 17.8% in the low- and high-risk groups, respectively. In some scenarios, an additional risk was also associated with MSM in the high-risk group due to EMIS-UK data suggesting that a greater proportion of these MSM either inject drugs (3.6% versus 1.0% among low-risk MSM), or undertake receptive (21.1% versus 8.6%) or insertive fisting (38.7% versus 14.0%) in the past year.6,13–15 Biological parameters were obtained from the literature. HCV treatment was not included in the baseline model because we were not aiming to closely model the precise HCV epidemic in the UK, and it was not considered to be an important determinant of observed epidemic patterns at existing treatment rates.5 All model parameters are outlined in Tables 1 and 2.
Table 1.
Model parameter values
| Parameter | Value | Source reference | Details/comments |
|---|---|---|---|
| Inflow and outflow rate due to entry and exit (annual) | 0.02 | – | Model age of sexual activity from 15 to 65 |
| Excess death rate due to chronic HCV mono-infection (annual) | 0.0014 | 44 | |
| Excess death rate due to mono-infection with HIV untreated (annual) | 0.089 | 24 | |
| Decreased mortality hazard ratio for HIV mono-infection due to ART treatment | 0.29 | 24,25 | |
| Excess death rate due to HIV in HIV-HCV co-infection with no HIV treatmenta (annual) | 0.089 | 24 | |
| Excess death rate due to HCV in HIV-HCV co-infection with no HIV treatmenta (annual) | 0.0035 | 27,44 | 2.5 times higher than the excess death rate in HCV mono-infected individuals. |
| Excess death rate due to HCV in HIV-HCV co-infection with ART treatmenta (annual) | 0.0024 | 27,44 | 1.7 times higher than the excess death rate in HCV mono-infected individuals |
| Transmission factor for HCV | Fit | 5 | Model calibrated to a 10% chronic HCV prevalence among HIV-positive MSM |
| Efficacy of HCV treatment | 90% | 30 | |
| Spontaneous clearance probability for HCV in HIV-negative MSM | 0.25 | 8 | |
| Odds ratio for spontaneous clearance probability for HCV in HIV-positive MSM compared with HIV-negative MSM | 0.68 | 9 | |
| Transmission factor for HIV | Fit | 29 | Model calibrated to a 5% HIV prevalence among MSM |
| Factor increase in HIV infectiousness during acute HIV phase compared with chronic HIV | 26 | 21 | |
| Duration in months of acute HIV phase of infection | 2.9 | 21 | |
| Relative transmissibility of HIV infection when on ART treatment compared with untreated HIV | 0.1 | 23,45 | |
| Percentage of diagnosed MSM on ART treatment | 83.2% | 30 | |
| Diagnosis rate of HIV | 1/3.2 years | 29 | Modelling approach to back-calculate diagnosis rates for HIV, range for 2010 |
| Factor increase in HCV infectiousness due to HIV co-infection | 2.35 | 10,11 | See Supplementary material for details |
| Percentage of MSM who mix non-randomly with MSM they assume have the same HIV status as themselves. Other MSM mix randomly | 35.2% | EMIS | See Supplementary material for details |
| Consistency of condom use between an HIV-diagnosed MSM and an assumed HIV-positive partner in latest sex act | 13% | EMIS | See Supplementary material for details |
| Consistency of condom use between all other MSM sexual pairings in latest sex act | 68% | EMIS | See Supplementary material for details |
| Efficacy of condoms per sex act | 70% | 47 | |
| Chance of error when evaluating HIV status of a sexual partnera | 24.9% | EMIS | See Supplementary material for details |
| Percentage of individuals in the low-risk sexual behaviour groupa | 82.2% | EMIS | EMIS data used to split the population into low- and high-risk, based on a number of partners for anal intercourse > 15 or < 15 in past year |
| Percentage of individuals in the high-risk sexual behaviour groupa | 1-Low | EMIS | |
| Mean number of sex partners for anal intercourse (when heterogeneity is turned on, low- and high-risk groups in brackets)a | 7.4 [2.9, 29.1] | EMIS | See Supplementary material for details |
| Increased overall risk ratio of HIV and HCV transmission due to injecting drugs and fisting between low- and high-risk groupa | 2.7 | EMIS [6,13–15] | See Supplementary material for details |
| Mixing parameter for choosing partners by risk behaviour categorya | 0.2 | EMIS | See Supplementary material for details |
See Supplementary material for details, available at IJE online.
Table 2.
Parameterization of the scenarios with point values shown for each model parameter, showing values used for different sub-scenarios in Figures 1 and 4, with range in [ ] being the +/-100% range used in Figures 2,3 and 5
| Scenario | Description | HCV mono-infection excess annual death rate | Co-infection excess annual death rate without HAART | Co-infection excess annual death rate on HAART | Risk ratio for HCV infectivity if HIV+ compared with if HIV- | High-/low- risk partner ratio | High-/low- risk fisting/IDU risk ratio | Proportion MSM mixing by HIV status | Error in HIV status judgements of sex partners | Ratio difference in condom use between assumed seroconcordant HIV+ MSM partnerships and all other MSM partnerships | Proportion MSM mixing by risk status | Risk ratio of spontaneous clearance if HIV+ compared with HIV- |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Baseline | No effects present | 0 | 0.089 (HIV-related death rate) | 0.0258 (HIV-related death rate) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| 2. Biological factors | HCV death rates, HCV spontaneous clearance and infectivity are dependent on HIV status | 0.0014 | 0.0925 | 0.0282 | 2.35 [1, 3.7] | 1 | 1 | 0 | 0 | 1 | 0 | 0.68 [1, 0.36] |
| 3. Mixing by HIV status | Biological factors with MSM preferentially selecting partners by HIV status, and sub-scenario with less condom use in assumed HIV+ pairingsc plus error in judgements of HIV status | 0.0014 | 0.0925 | 0.0282 | 2.35 [1, 3.7] | 1 | 1 | 35.2% [0%, 70.4%] | 24.9% | 1 or 5c [1, 9] | 0 | 0.68 [1, 0.36] |
| 4. Heteroge-neity in sexual risk behaviour | Biological factors with more sexual partners among high-risk MSM. Sub-scenarios consider effects of MSM selecting partners based on risk behavioura and including risk from fisting or IDUb | 0.0014 | 0.0925 | 0.0282 | 2.35 [1, 3.7] | 10.0 [1,20.0] | 1 or 2.7b [1, 4.4] | 0 | 0 | 1 | 0 or 0.2a[0,0.4] | 0.68 [1, 0.36] |
| 5. All effects | All the effects from the other scenarios | 0.0014 | 0.0925 | 0.0282 | 2.35 [1, 3.7] | 10.0 [1, 20.0] | 2.7 [1, 4.4] | 35.2% [0%, 70.4%] | 24.9% [0%, 49.8%] | 5 [1, 9] | 0.2 [0, 0.4] | 0.68 [1, 0.36] |
Sub-scenarios within the main scenarios take the parameter values corresponding to the values given by a,b and c in the table where relevant.
IDU, injecting drug use.
Model fitting and scenarios
For each different behavioural and biological risk factor scenario described below and in Table 2, the model was calibrated to a stable HIV and HCV prevalence. The model was run with a non-least squares fitting algorithm which took point values of all parameters relevant to the scenario (shown in Table 2), except the transmission parameters for HCV and HIV which were used to fit the simulation. We calibrated the model to a 5% HIV prevalence among MSM29 and a chronic HCV prevalence of 10% among HIV-infected MSM.5 This approach gives a simplified characterization of the HIV and HCV epidemic among MSM in the UK. We did not fit the HCV prevalence among HIV-uninfected MSM. Instead, we explored the effect of the different scenarios on the HCV prevalence among HIV-uninfected MSM, while assuming a 10% HCV prevalence among HIV-infected MSM. The scenarios are as follows.
Baseline. No effect of HIV infection on HCV progression, transmissibility or spontaneous clearance; no heterogeneity in sexual risk behaviour or HIV preferential mixing among MSM.
Biological factors only. Infection with HIV reduces HCV spontaneous clearance probability, increases HCV-related mortality and increases HCV infectivity.
Mixing by HIV status with biological factors. MSM select partners preferentially based on HIV status with errors in judgement, with an additional sub-scenario assuming less condom usage among partnerships where HIV-diagnosed individuals think their partner is also HIV-positive (irrespective of whether right or not). Biological factors included as above.
Heterogeneity in sexual risk behaviour with biological factors. Heterogeneity in sexual risk behaviour based on number of partners. Two additional sub-scenarios further assume that: (a) MSM select partners preferentially based on risk group; or (b) MSM select partners preferentially based on risk and assume further elevated transmission risk associated with high-risk MSM based on their higher prevalence of injecting drugs and fisting. Biological factors included as above.
All factors. Mixing is by HIV status and heterogeneity in sexual risk included as described above, with all associated effects from previous scenarios. Biological factors included as above.
Model analyses and sensitivity analyses
Impact on the HCV ratio: to explore the impact of these scenarios on the HCV relative burden among HIV-positive MSM, we define the HCV ratio as the chronic prevalence of HCV in HIV-positive MSM divided by the chronic prevalence of HCV in HIV-negative MSM. We first use point values for each parameter (Table 2) and assess whether each scenario produces an HCV ratio commonly observed in the UK and other settings (HCV ratio > 5).1,3,4,5 Then, to test the model’s sensitivity to parameter variation, for scenario 5 (all factors included) we undertook a univariate sensitivity analysis where we varied each scenario related parameter individually across +/-100% of their point value, and assessed the effect on the HCV ratio. These wide parameter uncertainty ranges were used to account for unknown biases and uncertainties in the data, with the same relative range being assumed for each parameter to see how each affected the results over the same relative range. We then performed bivariate sensitivity analyses on key parameters identified at the univariate level, quantifying their importance for three different levels of error in judgement of HIV status of sexual partners (-100%, 0% and +100% of point value).
Impact on HCV treatment-as-prevention initiatives: we explored the impact of HCV treatment-as-prevention for the different scenarios (Table 2). For each, we assessed the 10-year decrease in chronic HCV prevalence, among HIV-positive MSM and all MSM, achieved for an illustrative HCV treatment intervention that annually treated 10% of HIV-diagnosed HCV co-infected MSM. We assumed a 90% sustained viral response (SVR) with interferon-free direct acting antiviral therapy (DAA).30 By simultaneously sampling (5000 iterations) all the parameters varied for the univariate sensitivity analysis undertaken on scenario 5, we then considered the effect of variations in the HCV ratio on the impact of the illustrative HCV treatment-as-prevention strategy. Last, for scenario 5 (all factors), we individually varied key parameters across +/-100% of the point value, to assess their influence on the reduction in chronic HCV prevalence achieved with treatment.
Results
HCV ratio analysis
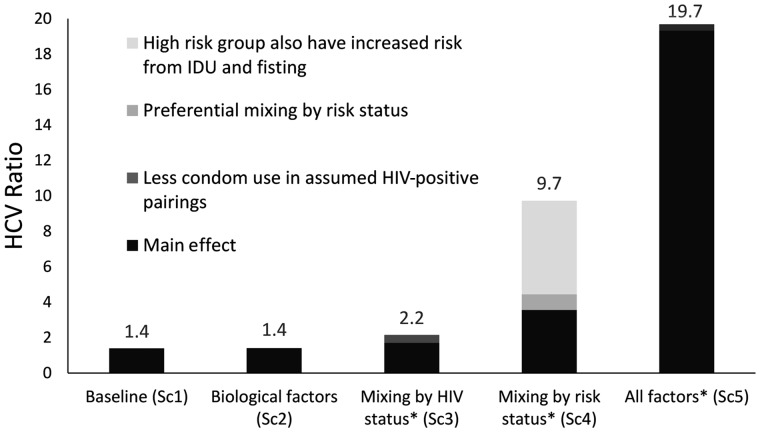
Model projections of the HCV ratio for the different scenarios in Table 2 are shown in Figure 1. If no biological or behavioural factors are included (scenario 1), the predicted HCV ratio is low but greater than one (1.39) due to MSM entering the model being susceptible to both diseases, so creating an increased proportion of HIV-negative MSM without HCV. Including biological factors only (scenario 2) marginally elevates the HCV ratio (1.41) because the greater HCV transmissibility in HIV-HCV co-infected MSM increases HCV transmission in both HIV-negative and HIV-positive MSM. Similarly, including preferential mixing by HIV status (scenario 3) cannot reproduce the high HCV ratio observed in the UK (HCV ratio of 5–20), with modelling projecting an HCV ratio of 1.7, which increases to 2.2 with inclusion of lower condom use in partnerships where HIV-diagnosed individuals assume their partner is HIV-positive.
Figure 1.
Modelled HCV ratio (ratio of HCV chronic prevalence among HIV-positive compared with HIV-negative MSM) for various scenarios incorporating different biological and/or behavioural factors as detailed in Table 2. *These scenarios also include the biological factors. IDU, injecting drug use; SC, scenario.
In contrast, higher and more commonly observed HCV ratios (> 5) are achieved through including heterogeneity in sexual risk behaviour (scenario 4). For instance, stratifying MSM into low- and high-risk groups based on the number of sexual partners, with greater injecting drug use and fisting among high-risk individuals, and preferential mixing between these groups produces an HCV ratio of 9.7.
Last, combining all behavioural and biological factors (scenario 5) amplifies the HCV ratio to 19.7, with different factors acting synergistically to transmit HCV among HIV-positive MSM but not HIV-negative MSM.
Univariate and bivariate sensitivity analyses on the HCV ratio
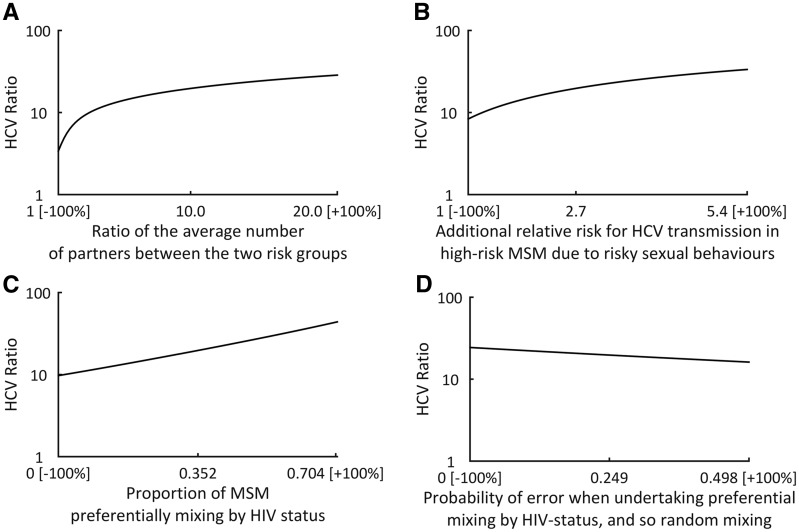
Univariate variations of parameters in scenario 5 around their point values (+/-100%; Table 2) identified four key parameters that have most effect on the HCV ratio (Figure 2): (i) proportion of individuals preferentially mixing by HIV status (HCV ratio varies from 9.7 to 43.9); (ii) error in HIV status judgements (HCV ratio varies 16.2–24.4); (iii) ratio difference in numbers of partners between low- and high-risk MSM groups (HCV ratio varies 3.4–28.6); and (iv) additional relative risk for HCV transmission in high-risk MSM due to risky sexual behaviours (HCV ratio varies 8.3–33.5). Parameters that did not affect the HCV ratio as much are shown in Supplementary Figure S3, available as Supplementary data at IJE online.
Figure 2.
Effect of univariate changes in individual parameters (A-D) on the HCV ratio for the ‘all effects’ scenario 5. All other parameters are set to their point values in Table 2. Only those parameters that markedly affect the HCV ratio are shown. Numbers shown on x axis are -100% of the point value, the point value and +100% of point value.
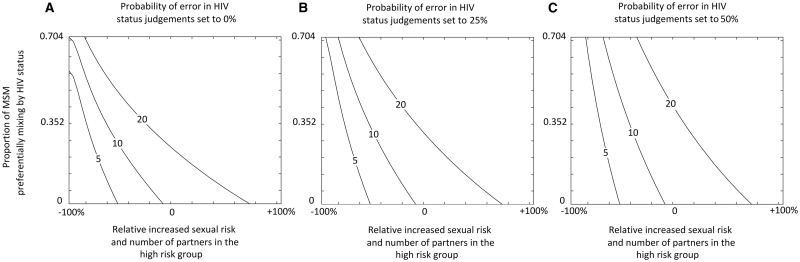
The bivariate sensitivity analysis explored the relationship between the four most influential parameters from the univariate analysis (Figure 3). The two risk heterogeneity parameters were varied simultaneously, forming one combined measure. The HCV ratio is sensitive to levels of heterogeneity in sexual risk behaviour and preferential mixing by HIV status, which amplify each other. Indeed, the figures illustrate that HCV ratios of 5–20 are possible with high levels of risk heterogeneity alone, or moderate levels of both preferential mixing by HIV status and risk heterogeneity with any level of error in HIV status judgements. Greater error in HIV status judgements dampens the HCV ratio.
Figure 3.
Contour maps showing how the HCV ratio (contour lines produced by the ‘all effects’ scenario 5) is affected by both the level of HIV preferential mixing (y axis) and sexual risk heterogeneity (x axis) for three levels of error in judging the HIV status of a sexual partner. (A) Zero error (e = 0%); (B) medium error (e = 25%); (C) and high error (e = 50%). All other parameters are set to their point values in Table 1. Sexual risk heterogeneity is the simultaneous variance of the ratio in the average number of partners between the low- and high-risk groups and the additional relative risk for HCV transmission in high-risk MSM due to risky sexual behaviours, from -100% to + 100% of their estimated point values, which vary respectively from 1 at -100% to 20.0 at + 100% and 1 at -100% to 5.4 at + 100%.
Impact of HCV treatment-as-prevention
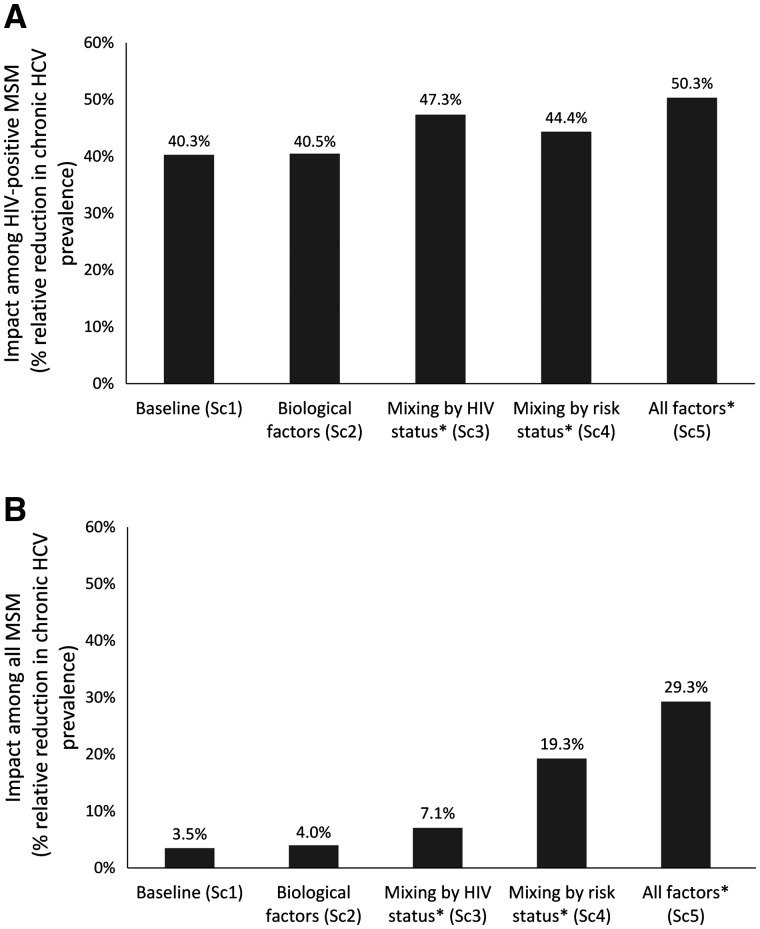
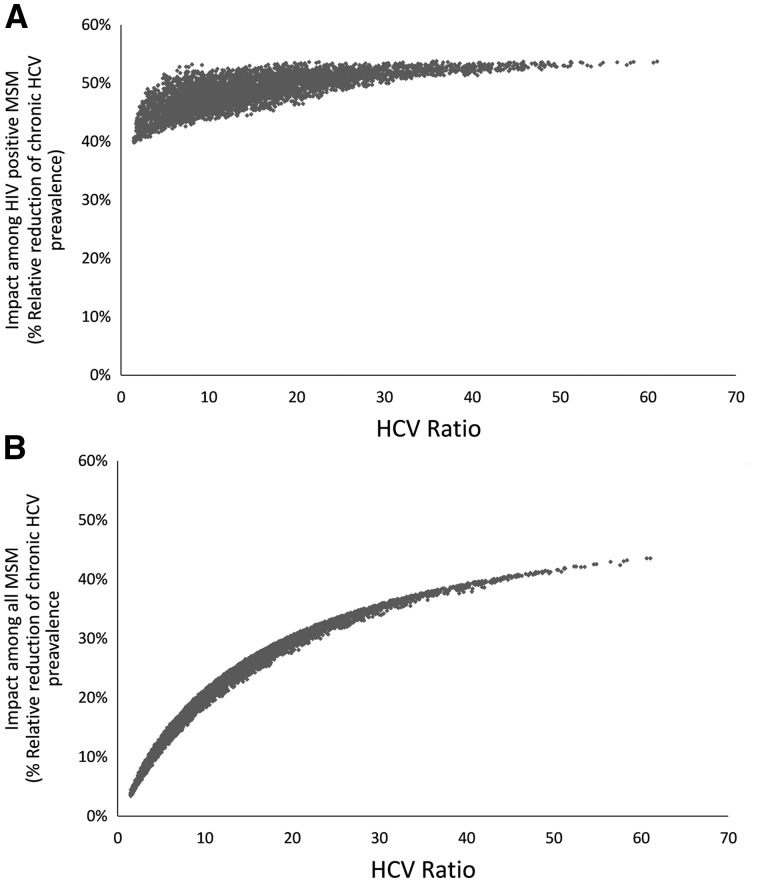
Annually treating 10% of HIV-diagnosed HCV co-infected MSM for HCV over 10 years reduces HCV chronic prevalence among HIV-positive MSM by a relative 40.3-50.3% across the different scenarios (Figure 4a). However, impact among the entire MSM population varies markedly, from a relative reduction in chronic HCV among MSM of 3.5% for scenario 1 to 29.3% for scenario 5 (Figure 4b). Figure 5 illustrates this effect further with the HCV ratio having a relatively small influence on the HCV treatment-as-prevention impact among HIV-positive MSM (Figure 5a), but a large influence among all MSM (Figure 5b). At higher HCV ratios, more of the epidemic is concentrated among HIV-positive MSM, so focusing treatment efforts on this population effectively combats the epidemic among all MSM. Univariate variations in parameters that have a large effect on the HCV ratio also affect the impact of HCV treatment among HIV-positive MSM on the overall HCV epidemic (Supplementary Figures S4 and S5, available as Supplementary data at IJE online).
Figure 4.
Impact of HCV treatment on the relative reduction in HCV chronic prevalence (%) among: (A) HIV-positive MSM; and (B) all MSM, achieved by treating 10% of HIV-diagnosed MSM with HCV per year for 10 years. Projections assume the point value of parameters for each scenario in Table 1 and assume 90% HCV treatment efficacy. *These scenarios also include the biological factors. SC, scenario.
Figure 5.
Effect of variations in the HCV ratio on the impact of HCV treatment-as-prevention (% relative reduction in chronic HCV prevalence at 10 years when treating at a rate of 10% of HIV-diagnosed HCV co-infected MSM annually, y axis) among: (A) HIV-positive MSM; and (B) all MSM. We assume 90% HCV treatment efficacy, and uniformly sampled other parameters randomly between +/-100% of their point values.
Discussion
We find that biological factors alone (lower spontaneous clearance rate and higher HCV infectivity and mortality among HIV-infected MSM) are unable to explain why the HCV epidemic is concentrated among HIV-positive MSM. Instead, we suggest that behavioural factors (heterogeneity in sexual risk behaviour alone or combined with preferential mixing by HIV status) are highly likely to account for the higher HCV burdens among HIV-positive MSM. Thus, HCV infection and co-infection should be seen as a marker of high sexual risk behaviours, which are preferentially undertaken within partnerships with other HIV-positive MSM.12 This is likely to have been aided by the scale-up of effective HIV treatment improving the survival of higher-risk MSM, paired with possible increases in risk behaviour due to ‘treatment optimism’.31
Importantly, these results highlight the possibility that changes in sexual behaviour or mixing patterns could reshape the HCV epidemic. For example, decreases in preferential mixing by HIV status could occur due to reductions in perceived risk resulting from widespread ART or pre-exposure prophylaxis (PrEP) use reducing HIV infectivity and susceptibility, which could increase HCV transmission among HIV-negative MSM. Alternatively, fewer high-risk MSM acquiring HIV (due to PrEP) might also raise the likelihood of HCV transmission among HIV-negative MSM, although this may be offset by increased HCV monitoring of MSM being prescribed PrEP.
Further, HCV treatment-as-prevention initiatives among HIV-diagnosed MSM will have greatest impact on overall levels of HCV transmission in settings where HCV is concentrated among HIV-positive MSM, as less of the epidemic is driven by HIV-negative MSM. Conversely, settings which have or develop a greater burden of HCV among HIV-negative MSM would also need to focus HCV treatment on the HIV-negative MSM.
Limitations
Our analysis has a number of limitations. First, we used a simplified model of HCV and HIV transmission and ART that was calibrated approximately to the UK without recreating historical epidemic trends, which suggest a slowly increasing HIV and HCV epidemic.5,29 This was done because our intention was to explore qualitatively how behavioural and biological factors contribute to HCV epidemic patterns, not make detailed predictions about the epidemics’ trajectory. Importantly, this simplification should not affect the degree to which HCV propagates preferentially among HIV-positive MSM. A further simplification of our model involved the incorporation of injecting drug use-related risk as an increased transmission risk among a subset of MSM,6,13-15 instead of explicitly modelling injecting. We made this simplification because, although injecting drug use is a risk factor for HIV/HCV acquisition among MSM, it is unclear the degree to which this is due to injecting drug use itself or co-occurring high-risk sexual behaviours. Also, datasets such as EMIS only ask basic questions about undertaking injecting drug use in the past year, so preventing any explicit modelling of its role in HCV transmission among MSM.
Second, there exists uncertainty in our parameters and variation across settings, most notably amongst those related to self-reported behavioural data. We performed extensive sensitivity and scenario analyses to explore the effect of varying different behavioural factors. As such, our analyses form a platform from which to explore how variations in parameter assumptions affect observed epidemic patterns and treatment-as-prevention impact. However, care should be taken in generalizing our results to non high-income settings where limited data suggest lower HCV-co-infection prevalences among MSM,32 and where differences in sexual behaviour and the underlying HIV and HCV epidemic are likely to heavily effect the HCV epidemic that occurs.
Third, although parameterizing our model to EMIS-UK data produced realistic projections for the HCV ratio (∼20), we advise caution regarding potential over-interpretation of the quantitative accuracy of our model. For instance, the model did not incorporate all sources of HCV infection, such as among migrants with historic HCV infection. Conversely, compared with a national probability survey on sexual behaviours, the EMIS-UK dataset used to parameterize our model was biased towards higher-risk MSM due to their web-based convenience sampling approach,34 as well as MSM with higher education levels.34 These MSM may have a greater interest in HIV prevention and so increased propensity to mix preferentially by HIV status and use condoms with perceived sero-discordant partners.
Finally, our estimate for the increased HCV infectiousness among HIV-positive MSM is uncertain, although data from vertical transmission studies11 suggest our assumption is reasonable. We assume that higher HCV viral load in blood samples among HIV-positive MSM translates to increased infectivity,10 but this may not be the case. However this should not be a concern, because this parameter had little effect on the resulting model projections.
Comparison with other publications
To our knowledge, this is the first modelling analysis of the joint epidemics of HIV and HCV among MSM, although numerous previous analyses have modelled just HIV17 and some have also modelled other sexually transmitted infections (STI) among MSM.17,34-37 However, existing HIV and STI co-infection models generally considered different questions, focusing primarily on the degree to which STIs contribute to HIV transmission and the possible impact of STI treatment on HIV epidemics. Previous analyses have also modelled the transmission of HIV and HCV among people who inject drugs.38-42 Importantly, existing work by our group and others has modelled the HCV epidemic among HIV-diagnosed MSM, and evaluated the impact of scaling up HCV treatment in this group.5,43 These studies were limited because they did not explicitly include HIV transmission. Our new analysis supports findings of these previous two studies by indicating that scaling up HCV treatment among HIV-positive MSM could have substantial prevention benefits among HIV-positive MSM.5 Additionally, it extends previous work by dynamically modelling the transmission of HCV to and from the HIV-negative population, assessing how different behavioural and biological factors could result in the observed epidemic patterns, and evaluating the implications for HCV treatment-as-prevention.
Concluding remarks
Overall, our work indicates that sexual risk behaviour heterogeneity and HIV preferential mixing likely explain why the HCV epidemic amongst MSM is strongly concentrated among HIV-positive MSM, with HCV co-infection possibly signifying high-risk behaviours as suggested by others.15 Targeted HCV treatment-as-prevention among HIV-diagnosed MSM could be an important tool for combating the current HCV epidemic, but will have less impact in settings which have or develop a substantial burden of HCV among HIV-negative MSM. This could occur if sexual risk behaviours increase among HIV-negative MSM or if higher-risk MSM do not become HIV-infected as readily. This underscores the importance of monitoring HCV among HIV-negative MSM, to assess any shifts in the patterns of the HCV epidemic.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by a research grant from Gilead Sciences. Gilead had no influence on the design, analysis or content of the study. This work was additionally supported by the National Institute for Drug Abuse [grant number R01 DA037773-01A1] to N.M. and P.V.; University of California San Diego Center for AIDS Research(CFAR), a National Institute of Health (NIH) funded programme [grant number P30 AI036214] to N.M.; National Institute for Health Research Health Protection Research Units (NIHR HPRU) in Evaluation of Interventions at University of Bristol to P.V. and M.H.; and HPRU in STIs and BBVs at London School of Hygiene and Tropical Medicine to P.V., F.H. and P.W. The Bill and Melinda Gates Foundation funded HIV modelling consortium to P.V. EMIS was funded by a grant of the European Commission under the EU Health Programme 2008-13. Further funding was received from the Department of Health for England. The views expressed are those of the author(s) and not necessarily those of the UK NHS, the UK NIHR or the UK Department of Health, the European Union or the EMIS Steering Group.
Supplementary Material
Acknowledgments
Author Contributions
P.V. and N.K.M. designed the study. C.M. undertook preliminary model analyses. L.M. undertook the statistical analyses, model development, simulations and analyses. P.V., N.K.M. and L.M. wrote the first draft of the article. L.M., C.M., F.H., P.W., M.H., N.K.M. and P.V. interpreted the data, edited the article and approved the final version.
Conflict of interest: NKM and PV have received research grants from Gilead, and NKM has received honoraria from Merck, AbbVie, and Gilead. LM has nothing to report.
Key Messages
Biological factors alone do not explain why the HCV epidemic is strongly concentrated among HIV-positive MSM.
Sexual behavioural risk heterogeneity and HIV preferential mixing among sexual partners is likely to explain this observation. Changes in sexual mixing patterns could reshape the epidemic.
Targeted HCV treatment-as-prevention amont HIV-diagnosed MSM could be an important tool for combating the current HCV epidemic, but will have less impact in settings which have or develop a substantial burden of HCV among HIV-negative MSM, underscoring the importance of HCV monitoring in this population.
References
- 1. Bradshaw D, Matthews G, Danta M.. Sexually transmitted hepatitis C infection: the new epidemic in MSM? Curr Opin Infect Dis 2013;26:66–72. [DOI] [PubMed] [Google Scholar]
- 2. Smith CJ, Ryom L, Weber R. et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014;384:241–48. [DOI] [PubMed] [Google Scholar]
- 3. Yaphe S, Bozinoff N, Kyle R, Shivkumar S, Pai NP, Klein M.. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect 2012;88:558–64. [DOI] [PubMed] [Google Scholar]
- 4. Price H, Gilson R, Mercey D. et al . Hepatitis C in men who have sex with men in London - a community survey. HIV Med 2013;14:578–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin NK, Thornton A, Hickman M. et al. Can hepatitis C virus (HCV) direct-acting antiviral treatment as prevention reverse the HCV epidemic among men who have sex with men in the United Kingdom? Epidemiological and modeling insights. Clin Infect Dis 2016;62:1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danta M, Brown D, Bhagani S. et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS 2007;21:983–91. [DOI] [PubMed] [Google Scholar]
- 7. van de Laar TJ, Matthews GV, Prins M, Danta M.. Acute hepatitis C in HIV-infected men who have sex with men: an emerging sexually transmitted infection. AIDS 2010;24:1799–812. [DOI] [PubMed] [Google Scholar]
- 8. Micallef JM, Kaldor JM, Dore GJ.. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 2006;13:34–41. [DOI] [PubMed] [Google Scholar]
- 9. Grebely J, Raffa JD, Lai C, Krajden M, Conway B, Tyndall MW.. Factors associated with spontaneous clearance of hepatitis C virus among illicit drug users. Can J Gastroenterol 2007;21:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas DL, Astemborski J, Vlahov D. et al. Determinants of the quantity of hepatitis C virus RNA. J Infect Dis 2000;181:844–51. [DOI] [PubMed] [Google Scholar]
- 11. Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ.. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis 2014;59:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eaton LA, Kalichman SC, O'Connell DA, Karchner WD.. A strategy for selecting sexual partners believed to pose little/no risks for HIV: serosorting and its implications for HIV transmission. AIDS Care 2009;21:1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koblin BA, Husnik MJ, Colfax G. et al. Risk factors for HIV infection among men who have sex with men. AIDS 2006;20:731–39. [DOI] [PubMed] [Google Scholar]
- 14. Macdonald N, Elam G, Hickson F. et al. Factors associated with HIV seroconversion in gay men in England at the start of the 21st century. Sex Transm Infect 2008;84:8–13. [DOI] [PubMed] [Google Scholar]
- 15. Marcellin F, Lorente N, Demoulin B. et al. Comparison of risk factors in HIV-infected men who have sex with men, co-infected or not with hepatitis C virus (ANRS VESPA2 French cross-sectional national survey). Sex Transm Infect 2015;91:21–23. [DOI] [PubMed] [Google Scholar]
- 16. Punyacharoensin N, Edmunds WJ, De Angelis D, White RG.. Mathematical models for the study of HIV spread and control amongst men who have sex with men. Eur J Epidemiol 2011;26:695–709. [DOI] [PubMed] [Google Scholar]
- 17. Baggaley RF, White RG, Boily MC.. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol 2010;39:1048–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vanhommerig JW, Lambers FA, Schinkel J. et al. Risk factors for sexual transmission of hepatitis C virus among human immunodeficiency virus-infected men who have sex with men: a case-control study. Open Forum Infect Dis 2015;2:ofv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wandeler G, Schlauri M, Jaquier ME. et al. Incident hepatitis C virus infections in the Swiss HIV Cohort Study: changes in treatment uptake and outcomes between 1991 and 2013. Open Forum Infect Dis 2015;2:ofv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Witt MD, Seaberg EC, Darilay A. et al. Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984-2011. Clin Infect Dis 2013;57:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hollingsworth TD, Anderson RM, Fraser C.. HIV-1 transmission, by stage of infection. J Infect Dis 2008;198:687–93. [DOI] [PubMed] [Google Scholar]
- 22. Anglemyer A, Rutherford GW, Baggaley RC, Egger M, Siegfried N.. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev 2011:CD009153. [DOI] [PubMed] [Google Scholar]
- 23. Rodger AJ, Cambiano V, Bruun T. et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016;316:171–81. [DOI] [PubMed] [Google Scholar]
- 24. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action: Concerted Action on SeroConversion to AIDS and Death in Europe. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Lancet 2000;355: 1131–37. [PubMed] [Google Scholar]
- 25. May MT, Gompels M, Delpech V. et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014;28:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vickerman P, Martin N, Turner K, Hickman M.. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction 2012;107:1984–95. [DOI] [PubMed] [Google Scholar]
- 27. Thein HH, Yi Q, Dore GJ, Krahn MD.. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 2008;22:1979–91. [DOI] [PubMed] [Google Scholar]
- 28. Weatherburn P, Schmidt AJ, Hickson F. et al. The European Men-Who-Have-Sex-With-Men Internet Survey (EMIS): Design and methods. Sex Res Soc Policy 2013;10:243–57. [Google Scholar]
- 29. Birrell PJ, Gill ON, Delpech VC. et al. HIV incidence in men who have sex with men in England and Wales 2001-10: a nationwide population study. Lancet Infect Dis 2013;13:313–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang HJ, Ryoo JY, Yoo BK.. Meta-analysis of the efficacy and safety of sofosbuvir for the treatment of hepatitis C virus infection. Int J Clin Pharm 2015;37:698–708. [DOI] [PubMed] [Google Scholar]
- 31. Crepaz N, Hart TA, Marks G.. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA 2004;292:224–36. [DOI] [PubMed] [Google Scholar]
- 32. Platt L, Easterbrook P, Gower E. et al . Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016;16:797–808. [DOI] [PubMed] [Google Scholar]
- 33. Prah P, Hickson F, Bonell C. et al. Men who have sex with men in Great Britain: comparing methods and estimates from probability and convenience sample surveys. Sex Transm Infect 2016;92:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gray RT, Hoare A, Prestage GP, Donovan B, Kaldor JM, Wilson DP.. Frequent testing of highly sexually active gay men is required to control syphilis. Sex Transm Dis 2010;37:298–305. [DOI] [PubMed] [Google Scholar]
- 35. Gray RT, Hoare A, McCann PD. et al. Will changes in gay men's sexual behavior reduce syphilis rates? Sex Transm Dis 2011;38:1151–58. [DOI] [PubMed] [Google Scholar]
- 36. Hoare A, Gray RT, Wilson DP.. Could implementation of Australia's national gay men's syphilis action plan have an indirect effect on the HIV epidemic? Sex Health 2012;9:144–51. [DOI] [PubMed] [Google Scholar]
- 37. Escobar E, Durgham R, Dammann O, Stopka TJ.. Agent-based computational model of the prevalence of gonococcal infections after the implementation of HIV pre-exposure prophylaxis guidelines. Public Health Inform 2015;7:e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwon JA, Iversen J, Maher L, Law MG, Wilson DP.. The impact of needle and syringe programs on HIV and HCV transmissions in injecting drug users in Australia: a model-based analysis. J Acquir Immune Defic Syndr 2009;51:462–69. [DOI] [PubMed] [Google Scholar]
- 39. Bayoumi AM, Zaric GS.. The cost-effectiveness of Vancouver's supervised injection facility. CMAJ 2008;179:1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vickerman P, Platt L, Hawkes S.. Modelling the transmission of HIV and HCV among injecting drug users in Rawalpindi, a low HCV prevalence setting in Pakistan. Sex Transm Infect 2009;85(Suppl 2):ii23–30. [DOI] [PubMed] [Google Scholar]
- 41. Vickerman P, Martin NK, Hickman M.. Understanding the trends in HIV and hepatitis C prevalence amongst injecting drug users in different settings - implications for intervention impact. Drug Alcohol Depend 2012;123:122–31. [DOI] [PubMed] [Google Scholar]
- 42. Vickerman P, Martin NK, Roy A. et al. Is the HCV-HIV co-infection prevalence amongst injecting drug users a marker for the level of sexual and injection related HIV transmission? Drug Alcohol Depend 2013;132:172–81. [DOI] [PubMed] [Google Scholar]
- 43. Salazar-Vizcaya L, Kouyos RD, Zahnd C. et al. Hepatitis C virus transmission among HIV-infected men who have sex with men: Modeling the effect of behavioral and treatment interventions. Hepatology 2016;64:1856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amin J, Law MG, Bartlett M, Kaldor JM, Dore GJ.. Causes of death after diagnosis of hepatitis B or hepatitis C infection: a large community-based linkage study. Lancet 2006;368:938–45. [DOI] [PubMed] [Google Scholar]
- 45. Cohen MS, Chen YQ, McCauley M. et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375:830–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nakagawa F, Lodwick RK, Smith CJ. et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS 2012;26:335–43. [DOI] [PubMed] [Google Scholar]
- 47. Smith DK, Herbst JH, Zhang X, Rose CE.. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2015;68:337–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.