Abstract
Evidence generated from randomized controlled trials forms the foundation of cardiovascular therapeutics and has led to the adoption of numerous drugs and devices that prolong survival and reduce morbidity, as well as the avoidance of interventions that have been shown to be ineffective or even unsafe. Many aspects of cardiovascular research have evolved considerably since the first randomized trials in cardiology were conducted. In order to be large enough to provide reliable evidence about effects on major outcomes, cardiovascular trials may now involve thousands of patients recruited from hundreds of clinical sites in many different countries. Costly infrastructure has developed to meet the increasingly complex organizational and operational requirements of these clinical trials. Concerns have been raised that this approach is unsustainable, inhibiting the reliable evaluation of new and existing treatments, to the detriment of patient care. These issues were considered by patients, regulators, funders, and trialists at a meeting of the European Society of Cardiology Cardiovascular Roundtable in October 2015. This paper summarizes the key insights and discussions from the workshop, highlights subsequent progress, and identifies next steps to produce meaningful change in the conduct of cardiovascular clinical research.
Keywords: Clinical trials, Pragmatic clinical trials, Randomized controlled trials, Cardiovascular diseases
Introduction
Randomized controlled trials generate evidence on the benefits and harms of therapeutic interventions. Regulations and guidelines that govern clinical trials are intended to protect the rights, safety and wellbeing of the study participants and to provide assurance that the evidence generated can be relied on for individual patient care and the broader public health. However, there are concerns that these objectives are not being met due to significant problems with the interpretation and implementation of current regulations and guidelines.1–5 Moreover, the over-interpretation of research governance requirements has inhibited methodological and technological innovation that could enhance the quality of cardiovascular trials. Moulding research to fit existing rules may not always be appropriate; instead regulations need to be flexible and allow proportionate approaches for each trial.6,7
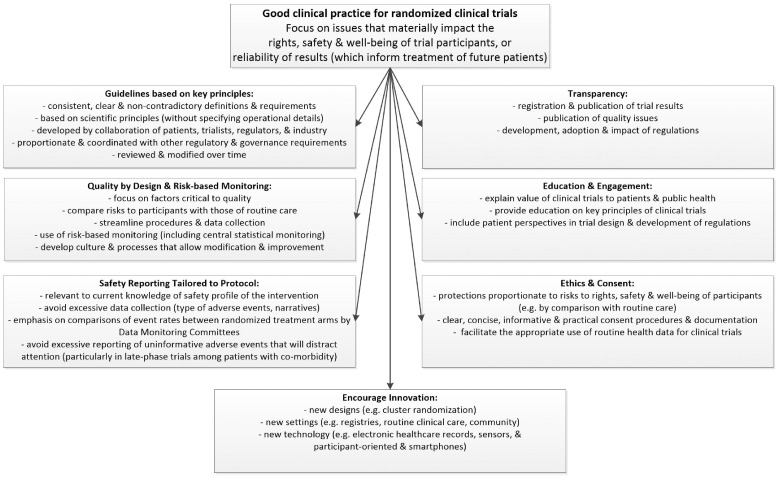
The Cardiovascular Round Table of the European Society of Cardiology (ESC) convened a workshop to engender dialogue about improving the regulation and governance of clinical trials. Representatives from groups interested in clinical cardiovascular research (including patients, clinicians, regulators, funders, and trialists) collaborated to generate recommendations for optimal research and regulatory methods that would support rapid, reliable, and cost-effective evidence generation, while protecting the safety of clinical trial participants (see Figure 1).
Figure 1.
Key elements of Good Clinical Practice for randomized clinical trials.
Research governance challenges facing clinical trials
The International Council for (formerly Conference on) Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice E6 (ICH-GCP) guideline was finalised in 1996 and has become established as the standard for the conduct of clinical trials worldwide.8 Developed by a select group of regulatory authorities and organizations representing the pharmaceutical industry (but without any input from non-commercial trialists or patient advocates), it was intended to provide consistency in the requirements for clinical trials conducted to support regulatory evaluations of new drugs across multiple countries. The guideline was not aimed at other types of clinical trials, such as non-registration trials, non-interventional studies, or trials of non-pharmacological interventions. However, it has been applied and, indeed, even mandated well beyond its original remit. For example, the European Union’s (EU) new Clinical Trials Regulation requires that trial sponsors and investigators take account of ICH-GCP in all clinical trials of any medicinal product.9 Similarly, the Gates Foundation requires grantees to adhere to ICH-GCP, even when they are conducting clinical trials in resource poor settings that are not intended for registration.10
Recently, ICH has acknowledged some of the problems with the GCP guideline11 and initiated a public consultation on an E6 (R2) integrated addendum in 2015. Following comments from ESC and many other organizations interested in clinical trials,12 ICH released a modified version in November 2016 for adoption and implementation.13 However, concerns remain that this revision does not address fundamental problems with the ICH-GCP guideline and does not correct errors and inconsistencies in the original text (see Table 1).14–16 ICH has also announced its intention to conduct a more substantial overhaul of guidelines that relate to GCP and clinical trial design, and published a reflection paper outlining their plans in January 2017.17
Table 1.
Examples of unclear, inconsistent and contradictory definitions within ICH-GCP (E6)
| Term | ICH-GCP definition | Concern |
|---|---|---|
| Adverse event | ‘Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment…’ | Implies that those not administered a pharmaceutical product (e.g. control group) cannot have adverse events |
| Adverse drug reaction | ‘…All noxious and unintended responses to a medicinal product related to any dose should be considered adverse drug reactions. The phrase responses to a medicinal product means that a causal relationship between a medicinal product and an adverse event is at least a reasonable probability, i.e. the relationship cannot be ruled out’ | The meaning of ‘is at least a reasonable probability’ is very different from ‘cannot be ruled out’ |
| Serious adverse event or serious adverse drug reaction | ‘Any untoward medicinal occurrence that at any dose results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, or is a congenital anomaly/birth defect’ | This is intended to define what is meant by ‘serious’. However, the text is confusing and can be interpreted as suggesting that Serious Adverse Event and Serious Adverse Reaction are synonymous. |
| Sponsor | ‘An individual, company, institution, or organization which takes responsibility for the initiation, management, and/or financing of a clinical trial.’ | Not consistent with other regulations:
|
| Note: EMA and FDA are both members of ICH | ||
Greater emphasis on the key scientific principles (e.g. maintaining the integrity of the randomization process, adherence to allocated study treatment, minimizing losses to follow-up) would have a greater impact on the quality of trial results than is achieved by the current focus on documentation and data checking in ICH-GCP,15,16 but these aspects are not included in the current addendum to E6 and are not a focus of GCP inspections by regulators.18 This failure can have serious detrimental effects; for example, it was found that researchers did not consider it to be critical to minimize losses to follow-up after randomization (which allows unbiased ‘intention-to-treat’ treatment comparisons) because it is not emphasized in ICH-GCP or included in ICH-GCP training.15
Quality assurance and risk-based monitoring
The ICH-GCP guideline is intended to ensure the credibility of clinical trial results. For example, it states that those responsible for the trial (i.e. the regulatory ‘sponsor’; which is not necessarily the funder) should ‘ensure that trials are adequately monitored’ and ‘determine the appropriate extent and nature of monitoring’, and it emphasizes that ‘in general there is a need for on-site monitoring’.8 These statements have been over-interpreted;18 consequently, site-based monitoring with extensive checking of source documentation is the prevailing method used in many trials and by many regulatory inspectors.18,19 On-site monitoring is amongst the most costly operational activities in a clinical trial,20 and there are serious concerns about its ability to detect important errors or improve quality, particularly of larger trials.21–26
Central statistical monitoring of trial-related data, in combination with targeted site monitoring informed by statistical analysis, has been proposed as a more effective and efficient method of detecting material errors during the conduct of a trial and identifying opportunities for improvement prospectively.26–28 Regulatory authorities, particularly in the US and Europe, have now issued guidance documents that focus on a risk-based approach to monitoring, emphasizing ‘quality-by-design’ concepts.29–31 The ICH-GCP Addendum includes similar language but the contradictory text in the original guideline remains.32 Widespread improvement seems unlikely unless consistency is achieved in the guidance across all regulatory agencies, as well as in the approach used by regulatory inspectors and those who conduct trial monitoring.
Safety reporting
A fundamental principle of clinical trials is the protection of clinical trial participants. However, the regulations and guidelines relating to safety reporting are unnecessarily complex and confusing, and frequently mis- or over-interpreted. Hence, important safety signals may get lost in the large volume of uninformative reports to regulatory authorities, ethics committees and investigators about adverse events.32 Recent EU and US legislation indicates that the nature and extent of adverse event reporting should be tailored to each trial protocol, and FDA guidance discourages excessive expedited adverse reaction reporting.9,33–35 However, this position is not well articulated in the ICH guidelines.36,37
In early phase trials of new treatments, rigorous ascertainment of adverse events is necessary37 but, as knowledge of the safety profile of the treatment increases, the level of adverse event recording should decrease.24 However, there is a widespread misunderstanding that it is required to record all non-serious adverse events even in late-stage trials of treatments when this may be neither scientifically justified nor required by regulators. Attempting to record information on all adverse events in a large late-stage trial may distract attention from systematic ascertainment of those serious health outcomes that might matter clinically and in public health terms.24,38,39 Furthermore, clinicians view excessive reporting activities (including the frequent demand from sponsors to provide detailed narrative descriptions for common events not believed to be related to the study treatment) as burdensome and a disincentive to participation, which may result in fewer, smaller trials and less reliable evidence to guide patient care.18
Much of the emphasis in clinical trial guidelines is on expedited reporting of individual serious adverse events that are believed to be due to the study treatment (‘reactions’) and not previously recognized as being caused by the treatment (‘unexpected’).36 There is good evidence that focus on these requirements, combined with the subjective nature of the attribution of adverse effects to the study treatment, can lead to excessive uninformative reporting.32 Reports of such suspected unexpected serious adverse reactions (SUSARs) only have to be expedited if they have occurred among patients who were allocated the active study drug, so it is hard to draw meaningful conclusions about causality. Attribution of individual suspected adverse reactions to a treatment is only likely to be a reliable source of evidence about causation when both the effect is large and the particular adverse event would be expected to occur rarely in the type of patient being studied.40,41 In all other circumstances, adverse events need to be compared collectively between the randomized treatment arms to determine their relationship to treatment.34,42 In on-going trials, such comparisons are best conducted by an unblinded Data Monitoring Committee (DMC), adequately firewalled from those responsible for conducting or overseeing the study in order to protect the integrity of the trial results.43,44
Despite introducing a new regulation that emphasised these points, a review conducted by the FDA’s Office of Hematology and Oncology Products found that there had been little improvement in the rate of expedited event reporting (with, if anything, an increase); only 14% of all such reports were considered to be appropriate, with the remainder not providing any useful information about the safety profile of the drug under investigation.32 Commercial sponsors have identified a lack of international harmonization, concerns about liability risks, and confusion about the rules for aggregated reporting as barriers to improving their adverse event reporting to regulatory authorities.45
Thus, although there have been advances in guidance about safety reporting issued by some regulatory authorities, modifications to ICH guidelines and the way that they are applied are clearly needed (see Figure 1). Changing guidance alone is unlikely to be sufficient; a more rational approach to safety monitoring will also need to be communicated widely and applied consistently by all involved—including trial sponsors, investigators, and regulatory authority reviewers, auditors, and inspectors—so that there is a change in the mind-set.
Promoting innovation
There is intense interest in the implementation of innovative clinical trial models for cardiovascular research. For example, many therapies for acute coronary syndromes have been developed in randomized effectiveness trials comparing a new treatment vs. the current standard treatment. Increasingly, randomized trials are using existing clinical infrastructure (including electronic healthcare records and registries)45–48 or collecting outcome information directly from patients (e.g. through smartphones and wearable sensors), without the involvement of a typical clinical research site. Overly cautious attitudes to innovation in trial design and the use of novel technologies may be the consequence of concerns about informed consent, privacy, information security, and data quality49 or uncertainty about whether such approaches will be accepted by regulators.50,51 However, it is important that clinical trial regulations (and the way in which they are interpreted and applied) keep pace with such innovation.52
Transparency
The public disclosure of clinical trial results ensures that the valuable contributions of study participants serve a meaningful purpose and advance the science and practice of medicine. Greater clinical trial transparency has been achieved through the use of clinical trial registries and requirements to report results.9 Although some trial funders and journal editors are keen to promote sharing of individual participant data,53–55 the potential benefits and challenges of doing so are the subject of ongoing debate.56–60 Access to patient-level data might offer opportunities for confirmatory or novel analyses, design of future trials, and methodological research. However, it also carries risks (for example, data-derived subgroup analyses may yield unreliable conclusions and lead to inappropriate treatment decisions) and opportunity costs (diverting resources away from new trials of cardiovascular treatments), so moves in this direction should be considered carefully.61,62
Education and engagement
The fundamental importance of conducting well-designed randomized trials in cardiovascular disease is often under-appreciated. Ensuring that the public, patients, physicians (particularly in medical school curricula or early career), and policy makers are better informed in the value and key principles of clinical trials is a priority. Such initiatives should emphasize both the value of integrating clinical trials into routine practice63,64 and the need to facilitate the reliable evaluation of existing treatments, some of which may not be as effective65 or safe66,67 as they are thought to be. Similarly, informing patients about the ways in which they can participate in clinical trials, the measures that are taken to ensure that their data are secure, and the value this information provides to the quality of care should help to reduce their concerns.
Patient advocacy groups can provide perspectives on disease or treatment burden and provide advice on the feasibility of specific aspects of a clinical trial, informing study design. Collaboration between patient groups and clinical trialists should be the norm rather than the exception. Likewise, patient perspectives should be included in the development of new guidelines and regulations, as has been done effectively in projects conducted by the FDA-funded Clinical Trial Transformation Initiative but is notably absent from ICH processes.
Ethics review and informed consent
The importance of ethics committees for the protection of the rights, safety and wellbeing of study participants is not a matter of debate. However, some of the other processes intended to achieve these protections are of questionable effectiveness or efficiency, especially for later phase studies of new drugs or pragmatic trials of well-known treatments. Informed consent is an essential component of recognizing patient autonomy and respect for a person’s right to make decisions about their participation in a clinical trial. However, in many cases, consent processes have become cumbersome, fail to provide study participants with the information necessary to allow them to make properly informed decisions, and are disproportionate to the level of risk involved. In particular, a streamlined approach should be adopted for pragmatic trials conducted in the setting of routine care. Such approaches are currently being considered in the proposed revisions to the Common Rule, which is the regulation that guides federally supported human research in the US.68Although the EU Clinical Trials Regulation includes provisions for low-(risk) intervention trials and cluster randomized trials,9 ICH-GCP does not currently address these issues.13
Conclusion
Cardiovascular therapeutics is built on a foundation of evidence-based practice created from decades of high-quality randomized trials. The ESC supports regulations and guidance that promote quality protections for clinical trial participants and meaningfully improve the reliability of the results of trials. However, regulations should be based on scientific principles, proportionate for the type of intervention and the extent of prior experience with it, and adaptable to the choice of trial design (including use of registry, electronic health record or sensor data). Regulations and guidance should also be internally consistent to avoid apparently conflicting requirements, which could lead to poor adoption of improved standards.
The ESC has set out a number of priority initiatives to improve the quality of GCP guidelines for clinical trials and their appropriate implementation (Table 2). The ESC is sharing the views generated by the workshop and has presented these with the public consultation on the ICH-GCP addendum. The ESC is committed to partnering with patients, investigators, sponsors, and regulators to create a clinical trial environment fit for the 21st Century, one that provides appropriate protection for trial participants, encourages innovation, operates efficiently, and leads to better care and improved outcomes for patients with cardiovascular disease.
Table 2.
Priority Initiatives of the European Society of Cardiology to improve the feasibility and quality of cardiovascular clinical trials
| Priority initiative | Aim |
|---|---|
| 1. Support research on the utility of clinical trial activities | Support approaches to evaluate specific clinical trial activities to determine their effectiveness, value, and impact on safety of trial participants and the reliability of the results. |
| 2. Make the case for improved regulation of clinical trials and participate in their development | Contribute actively to the development of regulations and guidance that facilitate high quality clinical trials, working in collaboration with all relevant stakeholders (including academic trialists, patient advocates, regulators, non-commercial funders, and industry) |
| 3. Share best practice for translating regulatory requirements to practice | Support collaborative efforts among academic trialists, patient advocates, regulators (including auditors and inspectors), non-commercial funders, and industry to establish a consensus on methods to translate regulatory guidance into modern clinical trials. |
| 4. Promote initiatives to reduce the over-interpretation and excessive application of reasonable regulatory requirements | Promote initiatives that encourage interaction among academic trialists, patient advocates, regulators (including auditors and inspectors), non-commercial funders, and industry to identify and rectify examples of over-interpretation of regulatory requirements (i.e. activities that are conducted out of conservative interpretation of regulations rather than actual requirements). |
| 5. Promote widespread understanding of the role of clinical trials in high quality cardiovascular healthcare | Provide mechanisms for educational initiatives targeting patients, practicing physicians, and policy makers on the importance of clinical trials for developing new therapies and for establishing the effectiveness of available therapies used in the setting of routine care. Through education, shift thinking towards a realization that, in the absence of such evidence, the most ethical approach is often to conduct a randomized trial. |
| 6. Encourage and facilitate effective engagement of patients and their advocates in the clinical trial enterprise | Encourage patients and patient advocacy groups to become involved in decisions related to clinical trial design (e.g. ensure that trials are answering questions relevant to patients) and/or regulatory standards (e.g. regulations that protect patients while also enabling quality research to be conducted) |
Acknowledgements
This paper was generated from discussions during a Cardiovascular Round Table Workshop organized on 09 October 2015 by the European Society of Cardiology (ESC). The Cardiovascular Round Table is a strategic forum for high-level dialogues between industry and ESC leadership to identify and discuss key strategic issues for the future of cardiovascular health in Europe. The authors acknowledge the contributions of Janet Wisely (Health Research Authority, National Health Service, London, United Kingdom), Patrick Archdeacon and Robert Califf (both United States Food and Drug Administration, Silver Spring, Maryland, USA) who were presenters at the workshop and participated in the discussions on which this manuscript is based. The opinions expressed in this paper are those of the authors and cannot be interpreted as the opinion of any of the organizations that employ the authors.
Conflict of interest: Martin Landray: Conducts clinical trials funded by charity, government, and industry in accordance with contracts through the University of Oxford (which acts as the regulatory sponsor) that ensure the independence of the research from the funders. Jeroen J. Bax: None declared. Laurence Alliot: Employee of Servier. Marc Buyse: Personal fees from IDDI and CluePoints (employee and stockholder); US Patent 9,092,566 on Central Statistical Monitoring of Research Trials (patent granted to IDDI on 28 July 2015). Adam Cohen: Vice Chairman of the Netherlands Clinical Trial Competent Authority and Central Ethics Committee; Personal fees from CHDR Leiden (full-time employee of the non-profit foundation CHDR and conducts trials funded by charity and industry in this capacity); Non-executive board position with Omnicomm, a company producing software for clinical trial data management. Rory Collins: Conducts clinical trials funded by charity, government, and industry in accordance with contracts through the University of Oxford (which acts as the regulatory sponsor) that ensure the independence of the research from the funders. Gerhard Hindricks: Research grants through the Heart Center Leipzig, with no personal payment received for services from St. Jude Medical, Boston Scientific. Stefan K. James: Institutional research grants from Astra Zeneca, The Medicines Company, Abbott Vascular, Boston Scientific, Janssen; personal fees from The Medicines Company and Bayer. Sile Lane: None declared. Aldo P. Maggioni: Research grant from Novartis, Cardiorentis, Bayer. Ann Meeker-O'Connell: Employee of Johnson & Johnson; Board of Directors for the Association for the Accreditation of Human Research Protection Programs, Inc. (AAHRPP). Gunnar O. Olsson: Board member for Athera Biotechnologies and Biocrine (both are biotechnology research startup companies with no products). Stuart J. Pocock: None declared. Michael Rawlins: None declared. Jonathan Sellors: Legal counsel of UK Biobank and Honorary Senior Research Fellow at the Nuffield Department of Health, Oxford University. Kaori Shinagawa: None declared. Karin R. Sipido: None declared. Liam Smeeth: Supported by a Wellcome Trust Senior Research Fellowship in Clinical Science grant number 098504/Z/12/Z; research grants from MRC, NIHR, GlaxoSmithKline, and European Union; personal fees from GlaxoSmithKline (advisory board unrelated to this work); unpaid chair of a steering committee for a randomized trial of a new drug (AstraZeneca) for diabetes; trustee of the British Heart Foundation. Richard Stephens: Personal fees from National Cancer Research Institute and BioMed Central (annual honoraria as an expert patient) and Astra Zeneca (consulting). Murray W. Stewart: Employee of GlaxoSmithKline. Wendy Gattis Stough: Personal fees for consulting to European Society of Cardiology, Heart Failure Association of the European Society of Cardiology, European Drug Development Hub, Relypsa, CHU Nancy, Heart Failure Society of America, Overcome, Stealth BioTherapeutics, University of Gottingen, University of North Carolina, Respicardia, and Celyad. Fergus Sweeney: None declared. Frans Van de Werf: Research grant from Merck and Boehringer Ingelheim; Personal fees (advisor, speaker’s bureau) from Merck, Boehringer Ingelheim, Astra Zeneca; European Society of Cardiology member of the Board and consulting editor of the European Heart Journal. Kerrie Woods: None declared. Barbara Casadei: Board Member and President-Elect of the European Society of Cardiology. Supported by a British Heart Foundation (BHF) Chair in Cardiovascular Medicine at the University of Oxford. Research support from BHF, NIHR Oxford Biomedical Research Centre, and the European Union.
References
- 1. Calvo G, McMurray JJ, Granger CB, Alonso-Garcia A, Armstrong P, Flather M, Gomez-Outes A, Pocock S, Stockbridge N, Svensson A, Van de Werf F.. Large streamlined trials in cardiovascular disease. Eur Heart J 2014;35:544–548. [DOI] [PubMed] [Google Scholar]
- 2. European Commission. Assessment of the functioning of the “Clinical Trials Directive” 2001/20EC public consultation paper. http://ec.europa.eu/health/files/clinicaltrials/docs/2009_10_09_public-consultation-paper.pdf (25 May 2016).
- 3. Jackson N, Atar D, Borentain M, Breithardt G, van Eickels M, Endres M, Fraass U, Friede T, Hannachi H, Janmohamed S, Kreuzer J, Landray M, Lautsch D, Le Floch C, Mol P, Naci H, Samani N, Svensson A, Thorstensen C, Tijssen J, Vandzhura V, Zalewski A, Kirchhof P.. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Roundtable of the European Society of Cardiology. Eur Heart J 2016;37:747–754. [DOI] [PubMed] [Google Scholar]
- 4. Komajda M, Coats A, Cowie MR, Jackson N, Svensson A, Vardas P.. Championing cardiovascular health innovation in Europe. Eur Heart J 2013;34:2630–2635. [DOI] [PubMed] [Google Scholar]
- 5. Roe MT, Mahaffey KW, Ezekowitz JA, Alexander JH, Goodman SG, Hernandez A, Temple T, Berdan L, Califf RM, Harrington RA, Peterson ED, Armstrong PW.. The future of cardiovascular clinical research in North America and beyond-addressing challenges and leveraging opportunities through unique academic and grassroots collaborations. Am Heart J 2015;169:743–750. [DOI] [PubMed] [Google Scholar]
- 6. Mentz RJ, Hernandez AF, Berdan LG, Rorick T, O'brien EC, Ibarra JC, Curtis LH, Peterson ED.. Good clinical practice guidance and pragmatic clinical trials: balancing the best of both worlds. Circulation 2016;133:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The Academy of Medical Sciences. A new pathway for the regulation and governance of health research. London: The Academy of Medical Sciences, 2011. [Google Scholar]
- 8. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). ICH Harmonised Tripartite Guideline: Guideline for good clinical practice (E6 [R1]). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (21 October 2015).
- 9. European Parliament and Council. Regulation (EU) No 536/2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. http://ec.europa.eu/health/files/eudralex/vol-1/reg_2014_536/reg_2014_536_en.pdf (25 May 2016).
- 10. Bill & Melinda Gates Foundation. Clinical studies and regulated research assurances. https://www.google.co.uk/url?sa= t&rct=j&q=& esrc=s& source= web& cd= 1& ved=0ahUKEwippdSz3fjQAhWM6iYKHQHQC1wQFggcMAA&url=https%3A%2F%2Fdocs.gatesfoundation.org%2Fdocuments%2Fregulated_research_module.doc&usg=AFQjCNHLGPCeiRA58aqZRFnAGR_P-3LZuQ&cad=rja (25 May 2016).
- 11. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Final business plan. Addendum for ICH E6: Guideline for Good Clinical Practice. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2_Business_Plan_July_2014.pdf (22 March 2016).
- 12.Moretrials: the public campaign for more, better, randomised trials. www.moretrials.net (22 March 2016).
- 13. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). ICH Harmonised Guideline: Integrated Addendum to ICD E6(R1): Guideline for good clinical practice E6 (R2). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4.pdf (22 November 2016).
- 14.Updated open letter to EMA & ICH: From 5 research organisations and an international consortium of 119 health researchers in 22 countries. http://moretrials.net/the-problem/ (25 May 2016).
- 15. Browne LH, Graham PH.. Good intentions and ICH-GCP: Trial conduct training needs to go beyond the ICH-GCP document and include the intention-to-treat principle. Clin Trials 2014;11:629–634. [DOI] [PubMed] [Google Scholar]
- 16. Grimes DA, Hubacher D, Nanda K, Schulz KF, Moher D, Altman DG.. The Good Clinical Practice guideline: a bronze standard for clinical research. Lancet 2005;366:172–174. [DOI] [PubMed] [Google Scholar]
- 17. International Council for Harmonisation. ICH Reflection on "GCP Renovation": Modernization of ICH E8 and Subsequent Renovation of ICH E6. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/GCP_Renovation/ICH_Reflection_paper_GCP_Renovation_Jan_2017_Final.pdf (24 February 2017).
- 18. Kramer JM, Smith PB, Califf RM.. Impediments to clinical research in the United States. Clin Pharmacol Ther 2012;91:535–541. [DOI] [PubMed] [Google Scholar]
- 19. Morrison BW, Cochran CJ, White JG, Harley J, Kleppinger CF, Liu A, Mitchel JT, Nickerson DF, Zacharias CR, Kramer JM, Neaton JD.. Monitoring the quality of conduct of clinical trials: a survey of current practices. Clin Trials 2011;8:342–349. [DOI] [PubMed] [Google Scholar]
- 20. Eisenstein EL, Collins R, Cracknell BS, Podesta O, Reid ED, Sandercock P, Shakhov Y, Terrin ML, Sellers MA, Califf RM, Granger CB, Diaz R.. Sensible approaches for reducing clinical trial costs. Clin Trials 2008;5:75–84. [DOI] [PubMed] [Google Scholar]
- 21. Andersen JR, Byrjalsen I, Bihlet A, Kalakou F, Hoeck HC, Hansen G, Hansen HB, Karsdal MA, Riis BJ.. Impact of source data verification on data quality in clinical trials: an empirical post hoc analysis of three phase 3 randomized clinical trials. Br J Clin Pharmacol 2015;79:660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clinical Trials Transformation Initiative. CTTI recommendations: Effective and efficient monitoring as a component of quality assurance in the conduct of clinical trials. http://www.ctti-clinicaltrials.org/files/Monitoring/Monitoring-Recommendations.pdf (18 December 2015).
- 23. Landray MJ, Grandinetti C, Kramer JM, Morrison BW, Ball L, Sherman RE.. Clinical trials: rethinking how we ensure quality. Drug Inf J 2012;46:657–660. [Google Scholar]
- 24. Reith C, Landray M, Devereaux PJ, Bosch J, Granger CB, Baigent C, Califf RM, Collins R, Yusuf S.. Randomized clinical trials–removing unnecessary obstacles. N Engl J Med 2013;369:1061–1065. [DOI] [PubMed] [Google Scholar]
- 25. Tudur SC, Stocken DD, Dunn J, Cox T, Ghaneh P, Cunningham D, Neoptolemos JP.. The value of source data verification in a cancer clinical trial. PLoS One 2012;7:e51623.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Venet D, Doffagne E, Burzykowski T, Beckers F, Tellier Y, Genevois-Marlin E, Becker U, Bee V, Wilson V, Legrand C, Buyse M.. A statistical approach to central monitoring of data quality in clinical trials. Clin Trials 2012;9:705–713. [DOI] [PubMed] [Google Scholar]
- 27. Buyse M, George SL, Evans S, Geller NL, Ranstam J, Scherrer B, Lesaffre E, Murray G, Edler L, Hutton J, Colton T, Lachenbruch P, Verma BL.. The role of biostatistics in the prevention, detection and treatment of fraud in clinical trials. Stat Med 1999;18:3435–3451. [DOI] [PubMed] [Google Scholar]
- 28. Clinical Trials Transformation Initiative. Quality objectives of monitoring: Workstream 2 final report. http://www.ctti-clinicaltrials.org/files/documents/MonitoringWS2FinalReport.pdf (25 May 2016).
- 29. European Medicines Agency. Reflection paper on risk based quality management in clinical trials. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/11/WC500155491.pdf (23 October 2015).
- 30. Medical Research Council, Department of Health, and Medicines and Healthcare Products Regulatory Agency Joint Project. Risk-adapted approaches to the management of clinical trials of investigational medicinal products. http://webarchive.nationalarchives.gov.uk/20141205150130/http://www.mhra.gov.uk/home/groups/l-ctu/documents/websiteresources/con111784.pdf (23 October 2015).
- 31. U.S. Department of Health and Human Services and Food and Drug Administration. Guidance for Industry. Oversight of Clinical Investigations - A Risk-Based Approach to Monitoring. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM269919.pdf (23 October 2015).
- 32. Jarow JP, Casak S, Chuk M, Ehrlich LA, Khozin S.. The majority of expedited investigational new drug safety reports are uninformative. Clin Cancer Res 2016;22:2111–2113. [DOI] [PubMed] [Google Scholar]
- 33. U.S. Department of Health and Human Services and Food and Drug Administration. Investigational new drug safety reporting requirements for human drug and biological products and safety reporting requirements for bioavailability and bioequivalence studies in humans, 21 CFR Parts 312 and 320. https://www.gpo.gov/fdsys/pkg/FR-2010-09-29/pdf/2010-24296.pdf (25 May 2016).
- 34. U.S. Department of Health and Human Services Food and Drug Administration. Guidance for industry and investigators: Safety reporting requirements for INDs and BA/BE studies. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM227351.pdf (25 May 2016).
- 35. U.S. Department of Health and Human Services Food and Drug Administration. Safety assessment for IND safety reporting: Guidance for industry. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM477584.pdf (25 May 2016).
- 36. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). ICH Harmonised Tripartite Guideline: Clinical safety data management - definitions and standards for expedited reporting (E2A). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf (21 October 2015).
- 37. Kenter MJ, Cohen AF.. The return of the prodigal son and the extraordinary development route of antibody TGN1. Br J Clin Pharmacol 2015;79:545–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. U.S. Department of Health and Human Services Food and Drug Administration. FDA Webinar: New draft guidance on safety data collection. http://www.fda.gov/Drugs/ucm296761.htm (25 May 2016).
- 39. US Department of Health and Human Services Food and Drug Administration. Guidance for clinical investigators, sponsors, and IRBs: Adverse event reporting to IRBs - improving human subject protection. http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM126572.pdf (25 May 2016).
- 40. Glasziou P, Chalmers I, Rawlins M, McCulloch P.. When are randomised trials unnecessary? Picking signal from noise. Bmj 2007;334:349–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rawlins M. De testimonio: on the evidence for decisions about the use of therapeutic interventions. Lancet 2008;372:2152–2161. [DOI] [PubMed] [Google Scholar]
- 42. U.S. Department of Health and Human Services Food and Drug Administration. Guidance for industry: Determining the extent of safety data collection needed in late stage premarket and postapproval clinical investigations. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291158.pdf (25 May 2016).
- 43. Archdeacon P, Grandinetti C, Vega JM, Balderson D, Kramer JM.. Optimizing expedited safety reporting for drugs and biologics subject to an investigational new drug application. Ther Innov Regul Sci 2013;doi:10.1177/2168479013509382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swedberg K, Borer JS, Pitt B, Pocock S, Rouleau J.. Challenges to data monitoring committees when regulatory authorities intervene. N Engl J Med 2016;374:1584. [DOI] [PubMed] [Google Scholar]
- 45. Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK.. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013;369:1587–1597. [DOI] [PubMed] [Google Scholar]
- 46. Cowie MR, Blomster JI, Curtis LH, Duclaux S, Ford I, Fritz F, Goldman S, Janmohamed S, Kreuzer J, Leenay M, Michel A, Ong S, Pell JP, Southworth MR, Stough WG, Thoenes M, Zannad F, Zalewski A.. Electronic health records to improve patient care and facilitate clinical research. Clin Res Cardiol 2016;doi: 10.1007/s00392-016-1025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hernandez AF, Fleurence RL, Rothman RL.. The ADAPTABLE Trial and PCORnet: shining light on a new research paradigm. Ann Intern Med 2015;163:635–636. [DOI] [PubMed] [Google Scholar]
- 48. Vickers AJ, Scardino PT.. The clinically-integrated randomized trial: proposed novel method for conducting large trials at low cost. Trials 2009;10:14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clinical Trials Transformation Initiative. Using mobile technology to facilitate clinical trials. http://www.ctti-clinicaltrials.org/what-we-do/ctti-projects/mobile-clinical-trials (25 May 2016).
- 50. Staa TP, Goldacre B, Gulliford M, Cassell J, Pirmohamed M, Taweel A, Delaney B, Smeeth L.. Pragmatic randomised trials using routine electronic health records: putting them to the test. Bmj 2012;344:e55.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Staa TP, Dyson L, McCann G, Padmanabhan S, Belatri R, Goldacre B, Cassell J, Pirmohamed M, Torgerson D, Ronaldson S, Adamson J, Taweel A, Delaney B, Mahmood S, Baracaia S, Round T, Fox R, Hunter T, Gulliford M, Smeeth L.. The opportunities and challenges of pragmatic point-of-care randomised trials using routinely collected electronic records: evaluations of two exemplar trials. Health Technol Assess 2014;18:1–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clinical Trials Transformation Initiative. Mobile clinical trials (MCT). https://www.ctti-clinicaltrials.org/programs/mobile-clinical-trials (6 December 2016).
- 53. www.clinicalstudydatarequest.com (25 May 2016).
- 54. Nisen P, Rockhold F.. Access to patient-level data from GlaxoSmithKline clinical trials. N Engl J Med 2013;369:475–478. [DOI] [PubMed] [Google Scholar]
- 55. Taichman DB, Backus J, Baethge C, Bauchner H, de Leeuw PW, Drazen JM, Fletcher J, Frizelle FA, Groves T, Haileamlak A, James A, Laine C, Peiperl L, Pinborg A, Sahni P, Wu S.. Sharing clinical trial data: a proposal from the International Committee of Medical Journal Editors. The Lancet 2016;387:e9–e11. [DOI] [PubMed] [Google Scholar]
- 56. Devereaux PJ, Guyatt G, Gerstein H, Connolly S, Yusuf S.. Toward fairness in data sharing. N Engl J Med 2016;375:405–407. [DOI] [PubMed] [Google Scholar]
- 57. Krumholz HM, Waldstreicher J.. The Yale Open Data Access (YODA) Project—a mechanism for data sharing. N Engl J Med 2016;375:403–405. [DOI] [PubMed] [Google Scholar]
- 58. Patel MR, Armstrong PW, Bhatt DL, Braunwald E, Camm AJ, Fox KA, Harrington RA, Hiatt WR, James SK, Kirtane AJ, Leon MB, Lincoff AM, Mahaffey KW, Mauri L, Mehran R, Mehta SR, Montalescot G, Nicholls SJ, Perkovic V, Peterson ED, Pocock SJ, Roe MT, Sabatine MS, Sekeres M, Solomon SD, Steg G, Stone GW, Van de Werf F, Wallentin L, White HD, Gibson M.. Sharing data from cardiovascular clinical trials—a proposal. N Engl J Med 2016;375:407–409. [DOI] [PubMed] [Google Scholar]
- 59. Warren E. Strengthening research through data sharing. N Engl J Med 2016;375:401–403. [DOI] [PubMed] [Google Scholar]
- 60. Horton R. Offline: data sharing-why editors may have got it wrong. Lancet 2016;388:1143.. [DOI] [PubMed] [Google Scholar]
- 61. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J.. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 62. The HPS2-THRIVE Collaborative Group. Supplementary appendix 2: THRIVE adverse events by MedDRA term. http://www.nejm.org/doi/suppl/10.1056/NEJMoa1300955/suppl_file/nejmoa1300955_appendix2.html (18 December 2015).
- 63. Califf RM, Platt R.. Embedding cardiovascular research into practice. Jama 2013;310:2037–2038. [DOI] [PubMed] [Google Scholar]
- 64. Institute of Medicine. The Learning Healthcare System: Roundtable on Evidence-based Medicine Workshop Summary. Washington DC: National Academies Press; 2007. [Google Scholar]
- 65. Bhatt DL, Kandzari DE, O'neill WW, D'agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL.. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 66. Clinical Trials Transformation Initiative. http://www.ctti-clinicaltrials.org/home (25 May 2016).
- 67. Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL.. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991;324:781–788. [DOI] [PubMed] [Google Scholar]
- 68. U.S. Department of Health and Human Services. Federal policy for the protection of human subjects: proposed rules. http://www.gpo.gov/fdsys/pkg/FR-2015-09-08/pdf/2015-21756.pdf (24 September 2015).
- 69. U.S. Department of Health and Human Services Food and Drug Administration. Code of Federal Regulations Title 21. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.3 (25 May 2016).