Abstract
Exposure to tobacco smoke, which contains several harmful and potentially harmful constituents such as acrolein increases cardiovascular disease (CVD) risk. Although high acrolein levels induce pervasive cardiovascular injury, the effects of low-level exposure remain unknown and sensitive biomarkers of acrolein toxicity have not been identified. Identification of such biomarkers is essential to assess the toxicity of acrolein present at low levels in the ambient air or in new tobacco products such as e-cigarettes. Hence, we examined the systemic effects of chronic (12 weeks) acrolein exposure at concentrations similar to those found in tobacco smoke (0.5 or 1 ppm). Acrolein exposure in mice led to a 2- to 3-fold increase in its urinary metabolite 3-hydroxypropyl mercapturic acid (3-HPMA) with an attendant increase in pulmonary levels of the acrolein-metabolizing enzymes, glutathione S-transferase P and aldose reductase, as well as several Nrf2-regulated antioxidant proteins. Markers of pulmonary endoplasmic reticulum stress and inflammation were unchanged. Exposure to acrolein suppressed circulating levels of endothelial progenitor cells (EPCs) and specific leukocyte subsets (eg, GR-1+ cells, CD19+ B-cells, CD4+ T-cells; CD11b+ monocytes) whilst other subsets (eg, CD8+ cells, NK1.1+ cells, Ly6C+ monocytes) were unchanged. Chronic acrolein exposure did not affect systemic glucose tolerance, platelet–leukocyte aggregates or microparticles in blood. These findings suggest that circulating levels of EPCs and specific leukocyte populations are sensitive biomarkers of inhaled acrolein injury and that low-level (<0.5 ppm) acrolein exposure (eg, in secondhand smoke, vehicle exhaust, e-cigarettes) could increase CVD risk by diminishing endothelium repair or by suppressing immune cells or both.
Keywords: aldehydes, cardiovascular disease, endothelial progenitor cells, oxidative stress, pulmonary injury, tobacco
Reactive aldehydes are generated during combustion of organic material in any form (eg, fossil fuels, wood, paper), and thus, are present in high concentrations in automobile exhaust, tobacco smoke, and smog. Such aldehydes constitute 1–2% of the volatiles in automobile exhaust (Feron et al., 1991). Indeed, these aldehydes are present in high concentrations in cigarette smoke (700–800 µg/cigarette in mainstream smoke; 50–70 ppm per puff) (Dong and Moldoveanu, 2004; Ghilarducci and Tjeerdema, 1995), cigars, water pipes (hookah) (Kassem et al., 2014), smokeless tobacco products (snus, snuff), and typically at lower levels in heated not burned cigarettes (deBethizy et al., 1990; Shihadeh et al., 2012; Stanfill et al., 2003; Stepanov et al., 2008) as well as in electronic cigarettes (McAuley et al., 2012). Yet, environmental levels of acrolein in the air are near limits of detection (Destaillats et al., 2002) but can be well above background (0.04–2.2 ppm) near petrochemical plants (Feron et al., 1991). Nonetheless, the relationship between smoking or exposure to secondhand smoke and CVD risk is well described, and the Institute of Medicine ranks aldehydes as the most significant cardiovascular toxins in tobacco smoke (Medicine, 2010). Indeed, a risk analysis suggests that aldehydes account for over 91% of the total non-cancer risk, which includes cardiovascular and pulmonary diseases of smoking (ie, acrolein, 88.5%; acetaldehyde, 2.4%; formaldehyde, 0.4%) (Haussmann, 2012). Moreover, despite extremely low levels in the environmental air, it remains difficult to set a no observable adverse effect level (NOAEL) of chronically inhaled acrolein for pulmonary (Woodruff et al., 2007) and cardiovascular disease risk (DeJarnett et al., 2014).
The cardiovascular effects of exogenous aldehydes in humans and animals have been studied for nearly a century. These effects include those of rapid onset and short-lived (eg, neurally mediated) or slower in onset (eg, transcriptional). Additionally, chronic exposure may produce completely different effects (eg, compensatory acclimation) than those observed in acutely exposed animals (eg, extreme sensitivity/toxicity). For example, acute exposure to 1 ppm acrolein increases aortic 1,N(2)-propanodeoxyguanosine acrolein adducts while chronic exposure to acrolein (0.52 ppm, 6 h/day, 5 days/week, 8 weeks) does not increase aorta lesion formation in cockerels (Penn et al., 2001). Moreover, 13-weeks of exposure to α-ethylacrolein induces cardiac weight gain in rats (Appelman et al., 1981). Acute feeding of acrolein increases pro-atherosclerotic cholesterol and triglycerides (Conklin et al., 2010, 2011), while chronic acrolein feeding enhances atherosclerosis and thrombosis markers in atherosclerosis-prone mice (apoE-null) (Srivastava et al., 2011). Short-term (3–4 days) inhalation exposure (6 h/day) of acrolein (1 ppm) decreases circulating endothelial progenitor cells (EPCs) (Wheat et al., 2011) and enhances thrombotic markers in mice (Sithu et al., 2010). The levels of the major acrolein urinary metabolite, 3-HPMA, are associated with increases in both platelet-mononuclear cell aggregates and CVD risk in humans (DeJarnett et al., 2014). Moreover, acrolein directly stimulates release of activated MMP-9 from murine aortic atherosclerotic plaques ex vivo—a process that may relate to plaque lesion degradation and rupture (O'Toole et al., 2009). Collectively, these data indicate acrolein is a consistent acute and chronic cardiovascular toxin in a variety of animal models and in humans.
Although numerous deleterious cardiovascular effects of acrolein exposure at high concentrations have been documented, there are limited data on the systemic toxicity of acrolein at levels found in tobacco smoke, ambient air or in new tobacco products such as e-cigarettes. Moreover, sensitive and persistent biomarkers of acrolein-induced toxicity under chronic exposure conditions have not been identified. Hence, we measured multiple cardiovascular biomarkers in a healthy, murine model (C57BL/6 male on normal chow) after chronic exposure (12 weeks) to acrolein. Interestingly, both EPCs and specific leukocyte subsets (eg, CD19+ B-cells) were suppressed by acrolein throughout 12 weeks of exposure without evidence of platelet activation and insulin resistance, and thus, the levels of these circulating cell types appear to be both robust and persistent biomarkers of cardiovascular injury. Moreover, these data suggest that chronic inhalation of acrolein, even at low levels, is likely to increase CVD risk.
MATERIALS AND METHODS
Materials
Ultra performance liquid chromatography (UPLC) grade acetonitrile, N-hexane, acetone, and pentafluorobenzyl bromide (PFBBr) were purchased from Fisher Scientific (Fairlawn, NJ). N-Acetyl-S-(3-hydroxypropyl)cysteine, Dicyclohexylammonium Salt (3HPMA); N-Acetyl-d3-S-(3-hydroxypropyl)cysteine, and Dicyclohexylammonium Salt 3HPMA-D3 (3HPMA-d3) were purchased from Toronto Research Chemicals (Downsview, Ontario, CAN). Malonaldehyde-1,3-d2 Bis(diethyl acetal) was purchased from CDN Isotopes (Pointe-Claire, Quebec, CAN). Antibodies for flow cytometric analyses: FITC-anti-Sca-1 (Ly-6A/E), APC-anti-Flk1 (CD309), APC-eFluor780-anti-CD41, PE-Cyanine7-anti-Sca-1, FITC-anti-Nk1.1, PE-anti-Ly6C, PerCPe710-anti-CD8, PECy7-anti-CD62, APC-anti-CD19, Alexa700-anti-Gr-1, APCe780-anti-CD3, eVolve605-CD11b, e650-anti-CD4, and OneComp eBeads, proprietary solution, and propidium iodide were purchased from eBioscience (San Diego, CA). Annexin V Buffer and Zenon Alexa Fluor488 labeling kit were obtained from Invitrogen (Waltham, MA). Sources for other reagents are: CD62E-PE (BD Bioscience; San Jose, CA), ACE ectodomain (R&D Systems, Minneapolis, MN), Fc Block (CD32/CD16) (Leinco Technologies; Fenton, MO), counting beads (Spherotech; Lake Forest, IL), PBS (Corning; Manassas, VA), BSA (Rockland Immunochemicals Inc.; Pottstown, PA). Sources for the ELISA kits are: insulin (Mercodia AB, Uppsala, SWE), endothelin-1 (Enzo Life Sciences Inc., Farmingdale, NY), vWF (My Biosource, San Diego, CA), PF4, MMP3, and MMP9 (R&D Systems), fibrinogen (Innovative Research, Novi, MI).
Primers for PCR Analyses
Primers for B2m, TNFα, IL-6, IL-8, GRP78, GRP94, and HERP were obtained from Qiagen, (Gaithersburgh, MD). Sources of antibodies for Western blotting: Anti-GSTP-1 (610719; 1:2500) was purchased from BD Bioscience (San Jose, CA); and anti-ALDH2 (LS-B7924; 1:1500) was bought from LifeSpan BioSciences (Seattle, WA). Other antibodies anti-AR (Sc-271007; 1:1500), anti-β-actin (Sc-47778; 1:1000), anti-Nrf2 (Sc-722; 1:1500), anti-COX-2 (Sc-376861;1:1500), anti-HO-1 (Sc-10789; 1:1500), anti-SOD-1 (Sc-11407; 1:2000), anti-SOD-2 (Sc-137254; 1:2000), anti-SOD-3 (Sc-101338; 1:2000), anti-Catalase (Sc271803; 1:2000), and anti-GAPDH (Sc-47724; 1:1000) were obtained from Santa Cruz Biotechnology (Dallas, TX).
Mice and Acrolein Exposure
Male C57BL/6J mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Mice were treated according to American Physiological Society Guiding Principles in the Care and Use of Animals, and all protocols were approved by University of Louisville Institutional Animal Care and Use Committee. Mice were housed under pathogen-free conditions in the University of Louisville vivarium under controlled temperature and 12 h light: 12 h dark cycle. Mice were provided a standard chow diet (Rodent Diet 5010, 4.5% fat by weight, LabDiet; St. Louis, MO). Mice (8 weeks old) were exposed to either HEPA- and charcoal-filtered air or acrolein (0.5 or 1 ppm; 6 h/day, 5 days/week) for 12 weeks using a custom exposure system and certified permeation tubes (Kin-Tek; LaMarque, TX) as described (O'Toole et al., 2014). Immediately after the end of the last exposure, mice were anesthetized with sodium pentobarbital (≈150 mg/kg) followed by ventral thoracotomy and exsanguination via cardiac puncture for blood collection in EDTA-coated syringes. Organs were removed, weighed, snap frozen in N2, and stored at −80°C.
Urine Collection and Acrolein Metabolism
Urine collection
On day 1, mice were weighed and briefly exposed to d-glucose/saccharin solution (w/v; 3.0%/0.125%; Sigma) immediately prior to HEPA- and charcoal-filtered air exposure (6 h), and then placed singly per metabolic cage (Harvard Apparatus) with glucose/saccharin solution in drinking water without food to collect urine (in graduated cylinders in 4°C water-jacketed organ baths) for continuous 3 h collection post-exposure (Conklin et al., 2009a; Wood et al., 2001). After urine collection, mice were placed in home cages overnight with food and water per normal housing arrangements. On day 2, the same mice were exposed to acrolein (0.5 or 1 ppm, 6 h) or KY Reference cigarette mainstream smoke (3R4F cigarettes: 12 cigarettes over 6 h) and then placed singly per metabolic cage with glucose/saccharin solution to collect urine (without food) in 1 h increments up to 3 h post-exposure followed by an overnight (O/N; with food) urine collection. Urine samples were centrifuged (600 × g, 5 min; to pellet any feces and food particles) in cold immediately after collection, decanted, and stored at −80°C.
Acrolein metabolite analysis
The major metabolite of acrolein, 3-hydroxypropylmercapturic acid (3-HPMA), was quantified in urine by mass spectrometry (negative ion mode). The following mass spectrometer settings were used: capillary voltage 0.8 kV, extractor lens 3 V, entrance 2. The interchannel delay was 5 ms and interscan delay was 6 ms. Urine (25 µl) was mixed with 15 mM ammonium acetate (975 µl) containing 3HPMA-d3 (400 ng) and filtered through a 0.2 mm PTFE membrane. Two microliters of the samples were applied on UPLC and the eluate was analyzed for the MRM traces. To account for urinary dilution, all values for urinary metabolites were normalized to urinary creatinine (mg/dl).
Pre-diabetes evaluation
A glucose tolerance test (GTT) was performed after a 6h exposure (ie, fast) in the 10th week by injecting d-glucose (1 g/kg; i.p., saline) (Sansbury et al., 2012).
Complete blood counts
CBC were measured (25 μl blood) with a hematology analyzer calibrated with multispecies hematological reference controls (Hemavet 950FS, Drew Scientific, Inc.).
Flow cytometry
Leukocyte subpopulations
To analyze circulating immune cell populations, 0.1 ml lysed whole blood was centrifuged and washed twice with PBS containing 1% BSA (PBS/BSA). The cell pellets were re-suspended in the same buffer and incubated with CD32/CD16 (0.25 μg) for 10 min at 4°C to prevent non-specific binding. The cells were then incubated with an antibody cocktail consisting of FITC-anti-Nk1.1 (0.5 μg), PE-anti-Ly6C (0.125 μg), PerCPe710-anti-CD8 (0.125 μg), PECy7-anti-CD62 (0.25 μg), APC-anti-CD19 (0.125 μg), Alexa 700-antiGr-1 (0.125 μg), APCe780-anti-CD3 (1 μg), eVolve605-CD11b (1 μg), and e650-anti-CD4 (1 μg). After 30 min on ice, the cells were washed, re-suspended, and analyzed on an LSR II flow cytometer (high speed, 90 s). Cell numbers were analyzed using the FlowJo software and normalized to sample volume. Monocytes double positive for Ly6C and CD62L were defined as Ly6high subpopulation.
Endothelial progenitor cells (EPCs)
Blood EPCs (Flk-1+/Sca-1+ cells) were identified by flow cytometry as described (Wheat et al., 2011). Blood (0.3 ml) was mixed with 3 ml proprietary solution to lyse RBCs. Cells were pelleted by centrifugation and washed twice with PBS containing 1% BSA (PBS/BSA). Pelleted cells were re-suspended in PBS/FCS incubated with Fc block for 10 min and then stained with a FITC-labeled Sca-1 antibody and an APC-labeled Flk-1 antibody for 30 min on ice. Cells were rinsed with 1% BSA/PBS and the pellets were re-suspended in 250 μl of 1% BSA/PBS containing propidium iodide (PI, 5 μl). Sample analysis was performed using an LSRII. EPCs were defined as the number of events double positive for Flk-1 and Sca-1 and counts were normalized to the volume used. Data were analyzed using FlowJo software. For the measurement of EPCs in bone marrow, the bone marrow was aspirated from tibia of right leg, and mononuclear cells were separated by Ficoll gradient centrifugation and analyzed as described (Haberzettl et al., 2012).
Microparticles (MP)
Plasma (110 µl) was centrifuged for 2 min (11 000 × g; 4 °C) to pellet residual cells and debris. Supernatant (100 µl) was aspirated and centrifuged for 45 min (17 000 × g; 4 °C). The resulting MP pellet was re-suspended in 70 µl 1.45× Annexin V Buffer pre-filtered through syringe filter (0.22 µm). Sample (35 µl) was mixed with Annexin-V Pacific Blue (2.5 μl), ACE ectodomain antibody (0.5 μg)—labeled with Zenon Alexa Fluor488 labeling kit, CD41 APC-eFluor780 (0.625 μl), CD309 APC (1.25 μl), CD62E-PE (2.5 μg), and Ly-6A/E (1.25 μg; Sca-1) PE-Cyanine7 for 30 min. The reaction was stopped with 235 µl Annexin V buffer (pre-filtered). Counting beads (10 µm, 15 µl) were added to the samples were analyzed on LSR II flow cytometer for 5 min. An identical sample without antibodies was used as a gating control. MP numbers were quantified in gated populations < 1 µm in size and positive for Annexin V staining using the FlowJo software. MP subpopulations were analyzed as the percentage of CD41- events in the MP gate (Pope et al., 2016).
Platelet-leukocyte aggregates
Platelet–leukocyte aggregates were identified by flow cytometry and quantified as events double positive for CD41 (platelets) and CD45 (leukocytes) as described (DeJarnett et al., 2014; Sithu et al., 2010).
Plasma biomarkers
Plasma lipoproteins, total protein, albumin, creatinine, aspartate transaminase (AST), alanine transaminase (ALT), and lactate dehydrogenase were measured on a Cobas Mira Plus Clinical chemistry autoanalyzer (Roche) as described previously (Conklin et al., 2009a; Srivastava et al., 2011). Levels of cytokines and chemokines in the plasma were measured by multiplex arrays (Eve Technologies; Calgary, Alberta, CAN) or by using commercially available ELISA kits according to manufacturer’s recommendations.
Pulmonary Oxidative Stress, Endoplasmic Reticulum Stress and Cytokines
MDA in lungs
Pulmonary malonaldialdehyde (MDA) levels were measured by gas chromatography-mass spectrometry (GC-MS). Lungs were pulverized and homogenized in phosphate buffered saline (1×, pH 7.4; 5% wt/vol). MDA-d2 (20 pmoles) obtained from hydrolysis of malonaldehyde-1,3-d2 bis(diethyl acetal) was used as an internal standard. Samples and standard were derivatized with 2,3,4,5-pentafluorobenzyle bromide (PFBBr; 130 µl) for 1 h at 80°C, extracted with hexane and analyzed by GC-MS in chemical ionization (CI) mode. A six-point calibration curve was used to quantify levels of MDA in lung homogenates.
Real time quantitative PCR analyses and Western blotting were performed on lung tissues as described (Vladykovskaya et al., 2012; Wheat et al., 2011). Expression of cytokine mRNA in the lungs of air and acrolein (1 ppm) exposed mice were measured by Qiagen RT2 profiler inflammatory cytokines and receptors PCR array. Fold regulation cut off and statistical significance were set at 2.0 and 0.05, respectively, as per Qiagen’s standard protocol.
Statistics
Data are presented as mean ± standard error of mean (SEM). Statistical significance was accepted at p < .05. Statistical analyses were performed on log transformed data using SAS 9.4 software. Student’s t-test was used for the data comparison between two groups, and one-way analysis of variance with Tukey’s correction was used when comparing more than two experimental groups.
RESULTS
Urinary Acrolein Metabolite in Cigarette Smoke- and Acrolein-Exposed Mice
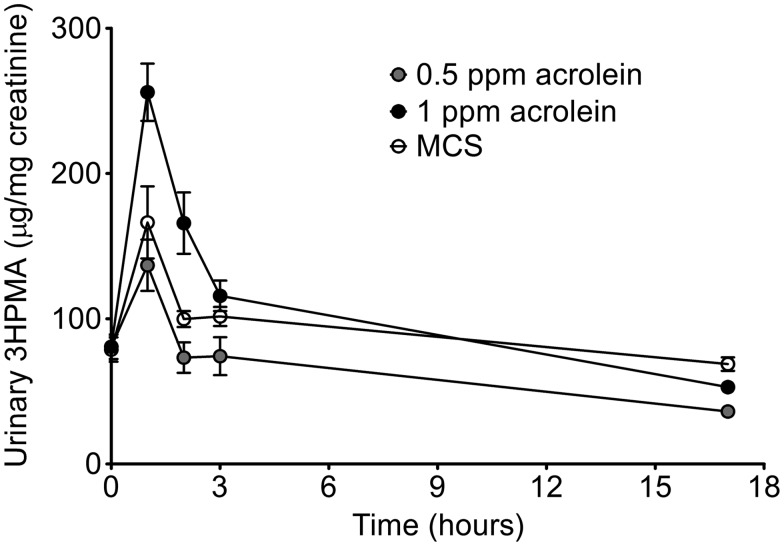
The levels of the primary urinary metabolite of acrolein, 3-HPMA, are positively associated with urine cotinine and with increased CVD risk in smokers (DeJarnett et al., 2014), hence we investigated the utility of 3-HPMA as a biomarker of tobacco exposure in mice. For this, we measured the level of 3-HPMA in the urine of mice exposed to mainstream cigarette smoke (MCS) from KY Reference cigarettes (3R4F) after a single exposure session (12 cigarettes in 6 h). As shown in Figure 1, urine 3HPMA was elevated rapidly from MCS-exposed mice compared with basal levels (after HEPA- and charcoal-filtered air exposure done the day before, ≈80 µg/mg creatinine). Moreover, the peak urinary 3-HPMA excreted after 0.5 ppm or 1 ppm acrolein exposure (6 h) bracketed the peak urinary 3-HPMA level after MCS exposure (Figure 1). All 3 excretion profiles were similar in appearance suggesting that nicotine and other constituents present in MCS do not noticeably influence acrolein metabolism or clearance. Thus, in order to better understand the independent contribution of acrolein as a harmful and potentially harmful constituent (HPHC) in tobacco smoke, we exposed mice to both 0.5 and 1 ppm acrolein alone and measured biomarkers of chronic toxicity in mice.
Figure 1.
Mass spectrometry analyses of primary acrolein metabolite in urine of acrolein- and mainstream cigarette smoke-exposed (MCS) mice. Mice were exposed to either acrolein (0.5 ppm or 1 ppm/6 h) or MCS (12 cigarettes/6 h) after which urine was collected at 0–1, 1–2, 2–3, and 3–17 h. Note: Baseline urine (t = 0) was collected after an air exposure (6 h) performed the day before acrolein or MCS exposure. Acrolein metabolite, 3-hydroxypropylmercapturic acid (3-HPMA), was measured by UPLC-MS/MS and normalized to urinary creatinine. Values are mean ± SEM. N = 5–6/group.
Acrolein, Pulmonary Oxidative Stress, and ER Stress Markers
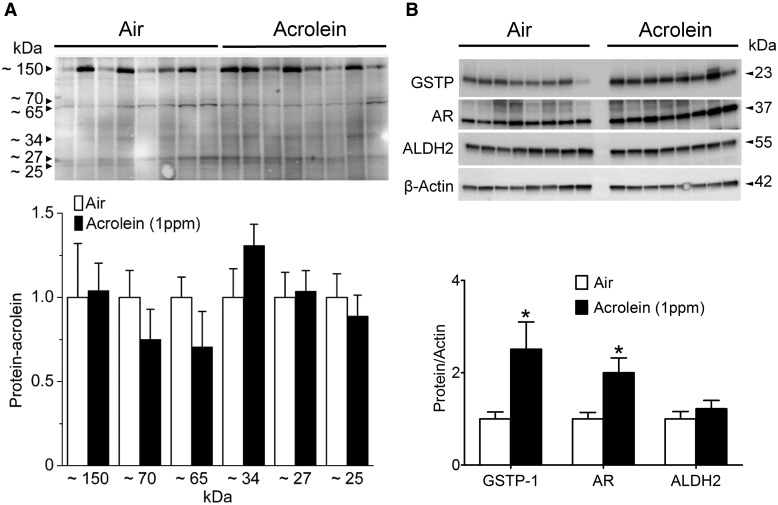
Formation of 3-HPMA and protection of the body from inhaled acrolein-induced toxicity are dependent on both of the enzymes, glutathione S-transferase P (GSTP; GSH conjugation), and aldose reductase (AR; reduction). For example, genetic deficiency of the Phase II acrolein-metabolizing enzyme, GSTP, promotes endothelium dysfunction (vascular injury) after 3-days (5 h/day) of environmental tobacco smoke (ETS) or acrolein (1 ppm) exposure (Conklin et al., 2009b). We also have shown that genetic deletion of AR increases the accumulation of aldehyde–protein adducts and exacerbates atherosclerosis in apoE-null mice (Srivastava et al., 2009). Because of the importance of the lungs as the port of entry of acrolein and the first line of enzymatic defense, we measured metabolic protection after 12 weeks of exposure. Acrolein rapidly adducts protein nucleophiles, so we measured the levels of protein-acrolein adducts in the lungs using Western blotting but observed no increase in the acrolein-exposed (1 ppm) mice (Figure 2A). In contrast, short-term exposure (5 h/day * 3 days, 1 ppm) to acrolein increases lung protein-acrolein adducts (Conklin et al., 2009b) indicating a compensatory response occurred after chronic acrolein exposure. As evidence of a compensatory response, both GSTP and AR proteins were up-regulated significantly and selectively in lungs (note: ALDH2 level was unchanged) by chronic acrolein exposure (Figure 2B).
Figure 2.
Pulmonary acrolein stress and induction of acrolein-metabolizing enzymes. Mice were exposed to HEPA-filtered air (n = 8) or acrolein (1 ppm, 6 h/day, 5 days/week; n = 8) for 12 weeks. Lung proteins were analyzed by Western blotting as described under Materials and Methods. A, Protein-acrolein adducts probed with anti-KLH-acrolein antibody (n = 8/group). B, Abundance of acrolein-metabolizing enzymes: GSTP, AR, and ALDH2 in air- and acrolein-exposed mice (n = 8/group). Values are mean ± SEM. *p < .05 vs. Air.
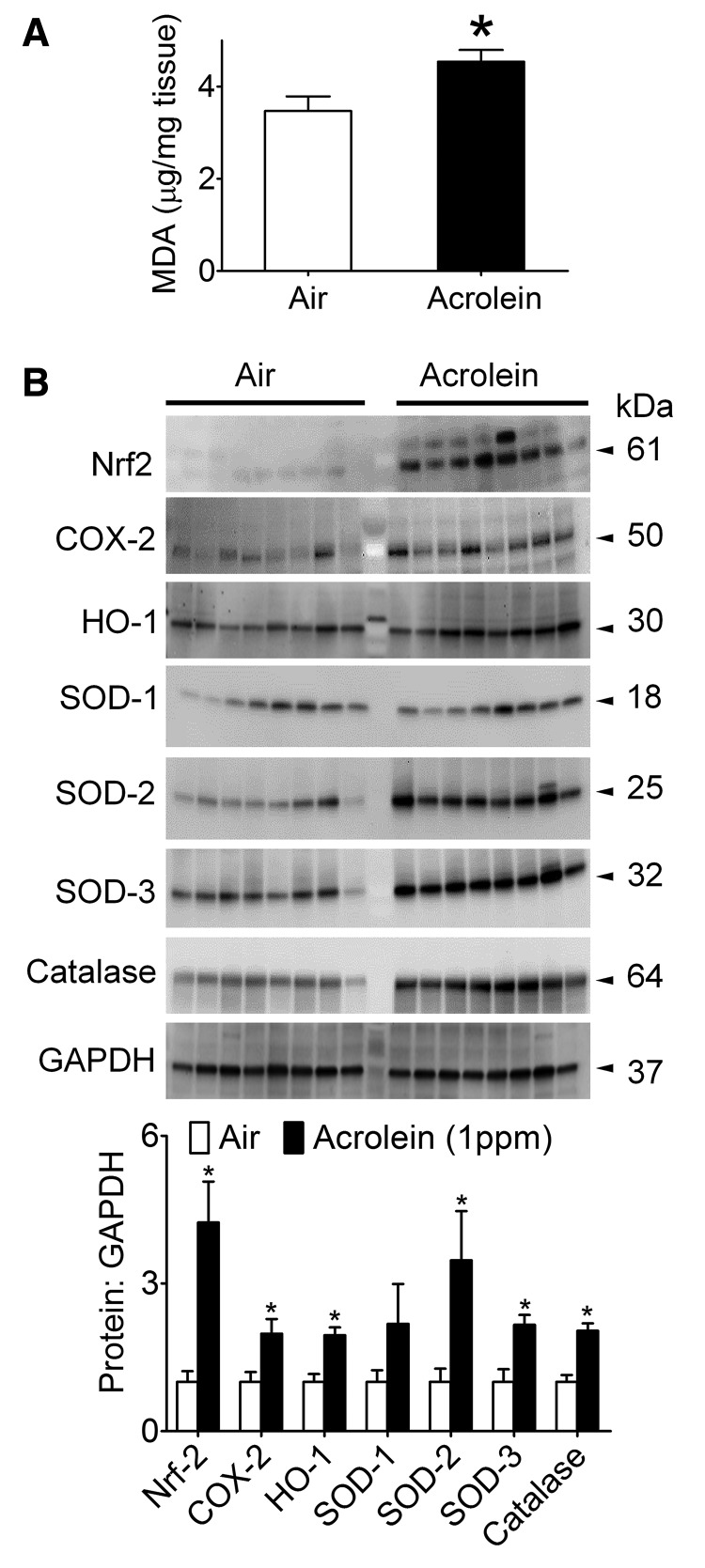
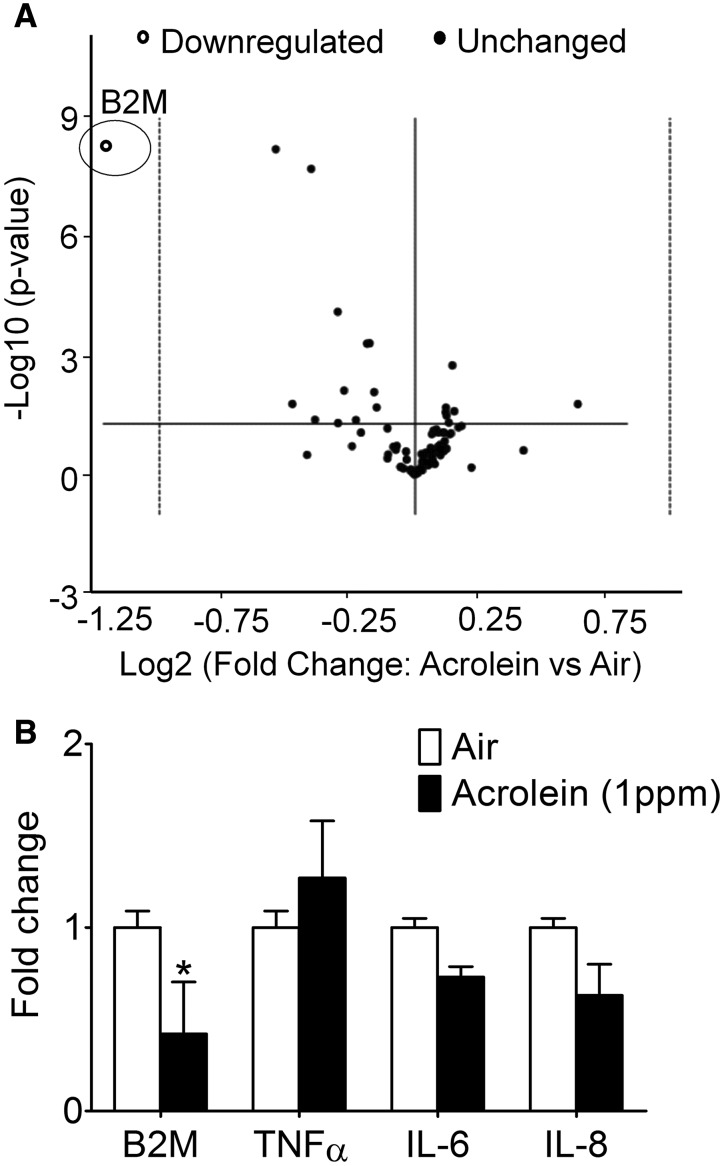
Because acrolein is known to deplete reduced GSH levels and trigger Nrf2 activation (Tirumalai et al., 2002) by an “oxidative stress” mechanism, we measured a stable marker of oxidative stress. Consistent with elevated lung oxidative stress, the levels of MDA were modestly (+10%), but significantly increased in the lungs of acrolein-exposed (1 ppm) mice relative to controls (Figure 3A). To assess the lung response, we also measured Nrf2 and antioxidant proteins (SOD1-3 and catalase) induced by oxidant exposure, including the highly inducible HO-1 (Zhang and Forman, 2008) and COX-2 (Mogel et al., 2011). Except SOD1 (unchanged abundance), all these proteins were induced significantly by chronic acrolein (1 ppm) exposure indicating an ‘oxidative stress’ response (Figure 3B). Because there appeared to be a compensatory response in the antioxidant proteins in the lungs, we screened, by gene array, whole lung derived gene transcripts for potential response patterns. Although gene analysis revealed distinct patterns of “hot spots” and “cold spots” between air- and acrolein-exposed mice (Supplementary Table 1), a volcano plot of principle component analysis showed that only the β-2 microglobulin (B2M) gene was down-regulated significantly (Figure 4A), and this change was confirmed by qRT-PCR (Figure 4B).
Figure 3.
Pulmonary oxidant stress and antioxidant enzymes. Mice were exposed to HEPA-filtered air or acrolein (1 ppm, 6 h/day, 5 days/week) for 12 weeks. A, Pulmonary malonaldialdehyde (MDA) levels in air- and acrolein-exposed mice (n = 8/group). B, Levels of antioxidant proteins: Nrf2, COX-2, HO-1, SOD-1 (CuZn-SOD), SOD-2 (Mn-SOD), SOD-3 (EC-SOD) and, catalase in air- and acrolein-exposed mice. Values are mean ± SEM. n = 8/group. *p < .05 vs. air.
Figure 4.
Unbiased pulmonary gene analysis. Mice were exposed to HEPA-filtered air or acrolein (1 ppm, 6 h/day, 5 days/week) for 12 weeks. A, Volcano plot of principle components of gene array displaying statistical significance versus fold-change on the y- and x-axes, respectively. B, Gene expression of B2M, TNFα, IL-6, and IL-8 confirmed by qRT-PCR (n = 8/group). Values are mean ± SEM. *p < .05 vs. Air.
Because our previous research had shown that acrolein induces endoplasmic reticulum (ER) stress in endothelial cells (Haberzettl et al., 2009) and cardiomyocytes (Keith et al., 2009), we tested whether markers of ER stress were induced in the lungs of acrolein-exposed mice. For this, we screened lung mRNAs for ER stress-induced chaperone proteins: GRP78, GRP94, and HERP. Surprisingly, none of the three chaperone mRNAs was upregulated following either 0.5 ppm or 1ppm acrolein exposure, and in fact, all the three chaperones were significantly downregulated (Supplementary Figure 1). These data suggest that chronic acrolein exposure does not induce sustained ER stress in the lungs.
Acrolein and Insulin Resistance
Cigarette smoking and intense use of moist snuff (150 g/week) increase the risk for type-2 diabetes (Persson et al., 2000). Some, but not all, studies have shown that smoking is associated with an increase in systemic insulin resistance (Eliasson et al., 1994; Facchini et al., 1992; Keith et al., 2016; Persson et al., 2000). We observed that fasting blood glucose, insulin and HOMA-IR levels in mice exposed to acrolein (1 ppm) for 12 weeks were comparable with controls. A glucose tolerance test (GTT) after 10 weeks of exposure revealed no difference in glucose handling between acrolein- and air-exposed mice (Supplementary Figure 2A and B).
Systemic Toxicity of Inhaled Acrolein
Chronic exposure to acrolein (0.5 or 1 ppm) did not affect overall body and organ weights (Supplementary Table 2) or plasma levels of liver (ALT, AST), muscle (CK), and lactate dehydrogenase (LDH) enzymes, total protein, albumin, and creatinine (Table 1) suggesting that inhaled acrolein (at 1 ppm) did not induce gross organ toxicity. Despite the lack of obvious effects on body growth and organ toxicity, the CBC in mice exposed to 1 ppm acrolein revealed a significant decrease in circulating leukocytes (−50%; p < .05) primarily a result of decreased lymphocytes (−48%; p < .05), neutrophils (−45%; p < .05), and monocytes (−40%; p < .05; Table 2) with little effect on RBCs suggesting effects on leukocytes were relatively selective.
Table 1.
Plasma Parameters of Male C57BL/6 Mice Exposed to Air or Acrolein
| Parameters | Air | Acrolein (1 ppm) |
|---|---|---|
| Blood glucose (mg/dl) | 259 ± 9 | 248 ± 6 |
| Cholesterol (mg/dl) | 117 ± 3 | 121 ± 5 |
| Triglycerides (mg/dl) | 34 ± 2 | 31 ± 1 |
| Total Protein (g/dl) | 4.0 ± 0.1 | 4.0 ± 0.1 |
| Albumin (g/dl) | 2.4 ± 0.04 | 2.4 ± 0.04 |
| Alanine aminotransferase (U/l) | 22 ± 1 | 25 ± 4 |
| Aspartate aminotransferase (U/l) | 45 ± 3 | 51 ± 4 |
| Creatine kinase (U/l) | 110 ± 13 | 110 ± 23 |
| Creatinine (mg/dl) | 0.27 ± 0.01 | 0.24 ± 0.01 |
| Lactate dehydrogenase (U/l) | 162 ± 10 | 172 ± 11 |
Values are mean ± SEM. No statistically significant differences between groups.
Table 2.
Complete Blood Counts (CBC) of C57BL/6 Mice Exposed to Air or Acrolein
| Parameters | Air | Acrolein (0.5 ppm) | Acrolein (1 ppm) |
|---|---|---|---|
| WBC/µl | 2256 ± 184 | 1791 ± 208 | 1187 ± 174* |
| Neutrophils/µl | 484 ± 69 | 444 ± 58 | 264 ± 31* |
| Lymphocytes/µl | 1658 ± 140 | 1227 ± 183 | 871 ± 148* |
| Monocytes/µl | 83 ± 13 | 58 ± 9 | 50 ± 8* |
| Eosinophils/µl | Traces | Traces | Traces |
| Basophils/µl | Traces | Traces | Traces |
| Platelets (103/µl) | 851 ± 57 | 750 ± 50 | 939 ± 72 |
| RBC (106/µl) | 7.8 ± 0.4 | 7.9 ± 0.6 | 7.4 ± 0.5 |
| Hemoglobin (g/dl) | 11.1 ± 0.4 | 11.2 ± 0.2 | 10.9 ± 0.8 |
| Hematocrit (%) | 39 ± 2 | 37 ± 3 | 42 ± 3 |
| Mean corpuscular volume (fl) | 51 ± 1 | 46 ± 1 | 57 ± 0.2 |
| Mean corpuscular hemoglobin (pg) | 13.8 ± 0.2 | 13.3 ± 0.1 | 14.8 ± 0.1 |
| Mean corpuscular hemoglobin concentration (g/dl) | 27.4 ± 0.3 | 28 ± 0.3 | 26 ± 0.1 |
| Red cell distribution (%) | 16.4 ± 0.4 | 17.5 ± 0.2 | 14.1 ± 0.2 |
Values are mean ± SEM.
p < .05 vs. Air.
Acrolein-Induced Leukocyte and Cytokine Suppression
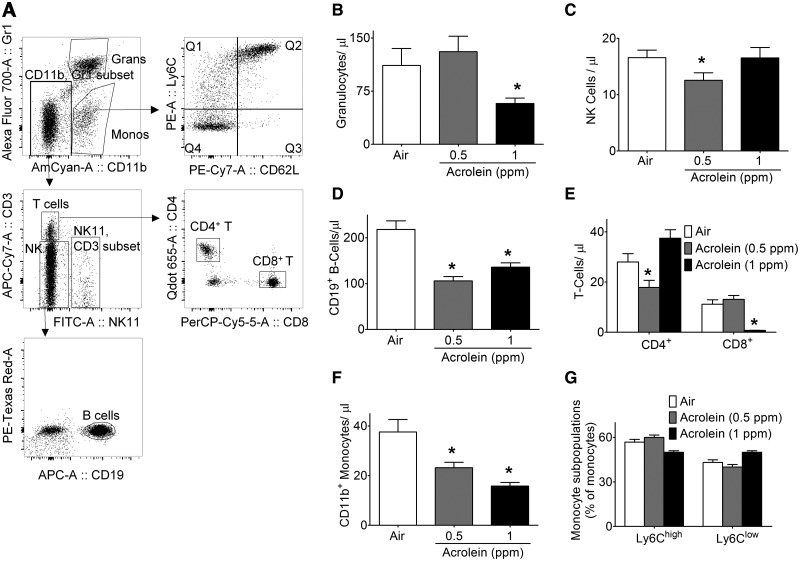
To better understand the selective hematological effects of acrolein, we quantified changes in leukocyte subpopulations by flow cytometry. Cells were gated as illustrated in Figure 5A. Flow cytometry revealed a dose-dependent suppression of overall leukocytes that confirmed CBC results (Table 2). Exposure to 0.5 ppm acrolein significantly decreased the abundance of NK1.1+-, CD19+B-, and CD4+T-cells and CD11b+ monocytes (Figure 5). Acrolein (1 ppm), in addition to CD19+B-cells and CD11b+ monocytes, also significantly decreased the levels of Gr1+ granulocytes, and CD8+ T-cells, but had no effect on the abundance of CD4+ T-cells and NK1.1+ cells (Figure 5). Despite the suppression of CD11b+ cells, acrolein did not alter the levels of either Ly6Chigh or Ly6Cl°w monocyte sub-populations (Figure 5G).
Figure 5.
Abundance of circulating blood immune cells of acrolein-exposed mice. Mice were exposed to HEPA-filtered air (open bars) or acrolein (0.5 [gray bars] or 1ppm [black bars], 6 h/day, 5 days/week) for 12 weeks. A, Flow cytometry gating scheme for immune cell differentials. Levels of: B, GR1+ granulocytes; C, NK1.1+ natural killer cells; D, CD19+ B-cell lymphocytes; E, CD4+ and CD8+ T-cell lymphocytes; F, CD11b+ monocytes; and G, Ly6Chigh and Ly6Clow subsets of monocytes in blood of mice exposed to air or acrolein for 12 weeks (n = 14–15/group). Values are mean ± SEM. *p < .05 vs. Air.
Because smoking induces systemic inflammation, and pro-inflammatory cytokines are strong predictors of future cardiovascular events (Tracy et al., 1997), we measured the levels of multiple cytokines and chemokines in the plasma of air- and acrolein-exposed mice. Surprisingly, none of the plasma cytokines measured were increased by acrolein (Table 3). In fact, IL-6 and KC (murine homolog of human IL-8) levels were significantly (p < .05) decreased at 1 ppm acrolein (Table 3).
Table 3.
Cytokines/Chemokines Measured in the Plasma of Air- and Acrolein-Exposed Mice
| Parameters | Air | Acrolein (0.5 ppm) | Acrolein (1 ppm) |
|---|---|---|---|
| TNFα (pg/ml) | 5.7 ± 0.8 | 3.2 ± 0.8 | 3.6 ± 0.9 |
| IL-6 (pg/ml) | 10 ± 2 | 7 ± 1 | 5 ± 1* |
| IL-10 (pg/ml) | 6 ± 1 | 10 ± 3 | 6 ± 1 |
| IL-17 (pg/ml) | 2.4 ± 0.5 | 4.2 ± 1.0 | 2.3 ± 0.5 |
| IFNγ (pg/ml) | 3.3 ± 0.5 | 4.3 ± 1.0 | 4.9 ± 1.0 |
| Eotaxin (pg/ml) | 666 ± 43 | 641 ± 47 | 760 ± 47 |
| MCP-1 (pg/ml) | 93 ± 10 | 91 ± 9 | 102 ± 12 |
| RANTES (pg/ml) | 19 ± 4 | 19 ± 4 | 19 ± 5 |
| PAI-1 (ng/ml) | 1.82 ± 0.22 | 2.30 ± 0.13 | 1.66 ± 0.17 |
| VEGF (pg/ml) | 1.03 ± 0.04 | 1.00 ± 0.03 | 1.02 ± 0.06 |
| KC (pg/ml) | 169 ± 33 | 158 ± 19 | 86 ± 11 |
| E-Selectin (ng/ml) | 406 ± 39 | 391 ± 43 | 221 ± 30* |
| P-Selectin (ng/ml) | 392±34 | 379±45 | 293 ± 26 |
| PF4 (pg/ml) | 65 ± 18 | Not measured | 32 ± 10* |
| Platelet–Leukocyte Aggregates (% of Leukocytes) | 27 ± 1 | 29 ± 2 | 29 ± 2 |
Values are mean ± SEM. *p < .05 vs. Air.
Acrolein and Endothelial Progenitor Cells
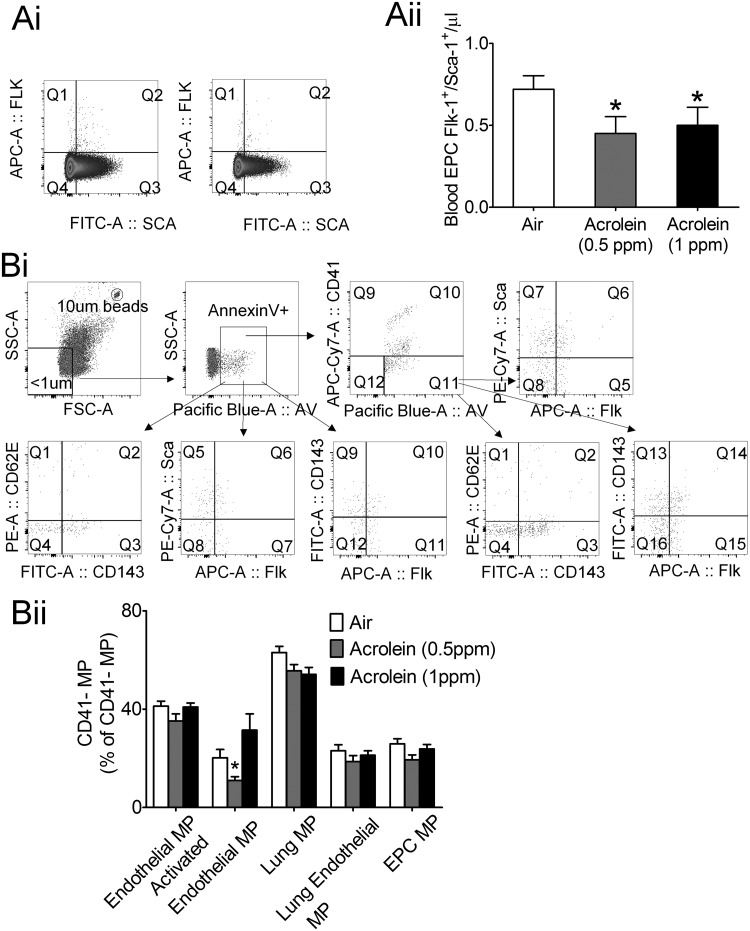
Decreased endothelial progenitor cell (EPC) levels are associated with an increased CVD risk (Fadini et al., 2012), and may predict future cardiovascular events (Werner et al., 2005). Secondhand smoke and smoking cessation are associated with altered EPC number and function (Heiss et al., 2008; Kondo et al., 2004), and our previous research show that just 4 consecutive days of exposure to 1 ppm acrolein suppresses circulating EPCs (Flk-1+/Sca-1+) by 50% in mice (Wheat et al., 2011). Consistent with acrolein-induced acute effects, chronic acrolein suppressed circulating EPCs by 40–50% (Figure 6A). Acute exposure to 1 ppm acrolein (4-days) increases the outgrowth of bone marrow-derived EPCs that likely reflects an increased number of bone marrow-resident EPCs (Wheat et al., 2011). In contrast, our current chronic (12 weeks) acrolein exposure (1 ppm) did not change the number of bone marrow resident EPCs relative to air controls (data not shown), which likely reflects an acclimation to chronic exposure.
Figure. 6.
Effect of acrolein on circulating endothelial progenitor cells (EPC) and endothelial cell-derived microparticles. Mice were exposed to HEPA-filtered air or acrolein (0.5 or 1 ppm, 6 h/day, 5 days/week) for 12 weeks. A (i), Flow cytometry gating scheme for blood EPC detection. A (ii), Summary data of circulating EPCs (Flk-1+/Sca-1+ cells) in blood of mice after 12 weeks of exposure to air or acrolein (n = 13–15/group). B (i), Flow cytometry gating scheme for the quantitation of endothelial cell-derived microparticles (MP). B (ii), Summary data of circulating endothelial cell-derived MP in air- and acrolein-exposed mice (n = 13–15/group). Values are mean ± SEM. *p < .05 vs. Air.
Acrolein and Microparticles
Another marker of endothelial activation is the level of circulating endothelial cell-derived microparticles (Boulanger et al., 2001). Increased levels of endothelial cell microparticles (MPs) have been observed in cardiovascular diseases characterized by impaired endothelium function such as in coronary artery disease and stroke (Simak et al., 2006; Werner et al., 2006). Chronic acrolein exposure, however, did not increase levels of circulating endothelial-derived MPs (<1 µm; Annexin V+/CD41−/Flk-1+), activated endothelial MPs (<1 µm; Annexin V+/CD41−/CD62E+), lung-derived MPs (<1 µm; Annexin V+/CD41−/CD143+), lung activated endothelial MPs (<1 µm; Annexin V+/CD41−/CD62E+/CD143+) or EPC-derived MPs (<1 µm; Annexin V+/CD41−/Flk-1+/Sca-1+; Figure 6B). Collectively, these data suggest that chronic exposure to acrolein (1 ppm) does not stimulate endothelial MP formation.
Acrolein and Platelet Activation
Although acute acrolein or cigarette smoke exposure increases platelet activation in mice (Sithu et al., 2008, 2010), chronic exposure to acrolein for 12 weeks neither affected total platelet counts (Table 2) nor the number of platelet–leukocyte aggregates (Table 3) as measured by CBC and flow cytometry, respectively. In fact, the platelet factor 4 (PF4) level in plasma was significantly depressed in chronic exposure to acrolein (1 ppm) (Table 3). Collectively, our data suggest that chronic exposure to acrolein does not induce persistent platelet activation in mice.
DISCUSSION
Acrolein is a ubiquitous pollutant present in air, water, food, and beverages. Its levels are dramatically increased by incomplete combustion of organic materials and acrolein is highest in smoke of smoldering cigarettes and structural fires (50–60 ppm) (Ghilarducci and Tjeerdema, 1995). Although acutely inhaled high-level acrolein (100–275 ppm) is toxic to cardiopulmonary targets (Conklin et al., 2016), acute exposure to lower levels (≤1 ppm) of acrolein selectively targets the cardiovascular system including induction of thrombosis (Sithu et al., 2010), arrhythmias (Hazari et al., 2011), cardiac dysfunction (Thompson et al., 2016), and diminished vascular repair (Wheat et al., 2011). Although one theoretical study estimates a measureable risk for pulmonary injury at ambient acrolein level (Woodruff et al., 2007), the chronic systemic effects of low level acrolein exposure have not been studied. Moreover, emerging tobacco products such as e-cigarettes that do not burn tobacco still generate acrolein during heating of the humectants including glycerol (Kosmider et al., 2014).
In this study we found that, as expected, exposures to 0.5 and 1 ppm acrolein increased the urinary levels of 3-HPMA in a dose-related manner. The level of 3-HPMA in urine of tobacco smoke-exposed mice was between that of urinary 3-HPMA of mice exposed to 0.5 and 1 ppm acrolein, respectively, suggesting that the level of acrolein in MCS, ie, 12 - 3R4F KY Reference cigarettes by ISO protocol over 6 h, is consistent with our chronic acrolein exposures. Two important inferences follow this observation: (1) time-dependent urine collection provides an estimate of acrolein clearance (ie, likely less than 90 min in mice). If this is comparable with clearance rate in humans, then random or convenience urine sampling from human subjects (eg, urine collected more than 3 h after exposure) likely will underestimate the contribution of 3-HPMA as a biomarker of acrolein exposure; and (2) our urine collection protocol reveals that the excretion pattern of 3-HPMA is similar following either acrolein alone or cigarette smoke exposure indicating that nicotine (or other constituents) likely has little effect on either the metabolism of acrolein or the clearance of 3-HPMA, respectively. This is important because it suggests that urinary 3-HPMA excretion is a useful and consistent biomarker of acute acrolein exposure (regardless of its source) (Nelson and Boor, 1982), and it certainly makes 3-HPMA a potentially viable biomarker of exposure to other acrolein-containing aerosols, eg, car exhaust, e-cigarettes or hookah (Kassem et al., 2014).
Our data also provide new evidence that chronic exposure of young, healthy mice to 0.5 ppm acrolein (low level) results in persistent effects in the cardiovascular compartment, eg, suppression of circulating EPCs and CD19+ B-cells and CD11b+ monocytes. Moreover, we show the lungs undergo persistent Nrf2 activation and induction of acrolein-metabolizing enzymes and antioxidant proteins in an apparent compensatory response to ongoing oxidative stress (albeit in the absence of either inflammatory or ER stress markers). From these data, we infer that low-level acrolein is sufficient to upregulate pulmonary defenses in metabolic protection (ie, GSTP and AR) that itself is a strong biomarker of acrolein exposure. Surprisingly, this antioxidant armamentarium appears insufficient, however, to quell acrolein-induced changes in circulating blood cells including chronic suppression of EPCs—a strong marker of CVD risk (Hill et al., 2003). Because circulating EPCs are suppressed by chronic exposure to acrolein (0.5 ppm) alone, we provide herein a biologically plausible mechanism to support the association between urinary 3-HPMA and increased CVD risk observed in human smokers and non-smokers alike (DeJarnett et al., 2014). Whether acrolein in cigarette smoke accounts for effects on circulating EPCs of acute (increased but dysfunctional) (Heiss et al., 2008) or chronic exposure (depressed but increased after cessation) (Kondo et al., 2004) remains to be seen.
Persistent biomarkers of harm prove useful both as a tool for evaluation of risk but also for insight into the mechanism of action. With regard to the latter, chronic acrolein persistently suppresses EPCs despite an upregulation of key metabolic enzymes, ie, GSTP and AR, for its detoxification. There are numerous examples of how these 2 specific enzymes provide acute and chronic protection to cardiovascular organs during acrolein exposure as occurs in ischemia-reperfusion of the heart (Conklin et al., 2015a; Wetzelberger et al., 2010), cardiotoxicity of cyclophosphamide (Conklin et al., 2015b), and cigarette smoke- or acrolein-induced endothelium dysfunction (Conklin et al., 2009b). It is expected that the induction of AR and GSTP collectively augment acrolein metabolism and detoxification at the level of entry of acrolein into the lung (although we did not measure in the nasal/upper airways). Yet, chronic acrolein exposure continually suppressed circulating EPCs. It is not clear why the robust induction of lung enzymes that metabolize acrolein, GSTP and AR, did not normalize EPC number. There are two likely explanations: (1) EPC downregulation occurs via a mechanism preceding acrolein metabolism; or (2) EPC downregulation is triggered by an acrolein metabolite. Future studies are required to distinguish between these and other possibilities.
Before the development of chronic lung diseases such as cancer, COPD and emphysema, cigarette smoking increases the risk for more frequent, and more severe upper respiratory infections (Arcavi and Benowitz, 2004; Trosini-Desert et al., 2004). Among the mechanisms considered responsible for the association between smoking and recurrent infections include suppression of lung defenses, eg, mucociliary transport and host immunity (Johnson et al., 1990). Acrolein is thought a likely constituent of cigarette smoke contributing to these effects, and indeed acrolein inhibits mucociliary transport, decreases immune cell function and induces alveolar macrophage apoptosis (Li et al., 1997). One mechanism by which acrolein suppresses immune function is by inhibiting NF-κB signaling (Lambert et al., 2005, 2007; Li and Holian, 1998; Li et al., 1999). Although we did not investigate pulmonary inflammatory response to an infectious challenge per se, we did not observe increased markers of inflammation (or ER stress) supporting the idea that acrolein alone (at 1 ppm or lower) does not induce pulmonary inflammation. Studies using higher levels of inhaled acrolein (2–3 ppm) that induce pulmonary epithelial damage and mucus hypersecretion observe increased inflammatory/immune responses (Borchers et al., 1999, 2009). Nonetheless, we observed broad-based immune cell suppression in the systemic circulation, which has major implications regarding systemic defenses against infectious agents—a condition present in cigarette smokers (Arcavi and Benowitz, 2004). Interestingly, recent investigations show e-cigarette users have strong suppression of pulmonary host defense as seen in cigarette smokers (Martin et al., 2016). Moreover, because many e-liquids contain flavorants, such as cinnamaldehyde, it is possible that host immune suppression is, in part, a collective effect of all the α,β-unsaturated aldehydes in e-cigarette aerosols. This possibility remains to be tested. Further studies also are required to: (1) establish the threshold dose of acrolein exposure for various biomarkers of harm; and (2) delineate the mechanisms of chronic acrolein inhalation-induced systemic toxicity. Nonetheless, our current results provide novel insights into the effects of chronic exposure to acrolein at levels likely encountered during exposure to aerosols of combusted and heated tobacco products including hookah (Kassem et al., 2014), e-cigarettes (Kosmider et al., 2014), and mainstream and secondhand cigarette smoke (see Figure 1) making the results of our study applicable to multiple scenarios of acrolein exposure. Thus, these findings implicate chronic low level acrolein (<0.5 ppm) exposure as conferring increased risk for cardiovascular and potentially pulmonary diseases, and as such, this may be a “benchmark level” useful in setting regulatory standards for acrolein generation of new and emerging tobacco products.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We thank K. Dixon, A. Hand, K. Moorman, D. Mosley, A. Ribble, M. Smith, W. Theis, P. Whang, and M.G. Winner for their technical assistance. Dr S. Rai partially supported by the Wendell Cherry Chair in Clinical Trial Research. The authors have declared that no conflicts of interest exist.
FUNDING
NIH (HL120163, HL120746, HL122676, and GM103492).
REFERENCES
- Appelman L. M., Hendriksen C. F., Feron V. J. (1981). Repeated exposure to alpha-ethylacrolein vapour: Subacute toxicity study in rats. Toxicology 22, 79–87. [DOI] [PubMed] [Google Scholar]
- Arcavi L., Benowitz N. L. (2004). Cigarette smoking and infection. Arch. Intern. Med. 164, 2206–2216. [DOI] [PubMed] [Google Scholar]
- Borchers M. T., Wesselkamper S., Wert S. E., Shapiro S. D., Leikauf G. D. (1999). Monocyte inflammation augments acrolein-induced Muc5ac expression in mouse lung. Am. J. Physiol. 277, L489–L497. [DOI] [PubMed] [Google Scholar]
- Borchers M. T., Wesselkamper S. C., Deshmukh H., Beckman E., Medvedovic M., Sartor M., Leikauf G. D. (2009). The role of T cells in the regulation of acrolein-induced pulmonary inflammation and epithelial-cell pathology. Res. Rep. Health Eff. Inst. 146, 5–29. [PubMed] [Google Scholar]
- Boulanger C. M., Scoazec A., Ebrahimian T., Henry P., Mathieu E., Tedgui A., Mallat Z. (2001). Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 104, 2649–2652. [DOI] [PubMed] [Google Scholar]
- Conklin D. J., Barski O. A., Lesgards J. F., Juvan P., Rezen T., Rozman D., Prough R. A., Vladykovskaya E., Liu S., Srivastava S., et al. (2010). Acrolein consumption induces systemic dyslipidemia and lipoprotein modification. Toxicol. Appl. Pharmacol. 243, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J., Guo Y., Jagatheesan G., Kilfoil P. J., Haberzettl P., Hill B. G., Baba S. P., Guo L., Wetzelberger K., Obal D., et al. (2015a). Genetic deficiency of glutathione S-transferase P increases myocardial sensitivity to ischemia-reperfusion injury. Circ. Res. 117, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J., Haberzettl P., Jagatheesan G., Baba S., Merchant M. L., Prough R. A., Williams J. D., Prabhu S. D., Bhatnagar A. (2015b). Glutathione S-transferase P protects against cyclophosphamide-induced cardiotoxicity in mice. Toxicol. Appl. Pharmacol. 285, 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J., Haberzettl P., Jagatheesan G., Kong M., Hoyle G. W. (2016). Role of TRPA1 in acute cardiopulmonary toxicity of inhaled acrolein. Toxicol. Appl. Pharmacol. 324, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J., Haberzettl P., Lesgards J. F., Prough R. A., Srivastava S., Bhatnagar A. (2009a). Increased sensitivity of glutathione S-transferase P-null mice to cyclophosphamide-induced urinary bladder toxicity. J. Pharmacol. Exp. Ther. 331, 456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J., Haberzettl P., Prough R. A., Bhatnagar A. (2009b). Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. Am. J. Physiol. Heart Circ. Physiol. 296, H1586–H1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J., Prough R. A., Juvan P., Rezen T., Rozman D., Haberzettl P., Srivastava S., Bhatnagar A. (2011). Acrolein-induced dyslipidemia and acute-phase response are independent of HMG-CoA reductase. Mol. Nutr. Food Res. 55, 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBethizy J. D., Borgerding M. F., Doolittle D. J., Robinson J. H., McManus K. T., Rahn C. A., Davis R. A., Burger G. T., Hayes J. R., Reynolds JHt., et al. (1990). Chemical and biological studies of a cigarette that heats rather than burns tobacco. J. Clin. Pharmacol. 30, 755–763. [DOI] [PubMed] [Google Scholar]
- DeJarnett N., Conklin D. J., Riggs D. W., Myers J. A., O'Toole T. E., Hamzeh I., Wagner S., Chugh A., Ramos K. S., Srivastava S., et al. (2014). Acrolein exposure is associated with increased cardiovascular disease risk. J. Am. Heart Assoc. 3, e000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaillats H., Spaulding R. S., Charles M. J. (2002). Ambient air measurement of acrolein and other carbonyls at the Oakland-San Francisco Bay Bridge Toll Plaza. Environ. Sci. Technol. 36, 2227–2235. [DOI] [PubMed] [Google Scholar]
- Dong J. Z., Moldoveanu S. C. (2004). Gas chromatography-mass spectrometry of carbonyl compounds in cigarette mainstream smoke after derivatization with 2,4-dinitrophenylhydrazine. J. Chromatogr. A 1027, 25–35. [DOI] [PubMed] [Google Scholar]
- Eliasson B., Attvall S., Taskinen M. R., Smith U. (1994). The insulin resistance syndrome in smokers is related to smoking habits. Arterioscler. Thromb. 14, 1946–1950. [DOI] [PubMed] [Google Scholar]
- Facchini F. S., Hollenbeck C. B., Jeppesen J., Chen Y. D., Reaven G. M. (1992). Insulin resistance and cigarette smoking. Lancet 339, 1128–1130. [DOI] [PubMed] [Google Scholar]
- Fadini G. P., Losordo D., Dimmeler S. (2012). Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 110, 624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron V. J., Til H. P., de Vrijer F., Woutersen R. A., Cassee F. R., van Bladeren P. J. (1991). Aldehydes: Occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res. 259, 363–385. [DOI] [PubMed] [Google Scholar]
- Ghilarducci D. P., Tjeerdema R. S. (1995). Fate and effects of acrolein. Rev. Environ. Contam. Toxicol. 144, 95–146. [DOI] [PubMed] [Google Scholar]
- Haberzettl P., Lee J., Duggineni D., McCracken J., Bolanowski D., O'Toole T. E., Bhatnagar A., Conklin D. J. (2012). Exposure to ambient air fine particulate matter prevents VEGF-induced mobilization of endothelial progenitor cells from the bone marrow. Environ. Health Perspect. 120, 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl P., Vladykovskaya E., Srivastava S., Bhatnagar A. (2009). Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicol. Appl. Pharmacol. 234, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann H. J. (2012). Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem. Res. Toxicol. 25, 794–810. [DOI] [PubMed] [Google Scholar]
- Hazari M. S., Haykal-Coates N., Winsett D. W., Krantz Q. T., King C., Costa D. L., Farraj A. K. (2011). TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ. Health Perspect. 119, 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss C., Amabile N., Lee A. C., Real W. M., Schick S. F., Lao D., Wong M. L., Jahn S., Angeli F. S., Minasi P., et al. (2008). Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: Sustained vascular injury and blunted nitric oxide production. J. Am. Coll. Cardiol. 51, 1760–1771. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Zalos G., Halcox J. P., Schenke W. H., Waclawiw M. A., Quyyumi A. A., Finkel T. (2003). Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 348, 593–600. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Houchens D. P., Kluwe W. M., Craig D. K., Fisher G. L. (1990). Effects of mainstream and environmental tobacco smoke on the immune system in animals and humans: A review. Crit. Rev. Toxicol. 20, 369–395. [DOI] [PubMed] [Google Scholar]
- Kassem N. O., Daffa R. M., Liles S., Jackson S. R., Kassem N. O., Younis M. A., Mehta S., Chen M., Jacob P. III, Carmella S. G., et al. (2014). Children's exposure to secondhand and thirdhand smoke carcinogens and toxicants in homes of hookah smokers. Nicotine Tob. Res. 16, 961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith R. J., Al Rifai M., Carruba C., De Jarnett N., McEvoy J. W., Bhatnagar A., Blaha M. J., Defilippis A. P. (2016). Tobacco use, insulin resistance, and risk of type 2 diabetes: Results from the multi-ethnic study of atherosclerosis. PLoS One 11, e0157592.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith R. J., Haberzettl P., Vladykovskaya E., Hill B. G., Kaiserova K., Srivastava S., Barski O., Bhatnagar A. (2009). Aldose reductase decreases endoplasmic reticulum stress in ischemic hearts. Chem. Biol. Interact. 178, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Hayashi M., Takeshita K., Numaguchi Y., Kobayashi K., Iino S., Inden Y., Murohara T. (2004). Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler. Thromb. Vasc. Biol. 24, 1442–1447. [DOI] [PubMed] [Google Scholar]
- Kosmider L., Sobczak A., Fik M., Knysak J., Zaciera M., Kurek J., Goniewicz M. L. (2014). Carbonyl compounds in electronic cigarette vapors-effects of nicotine solvent and battery output voltage. Nicotine Tob. Res. 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C., Li J., Jonscher K., Yang T. C., Reigan P., Quintana M., Harvey J., Freed B. M. (2007). Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J. Biol. Chem 282, 19666–19675. [DOI] [PubMed] [Google Scholar]
- Lambert C., McCue J., Portas M., Ouyang Y., Li J., Rosano T. G., Lazis A., Freed B. M. (2005). Acrolein in cigarette smoke inhibits T-cell responses. J. Allergy Clin. Immunol. 116, 916–922. [DOI] [PubMed] [Google Scholar]
- Li L., Hamilton R. F. Jr, Holian A. (1999). Effect of acrolein on human alveolar macrophage NF-kappaB activity. Am. J. Physiol. 277, L550–L557. [DOI] [PubMed] [Google Scholar]
- Li L., Hamilton R. F. Jr., Taylor D. E., Holian A. (1997). Acrolein-induced cell death in human alveolar macrophages. Toxicol. Appl. Pharmacol. 145, 331–339. [DOI] [PubMed] [Google Scholar]
- Li L., Holian A. (1998). Acrolein: A respiratory toxin that suppresses pulmonary host defense. Rev. Environ. Health 13, 99–108. [PubMed] [Google Scholar]
- Martin E. M., Clapp P. W., Rebuli M. E., Pawlak E. A., Glista-Baker E., Benowitz N. L., Fry R. C., Jaspers I. (2016). E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am. J. Physiol. Lung Cell Mol. Physiol. 311, L135–L144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley T. R., Hopke P. K., Zhao J., Babaian S. (2012). Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal. Toxicol. 24, 850–857. [DOI] [PubMed] [Google Scholar]
- Medicine I. O. 2010. Secondhand Smoke Exposure and Cardiovascular Effects. The National Academies, Washington, D.C. [PubMed] [Google Scholar]
- Mogel I., Baumann S., Bohme A., Kohajda T., von Bergen M., Simon J. C., Lehmann I. (2011). The aromatic volatile organic compounds toluene, benzene and styrene induce COX-2 and prostaglandins in human lung epithelial cells via oxidative stress and p38 MAPK activation. Toxicology 289, 28–37. [DOI] [PubMed] [Google Scholar]
- Nelson T. J., Boor P. J. (1982). Allylamine cardiotoxicity–IV. Metabolism to acrolein by cardiovascular tissues. Biochem. Pharmacol. 31, 509–514. [DOI] [PubMed] [Google Scholar]
- O'Toole T. E., Abplanalp W., Li X., Cooper N., Conklin D. J., Haberzettl P., Bhatnagar A. (2014). Acrolein decreases endothelial cell migration and insulin sensitivity through induction of let-7a. Toxicol. Sci. 140, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T. E., Zheng Y. T., Hellmann J., Conklin D. J., Barski O., Bhatnagar A. (2009). Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicol. Appl. Pharmacol. 236, 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn A., Nath R., Pan J., Chen L., Widmer K., Henk W., Chung F. L. (2001). 1,N(2)-propanodeoxyguanosine adduct formation in aortic DNA following inhalation of acrolein. Environ. Health Perspect. 109, 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson P. G., Carlsson S., Svanstrom L., Ostenson C. G., Efendic S., Grill V. (2000). Cigarette smoking, oral moist snuff use and glucose intolerance. J. Intern. Med. 248, 103–110. [DOI] [PubMed] [Google Scholar]
- Pope C. A. III, Bhatnagar A., McCracken J. P., Abplanalp W., Conklin D. J., O'Toole T. (2016). Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ. Res. 119, 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansbury B. E., Cummins T. D., Tang Y., Hellmann J., Holden C. R., Harbeson M. A., Chen Y., Patel R. P., Spite M., Bhatnagar A., et al. (2012). Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circ. Res. 111, 1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihadeh A., Salman R., Jaroudi E., Saliba N., Sepetdjian E., Blank M. D., Cobb C. O., Eissenberg T. (2012). Does switching to a tobacco-free waterpipe product reduce toxicant intake? A crossover study comparing CO, NO, PAH, volatile aldehydes, “tar” and nicotine yields. Food Chem. Toxicol. 50, 1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simak J., Gelderman M. P., Yu H., Wright V., Baird A. E. (2006). Circulating endothelial microparticles in acute ischemic stroke: A link to severity, lesion volume and outcome. J. Thromb. Haemost. 4, 1296–1302. [DOI] [PubMed] [Google Scholar]
- Sithu S. D., Srivastava S., Conklin D. J., O'Toole T. E., Bhatnagar A., D'Souza S. E. (2008). Platelet Sensitivity is Increased by Acrolein. FASEB J. 22, 897–896. [Google Scholar]
- Sithu S. D., Srivastava S., Siddiqui M. A., Vladykovskaya E., Riggs D. W., Conklin D. J., Haberzettl P., O'Toole T. E., Bhatnagar A., D'Souza S. E. (2010). Exposure to acrolein by inhalation causes platelet activation. Toxicol. Appl. Pharmacol. 248, 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Sithu S. D., Vladykovskaya E., Haberzettl P., Hoetker D. J., Siddiqui M. A., Conklin D. J., D'Souza S. E., Bhatnagar A. (2011). Oral exposure to acrolein exacerbates atherosclerosis in apoE-null mice. Atherosclerosis 215, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Vladykovskaya E., Barski O. A., Spite M., Kaiserova K., Petrash J. M., Chung S. S., Hunt G., Dawn B., Bhatnagar A. (2009). Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein E-null mice. Circ. Res. 105, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfill S. B., Calafat A. M., Brown C. R., Polzin G. M., Chiang J. M., Watson C. H., Ashley D. L. (2003). Concentrations of nine alkenylbenzenes, coumarin, piperonal and pulegone in Indian bidi cigarette tobacco. Food Chem. Toxicol. 41, 303–317. [DOI] [PubMed] [Google Scholar]
- Stepanov I., Jensen J., Hatsukami D., Hecht S. S. (2008). New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. Nicotine Tob. Res. 10, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. C., Ledbetter A. D., Haykal-Coates N., Cascio W. E., Hazari M. S., Farraj A. K. (2016). Acrolein inhalation alters myocardial synchrony and performance at and below exposure concentrations that cause ventilatory responses. Cardiovasc. Toxicol. 17, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirumalai R., Rajesh Kumar T., Mai K. H., Biswal S. (2002). Acrolein causes transcriptional induction of phase II genes by activation of Nrf2 in human lung type II epithelial (A549) cells. Toxicol. Lett. 132, 27–36. [DOI] [PubMed] [Google Scholar]
- Tracy R. P., Lemaitre R. N., Psaty B. M., Ives D. G., Evans R. W., Cushman M., Meilahn E. N., Kuller L. H. (1997). Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler. Thromb. Vasc. Biol. 17, 1121–1127. [DOI] [PubMed] [Google Scholar]
- Trosini-Desert V., Germaud P., Dautzenberg B. (2004). Tobacco smoke and risk of bacterial infection. Rev. Mal. Respir. 21, 539–547. [DOI] [PubMed] [Google Scholar]
- Vladykovskaya E., Sithu S. D., Haberzettl P., Wickramasinghe N. S., Merchant M. L., Hill B. G., McCracken J., Agarwal A., Dougherty S., Gordon S. A., et al. (2012). Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J. Biol. Chem. 287, 11398–11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner N., Kosiol S., Schiegl T., Ahlers P., Walenta K., Link A., Bohm M., Nickenig G. (2005). Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 353, 999–1007. [DOI] [PubMed] [Google Scholar]
- Werner N., Wassmann S., Ahlers P., Kosiol S., Nickenig G. (2006). Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 26, 112–116. [DOI] [PubMed] [Google Scholar]
- Wetzelberger K., Baba S. P., Thirunavukkarasu M., Ho Y. S., Maulik N., Barski O. A., Conklin D. J., Bhatnagar A. (2010). Postischemic deactivation of cardiac aldose reductase: Role of glutathione S-transferase P and glutaredoxin in regeneration of reduced thiols from sulfenic acids. J. Biol. Chem. 285, 26135–26148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat L. A., Haberzettl P., Hellmann J., Baba S. P., Bertke M., Lee J., McCracken J., O'Toole T. E., Bhatnagar A., Conklin D. J. (2011). Acrolein inhalation prevents vascular endothelial growth factor-induced mobilization of flk-1+/sca-1+ cells in mice. Arterioscler. Thromb. Vasc. Biol. 31, 1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R., Eichel L., Messing E. M., Schwarz E. (2001). Automated noninvasive measurement of cyclophosphamide-induced changes in murine micturition frequency and volume and demonstration of pharmacologic sensitivity. Urology 57, 115–116. [DOI] [PubMed] [Google Scholar]
- Woodruff T. J., Wells E. M., Holt E. W., Burgin D. E., Axelrad D. A. (2007). Estimating risk from ambient concentrations of acrolein across the United States. Environ. Health Perspect. 115, 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Forman H. J. (2008). Acrolein induces heme oxygenase-1 through PKC-delta and PI3K in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 38, 483–490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.