Abstract
Aims
CT calcium scoring (CTCS) and CT cardiac angiography (CTCA) are widely used in patients with stable chest pain to exclude significant coronary artery disease (CAD). We aimed to resolve uncertainty about the prevalence of obstructive coronary artery disease and long-term outcomes in patients with a zero-calcium score (ZCS).
Methods and results
Consecutive patients with stable cardiac symptoms referred for CTCS or CTCS and CTCA from chest pain clinics to a tertiary cardiothoracic centre were prospectively enrolled. In those with a ZCS, the prevalence of obstructive CAD on CTCA was determined. A follow-up for all-cause mortality was obtained from the NHS tracer service. A total of 3914 patients underwent CTCS of whom 2730 (69.7%) also had a CTCA. Half of the patients were men (50.3%) with a mean age of 56.9 years. Among patients who had both procedures, a ZCS was present in 52.2%, with a negative predictive value of 99.5% for excluding ≥70% stenosis on CTCA. During a mean follow-up of 5.2 years, the annual event rate was 0.3% for those with ZCS compared with 1.2% for CS ≥1. The presence of non-calcified atheroma on CTCA in patients with ZCS did not affect the prognostic value (P = 0.98).
Conclusion
In patients with stable symptoms and a ZCS, obstructive CAD is rare, and prognosis over the long-term is excellent, regardless of whether non-calcified atheroma is identified. A ZCS could reliably be used as a ‘gatekeeper’ in this patient cohort, obviating the need for further more expensive tests.
Keywords: coronary artery calcification , stable angina , coronary CT angiography , coronary artery disease , prognosis
Introduction
Computed tomography has become a cost-effective, mainstream technique for excluding coronary artery disease (CAD) in patients with stable symptoms.1–3 Non-contrast CT calcium scoring (CTCS) allows detection and quantification of coronary artery calcification (CAC), but CT coronary angiogram (CTCA) is required for imaging of calcified and non-calcified plaques as well as quantification of luminal stenoses.4 Often both techniques are performed sequentially. Numerous studies have demonstrated the very high negative predictive value of a normal CTCA in excluding significant CAD, which is now recommended in national and international guidelines, including recently updated UK’s NICE (National Institute for Health and Care Excellence) guidance.2,5 Based on the findings of landmark trials,6–8 a recent review has also concluded that CTCA should have a yet greater role in the diagnostic pathway of patients with stable chest pain.9 However, CTCA is not only more time consuming and costly when compared with CTCS, but also requires injection of a contrast agent, often with administration of beta-blockers adding to the procedural risk.
A zero-calcium score (ZCS) is associated with an excellent prognosis in asymptomatic people.10,11 However, in symptomatic populations with stable symptoms and a ZCS, there remains uncertainty about the prevalence of obstructive CAD and longer term outcomes. It is further uncertain how prognosis is affected by additional CTCA.12
Early studies of CTCS in patients with angina found that a ZCS was associated with a 2% prevalence of obstructive CAD (>50% stenosis) on invasive coronary angiography (ICA).11 But, more recent studies have reported up to 19% prevalence rate using CTCA.13,14 This has led to recommendations of using CTCA despite a ZCS.2 Although a large multi-centre study reported a low prevalence (3.5%) of more than 50% stenosis in patients with a ZCS and no effect on mortality at 2 years compared with patients with obstructive disease, there was increase in composite end-point driven with revascularisation leading the authors to conclude that a ZCS is associated with obstructive CAD and increased cardiovascular events.15
The main objective of this study was to determine the prevalence of significant CAD (≥70% diameter stenosis) in patients with ZCS and prognostic value of the latter in a large cohort of patients with stable symptoms over a long-term follow-up. A secondary objective was to determine prognosis stratified by calcium score in groups with and without significant CAD on CTCA.
Methods
This is an observational cohort study from prospectively collected data in Harefield Hospital Cardiac CT Registry from patients referred to a tertiary cardiac centre from six Rapid Access Chest Pain clinics (RACPC) in different hospitals for a cardiac CT. Consecutive patients referred for cardiac CT who presented with stable chest pain or dyspnoea with no prior history of CAD were included in the study. Those with previous percutaneous intervention, coronary artery bypass surgery, or known conditions in an advanced stage such as heart failure, arrhythmia, cancer, valve disease, and pulmonary disease were excluded. Also, patients who had a CTCA but images were non-interpretable due to artefacts (n = 22) were excluded from the analysis of CAD, but the calcium score was included for the purpose of prognosis. Appropriate NHS (National Health Service) research ethics approval was obtained (IRAS Project ID: 199531).
The data was divided into two groups: Group A, containing patients from 2007 to 2015 who underwent both CTCS and CTCA, and Group B, containing patients from 2003 to 2015, who underwent only CTCS. The decision to perform a CTCA after CTCS was based on NICE (National Institute for Health and Care Excellence) guidance for local patients,16 while patients from other hospitals had both scans performed at the same time as requested by the referring cardiologists. Generally, patients with low to intermediate pre-test probability were referred for cardiac CT (to Harefield Hospital) but some patients with higher probability but atypical symptoms or those who preferred a non-invasive test were also referred. In both groups, further investigations in the form of stress imaging or ICA were performed, as clinically required, but only analysed in those patients with a ZCS.
The chest pain was labelled as non-cardiac, atypical, or typical angina as per standard criteria.2 History of exertional dyspnoea was classified as typical chest pain for the calculation of pre-test probability (PTP).17 The PTP of each patient was calculated using the Duke’s criteria18 and categorised as very low (<10%), low (10–29%), intermediate (30–60%), high (61–90%), and very high risk (>90%) on the basis of type of chest pain, age, sex, and presence or absence of high cholesterol, diabetes, and history of smoking.16 Smokers were defined as those who were currently smoking or have quit within the last 3 years. Hypercholesterolaemia was defined as total serum cholesterol of greater than 5.5mmol/L, or if the patient was on statin therapy. Patients were considered hypertensive or diabetic if they had an extant diagnosis or were on anti-hypertensive or diabetic medication. The family history of premature CAD was considered to be present if this was known in a first-degree male relative age <55 years or female relative age <60 years.
The CTCS studies were performed on either a 4-slice (Siemens Volume Zoom) (from 2003 to 2006) or a 64-slice CT scanner (Toshiba Aquilion) (from 2007 to 2015) with images acquired using 120 kVp, 300–600 mAs, prospective ECG gating, and 3/3 mm reconstructions. Standard Agatston’s method was used to calculate the calcium score (CS).19 The analysis was performed on either a Siemens’ Virtuoso (Siemens Medical Systems, Germany) or Vitrea (Vital Images, Minnesota) workstation. The mean effective radiation dose was 0.9 mSv for men and 1.4mSv for women, using a conversion factor of 0.014. The calcium scores were categorised as 0, between 1–100, 101–400, and >400 for the purpose of analysis. The patients who only had a CTCS scan were included for follow-up purpose but not for analysing the presence or absence of obstructive CAD.
All CTCA scans were performed on a 64-slice multi-detector CT scanner (Toshiba Aquilion) using 100 or 120 kVp and 400–600 mAs depending upon patient’s body weight. Patients received oral or intravenous metoprolol to reduce the heart rate to <65 bpm and 800 µg of sublingual glyceryl trinitrate for vasodilatation in the absence of contraindications. 70–90 mL of non-ionic, low osmolar contrast (iopromide, Ultravist 370, Bayer Healthcare) was administered intravenously as the contrast media. The scanner generated the best phase image data set with least motion automatically, and further reconstructions were performed, as required, using 0.5 collimation and 0.3 mm slice-interval. The mean radiation dose was 7.2 and 13.1 mSv for prospective and retrospective gated scans respectively. Interpretation of CTCA was performed as per SCCT guidelines20 by level 3 trained and experienced cardiac radiologists and cardiologists. All plaques were evaluated using orthogonal and curved multi-planar reformats (MPRs) and considered to be present if visually encroaching into the lumen of coronary artery. Plaques were classified as calcified, partially-calcified, or non-calcified and described in a modified 16-segment coronary artery model.20 The degree of CAD was classified as being absent, mild (<50% in luminal diameter), moderate (50–69% stenosis), and severe (≥50 in the left main stem and ≥70% in other coronary arteries) stenosis.
Patients found to have moderate or severe stenosis on CTCA were recommended to have further evaluation with stress imaging or ICA respectively, but the final decision was left to their cardiologist/physician.
All patients were followed up for all-cause mortality through the UK’s Health & Social Care Information Centre via the NHS tracing service, which was available for 97% of the patients. Further follow-up of patients with ZCS who died was obtained from their general practitioners and hospital records.
Statistical analysis
The continuous variables are presented as mean ± SD or median (inter-quartile range), and comparison between groups was performed using the unpaired t-test or the Mann–Whitney test. The categorical variables are presented as frequencies with percentages, and the χ2 or Fisher’s exact test was used to compare between groups. A survival analysis was performed as percentage surviving at 5 and 13 years using the Kaplan–Meier method. Kaplan–Meier survival curves were drawn by presence or absence of CS, presence or absence of CAD on CTCA in ZCS group, CS categories, and CTCA stenosis severity.
Cox regression was performed in Group A patients for varying degree of CS and CTCA stenosis severity to assess their association with survival times. Any CS of >100 was considered as one category for this purpose due to a smaller number of patients with CS >400. The multivariate analysis was performed after adjusting for age, gender, and risk factors (hypertension, diabetes, smoking, high cholesterol, and family history) as three different models: Model 1, including patients with CS ≥1 and CAD ≥25%; Model 2, including patients with CS ≥100 and CAD ≥50%; and model 3 including patients with CS ≥100 and CAD >70%.
All analysis was performed using STATA version 13.0 (StataCorp, College Station, Texas). A two-tailed P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 3914 patients fulfilled the inclusion criteria (Table 1). The mean age was 56.9 years (±12.4), and 50.3% of patients were men. Half of the patients (50.5%) had a ZCS, but the prevalence varied inversely with the PTP of disease, being highest (58.9%) in those with PTP of <30% and lowest (14%) in PTP of >60%. Patients with ZCS were less likely to be male, or to have hypertension, diabetes, high cholesterol, or typical chest pain.
Table 1.
Baseline characteristics of patients with a zero-calcium score and those with a calcium score of ≥1 in both groups and CTCA stenosis severity in group A patients
| Variable | All patients | CS = 0 | CS ≥ 1 | P-value |
|---|---|---|---|---|
| Number (%) | 3914 | 1978 (50.5) | 1936 (49.5) | |
| Age (years), Mean ± SD | 56.9 ± 12.4 | 51.2 ± 11.4 | 62.8 ± 10.5 | <0.001 |
| Male sex (%) | 1969 (50.3) | 862 (43.6) | 1107 (57.2) | <0.001 |
| BMI (kg/m2), Mean ± SD | 28.8 ± 5.9 | 28.6 ± 6.0 | 29.0 ± 6.0 | 0.17 |
| Hypertension—N (%) | 1563 (39.9) | 574 (29.0) | 989 (51.1) | <0.001 |
| Diabetes—N (%) | 486 (12.4) | 150 (7.6) | 336 (17.4) | <0.001 |
| High cholesterol—N (%) | 1664 (42.5) | 689 (34.8) | 975 (50.4) | <0.001 |
| Current smoker—N (%) | 595 (15.2) | 299 (15.1) | 296 (15.3) | 0.88 |
| Family history—N (%) | 1258 (38.8) | 665 (40.4) | 593 (37.1) | 0.06 |
| Chest pain | <0.001 | |||
| Typical—N (%) | 723 (18.5) | 303 (15.3) | 420 (21.7) | |
| Atypical—N (%) | 1614 (41.2) | 876 (44.3) | 738 (38.1) | |
| Non-anginal—N (%) | 992 (25.3) | 533 (26.9) | 459 (23.7) | |
| Dyspnoea—N (%) | 585 (14.9) | 266 (13.5) | 319 (16.4) | |
| PTP %—Median [IQR] | 37 (16, 67) | 22 [10, 46] | 58 [31, 79] | <0.001 |
| PTP category | <0.001 | |||
| Very low—N (%) | 578 (14.8) | 488 (24.7) | 90 (4.7) | |
| Low—N (%) | 1042 (26.6) | 677 (34.2) | 365 (18.9) | |
| Intermediate—N (%) | 1086 (27.8) | 537 (27.2) | 549 (28.4) | |
| High—N (%) | 936 (23.9) | 241 (12.2) | 695 (35.8) | |
| Very high—N (%) | 272 (6.9) | 35 (1.8) | 237 (12.2) | |
| Calcium score— Median [IQR] | 0 (0, 96) | 0 [0, 0] | 97 [24, 338] | – |
| Group A Stenosis severity | 2730 (69.7) | 1426 (52.2) | 1304 (47.8) | <0.001 |
| Absent—N (%) | 1320 (48.4) | 1282 (89.9) | 38 (3.0) | |
| Mild—N (%) | 935 (34.3) | 120 (8.4) | 815 (62.5) | |
| Moderate—N (%) | 195 (7.1) | 17 (1.2) | 178 (13.7) | |
| Severe—N (%) | 278 (10.2) | 7 (0.5) | 271 (20.8) | |
| Died, N (%) | 147 (3.8) | 28 (1.4) | 119 (6.1) | <0.0001 |
CS, calcium score; CTCA, CT cardiac angiogram; BMI, body mass index; PTP, pre-test probability.
Among the study cohort, 2730 (69.7%) had both CTCS and CTCA (Group A). In this group, a ZCS was seen in 1426 (52.2%) of whom 17 (1.2%) had moderate stenoses, and 7 (0.5%) had severe stenoses on CTCA. In women, who had higher prevalence of a ZCS (56.4%, P < 0.0001), no difference was seen in the prevalence of moderate or severe stenosis compared with man (12 cases each, P = 0.493) All patients with >50% stenosis underwent subsequent stress imaging or ICA confirming flow limiting stenosis in only four patients (0.3%). The negative predictive value of ZCS for excluding severe CTCA stenosis was 99.5%.
There were 1408 (51.6%) patients who had some degree of atheromatous plaques in the coronary arteries on CTCA (Table 1) causing varying degree of stenosis. Detailed plaque analysis was available in 2704 patients. There were 3889 coronary artery segments (9.6%) containing predominantly calcified, partially-calcified, and non-calcified plaques in 50.6, 39.5, and 9.9% segments respectively.
The median PTP of coronary disease in Group A patients was 37% (IQR = 14–66%) with severe CAD identified in 10.2% patients on CTCA. The seven patients with ZCS but severe CTCA stenosis were distributed across all PTP categories (P = 0.732). In patients with a ZCS, no significant difference was found in the baseline characteristics or risk factors in those with or without ≥50% CTCA stenosis (Table 2).
Table 2.
Baseline characteristics of patients with a zero calcium score and absence or presence of ≥50% stenosis on CTCA
| Variable | CAD <50% | CAD ≥50% | P-value |
|---|---|---|---|
| (n = 1402) | (n = 24) | ||
| Age (years), Mean ± SD | 49.4 ± 11.4 | 52.9 ± 9.3 | 0.08 |
| Male sex (%) | 603 (43) | 12 (50) | 0.49 |
| Typical chest pain or dyspnoea, N (%) | 472 (34) | 11 (46) | 0.21 |
| Hypertension—N (%) | 402 (29) | 9 (38) | 0.34 |
| Diabetes—N (%) | 104 (7.4) | 3 (13) | 0.35 |
| High cholesterol—N (%) | 471 (34) | 7 (29) | 0.65 |
| Current smoker—N (%) | 223 (16) | 7 (29) | 0.08 |
| Family history—N (%) | 526 (38) | 8 (33) | 0.70 |
| PTP (%)—Mean ± SD | 28.9 ± 24.7 | 40.8 ± 29.8 | 0.06 |
CAD, coronary artery disease; PTP, pre-test probability.
Follow-up and survival
In the 13-year follow-up period (mean = 5.2 ± 2.8 years), a total of 147 deaths (3.8%) were observed from any cause. There were 28 deaths in patients with ZCS (1.4%; annual event rate, 0.3) compared with 119 deaths (6.1%; annual event rate, 1.2) in those with a CS ≥1 (OR = 4.6, 95% CI = 3.0, 6.0; P < 0.0001). None of the patients with ZCS died of a coronary event.
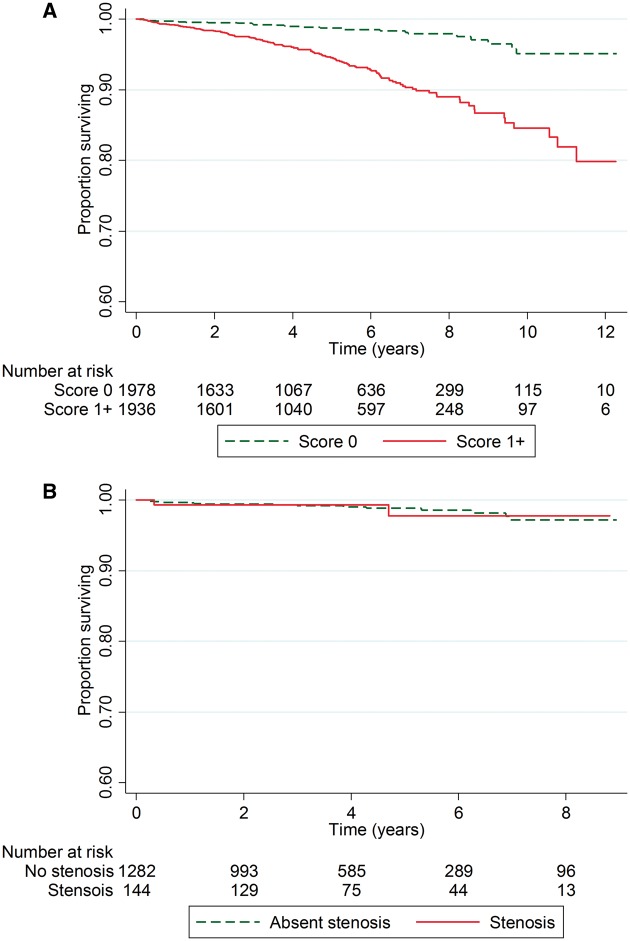
Kaplan–Meier survival estimates in groups with a ZCS and a calcium score ≥1 were 99.0% (95% CI −98.3, 99.4) and 94.5% (95% CI −92.9, 95.5, P < 0.001) at 5 years, and 95.5% (95% CI −92.1, 97.5) and 84.0% (95% CI −78.6, 88.2) at 13 years (Figure 1A). Among patients with a ZCS, Kaplan–Meier survival estimates were unaffected by the presence of non-calcified atheroma on CTCA (P = 0.98, Figure 1B).
Figure 1.
Kaplan–Meier survival curves by (A) presence or absence of any coronary artery calcium score, and (B) presence or absence of any degree of non-calcified atheroma on CTCA in patients with zero calcium score.
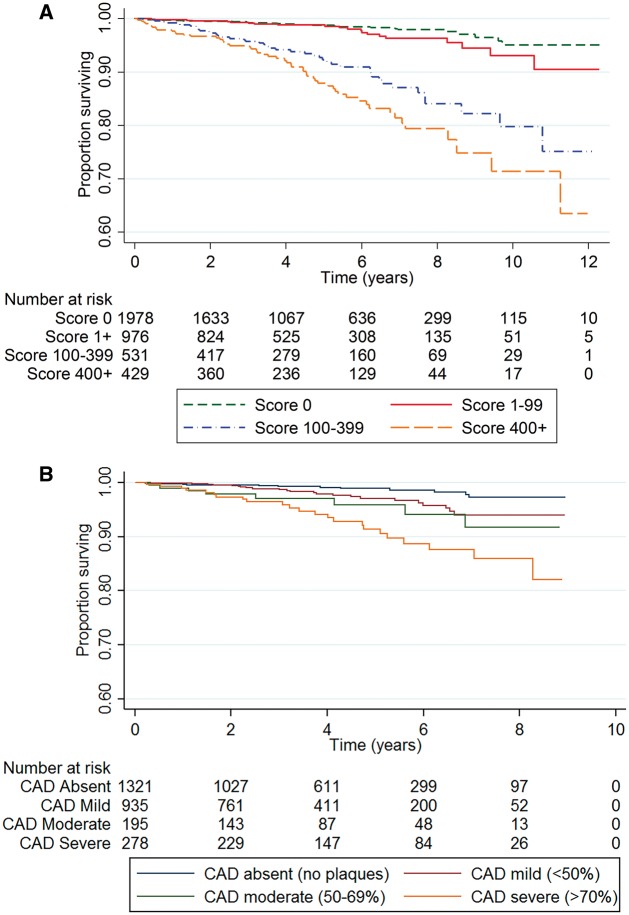
Among Group A patients, both calcium score (Figure 2A) and CTCA stenosis severity (Figure 2B) were inversely related to survival. Cox-regression analysis confirmed stepwise associations of increasing calcium score and increasing CAD severity with the hazard of death (Table 3). However, in the adjusted Cox analysis only the calcium score showed significant association with the hazard of death.
Figure 2.
Kaplan–Meier survival curves by (A) calcium score category, and (B) CTCA stenosis severity.
Table 3.
Cox regression analysis demonstrating hazard ratios (95% confidence intervals) for mortality stratified by calcium score and severity of obstructive coronary artery disease
| Univariable analysis |
Multivariable analysisa |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Model 1: CS ≥ 1 and CAD ≥ 25% | ||||
| CS ≥ 1 | 3.4 (1.9, 5.8) | <0.0001 | 1.9 (1.2, 3.1) | 0.004 |
| CAD ≥ 25% | 1.1 (1.0, 1.1) | 0.026 | 1.0 (0.9, 1.0) | 0.41 |
| Model 2: CS ≥ 100 and CAD ≥ 50% | ||||
| CS ≥ 100 | 5.3 (3.2, 8.5) | <0.0001 | 2.4 (1.3, 4.3) | 0.006 |
| CAD ≥ 50% | 3.6 (2.2, 5.8) | <0.0001 | 1.5 (0.9, 2.7) | 0.14 |
| Model 3: CS ≥ 100 and CAD ≥ 70% | ||||
| CS ≥ 100 | 5.3 (3.2, 8.5) | <0.0001 | 2.4 (1.3, 4.4) | 0.005 |
| CAD ≥ 70% | 3.9 (2.4, 6.4) | <0.0001 | 1.6 (0.9, 2.9) | 0.11 |
CS, calcium score; CAD, coronary artery disease severity on CT cardiac angiogram.
Multivariable analysis includes an adjustment for age, sex, chest pain typicality, hypertension, diabetes mellitus, high cholesterol, current smoking, and family history.
Discussion
In this large cohort study of patients with suspected stable coronary artery disease, a zero-calcium score reliably excluded obstructive coronary artery disease with a negative predictive value of 99.5%. It also predicted an excellent long-term prognosis, effectively ruling out the risk of coronary events during follow-up for 13 years. These anatomic and prognostic data show that CT calcium scoring is a robust method for identifying low-risk patients and question the need for further testing when the score is zero.
Among patients with a ZCS, the prevalence of obstructive CAD, with >50% luminal narrowing, has been the subject of debate. The prevalence by invasive coronary angiography is very low,11 yet in two CTCA cohorts, comprising 668 and 291 patients, prevalence rates of 7 and 19% were reported.13,14 In these studies, however, up to 38% of the patients had presented acutely, unlike more recent CTCA studies12,15,21 that exclusively included stable patients when the prevalence of obstructive lesions (>50% luminal narrowing) in patients with a ZCS was much lower. Indeed, allowing for the low positive predictive value of CTCA,22 the prevalence data in these more recent studies are comparable to those we report. In our patients, we could confirm the diagnosis of obstructive disease by invasive coronary angiography or stress imaging, lending further weight to the validity of our findings. The 99.5% negative predictive value of a zero calcium score compares favourably with CTCA23 and is considerably higher compared to stress imaging tests,24 questioning the need for further testing to rule out coronary disease when the calcium score is zero.
The proportion of patients with stable symptoms having a ZCS largely depends upon the pre-test probability as shown in this study. While in patients with typical angina undergoing ICA, a ZCS was present in up to 20% patients,11 the recent CTCA studies12,15,21,25,26 have demonstrated a prevalence of between 40 and 53% in patients with a mean PTP of between 40 and 45%. It is also now being realised that the PTP calculation methods developed in 1980’s and 1990’s overpredict the prevalence of significant CAD based on both newer ICA27 as well as CTCA data,28 at least in the developed countries. Downgrading the PTP by newer methods may result in increased proportion of ZCS in the low to intermediate PTP groups.
It was a strength of our study that medium and long-term prognostic data were available showing that a ZCS in patients with stable chest pain was associated not only with a very low prevalence of obstructive coronary disease but also with excellent long-term survival. Previous studies in asymptomatic patients have also reported favourable survival but in symptomatic patients, the prognostic data are restricted to the first 2 years when survival exceeds 99% in patients with a ZCS.15 We now confirm that estimated survival remains at this level after 5 years, falling to only 95.5% after 13 years. Importantly, these survival data in patients with a zero calcium score are unaffected by the presence of non-calcified atheroma on the CT coronary angiogram, a finding that is consistent with previous reports, the CONFIRM study, for example, reporting a 0.4% 2-year mortality irrespective of CTCA findings.15 Although a subsequent analysis of CONFIRM data revealed a trend towards increasing combined event rate of all-cause mortality and myocardial infarction over a shorter median follow-up period of 25 months, it was not statistically significant (P = 0.07).29 These findings reassure that in patients with chest pain and a ZCS, the long-term prognosis is excellent and unlikely to be improved by further cardiac investigation and treatment. As the calcium score rises above zero, however, so does the risk of obstructive coronary disease and death.12,15,25,29–32 Genders et al.27 found the calcium score improved probability estimates of obstructive coronary disease, but our findings suggest that the calcium score may be a stronger predictor of events, perhaps reflecting the limitations of CTCA for quantifying lesion severity.
There is no doubt that CTCA provides comprehensive assessment of CAD with demonstration of plaques with quantification of stenosis, thus providing greater accuracy for diagnosis and prognosis. CTCS, on the other hand is a relatively crude technique, but is much simpler to perform without the need for contrast and beta-blockers, as well requiring less time for reporting. The 2010 NICE chest pain guideline16 had recommended CTCS as the initial test to rule out coronary disease in low-risk individuals, but the recently updated guidance5 advises CTCA as the first-line investigation for all patients with angina, independently of CTCS. Our data suggest that even amongst patients with typical or atypical angina, as many as 50% will have a ZCS with an excellent prognosis. As CTCS is easy to perform, it can be readily integrated into busy outpatient care and we would argue that it can reasonably be retained as a gatekeeper to CTCA when implementing the new NICE guideline.
Strengths of this study include the large cohort size and the long follow-up period. However, it was an observational study based in a single-centre, and this limitation must be acknowledged. A further limitation was the self-reporting of risk factors with the potential to undermine the accuracy of the pre-test probability of disease estimates. We were unable to determine the cause of death in all cases, except in those with ZCS, and have thus used all-cause mortality as the main outcome. We were also not able to identify non-fatal events. Like in other similar studies, the outcome results did not exclude those who underwent revascularisation and did not consider the effect of any preventative medical therapy. This could affect the outcome, particularly in those in ZCS group who were found to have flow limiting stenosis and underwent revascularisation.
In conclusion, patients with stable symptoms and a zero-calcium score have a very low prevalence of obstructive CAD and an excellent prognosis over the medium to long-term. As the calcium score rises above zero, so does the prevalence of coronary disease and the risk of death. Calcium scoring has the potential to enhance risk management in patients with undiagnosed chest pain, a zero-score questioning the need for a further cardiac investigation. Indeed, a zero-calcium score might be seen as a gatekeeper, with further testing reserved for patients with positive scores.
Acknowledgements
Authors would like to acknowledge gratefully the statistical assistance provided by Paul Bassett and Winston Banya for this study.
Conflict of interest: None declared.
Funding
No specific funding was received for this study.
References
- 1. Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM. et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;63:380–406. [DOI] [PubMed] [Google Scholar]
- 2. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A. et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 3. van Waardhuizen CN, Khanji MY, Genders TSS, Ferket BS, Fleischmann KE, Hunink MGM. et al. Comparative cost-effectiveness of non-invasive imaging tests in patients presenting with chronic stable chest pain with suspected coronary artery disease: a systematic review. Eur Heart J Qual Care Clin Outcomes 2016;2:245–60. [DOI] [PubMed] [Google Scholar]
- 4. Suhny A, Armin A-Z, Tracy QC, Milind YD, Wilfred M, Louise T. et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. 2009;3:190–204. [DOI] [PubMed] [Google Scholar]
- 5.NICE. Chest pain of recent onset: assessment and diagnosis, Clinical guidance [CG95] update 2016. www.nice.org.uk/guidance/cg95 (22 December 2016, date last accessed).
- 6. The SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. The Lancet 2015;385:2383–91. [DOI] [PubMed] [Google Scholar]
- 7. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B. et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dewey M, Rief M, Martus P, Kendziora B, Feger S, Dreger H. et al. Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial. BMJ 2016;355:i5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doris MK, Newby DE.. How should CT coronary angiography be integrated into the management of patients with chest pain and how does this affect outcomes? Eur Heart J Qual Care Clin Outcomes 2016;2:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaw LJ, Giambrone AE, Blaha MJ, Knapper JT, Berman DS, Bellam N. et al. Long-term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med 2015;163:14–21. [DOI] [PubMed] [Google Scholar]
- 11. Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Cury RC. et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009;2:675–88. [DOI] [PubMed] [Google Scholar]
- 12. Hulten E, Bittencourt MS, Ghoshhajra B, O'Leary D, Christman MP, Blaha MJ. et al. Incremental prognostic value of coronary artery calcium score versus CT angiography among symptomatic patients without known coronary artery disease. Atherosclerosis 2014;233:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rubinshtein R, Gaspar T, Halon DA, Goldstein J, Peled N, Lewis BS.. Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64-slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol 2007;99:472–5. [DOI] [PubMed] [Google Scholar]
- 14. Gottlieb I, Miller J, Arbab-Zadeh A, Dewey M, Clouse M, Sara L. et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol 2010;55:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Villines T, Hulten E, Shaw L, Goyal M, Dunning A, Achenbach S. et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol 2011;58:2533–40. [DOI] [PubMed] [Google Scholar]
- 16. Cooper A, Timmis A, Skinner J.. Assessment of recent onset chest pain or discomfort of suspected cardiac origin: summary of NICE guidance. BMJ 2010;340:c1118. [DOI] [PubMed] [Google Scholar]
- 17. Abidov A, Rozanski A, Hachamovitch R, Hayes SW, Aboul-Enein F, Cohen I. et al. Prognostic significance of dyspnea in patients referred for cardiac stress testing. N Engl J Med 2005;353:1889–98. [DOI] [PubMed] [Google Scholar]
- 18. Pryor DB, Shaw L, Harrell FE Jr., Lee KL, Hlatky MA, Mark DB. et al. Estimating the likelihood of severe coronary artery disease. Am J Med 1991;90:553–62. [PubMed] [Google Scholar]
- 19. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R.. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 20. Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ. et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr 2009;3:122–36. [DOI] [PubMed] [Google Scholar]
- 21. Chaikriangkrai K, Velankar P, Schutt R, Alchalabi S, Nabi F, Mahmarian J. et al. Additive prognostic value of coronary artery calcium score over coronary computed tomographic angiography stenosis assessment in symptomatic patients without known coronary artery disease. Am J Cardiol 2015;115:738–44. [DOI] [PubMed] [Google Scholar]
- 22. Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E. et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–32. [DOI] [PubMed] [Google Scholar]
- 23. Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA. et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–44. [DOI] [PubMed] [Google Scholar]
- 24. Takx RA, Blomberg BA, El Aidi H, Habets J, de Jong PA, Nagel E. et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2015;8:e002666. [DOI] [PubMed] [Google Scholar]
- 25. Bittencourt MS, Hulten E, Ghoshhajra B, O'Leary D, Christman MP, Montana P. et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging 2014;7:282–91. [DOI] [PubMed] [Google Scholar]
- 26. Lubbers M, Dedic A, Coenen A, Galema T, Akkerhuis J, Bruning T. et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J 2016;37:1232–43. [DOI] [PubMed] [Google Scholar]
- 27. Genders TSS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K. et al. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J 2011;32:1316–30. [DOI] [PubMed] [Google Scholar]
- 28. Min JK, Dunning A, Gransar H, Achenbach S, Lin FY, Al-Mallah M. et al. Medical history for prognostic risk assessment and diagnosis of stable patients with suspected coronary artery disease. Am J Med 2015;128:871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Mallah MH, Qureshi W, Lin FY, Achenbach S, Berman DS, Budoff MJ. et al. Does coronary CT angiography improve risk stratification over coronary calcium scoring in symptomatic patients with suspected coronary artery disease? Results from the prospective multicenter international CONFIRM registry. Eur Heart J Cardiovasc Imaging 2014;15:267–74. [DOI] [PubMed] [Google Scholar]
- 30. Ostrom MP, Gopal A, Ahmadi N, Nasir K, Yang E, Kakadiaris I. et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol 2008;52:1335–43. [DOI] [PubMed] [Google Scholar]
- 31. Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC.. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol 2011;57:1237–47. [DOI] [PubMed] [Google Scholar]
- 32. Hadamitzky M, Freissmuth B, Meyer T, Hein F, Kastrati A, Martinoff S. et al. Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease. JACC Cardiovasc Imaging 2009;2:404–11. [DOI] [PubMed] [Google Scholar]