Abstract
Aims
Systemic levels of trimethylamine N-oxide (TMAO), a pro-atherogenic and pro-thrombotic metabolite produced from gut microbiota metabolism of dietary trimethylamine (TMA)-containing nutrients such as choline or carnitine, predict incident cardiovascular event risks in stable primary and secondary prevention subjects. However, the prognostic value of TMAO in the setting of acute coronary syndromes (ACS) remains unknown.
Methods and results
We investigated the relationship of TMAO levels with incident cardiovascular risks among sequential patients presenting with ACS in two independent cohorts. In the Cleveland Cohort, comprised of sequential subjects (n = 530) presenting to the Emergency Department (ED) with chest pain of suspected cardiac origin, an elevated plasma TMAO level at presentation was independently associated with risk of major adverse cardiac events (MACE, including myocardial infarction, stroke, need for revascularization, or death) over the ensuing 30-day (4th quartile (Q4) adjusted odds ratio (OR) 6.30, 95% confidence interval (CI), 1.89–21.0, P < 0.01) and 6-month (Q4 adjusted OR 5.65, 95%CI, 1.91–16.7; P < 0.01) intervals. TMAO levels were also a significant predictor of the long term (7-year) mortality (Q4 adjusted HR 1.81, 95%CI, 1.04–3.15; P < 0.05). Interestingly, TMAO level at initial presentation predicted risk of incident MACE over the near-term (30 days and 6 months) even among subjects who were initially negative for troponin T (< 0.1 ng/mL) (30 days, Q4 adjusted OR 5.83, 95%CI, 1.79–19.03; P < 0.01). The prognostic value of TMAO was also assessed in an independent multicentre Swiss Cohort of ACS patients (n = 1683) who underwent coronary angiography. Trimethylamine N-oxide again predicted enhanced MACE risk (1-year) (adjusted Q4 hazard ratios: 1.57, 95% CI, 1.03–2.41; P <0.05).
Conclusion
Plasma TMAO levels among patients presenting with chest pain predict both near- and long-term risks of incident cardiovascular events, and may thus provide clinical utility in risk stratification among subjects presenting with suspected ACS.
Keywords: Gut microbiota, Trimethylamine N-oxide, Acute coronary syndrome, Choline, Risk stratification, Incident major adverse cardiac events, All-cause mortality
Introduction
Recent studies highlight the participation of gut microbes in the pathogenesis of both atherosclerotic heart disease1–4 and its adverse thrombotic events.5 Trimethylamine N-oxide (TMAO) is a plasma metabolite shown to be formed through a metaorganismal pathway involving nutrient precursors abundant in a Western diet (choline, phosphatidylcholine, and L-carnitine) and the sequential action of both gut microbiota, initially forming trimethylamine (TMA), followed by host hepatic flavin monooxygenase-dependent conversion into trimethylamine N-oxide (TMAO).1,6 Numerous studies reveal an association between systemic TMAO levels and cardiovascular risks in a variety of stable cohorts.1–3,5,7–20
The relationship between TMAO levels and incident risks in subjects with acute coronary syndromes has not yet been examined. This is particularly relevant given that recent human platelet and animal model studies reveal that TMAO interacts with platelets, altering stimulus-dependent calcium signalling, fostering platelet hyperreactivity and enhanced thrombosis potential in vivo.5 Numerous studies have reported platelet hyperreactivity is a risk factor for incident cardiovascular events.21,22 Trimethylamine N-oxide was also recently shown in animal models to promote vascular inflammation, inducing aortic endothelial cell activation and up-regulation of adhesion proteins.23 Finally, recent clinical studies have shown that elevated systemic levels of TMAO among stable subjects (n = 4007) undergoing elective diagnostic cardiac evaluations predict incident thrombotic event risks.5 A rational hypothesis is thus that circulating levels of TMAO predict incident risks for thrombotic and cardiovascular events among subjects presenting with acute coronary syndrome (ACS). Herein, we sought to explore the relationship between systemic levels of TMAO and incident major adverse cardiovascular events (MACE, including myocardial infarction (MI), stroke, need for revascularization, or death) and mortality among subjects presenting with ACS in two independent prospective cohorts.
Methods
Study populations
Plasma samples and associated clinical data were collected from two independent populations, each of which was divided into a case: control cohort based upon clinical evidence of MACE. The Cleveland Cohort (n = 530) was a single-center, prospective cohort study approved by the Cleveland Clinic Institutional Review Board. Sequential consenting adult subjects (ages 18 years and above) who presented to the emergency department (ED) with chest pain of suspected cardiac origin within 24 h of onset were eligible. Blood samples were drawn at the time of presentation to the emergency room (baseline) and 4, 8 and 16 h later. TMAO levels were examined, and baseline and serial cardiac troponin (cTnT) testing were also performed. Of the initial 530 patients, 112 were TnT-positive (cTnT ≥ 0.1 ng/mL) at initial presentation (baseline), and the 418 initially negative for cTnT were monitored for outcomes (MACE = MI, stroke, need for revascularization or death at 1 month and 6 month; and all-cause mortality annually) in the continuing TMAO study. All events were adjudicated with medical record checks.
The Swiss ACS Cohort is part of the Special Program University Medicine (SPUM), including all patients who underwent coronary angiography for ACS at one of the participating University Hospitals (Zurich, Bern, Lausanne, Geneva; see www.spum-acs.ch). This prospective multi- centre cohort was based on consecutive recruitment and follow-up performed at 30 days (phone call) and 1 year (clinical visit) of subjects with adjudicated ACS. Inclusion criteria where: (1) patients older than 18 years being admitted within 5 days (preferably within 72 h) after pain onset with the main diagnosis of ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI) or unstable angina (UA); (2) persistent ST-segment elevation or depression, T-inversion or dynamic Electrocardiogram (ECG) changes, new left bundle branch block (LBBB) or (3) evidence of positive troponin by local laboratory reference values with a rise and/or fall in serial troponin levels; (4) known coronary artery disease, specified as status after (MI), coronary artery bypass graft (CABG), or Percutaneous Coronary Intervention or newly documented ≥ 50% stenosis of an epicardial coronary artery during the initial catheterization. Exclusion criteria comprised (1) severe physical disability; (2) inability to comprehend study; (3) less than 1 year of life expectancy for non-cardiac reasons. All subjects gave written informed consent, and the study was approved by the institutional review board (Ethical Committee of the Canton of Zurich, Switzerland).
Medications
For the Cleveland Cohort, patients were treated during their hospital stay and after discharge according to standard of care by their treating physicians. For the Swiss ACS Cohort, the protocol for medications during hospital stay and follow-up comprised administration of aspirin and an additional platelet inhibitor (prasugrel or ticagrelor) for 1 year after the ACS unless an indication for oral anticoagulation was present. Treating physicians were advised to administer a statin (rosuvastatin 20 mg/d), an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker and a beta-blocker immediately after the ACS as tolerated by the patient.
Laboratory testing
For the Cleveland Cohort, routine laboratory testing was performed, including the Roche cTnT (4th generation) assay. For the Swiss ACS multicentre cohort, routine laboratory tests were performed according to laboratories of each Institution. Given the different assays used for troponin, ratios of values on upper limit of reference (URL) were reported. Estimated glomerular filtration rate (eGFR; in mL/min per 1.73 m2) was calculated for each cohort using the modification of Diet in Renal Disease study equation.24
Quantification of plasma trimethylamine N-oxide levels
Trimethylamine N-oxide levels in plasma were determined using stable isotope dilution high-performance liquid chromatography with on line electrospray ionization tandem mass spectrometry (LC/MS/MS) using d9-(trimethyl)-labelled internal standard as described previously,25 but on a Shimadzu 8050 triple quadrupole mass spectrometer interfaced to a Shimadzu Nexera Ultra High Performance Liquid Chromatograph (UHPLC) system.
Clinical diagnosis
Positive troponin was defined by local laboratory reference values (e.g. myocardial necrosis was defined by troponin T levels ≥ 0.1 ng/mL in the Cleveland Cohort). Unstable angina was ascertained on the basis of the presence of angina at rest, a sudden increase in episodes of previously stable angina, ST-segment depression, or T-wave inversions, as described previously.26 For both the Cleveland Cohort and the Swiss ACS Cohort, electrocardiographic data were verified independently by personnel at electrocardiography core facilities who were unaware of the patients’ diagnoses. The diagnosis of an acute coronary syndrome (ACS) was based on the presence of MI or unstable angina, as defined in the protocol, and confirmed by a chart review by an investigator who was unaware of the patients’ diagnoses.
Definitions of outcomes
All-cause mortality included cardiac, vascular and non-cardiovascular causes of death. Major adverse cardiovascular events (MACE) were defined as a composite of MI, stroke, revascularization, or all-cause mortality. Reviews of medical records and follow-up telephone interviews or clinical visit were conducted for the 30-day, 6-month and 1 year outcomes. The Cleveland Cohort was followed annually for all-cause mortality. Repeat revascularization included any repeat coronary revascularization (target and non-target vessel); clinically indicated repeat revascularization included any clinically driven repeat coronary revascularization (target and non-target vessel)
Statistical analysis
Student’s t-test or Wilcoxon-rank sum test for continuous variables and χ2 test for categorical variables were used to examine the difference between groups. Odds ratio (OR) for MACE and corresponding 95% confidence interval (CI) were calculated using both univariable (unadjusted) and multivariable (adjusted) logistic regression models. Hazards ratio (HR) for incident MACE and corresponding 95% CI were calculated using both univariable (unadjusted) and multivariable (adjusted) Cox models. Kaplan–Meier analysis with Cox proportional hazards regression was used for time-to-event analysis to determine HR and 95% CI for MACE and all-cause mortality. Adjustments were made for individual traditional cardiac risk factors including but not limited to age, gender, HDL, LDL, smoking, presence or absence of a history of diabetes mellitus, hypertension, hyperlipidaemia, revascularization or coronary artery disease, high sensitivity C-reactive protein level, eGFR, initial cTnT level, and diagnosis of either STEMI, NSTEMI or unstable angina. Improvement in model performance that was introduced by the inclusion of TMAO levels was evaluated with the use of net reclassification improvement (NRI), we also calculated the C-statistic using the area under the receiver-operating-characteristic (ROC) curve.27,28 Model validation and calibration are shown in the Supplemental Statistical Methods section (Supplementary material online, Table S1 and Figures S1–S3). All analyses were performed using R 3.1.3 (Vienna, Austria) and P-values < 0.05 were considered statistically significant.
Results
Characteristics of patients in both Cleveland and Swiss ACS Cohorts
The Cleveland and Swiss ACS Cohorts examined comprised a total of 2213 patients with baseline clinical characteristics summarized in Tables1and2 according to cohorts and stratified by MACE status. The Cleveland Cohort was composed of 530 sequential patients who presented to the emergency department with complaint of chest pain. The average time from the onset of chest pain to presentation and blood draw was 4.0 h. The Swiss ACS population included 1683 patients with ACS and onset of symptoms within 72 h. Patients who experienced MACE following enrolment in either the Cleveland Cohort (learning cohort) or the Swiss ACS Cohort (validation cohort) were older, had a greater prevalence of revascularization and diabetes, used aspirin and beta-blockers more frequently, and were more likely to have higher C-reactive protein levels. In the Cleveland Cohort, 112 patients (21.1%) were noted to be cTnT positive in the initial evaluation period and 418 patients remained persistently negative for cTnT testing. In addition, 88 patients (16.6%) had unstable angina and 199 patients (37.6%) had suspected ACS. Follow-up in the Cleveland Cohort was completed for 30-day and 6-month outcomes. All-cause mortality at 7 years was available in 89% (n = 474) of the Cleveland Cohort subjects. Outcomes at 6 months included MI in 117 patients, stroke in 6 patients, revascularization in 163 patients, death in 29 patients, and the composite MACE in 220 patients (41.5%). In initial cTnT negative patients(n = 418), outcomes at 6 months comprised MI in 5 patients, stroke in 4 patients, revascularization in 92 patients, death in 13 patients, and the composite MACE in 108 patients (25.8%). In the Swiss ACS Cohort, outcomes at 1 year included MI in 52 patients, stroke in 21 patients, revascularization in 99 patients, death in 71 patients, and the composite MACE in 190 patients (11.3%).
Table 1.
Baseline characteristics of the patients in Cleveland acute coronary syndrome cohort
| Characteristics | All patients | MACE at 6 months |
P Value | |
|---|---|---|---|---|
| (n = 530) | No (n = 310) | Yes (n = 220) | ||
| Age (yr) (mean ± sd) | 62.4 ± 13.9 | 60.4 ±14.3 | 65.3 ± 12.7 | <0 .001 |
| Sex (% male) | 57.5 | 49.7 | 68.6 | <0 .001 |
| C-reactive protein (mg/L) | 5.60 (1.90–11.9) | 5.00 (1.50–10.9) | 6.10 (2.40–13.5) | 0 .01 |
| History of hyperlipidaemia (%) | 50.0 | 42.1 | 60.9 | <0 .001 |
| History of Diabetes (%) | 27.2 | 23.7 | 32.1 | 0 .04 |
| History of hypertension (%) | 65.6 | 63.5 | 68.5 | 0 .28 |
| History of revascularization (%) | 33.9 | 27.1 | 43.2 | <0 .001 |
| History of CAD (%) | 48.9 | 44.4 | 54.9 | 0 .03 |
| History of smoking (%) | 61.1 | 56.4 | 67.6 | 0 .01 |
| HDL (mg/dL) | 39.0 (32.0–47.0) | 40.0 (33.0–49.0) | 38.0 (30 .0–44.0) | 0 .001 |
| LDL(mg/dL) | 104.5 (80 .0–133.2) | 102.0 (79.0–133.0) | 108.0 (83.5–134.5) | 0 .24 |
| Adjudicated ACS (%) | 37.6 | 6.15 | 81.8 | <0 .001 |
| eGFR (mL/min/1.73 m2) | 75.8 (54.7–91.2) | 78.2 (60 .3–92.9) | 70.1 (47.3–89.2) | <0 .01 |
| TMAO (µM) | 4.28 (2.55–7.91) | 3.73 (2.37–6.91) | 5.09 (3.11–9.42) | <0 .001 |
| Baseline hs-TnT(ng/mL) | 0.018 (0.009–0.045) | 0.013 (0.007–0.026) | 0.035 (0.014–0.12) | <0 .001 |
| Baseline medications (%) | ||||
| Aspirin | 36.5 | 30.3 | 45.3 | <0 .01 |
| ACE inhibitors | 25.4 | 23.2 | 28.4 | 0 .24 |
| Statin | 13.0 | 10.6 | 16.4 | 0 .08 |
| Beta-blockers | 23.1 | 15.4 | 31.8 | <0 .001 |
Continuous data are presented as mean ± standard deviation or median (interquartile range), categorical variables are presented as %.
Table 2.
Baseline characteristics of the patients in Swiss ACS cohort
| Characteristics | All patients | MACE at 1 year |
P Value | |
|---|---|---|---|---|
| (n = 1683) | No (n = 1493) | Yes (n = 190) | ||
| Age (yr) (mean ± sd) | 63.9 ± 12.4 | 63.3 ± 12.3 | 68.7 ± 12.4 | <0 .001 |
| Sex (% male) | 77.8 | 77.8 | 77.4 | 0 .96 |
| C-reactive protein (mg/L) | 2.80 (1.10–8.00) | 2.7 (1.10–7.60) | 4.40 (1.70–15.05) | <0 .001 |
| History of hyperlipidaemia (%) | 62.6 | 62.7 | 62.1 | 0 .94 |
| History of Diabetes (%) | 17.5 | 16.3 | 26.3 | <0 .01 |
| History of hypertension (%) | 59.3 | 58.5 | 65.3 | 0 .09 |
| History of revascularization (%) | 17.4 | 15.9 | 29.1 | <0 .001 |
| History of CAD (%) | 36.8 | 36.4 | 39.8 | 0 .41 |
| History of smoking (%) | 67.8 | 68.4 | 63.2 | 0 .17 |
| HDL (mg/dL) | 43.3 (36.3–53.4) | 43.3 (36.3–53.0) | 44.5 (35.2–54.1) | 0 .93 |
| LDL (mg/dL) | 118.7 (90 .1–148.5) | 119.5 (92.4–148.9) | 107.9 (81.2–144.2) | <0 .01 |
| eGFR (mL/min/1.73 m2) | 100.2 (74.2–126.9) | 102.5 (77.6–129.0) | 83.7 (54.8–111.5) | <0 .001 |
| TMAO (µM) | 2.87 (1.94–4.85) | 2.80 (1.91–4.55) | 3.75 (2.14–7.04) | <0 .001 |
| Hs-TnT (ng/mL) | 0.20 (0 .056–0 .71) | 0.20 (0 .054–0 .69) | 0.28 (0 .067–0 .83) | 0 .12 |
| Baseline medications (%) | ||||
| Aspirin | 33.3 | 31.9 | 44.9 | <0 .001 |
| ACE inhibitors | 18.3 | 17.7 | 23.0 | 0 .10 |
| Statin | 31.4 | 30.4 | 39.0 | 0 .02 |
| Beta-blockers | 26.3 | 24.7 | 39.6 | <0 .001 |
Continuous data are presented as mean ± standard deviation or median (interquartile range), categorical variables are presented as %.
The association of trimethylamine N-oxide levels with incident major adverse cardiovascular events and long-term mortality in the Cleveland Cohort
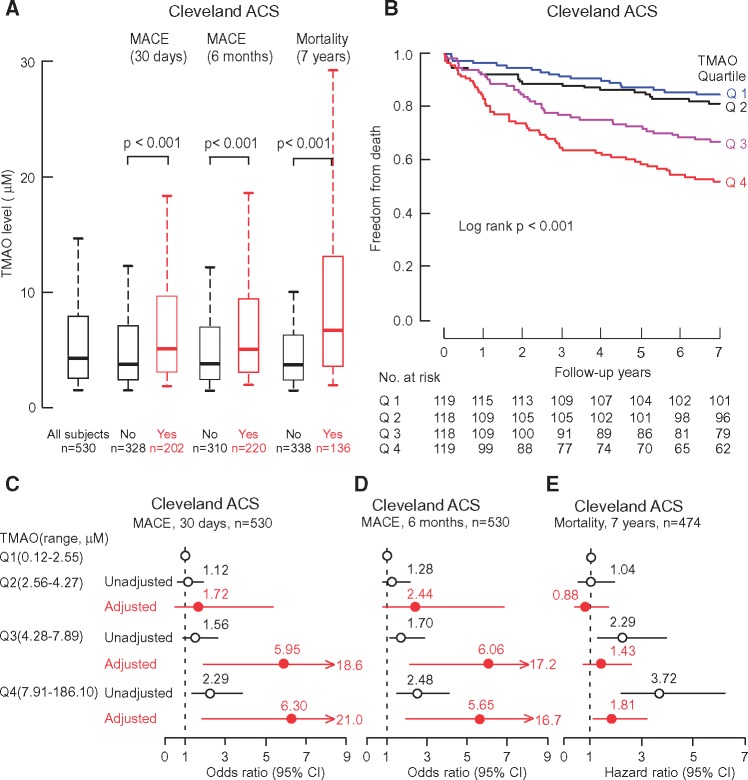
Subjects with a higher plasma TMAO level at presentation were more likely to experience incident MACE in the ensuing 30-day and 6-month period. TMAO levels were also higher among subjects who died in the long-term (7-year) follow-up period (Figure 1A). Kaplan-Meier analyses of baseline TMAO levels also revealed dose-dependent increases in mortality rates among subjects (log rank P < 0.001) (Figure 1B). We next explored the relationship between plasma TMAO levels with incident MACE risk. The frequency of experiencing a MACE at 30 days and 6 months following presentation increased with increasing quartiles of TMAO levels as follows: 30-day and 6-month frequency of 30.1% and 31.6% in quartile 1(< 2.56 µM), 32.6% and 37.1% in quartile 2 (2.56–4.27 µM), 40.2% and 43.9% in quartile 3 (4.28–7.89 µM), and 49.6% and 53.4% in quartile 4 (> 7.9 µM) (P < 0.01 for trend). As compared with subjects in the lowest quartile of TMAO levels, patients in the highest quartile (Q4) demonstrated a significantly increased 2.29-fold risk of incident MACE at 30 days (OR, 2.29, 95% CI, 1.39–3.79; P < 0.01) (Figure 1C). Trimethylamine N-oxide levels also predicted the risk of MACE over the following 6-month (Q4: OR 2.48, 95% CI, 1.51–4.09; P < 0.001), as well as 7 year mortality risk (Q4 HR, 3.72, 95% CI, 2.23–6.20; P < 0.001) (Figure 1D and E). To ascertain whether plasma TMAO levels independently predict incident major adverse cardiac events and long-term mortality, adjustments were made by using multivariable logistic-regression or Cox models for variables associated with TMAO levels or outcomes in univariable models (age, gender, HDL, LDL, smoking, presence or absence of a history of diabetes mellitus, hypertension, hyperlipidaemia, revascularization or coronary artery disease, C-reactive protein level, eGFR, initial cTnT level, diagnosis of either STEMI, NSTEMI or unstable angina). Following adjustments, elevated plasma levels of TMAO remained a significant predictor of risks of incident MACE at 30 days (Q4 OR 6.30, 95% CI, 1.89–21.00; P < 0.01), 6 months (Q4 OR 5.65, 95% CI, 1.91–16.74; P < 0.01) and all-cause mortality at 7 years (Q4 HR 1.81, 95% CI, 1.04–3.15; P < 0.05) (Figure 1C–E). The inclusion of TMAO as a variable resulted in a significant improvement in risk estimation over traditional risk factors for MACE at 30 days as monitored by net reclassification improvement (NRI, 0.36 (0.19–0.53), P < 0.001; C statistic, 0.94 (0.92–0.96) (with TMAO) vs. 0.92 (0.88–0.96) (without TMAO), P < 0.05), and similar results at 6 months (NRI, 0.34 (0.17-0.5), P < 0.001; C statistic, 0.92 (0.9–0.94) vs. 0.91 (0.87–0.95), P = 0.08) and 7-year mortality (NRI, 0.52 (0.33–0.71), P < 0.001; C statistic, 0.82 (0.78–0.86) vs. 0.81 (0.77–0.85), P = 0.09) (Supplementary material online, Table S2).
Figure 1.
The association of TMAO levels with clinical outcomes in the Cleveland Cohort. Box-Whisker plots of TMAO levels among patients with complaint of chest pain with (Yes) and without (No) incident major adverse cardiac events (MACE) (MI, stroke, the need for revascularization, or death) and mortality over follow-up periods (A); Kaplan-Meier estimates and the risk of all-cause mortality ranked by quartile of TMAO levels (B); Forest plots indicating the risks of incident major adverse cardiac events (MACE) at 30 days and 6 months (C and D) and all-cause mortality by 7 years (E) according to the quartiles of TMAO levels, multivariable logistic regression model for odds ratio or multivariable Cox model for hazard ratio included adjustments for age, gender, HDL, LDL, smoking, presence or absence of a history of diabetes mellitus, hypertension, hyperlipidaemia, revascularization or coronary artery disease, C-reactive protein level, estimated glomerular filtration rate, initial troponin T level and diagnosis of either STEMI, NSTEMI or unstable angina. The 5–95% confidence interval is indicated by line length.
The relation of trimethylamine N-oxide levels and incident major adverse cardiovascular events risks among patients initially negative for troponin T at presentation
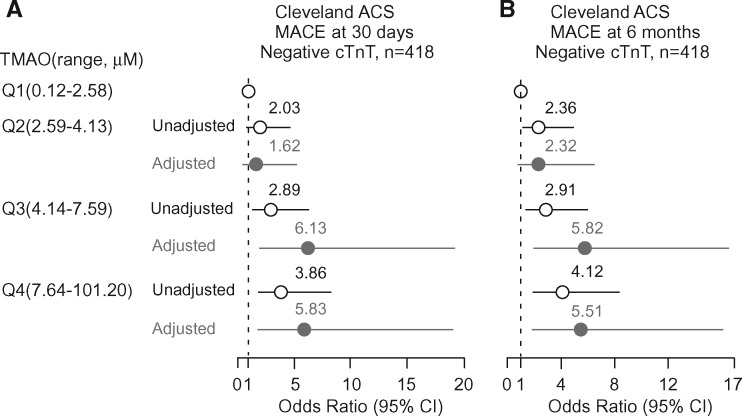
Given the recent demonstration that TMAO impacts platelet function, fostering hyperreactivity and enhanced thrombosis potential,5 we sought to evaluate whether knowledge of TMAO levels at initial presentation may provide added potential clinical utility in risk stratification among patients initially cTnT negative at presentation. Of the 530 subjects enrolled, 418 were persistently cTnT negative during the initial evaluation period. Within this sub-cohort, TMAO levels were higher amongst those who subsequently experienced an incident MACE at 30 days (median (IQR) 5.5 µM (3.5–9.9) vs. 3.7 µM (2.4–6.9); P < 0.001) and 6 months (median (IQR) 5.3 µM (3.3–9.6) vs. 3.7 µM (2.4–6.8); P < 0.0001) compared with those who did not. For patients who were initially negative for troponin T, the frequency of subsequent MACE at 30 days and 6 months, respectively, increased with increasing quartiles of TMAO (e.g. 10.5% and 12.4% in Q1 (< 2.59 µM); 19.2% and 25.0% in Q2(2.59–4.13 µM); 25.2% and 29.1% in Q3 (4.14–7.59 µM); and 31.1% and 36.8% in Q4 (> 7.59 µM) (P < 0.05 for trend)). The relationship of TMAO quartiles as a predictor for MACE among patients initially (baseline sample) cTnT negative are shown in Figure 2. Trimethylamine N-oxide levels remained a significant predictor of MACE among these subjects at both 30 days (Q4 OR 5.83, 95% CI, 1.79–19.03; P < 0.01) and 6 months (Q4 OR 5.51, 95%CI, 1.90–16.01; P < 0.001) after adjusting for risk factors including age, gender, HDL, LDL, smoking, presence or absence of a history of diabetes mellitus, hypertension, hyperlipidaemia, revascularization or coronary artery disease, C-reactive protein level, eGFR, initial cTnT level and diagnosis of either STEMI, NSTEMI or unstable angina. The inclusion of TMAO as a variable resulted in a significant improvement in risk estimation over traditional risk factors for MACE at 30 days (NRI, 0.41 (0.19-0.63), P < 0.001; C statistic, 0.89 (0.85–0.93) (with TMAO) vs. 0.84 (0.78–0.9) (without TMAO), P < 0.01) and 6 months (NRI, 0.33 (0.12–0.54), P < 0.01; C statistic, 0.87 (0.83–0.91) vs. 0.84 (0.78–0.9), P < 0.05)) (Supplementary material online, Table S2).
Figure 2.
Risks of incident major adverse cardiac events (MACE) at 30 days (A) and 6 months (B) for patients initially negative for Troponin T test according to TMAO levels ranked by quartiles in the Cleveland Cohort. Multivariable logistic regression model included adjustments for age, gender, HDL, LDL, smoking, presence or absence of a history of diabetes mellitus, hypertension, hyperlipidaemia, revascularization or coronary artery disease, C-reactive protein level, estimated glomerular filtration rate, initial troponin T level and diagnosis of either STEMI, NSTEMI or unstable angina. The 5–95% confidence interval is indicated by line length.
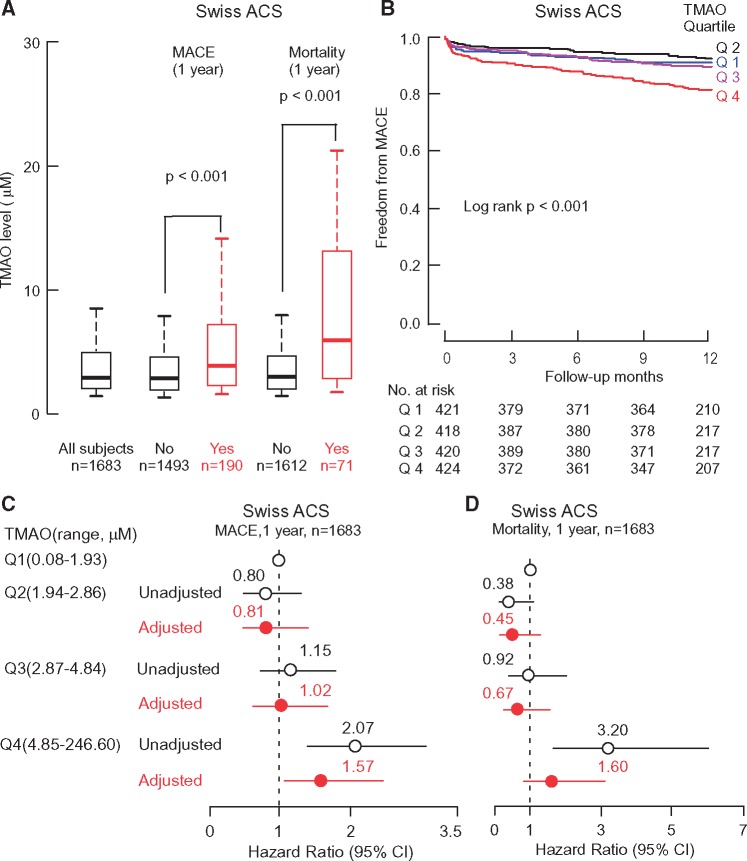
The association of trimethylamine N-oxide levels with incident major adverse cardiovascular events and mortality in the Swiss Acute Coronary Syndrome Cohort
We next sought to determine whether the strong prognostic value of TMAO in ACS subjects observed in the single center Cleveland Cohort could be replicated in an independent multi-site cohort. Plasma TMAO levels in the Swiss ACS Cohort similarly showed higher TMAO levels amongst patients who experienced adverse outcomes, including 1 year MACE (3.75 µM(2.14–7.04), n = 190, vs. 2.80 μM (1.91–4.55), n = 1493; P <0.001), and 1 year mortality (5.95 µM (2.79–13.08), n = 71, vs. 2.82 µM(1.92–4.61), n = 1612; P <0.001), respectively (Figure 3A). Kaplan–Meier survival analyses revealed quartile levels of TMAO were associated with a graded increase (log rank P < 0.001) in incident MACE risk among patients in the Swiss ACS Cohort, with highest cumulative MACE rate noted especially in the 4th quartile (Figure 3B). Multilogistic regression analyses of the relationship between TMAO levels and adverse events at 1 year (adjustments for age, gender, HDL, LDL, smoking, presence or absence of a history of diabetes mellitus, hypertension, hyperlipidaemia, revascularization or coronary artery disease, C-reactive protein level, eGFR, and diagnosis of either STEMI, NSTEMI or unstable angina) similarly revealed elevated levels of TMAO remained a significant predictor of MACE (Q4 adjusted HR 1.57, 95% CI, 1.03–2.41, P < 0.05), while the association of TMAO levels with mortality was attenuated following the adjustments (Figure 3C and D). The inclusion of TMAO as a variable to the model prediction of MACE resulted in a significant improvement in risk estimation over traditional risk factors (NRI, 0.3 (0.16–0.44), P < 0.001); but no significant improvement in C-statistic (C-statistic, 0.66 (0.62–0.7) (with TMAO) vs. 0.64 (0.6–0.68) (without TMAO); P = 0.15). Inclusion of TMAO in the model for predicting 1-year mortality resulted in significant improvement in NRI (0.45 (0.25–0.65), P < 0.001), but no significant improvement in C-statistic (0.78 (0.72–0.84) with TMAO vs. 0.77 (0.71–0.83) without TMAO; P = 0.26) (Supplemental material online, Table S2).
Figure 3.
The association of TMAO levels with clinical outcomes in the Swiss ACS Cohort. Box-Whisker plots of TMAO levels in ACS patients with (Yes) and without (No) incident major adverse cardiac events (MACE) (MI, stroke, the need for revascularization, or death) and mortality over follow-up periods (A); Kaplan-Meier estimates and the risk of MACE ranked by quartile of TMAO levels (B); Forest plots indicating the risks of incident major adverse cardiac events (MACE)(C) and all-cause mortality(D) by 1 year according to the quartiles of TMAO levels; The multivariable Cox model included adjustments for age, gender, HDL, LDL, smoking, presence or absence of a history of diabetes mellitus, hypertension, hyperlipidaemia, revascularization or coronary artery disease, C-reactive protein level, estimated glomerular filtration rate, and diagnosis of either STEMI, NSTEMI or unstable angina. The 5–95% confidence interval is indicated by line length.
Plasma TMAO levels were higher on average in the Cleveland cohort compared with the Swiss cohort, and the quartile cut-offs used for each cohort were thus different. In a final set of analyses we further examined the impact of TMAO cut point selection across both cohorts. We stratified each cohort based on TMAO levels ranging from 2.4 to 12.6 μM at every 0.2 μM increment to define high vs. low TMAO level (Supplemental material online, Tables S3 and 4). In both the Cleveland and Swiss cohorts, regardless of the TMAO cut point, the association between adverse outcome (either mortality, or MACE) and TMAO levels remained robustly strong (all P < 0.01).
Discussion
The accurate diagnosis of cardiovascular events in patients presenting with acute coronary syndromes by electrocardiography (ECG) and conventional laboratory tests is challenging. The recent demonstration that the TMAO pathway impacts multiple aspects of ‘patient vulnerability’, including development of atherosclerotic plaque,1–4 alteration in macrophage and endothelial cell phenotype,3,23 and promotion of platelet hyperresponsiveness,5 makes this circulating metabolite particularly interesting to explore in the setting of subjects presenting with chest pain and suspected ACS. The present study demonstrates for the first time that systemic TMAO levels are associated with both near-term risk of MACE and long term mortality, among sequential patients presenting with complaint of chest pain and suspected ACS in two independent ACS cohorts. TMAO levels showed a dose-dependent relationship between increase in risk of incident MACE and higher TMAO levels in both the Cleveland and Swiss cohorts (Supplementary material online Figure S4). These studies thus add to the growing body of data demonstrating that the gut microbiota dependent metabolite, TMAO, may serve as a clinically useful and potentially modifiable prognostic marker for both near-term and long-term cardiovascular outcomes beyond currently used risk factors and laboratory testing. These findings not only broaden the potential clinical utility of TMAO as an independent prognostic marker to those presenting with suspected ACS, but also suggest, in combination with the recent studies linking this metabolite to platelet function, a potential basis for a novel therapeutic target in the management of ACS patients.
In the quest to improve outcomes in patients with cardiovascular heart disease, which still remains the leading cause of death worldwide,29–31 research has focused on novel modifiable risk factors, with a special attention to environmental sources.32,33 Although nutrition has always been linked to outcomes of cardiovascular patients, the pivotal role of the gut microbiome has only recently been recognized in this context. Hence, we have sought to assess the impact of the gut microbiome dependent metabolite, TMAO, on cardiovascular outcomes in patients with ACS. To strengthen the impact of our findings, after initial observation of associations with MACE risk in the Cleveland cohort, we sought to validate these findings by exploring the relationship between TMAO levels and adverse outcomes in an independent large prospective multi-center Swiss ACS registry with independent events adjudication. The prognostic value of TMAO has thus far predominantly been examined in lower risk community based or stable cardiac cohorts.1–3,5,7–20 Our results are consistent with these other clinical studies in that they show that elevated levels of TMAO may serve as an independent risk factor of MACE and long-term all-cause mortality, including in those presenting with ACS. The impact of risk stratification by TMAO quartiles was most strikingly evident in patients belonging to the 4th quartile (Q4), where they faced an approximate two-fold increased hazard of 7-year mortality when compared with those in the lowest quartile. It should be noted, however, that while the majority of studies in different cohorts have observed a positive association between systemic TMAO levels and incident cardiovascular risks,1–3,5,7–20,34 others have not.35,36 The reasons for these differences are unclear, but it is noteworthy that the negative studies have tended to be both smaller than comparable alternative positive studies, and in general to have lower event rates (and often TMAO levels). Interestingly, a recent large (n > 1000) clinical study exploring the prognostic value of TMAO in subjects on haemodialysis observed that the strength of the association between TMAO and adverse events differed somewhat by race, with whites showing a significant association between higher TMAO levels and heightened incident risk of cardiac death, sudden cardiac death, first cardiovascular event, and any-cause death, whereas in blacks, elevated TMAO levels were only significantly associated with cardiac death.34 It is also noteworthy that in an alternative recent study, genetic variants in the flavin monooxygenase 3 gene, the host hepatic enzyme primarily responsible for conversion of TMA into TMAO,6 were reported to be associated with both rate of chronic kidney disease progression and mortality risk in patients with impaired renal function.37 Trimethylamine N-oxide exerts pro-atherosclerotic effects via a variety of effects, including changes in macrophage and tissue sterol metabolism, and endothelial cell activation1,3,23. In line with these findings, recent studies reveal that TMAO levels are highly related to the coronary atherosclerotic burden and plaque complexity as monitored by the SYNTAX score.19 In addition, TMAO has a pro-thrombotic effect by altering platelet calcium signalling, rendering platelets more responsive to agonists, increasing the risk of in vivo thrombus formation.5 All these effects are crucial mechanisms of acute coronary syndromes and their complications during follow-up. Furthermore, experimental evidence suggests that the use of a drug that selectively targets microbial TMA production and lowers plasma TMAO levels in the host can serve as a means of inhibiting diet-induced atherosclerosis in animal models.38 Similarly, use of antisense oligonucleotide approaches to knock down flavin monooxygenase 3, the major hepatic enzyme responsible for the conversion of microbial-generated TMA into TMAO,6 suppresses TMAO levels, inhibits atherosclerosis in animal models, and reveals the TMAO metaorganismal pathway as a central regulator of tissue cholesterol cholesterol balance.39–41 Thus, TMAO appears to not only be a risk marker, but also may serve as a potential therapeutic target to improve outcomes in patients, including subjects with ACS. Moreover, as suggested by previous work, TMAO also appears to exert a particularly profound impact on outcomes in patients developing post-infarction heart failure, and in animal models was shown to accelerate adverse ventricular remodeling and dysfunction.8,42 It is also of interest that in the present study, in the sub analysis involving patients with chest pain and negative troponin, plasma levels of TMAO maintained strong prognostic significance beyond traditional risk factors, biomarkers and electrocardiographic data. It is therefore tempting to speculate that the ability to generate rapid and accurate TMAO results through point-of-care testing could significantly improve rapid triaging and risk stratification amongst subjects presenting with the complaint of chest pain and suspected ACS.
As again exemplified in the patient cohorts of this study, event rates in ACS remain high in spite of all the progress made in the management and secondary prevention of ACS over the last few decades. It is of note that both the plasma levels of TMAO and the event rates were higher in the US compared with the Swiss patients, and that incident event rates increased dose dependently in both cohorts. The burden of comorbidities was significantly higher within the Cleveland cohort, and this may account in part for these observations. Whether different dietary behaviours may also be involved in these differences is unknown. As a metabolite produced as a result of gut microbe action of specific dietary nutrients, TMAO is uniquely positioned at the interface between our diet and cardiovascular disease pathogenesis. It is therefore interesting to note that recent large-scale epidemiological studies examining phosphatidylcholine ingestion, a nutrient that is more abundant in animal products, and mortality risks in >100 000 subjects with over a quarter century of follow-up, revealed phosphatidylcholine consumption is associated with increased all-cause and cardiovascular mortality independent of traditional risk factors.43 Moreover, adherence to a vegetarian or vegan diet compared with an omnivorous diet is reported to be associated with reduced plasma and urinary levels of TMAO.3,44 These results suggest that studies are needed in patients presenting with ACS to test whether dietary modification efforts coupled with monitoring TMAO levels can serve as an additional potential intervention to help reduce long term adverse risks. For example, our current understanding of the biosynthetic pathway of TMAO1,3,45 can provide additional hints on how one could possibly intervene. Indeed, consumption of choline and L-carnitine rich diets offer the necessary precursors for the production of TMAO.2,3 Hence, a change in dietary habits, specifically to that of the Mediterranean type,46 which has been shown to decrease the risk of adverse cardiovascular events in both observational and prospective studies,47,48 could be tested to determine whether diet-induced reduction in TMAO enhances secondary prevention in patients after ACS. Animal model studies suggest another approach to the high TMAO ACS patient could involve inhibition of the gut microbial participants involved in metabolic production of TMAO.49 Particularly promising is the effective experimental use of 3,3-dimethyl-1-butanol (DMB), which by inhibiting microbial choline and carnitine TMA lyase activities in vivo appears to prevent foam cell formation and aortic atherosclerotic plaque formation, while maintaining a normal gut flora.38
Limitations
This study provides the basis for future studies investigating interventions to lower TMAO levels in ACS patients to confirm a cause effect relationship. On its own, our findings are hypothesis generating for such trials, but establish the prognostic information provided by TMAO in ACS patients. Cause of death was not available for the Cleveland cohort, so all-cause death was used as the mortality end point. Another limitation of the present study is that plasma samples from subjects presenting with suspected ACS were not necessarily fasting, and no information was collected on timing of presentation relative to last meal, or dietary patterns preceding presentation in either the Cleveland or Swiss ACS cohorts. Nonetheless, the present studies reveal that the prognostic value of TMAO in the ACS setting appears to be reproducible in both the US (single site) and Swiss (multi-site) cohorts.
Conclusion
To the best of our knowledge, we were able to show for the first time that TMAO is an important prognostic marker in predicting both near- and long-term adverse cardiovascular events beyond traditional risk factors and laboratory tests in the setting of acute coronary syndrome. This was not only true for patients with elevated troponins due to a STEMI or NSTEMI, but also was observed for patients who did not suffer an infarction, but rather, were negative for troponin at initial presentation. The importance of our findings is underlined by the modifiable nature of TMAO, both with diet, and with potential therapeutics under development, offering new prospects for treatment strategies at both levels of primary and secondary prevention.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors are grateful to Anika Adam for helping to build the ACS SPUM registry and to Dr Milosz Jaguszewski, Philipp Jakob and Sebastian Schlager for their assistance. This work was supported by the National Institute of Health (NIH) and the Office of Dietary Supplements (R01HL103866, P20HL113452, R01DK106000, R01HL126827A1), and the Swiss National Research Foundation (Special Programme University Medicine ‘Acute coronary syndromes and inflammation’ Nr. 33CM30-124112 to TFL). Mass spectrometry studies were performed on instruments housed in a facility supported in part by a Center of Excellence Award by Shimadzu Scientific Instruments. Dr Wang was partially supported by NIH grant R01HL130819. Dr Hazen was partially supported by a gift from the Leonard Krieger endowment. This work was also supported by the Foundation for Cardiovascular Research – Zurich Heart House, Zurich, Switzerland. In part an unrestricted research grant of AstraZeneca, Zug, Switzerland and Eli Lilly, Indianapolis, Indiana, USA helped building the Swiss ACS registry.
Conflict of interest: Drs Hazen and Wang are named as co-inventors on patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr Hazen is a paid consultant for Esperion and P&G; has received research funds from P&G, Pfizer Inc., Roche Diagnostics, and Takeda; and is eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, Siemens, Esperion, and Frantz Biomarkers, LLC. Dr Lüscher reports grants from AstraZeneca, grants from Biotronik, grants from Boston Scientific, grants from Eli Lilly, grants from St. Jude, outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
The authors do hereby declare that all materials used herein are original and do not require permission for reprinting.
References
- 1. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL.. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL.. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL.. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL.. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem 2015;290:5647–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL.. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ.. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL.. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 2014;35:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL.. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 2014;64:1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL, Hazen SL.. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail 2015;21:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL.. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 2015;116:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mente A, Chalcraft K, Ak H, Davis AD, Lonn E, Miller R, Potter MA, Yusuf S, Anand SS, McQueen MJ.. The relationship between trimethylamine-n-oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can J Cardiol 2015;31:1189–1194. [DOI] [PubMed] [Google Scholar]
- 12. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD, Spertus JA, Yu AS.. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 2016;27:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mafune A, Iwamoto T, Tsutsumi Y, Nakashima A, Yamamoto I, Yokoyama K, Yokoo T, Urashima M.. Associations among serum trimethylamine-N-oxide (TMAO) levels, kidney function and infarcted coronary artery number in patients undergoing cardiovascular surgery: a cross-sectional study. Clin Exp Nephrol 2016;20:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL.. Trimethylamine N-oxide and prognosis in acute heart failure. Heart 2016;102:841–848. [DOI] [PubMed] [Google Scholar]
- 15. Missailidis C, Hallqvist J, Qureshi AR, Barany P, Heimburger O, Lindholm B, Stenvinkel P, Bergman P.. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One 2016;11:e0141738.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Randrianarisoa E, Lehn-Stefan A, Wang X, Hoene M, Peter A, Heinzmann SS, Zhao X, Konigsrainer I, Konigsrainer A, Balletshofer B, Machann J, Schick F, Fritsche A, Haring HU, Xu G, Lehmann R, Stefan N.. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep 2016;6:26745.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dambrova M, Latkovskis G, Kuka J, Strele I, Konrade I, Grinberga S, Hartmane D, Pugovics O, Erglis A, Liepinsh E.. Diabetes is associated with higher trimethylamine N-oxide plasma levels. Exp Clin Endocrinol Diabetes 2016;124:251–256. [DOI] [PubMed] [Google Scholar]
- 18. Kim RB, Morse BL, Djurdjev O, Tang M, Muirhead N, Barrett B, Holmes DT, Madore F, Clase CM, Rigatto C, Levin A, Can PI.. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int 2016;89:1144–1152. [DOI] [PubMed] [Google Scholar]
- 19. Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison B, Wang Z, Hazen SL, Tang WH.. Plasma trimethylamine N-Oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol 2016;67:2620–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WH, Hazen SL.. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc 2016;5:pii: e002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trip MD, Cats VM, van Capelle FJ, Vreeken J.. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med 1990;322:1549–1554. [DOI] [PubMed] [Google Scholar]
- 22. Marcucci R, Valente S, Gori AM, Chiostri M, Paniccia R, Giusti B, Cau V, Lazzeri C, Gensini GF, Abbate R.. Global platelet hyperreactivity and elevated C-reactive protein levels predict long term mortality in STEMI patients. Thromb Res 2014;134:884–888. [DOI] [PubMed] [Google Scholar]
- 23. Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM.. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappaB. J Am Heart Assoc 2016;5:pii: e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL.. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL.. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 2003;349:1595–1604. [DOI] [PubMed] [Google Scholar]
- 27. Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr., Vasan RS.. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212. [DOI] [PubMed] [Google Scholar]
- 28. Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW.. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med 2014;160:122–131. [DOI] [PubMed] [Google Scholar]
- 29. Townsend N, Nichols M, Scarborough P, Rayner M.. Cardiovascular disease in Europe–epidemiological update 2015. Eur Heart J 2015;36:2696–2705. [DOI] [PubMed] [Google Scholar]
- 30. Vedanthan R, Seligman B, Fuster V.. Global perspective on acute coronary syndrome: a burden on the young and poor. Circ Res 2014;114:1959–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heron M. Deaths: leading causes for 2013. Natl Vital Stat Rep 2016;65:1–95. [PubMed] [Google Scholar]
- 32. Yamashita T, Kasahara K, Emoto T, Matsumoto T, Mizoguchi T, Kitano N, Sasaki N, Hirata K.. Intestinal immunity and gut microbiota as therapeutic targets for preventing atherosclerotic cardiovascular diseases. Circ J 2015;79:1882–1890. [DOI] [PubMed] [Google Scholar]
- 33. Org E, Mehrabian M, Lusis AJ.. Unraveling the environmental and genetic interactions in atherosclerosis: Central role of the gut microbiota. Atherosclerosis 2015;241:387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shafi T, Powe NR, Meyer TW, Hwang S, Hai X, Melamed ML, Banerjee T, Coresh J, Hostetter TH.. Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J Am Soc Nephrol 2016; pii: ASN.2016030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A.. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis 2015;243:638–644. [DOI] [PubMed] [Google Scholar]
- 36. Kaysen GA, Johansen KL, Chertow GM, Dalrymple LS, Kornak J, Grimes B, Dwyer T, Chassy AW, Fiehn O.. Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr 2015;25:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robinson-Cohen C, Newitt R, Shen DD, Rettie AE, Kestenbaum BR, Himmelfarb J, Yeung CK.. Association of FMO3 variants and trimethylamine N-oxide concentration, disease progression, and mortality in CKD patients. PLoS One 2016;11:e0161074.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL.. Non-lethal Inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015;163: 1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, Croce KJ, Esquejo RM, Clish CB Morbid Obesity Study G Vicent D, Biddinger SB.. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun 2015;6:6498.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ.. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res 2015;56:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM.. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep 2015;10:326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WH, Wu Y, Hazen SL, Lefer DJ.. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail 2016;9:e002314.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zheng Y, Li Y, Rimm EB, Hu FB, Albert CM, Rexrode KM, Manson JE, Qi L.. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am J Clin Nutr 2016;104:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O'toole PW, Ercolini D.. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016;65:1812–1821. [DOI] [PubMed] [Google Scholar]
- 45. Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, Tang WH, DiDonato JA, Lusis AJ, Hazen SL.. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab 2014;20:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martinez-Gonzalez MA, Ruiz-Canela M, Hruby A, Liang L, Trichopoulou A, Hu FB.. Intervention trials with the Mediterranean diet in cardiovascular prevention: understanding potential mechanisms through metabolomic profiling. J Nutr 2016;146(suppl):913S–919S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Martinez-Gonzalez MA, Bes-Rastrollo M.. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr Opin Lipidol 2014;25:20–26. [DOI] [PubMed] [Google Scholar]
- 48. Martinez-Gonzalez MA, Salas-Salvado J, Estruch R, Corella D, Fito M, Ros E, Predimed I.. Benefits of the Mediterranean diet: insights from the PREDIMED study. Prog Cardiovasc Dis 2015;58:50–60. [DOI] [PubMed] [Google Scholar]
- 49. Brown JM, Hazen SL.. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med 2015;66:343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.