Why was the cohort set up?
Early life environmental factors may increase the risk of disease in childhood or adulthood. Well-established examples of early life environmental factors affecting later life health include the increased risk of vaginal clear cell carcinoma and several reproductive disorders following in utero diethylstilbestrol exposure, and cognitive decrements in children with prenatal mercury or childhood lead exposure.1–4 These studies indicate that the effect of some environmental factors may depend on the timing of exposure relative to sensitive developmental windows.
There is concern that exposure to environmental chemicals during gestation, infancy or childhood may increase the risk of neurodevelopmental disorders, obesity or allergic diseases.5 However, we know little about the health effects of individual chemicals and even less about mixtures of environmental chemicals.6,7 Thus, prospective and longitudinal cohort studies with repeated exposure and outcome assessments offer the opportunity to determine whether and when human health is affected by environmental chemical exposures.
We established the Health Outcomes and Measures of the Environment (HOME) Study, a prospective pregnancy and birth cohort in the greater Cincinnati OH metropolitan area, to determine whether early life environmental chemical exposures influence children’s health. The National Institute of Environmental Health Sciences and the United States Environmental Protection Agency originally funded the HOME Study through their Children’s Environmental Health Center Program to examine the relationship of prenatal lead, tobacco smoke, mercury, polychlorinated biphenyl (PCB) and pesticide exposures with children’s cognitive and behavioural development between birth and 3 years of age. We nested a randomized trial within the cohort to assess the efficacy of lead and injury hazard controls on children’s blood lead levels, cognitive and behavioural development and risk of household injury. Continued funding from the National Institutes of Health and various foundations has allowed us to measure a multitude of chemical exposures and conduct additional assessments through 8 years of age. Anticipated funding will allow us to conduct follow-up when children are 12 years old.
Who is in the cohort?
Between March 2003 and January 2006, we recruited pregnant women to participate in a longitudinal pregnancy and birth cohort study. We identified women living in a nine-county region of the Cincinnati OH metropolitan area (Brown, Butler, Campbell, Clermont, Hamilton and Warren counties) and Northern Kentucky (Campbell, Brenton and Boone counties) using the medical scheduling systems of nine prenatal practices affiliated with three hospitals.
Eligibility was determined using clinic records and phone interviews with women. Eligibility criteria included: living in the study region, < 19 weeks pregnant, > 18 years old, residing in a home built in or before 1978, not living in a mobile or trailer home, HIV-negative, not taking medications for seizures or thyroid disorders, planning to continue prenatal care and deliver at the collaborating clinics and hospitals, planning to live in the greater Cincinnati area for the next year, fluent in English and no diagnosis of diabetes, bipolar disorder, schizophrenia or cancer that resulted in radiation treatment or chemotherapy. To target children at increased risk of lead exposure, we enrolled women living in homes built before 1978 and stratified enrolment so that approximately 50%, 38% and 12% of women would be from the city of Cincinnati, surrounding suburbs and surrounding rural areas, respectively.8 We oversampled women who self-identified as Black (31%) to investigate potential health disparities.
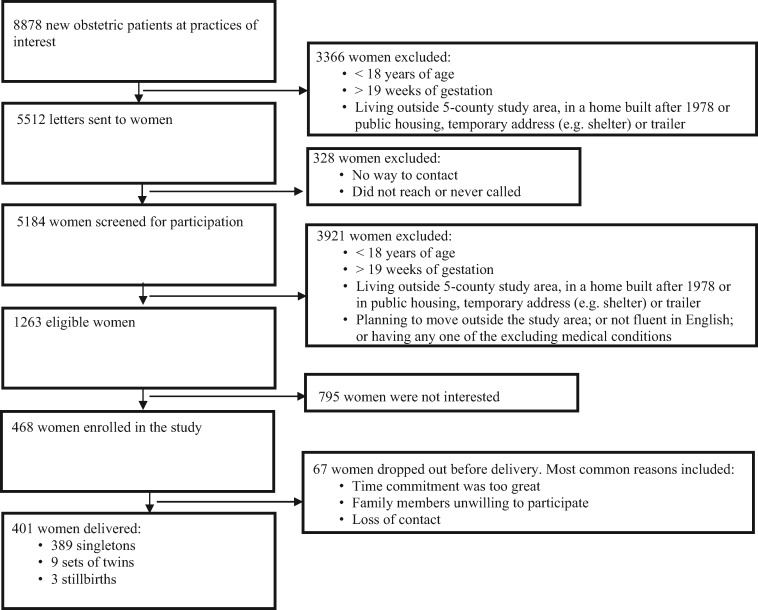
Of 8878 women in the sampling frame, we excluded 3366 who were ineligible based on information from clinic records and the housing assessors’ database (Figure 1 ). We mailed letters to 5512 women and surveyed 5184 (94%) to determine eligibility. Of 1263 eligible women (15% of those screened for eligibility), 468 (37% of those eligible) agreed to participate. Our Community Advisory Board recommended instituting a ‘run-in’ period so women could discuss the study with family and friends before committing to participate; 67 women dropped out during this period, leaving 401 women who delivered 389 singleton infants, nine sets of twins, and three stillbirths. Our final sample included women from seven prenatal care practices in five counties in OH (none from Brown County) and Campbell County in Kentucky.
Figure 1.
Eligibility and enrolment flowchart for the HOME Study.
We compared HOME Study participants with women > 18 years of age who delivered infants in the study region from 2003 to 2004 (Table 1). Compared with women in the study region, participants were similar in terms of marital status and smoking during pregnancy, but were older at delivery (mean: 29.4 vs 27.9 years) and more likely to be non-Hispanic Black (31 vs 19%) and have a college education or greater (51 vs 34%).
Table 1.
Comparison of sociodemographic characteristics of HOME Study participants with a live birth (2003–06) and women in the study region with live births (2003–04)
| Births in the study area, n = 42828 (%)a | HOME Study participants, n = 392 (%)b | |
|---|---|---|
| Race | ||
| Non-Hispanic White | 33365 (77.9) | 244 (62.2) |
| Non-Hispanic Black | 8163 (19.1) | 121 (30.9) |
| Other | 1300 (3.0) | 27 (6.9) |
| Maternal education | ||
| < 12 years | 6231 (14.6) | 41 (10.5) |
| 12 years | 12596 (29.5) | 54 (13.8) |
| 13–15 years | 8262 (19.3) | 99 (25.3) |
| > 16 years | 14464 (33.8) | 198 (50.5) |
| Not classifiable | 1199 (2.8) | |
| Marital status | ||
| Married | 27631 (63.2) | 252 (64.3) |
| Not married | 16087 (36.8) | 140 (35.7) |
| Tobacco use | ||
| No | 36819 (84.4) | 343 (87.5) |
| Yes | 6654 (15.3) | 49 (12.5) |
| Don’t know | 136 (0.3) | |
| Mean maternal age in years (range) | 27.9 (18–50) | 29.4 (18–45) |
aStudy region originally included Brown, Butler, Hamilton, Clermont and Warren counties in Ohio, and Campbell, Brenton and Boone counties in Kentucky. We show study region data for Butler, Hamilton, Clermont and Warren counties since no participating women enrolled from Brown county and only two women enrolled from Kentucky.
bSix women were missing sociodemographic information.
cStudy region data come from the National Center for Health Statistics Birth Data. Data 2005–06 were not publicly available.32
How often have they been followed up?
We met women at their prenatal clinic appointments at an average of 16.0 and 26.4 weeks of gestation and at the hospital within 48 h of delivery (Table 2). We also conducted home visits at an average of 20.7 weeks of gestation and when children were an average of 4.9 weeks and 1.1, 2.1 and 3.1 years old. On average, women and their children returned for clinic visits when children were 1.1, 2.1, 3.1, 4.1, 5.2 and 8.2 years old. We conducted telephone interviews quarterly until the child’s first birthday and twice yearly thereafter until children were 6 years old. Among live-born children (n = 407), follow-up rates at the 1-, 2-, 3-, 4-, 5- and 8-year clinic visits were 83%, 70%, 65%, 48%, 54% and 58%, respectively. A total of 391 (96%) children completed at least one follow-up visit between birth and 8 years of age (median number of visits: 5) (Table 3).
Table 2.
Summary of measurements collected in HOME Study women and children (Cincinnati, OH, 2003–14)
| Measurement category | Measurement or sample | 16-W and 26-W CV | 20-W HV | Deliverya | 4-W HV | 1‐3-Y CV | 1‐3-Y HV | 4-Y CV | 5-Y CV | 8-Y CV |
|---|---|---|---|---|---|---|---|---|---|---|
| Biospecimens | Urine | X | Xb | Xb | X | X | X | X | ||
| Blood | X | Xc | X | X | X | X | ||||
| Hair | X | X | X | X | X | X | ||||
| Meconium | X | |||||||||
| Saliva | X | |||||||||
| Breast milk or formula | X | |||||||||
| Shed deciduous teeth | X | |||||||||
| Leukocyte DNA | Xd | Xd | ||||||||
| Clotted red blood cells | X | |||||||||
| Environmental samples | Dust and dust wipes | X | X | |||||||
| Tap water | X | |||||||||
| Soil samples | X | |||||||||
| Neurobehaviour | Infant neurobehaviour | X | X | |||||||
| Mental/psychomotor development | X | |||||||||
| IQ | X | X | ||||||||
| Behaviour and mental health | X | X | X | X | ||||||
| Executive function | X | X | X | X | ||||||
| Visuospatial ability | X | X | ||||||||
| Language/reading readiness | X | X | ||||||||
| Academic achievement | X | |||||||||
| Anxiety and depression | X | |||||||||
| Play behaviour and gender identity | X | |||||||||
| Anthropometry | Weight, length/height, and head circumference | X | X | X | X | X | X | |||
| Waist circumference | X | X | X | |||||||
| Body fat | X | |||||||||
| Allergy, asthma and respiratory system | Asthma, allergy, and respiratory symptoms | X | X | X | X | |||||
| Spirometry | X | X | ||||||||
| Exhaled nitric oxide | Xe | X | X | |||||||
| Injuries | Parent-reported injuries | X | X | X | ||||||
| Linkage to emergency room records | X | X | X | |||||||
| Questionnaires | Maternal and Neonatal Medical Chart Review | X | ||||||||
| Breastfeeding | X | X | ||||||||
| Infant/Child Sleep | X | X | X | X | ||||||
| Sociodemographic Factors | X | X | X | X | X | X | ||||
| Environmental Exposure Questionnaire | X | X | X | X | X | X | ||||
| Parent Mental Health and ADHD Symptoms | X | X | X | |||||||
| Maternal Depression | X | X | X | X | X | X | ||||
| Parenting Stress | X | X | X | X | ||||||
| Caregiving Environment | X | |||||||||
| Maternal/Paternal IQ | X | X | ||||||||
CV, clinic visit; HV, home visit; W, week; Y, year.
aMeasures collected within 48 h of delivery.
bMaternal urine.
cWe collected both cord blood and maternal blood.
dMaternal DNA collected from 26-week blood sample and child DNA collected from cord blood sample.
e3 year visit only.
Table 3.
Sociodemographic, perinatal and infant characteristics of HOME Study women who had a live birth (n = 398) and those who completed follow-up with their child at 8 years of age (n = 233)a
| Characteristic | N (%)/mean [SD] at enrolment | N (%)/mean [SD] at 8-year visit | Median (25th, 75th) number of follow-up visits completed |
|---|---|---|---|
| Maternal race | |||
| Non-Hispanic White | 244 (62.2) | 141 (60.5) | 6 (4, 7) |
| Non-Hispanic Black | 121 (30.9) | 79 (33.9) | 4 (2, 7) |
| American Indian, Asian/Pacific/Hispanic | 27 (6.9) | 13 (5.6) | 5 (3, 7) |
| Maternal age at delivery (years) | |||
| 18–< 25 | 97 (24.4) | 62 (26.6) | 4 (2, 7) |
| 25–< 30 years | 114 (28.6) | 65 (27.9) | 6 (4, 7) |
| 30–< 35 years | 124 (31.2) | 70 (30.0) | 6 (4, 7) |
| > 35 years | 63 (15.8) | 36 (15.5) | 5 (3, 7) |
| Maternal education | |||
| Some high school | 41 (10.5) | 24 (10.3) | 5 (2, 6) |
| High school or Equivalent | 54 (13.8) | 35 (16.8) | 4 (2, 7) |
| Some college or technical school | 99 (25.3) | 65 (31.1) | 6 (3, 7) |
| > Bachelor’s degree | 198 (50.5) | 109 (46.8) | 6 (4, 7) |
| Parity | |||
| Nulliparous | 174 (43.9) | 106 (45.5) | 6 (3, 7) |
| Multiparous | 222 (56.1) | 127 (54.5) | 5 (3, 7) |
| Prenatal vitamin use | |||
| Never | 46 (11.7) | 28 (12.0) | 5 (3, 7) |
| Any | 346 (88.3) | 205 (88.0) | 5 (4, 7) |
| Household income (US$ per year) | |||
| < 20 000 | 88 (22.4) | 62 (26.6) | 5 (2, 7) |
| 20 000–< 40 000 | 68 (17.4) | 35 (15.0) | 4 (3, 6) |
| 40 000–< 80 000 | 131 (33.4) | 77 (33.1) | 6 (4, 7) |
| > 80 000 | 105 (26.8) | 59 (25.3) | 6 (4, 7) |
| Smoking during pregnancyc | |||
| None | 344 (86.4) | 202 (86.7) | 6 (4, 7) |
| Any | 54 (13.6) | 31 (13.3) | 4 (2, 6) |
| Infant sex | |||
| Female | 220 (54.0) | 134 (56.5) | 6 (4, 7) |
| Male | 187 (46.0) | 103 (43.5) | 5 (3, 7) |
| Infant birthweight (g) | 3322 [649] | 3306 [633] | |
| Gestational duration (weeks) | 38.9 [1.9] | 38.9 [1.8] |
aOf the 398 women with a live birth, 6 women dropped out before we collected sociodemographic information. Thus, 2 to 6 women are missing data for race, parity, prenatal vitamin use, and household income.
bThe median, 25th percentile and 75th percentile of the number of visits was calculated by summing the number of follow-up visits a mother-child pair completed at 4 weeks and 1, 2, 3, 4, 5 and 8 years of age. We counted clinic and home visits conducted at 1, 2 and 3 years of age as one visit for each year, even for those completing both a clinic and home visit in a given year.
cDetermined using serum cotinine concentrations at 16 or 26 weeks of gestation or at delivery. Women with a serum cotinine > 3 ng/ml at any visit were classified as smokers.33
Participating women who had a live birth were predominately non-Hispanic White (62%), 30 to < 35 years old at delivery (31%), multiparous (56%), non-smokers (86%) and had a bachelor’s degree or greater (51%) (Table 3). In baseline characteristics, women who returned to the clinic with their child at 8 years of age (n = 233 women, n = 237 children) were similar to the original cohort (Table 3). However, compared with the original cohort, a slightly greater proportion of women who were non-Hispanic White, well educated and had higher household income returned for visits from 1 to 5 years following delivery (results not shown). In addition, women with these characteristics returned to our study clinic for more visits (Table 3). All of the 407 live-born children are still eligible for follow-up. In addition, beginning with the 8-year visit, we attempted to re-engage children born to women who dropped out during the ‘run-in’ period. Threeof these children (one set of twins and one singleton) returned for the 8-year visit.
What has been measured?
We have extensive, multimodal and repeated assessments of environmental chemical exposures, child health and confounders in both mothers and children (Table 2). If possible, we used measures used by other birth cohorts to facilitate pooled analyses.9
Environmental chemical biomarkers
We designed our biospecimen collectionto identify unique windows of vulnerability to environmental chemicals during gestation or childhood. In addition, we lot-tested our collection materials for contamination by chemicals that might be used in the secollection supplies (e.g. phthalates in diaper inserts).10 We collected > 37 000 biological specimens at multiple time points from women during pregnancy and the postpartum period and from children from 1 to 8 years of age (Table 2). Unique biospecimens include neonatal meconium, vernix from a subset of 122 infants, breast milk from mothers of breastfed infants and formula from weaned or formula-fed infants. We also collected clotted red blood cells and shed deciduous teeth from children at 8 years of age.
To date, we have measured > 100 different chemicals in our participants’ biospecimens. These include lead, cadmium, mercury, arsenic, tobacco smoke metabolites, PCBs, organochlorine pesticides, polybrominated diphenyl ethers (PBDEs), phthalate metabolites, environmental phenols, perfluoroalkyl substances and organophosphorous/pyrethroid pesticide metabolites. For many chemicals, we have multiple measures during pregnancy and childhood (Supplementary Table 1, available as Supplementary data at IJE online). We measured whole-blood folate, urinary iodine and serum thyroid hormone concentrations during pregnancy, as well as cord blood thyroid hormone concentrations. Finally, we extracted DNA from maternal and child samples.
Environmental samples
We collected > 15 000 environmental samples from participants’ homes at 20 weeks of gestation and when children were 1, 2 and 3 years old. We collected outdoor soil samples at 20 weeks of gestation and dust wipes of floors, window troughs and windowsills during the first 3 years of life. We collected tap water at 20 weeks of gestation and floor or carpet dust with a high volume small surface sampler at all visits. We measured dust lead loadings in house dust and lead concentrations in soil, water and paint. We quantified organohalogen flame retardant concentrations in a subset of homes.
Chemical exposure questionnaire
We administered standardized questionnaires about pregnant women or children’s exposure to environmental chemicals. We initially designed our questionnaires to identify sources of exposure to pesticides, mercury, lead and tobacco smoke. We added questions about exposures to phthalates, bisphenol A, parabens, perfluoroalkyl substances and flame retardants when children were 5 and 8 years old.
Neurodevelopmental assessments
We used examiner-administered tests and parent-reported surveys to assess several neurobehavioural domains that might be affected by environmental chemical exposures.11 We developed our protocols to accommodate the unique aspects of conducting neurobehavioural assessments in children. All examiners underwent intensive training including discussions of test goals, psychometric properties, directions for proper administration and scoring, and strategies for testing young children. Examiners completed a prescribed number of practice examinations on age-appropriate pilot subjects and received frequent feedback before presenting for a ‘certification’ test. Once certified, our developmental psychologist (K.Y.) conducted quality checks every 6 months to ensure continued reliability. Quality control procedures also included observation of test administration and review of scoring forms. We conducted all neurobehavioural assessments at > 1 year of age in a clinic setting to reduce the influence of different environments or distractions on children’s performance.
We assessed infant neurological and behavioural function within 48 h of delivery in the delivery hospital and at 4 weeks of age in the home using the NICU Network Neurobehavioral Scale. Between 1 and 8 years of age, we administered the Bayley Scales of Infant Development-II, Wechsler Preschool and Primary Scales of Intelligence-III and the Wechsler Intelligence Scales for Children-IV to assess mental and psychomotor development and cognitive abilities [i.e. intelligence quotient [IQ]). We also repeatedly assessed continuous and quantitative phenotypes of clinical disorders using parent-reported measures of child behaviour. These included the Social Responsiveness Scale to assess features of autism spectrum disorders and the Behavior Assessment System for Children-2 to assess features of attention-deficit/hyperactivity disorder (ADHD), conduct disorder, anxiety and depression.
We assessed children’s executive function with the Behavior Rating Inventory of Executive Function, Conner’s Continuous Performance Task, Shape School, TRAILS-P, delay of gratification test and NEPSY. We assessed children’s language abilities, reading readiness and academic achievement with the Clinical Evaluation of Language Fundamentals, Woodcock-Johnson-III, and Wide Range Achievement Test-4, respectively. At 8 years of age, we assessed children’s play behaviours/preferences, gender identity and anxiety. Finally, we assessed children’s visual-spatial abilities using the Virtual Morris Water Maze, a computerized version of a rodent test used in toxicology studies.
Anthropometry
We abstracted neonatal anthropometry from medical records. Study staff measured weight, length/height and head circumference when children were 4 weeks and 1, 2, 3, 4, 5 and 8 years old. We measured weight using a digital scale with children dressed in undergarments or a dry diaper, except at 4 weeks when we weighed infants while they were naked. We measured recumbent length with a length board and standing height with a wall-mounted stadiometer. We assessed head circumference using a paper tape measure. At 4, 5 and 8 years of age, we measured waist circumference around a horizontal plane defined by the left and right iliac crests using a plastic measuring tape. Finally, we measured children’s body fat using a Tanita children’s body fat monitor at 8 years of age.
Respiratory function and asthma/allergy symptoms
We used standardized questions based on those from the National Health and Nutrition Examination Survey to assess wheeze, eczema, allergy and asthma symptoms at 6-month intervals between 6 months and 5 years of age and again when children were 8 years old. When children were 4 and 5 years old, we attempted to collect at least three acceptable forced expiratory volume (FEV)-1 measurements using a portable spirometer. At the 3-, 4- and 5-year visit, we measured exhaled nitric oxide, a marker of airway inflammation.
Child injuries
We asked parents about child injuries in the home at between 3 months and 6 years of age. We provided parents with calendars to record events as they occurred. Medically attended injuries were those that prompted parents to call or visit a physician’s office, urgent care or emergency department. We classified injuries as modifiable if one of the installed interventions (e.g. wall-mounted stair gate) could have prevented the hazard or mechanism from causing the injury. We confirmed emergency room visits for residential injuries using the Hamilton County Injury Surveillance System.
Randomized trial
Because low-level lead exposure is a risk factor for intellectual deficits and behavioural problems in children, we conducted a randomized controlled trial in 355 participants to test the efficacy of lead abatement techniques on lead-contaminated house dust, blood lead concentrations and children’s neurodevelopment. Families were randomly assigned to receive either lead hazard controls (intervention group) before the child’s birth or equipment to reduce residential injuries (control group). The intervention consisted of reducing lead hazards (e.g, paint stabilization, window repair and extensive dust control). The control group received injury prevention devices (e.g. stair gates) or home modifications (e.g. reducing water heater temperature).
Confounders
Because numerous factors could confound the association between chemical exposures and child health, we collected an extensive set of covariates. We measured sociodemographic factors including maternal race/ethnicity, age, education, marital status, employment, insurance and household income. We assessed maternal depressive symptoms, ADHD, mental health and intelligence. We collected peri- and antenatal factors like maternal anthropometry, parity, breastfeeding and alcohol/drug/tobacco use. We abstracted clinical information from the mother and infant’s medical records. We measured some dietary behaviours during pregnancy (e.g. prenatal vitamin intake) and dietary/lifestyle factors in childhood (e.g. fresh fruit and vegetable consumption and physical/sedentary activity). Finally, we assessed parenting stress, caregiving environment, child sleep and child mouthing behaviours.
What has it found? Key findings and publications:
Below we summarize some of our key findings related to associations between specific environmental chemicals and children’s health. A full list of our publications can be found at: [http://www.cincinnatichildrens.org/research/divisions/g/pediatrics/labs/yolton/publications/].
Bisphenol A
Prenatal urinary bisphenol A (BPA) concentrations were associated with increased behaviour problems and decrements in executive function in girls, but not boys, at 2 and 3 years of age.12,13 In addition, urinary BPA concentrations during pregnancy were associated with lower cord blood thyroid stimulating hormone concentrations in girls.14 Prenatal BPA concentrations were also associated with increased risk of wheeze and decreased lung function at 2–5 years of age.15,16 However, prenatal BPA concentrations were not associated with infant neurobehaviour, autistic behaviours at 4–5 years of age or body mass index at 2–5 years of age.17–19
Polybrominated diphenyl ethers
Serum PBDE concentrations during pregnancy were associated with decreased cognitive abilities, increased behaviour problems and increased autistic behaviours in children at 2–5 years of age.18,20 In addition, maternal PBDE concentrations during pregnancy were associated with increased maternal thyroxine and triiodothyronine concentrations in pregnancy.21 There were no associations between maternal PBDEs and infant neurobehaviour.22
Perfluoroalkyl substances
Serum perfluorooctanoic acid (PFOA) concentrations among HOME Study women were over twice as high as concentrations in US pregnant women (median: 5.3. vs 2.3 ng/ml).23 We observed that higher maternal PFOA concentrations during pregnancy were associated with increased hypotonicity in infants, increased adiposity at 8 years of age in a non-monotonic fashion and excess adiposity gain between 2 and 8 years of age.22,23
Chemical mixtures
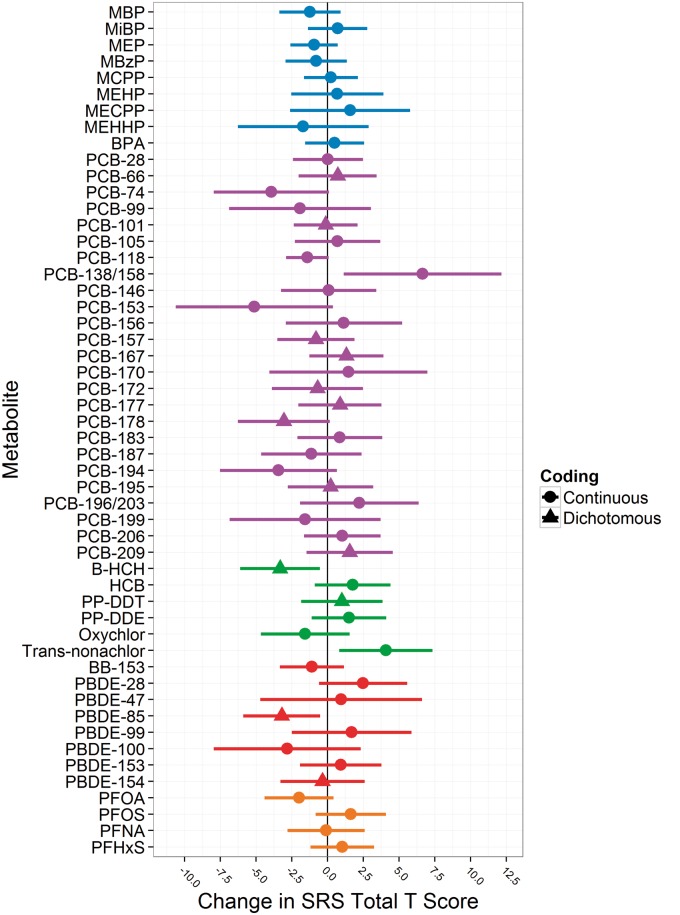
We used Bayesian methods to identify individual prenatal chemical exposures, among a mixture of 52 associated with autistic behaviours (Figure 2 ).18 Serum PBDE and trans-nonachlor concentrations were positively associated with autistic behaviours, but most exposures were not.
Figure 2.
Confounder- and exposure-adjusted associations between maternal gestational urinary or serum endocrine disrupting chemical concentrations and Social Responsiveness Scale (SRS) total t-scores in 4- and 5-year-old Cincinnati children. PCB-66, PCB-101, PCB-157, PCB-167, PCB-172, PCB-177, PCB-178, PCB-195, PCB-209, β-HCH, DDT, PBDE-85, PBDE-154,and PBDE 183 are coded as detected vs non-detectable. The displayed betas are the change in SRS scores among children born to women with detectable vs non-detectable levels of these chemicals. All other chemicals were treated as continuous log10-transformed variables that are divided by two times their standard deviation to put them on a comparable scale to the dichotomous variables. Adjusted models include maternal age, race, marital status, education, parity, insurance status during pregnancy, prenatal vitamin use, employment during pregnancy, IQ, household income, depressive symptoms during pregnancy, caregiving environment score, gestational serum cotinine concentration and all chemical biomarker concentrations. Horizontal lines indicate the 95% confidence interval around the point estimate.
Tobacco smoke
Higher maternal serum cotinine concentrations during pregnancy, a biomarker of tobacco smoke exposure, were associated with decreased birthweight, altered neurobehaviour in infancy, increased wheeze in infancy and increased body mass index (BMI) in children at ages 2 and 3 years.24–27
Child injury
We found that the in-home injury intervention was associated with a 70% reduction in medically attended injuries through 2 years of age.28 Children receiving this intervention were the control arm of the randomized trial to reduce residential lead hazards.
What are the main strengths and weaknesses?
One of the major strengths of the HOME Study is the longitudinal and multimodal assessment of environmental chemical exposures during gestation and childhood. For many chemicals, we measured exposure up to twice during pregnancy and up to six times during childhood (Supplementary Table S1, available as Supplementary data at IJE online). Another strength is the repeated assessment of several prevalent child health outcomes, which allows us to examine the persistence of any associations, as well as trajectories of child health. Finally, we collected detailed measures of many potential confounders.
One limitation of the HOME Study is the modest sample size, reducing the statistical power to detect relatively small effect sizes and precluding us from examining clinical diagnoses (e.g. ADHD). Another limitation, which is not unique to the HOME Study, is loss to follow-up (58% follow-up at 8 years of age). However, measured sociodemographic factors were not substantially different among participants that did and did not complete follow-up at 8 years of age. In addition, follow-up at 4–5 years of age was unrelated to maternal chemical biomarker concentrations at baseline.18 Finally, our findings may not be generalizable to other populations since we employed specific eligibility criteria, resulting in our cohort not being representative of births in the study region. Reassuringly, most chemical biomarker concentrations among women in our cohort are similar to those of pregnant women in the USA.29
Can I get hold of the data? Where can I find out more?
The HOME Study principal investigators have actively engaged in collaborative data-sharing projects.30,31 We welcome new collaborations with other investigators. Interested investigators should contact Drs Joseph M. Braun and Kimberly Yolton [joseph_braun_1@brown.edu] and [kimberly.yolton@cchmc.org] to discuss collaborative opportunities, obtain additional information about the HOME Study and request a project proposal form. The HOME Study Data Sharing Committee meets regularly to review proposed research projects and ensure that they do not overlap with extant projects and are an efficient use of scarce resources (e.g. cord blood).
Profile in a nutshell
The HOME Study is a prospective pregnancy and birth cohort designed to investigate the influence of environmental chemical exposures on children’s growth and development.
Between March 2003 and January 2006, we recruited 401 pregnant women from a 5-county region surrounding Cincinnati, OH.
Longitudinal follow-up included 1 home and 2 clinic visits during pregnancy, a visit within 48 h of delivery, and 10 home or clinic visits when children were 4 weeks and 1, 2, 3, 4, 5 and 8 years of age. Of 407 live-born children, all are eligible for follow-up and 237 children completed follow-up at 8 years of age.
Data include longitudinal and repeated measures of chemical exposures and child health. This includes 100+ environmental chemical exposures measured in women and children, as well as repeated measures of child neurodevelopment, anthropometry, respiratory and allergy outcomes and injuries.
Investigators interested in learning more about The HOME Study can e-mail [joseph_braun_1@brown.edu] and [kimberly.yolton@cchmc.org].
Supplementary Data
Supplementary data are available at IJE online.
Funding
The National Institutes of Health (National Institute of Environmental Health Sciences), Environmental Protection Agency, Department of Housing and Urban Development, Brown University School of Public Health, Flight Attendant Medical Research Institute and Passport Foundation provided funding for the HOME Study.
Conflict of interest: The authors declare no competing interests.
Supplementary Material
References
- 1. Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 1971;284:878–81. [DOI] [PubMed] [Google Scholar]
- 2. Hoover RN, Hyer M, Pfeiffer RM et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med 2011;365:1304–14. [DOI] [PubMed] [Google Scholar]
- 3. Lanphear BP, Hornung R, Khoury J et al. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect 2005;113:894–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Axelrad DA, Bellinger DC, Ryan LM, Woodruff TJ. Dose-response relationship of prenatal mercury exposure and IQ: an integrative analysis of epidemiologic data. Environ Health Perspect 2007;115:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heindel JJ, Balbus J, Birnbaum L et al. Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology 2015;156:3416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Claus Henn B, Coull BA, Wright RO. Chemical mixtures and children's health. Curr Opin Pediatr 2014;26:223–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol 2014;13:330–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobs DE, Clickner RP, Zhou JY et al. The prevalence of lead-based paint hazards in U.S. housing. Environ Health Perspect 2002;110:A599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen A, Dietrich KN, Ware JH, Radcliffe J, Rogan WJ. IQ and blood lead from 2 to 7 years of age: are the effects in older children the residual of high blood lead concentrations in 2-year-olds? Environ Health Perspect 2005;113:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watkins DJ, Eliot M, Sathyanarayana S et al. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol 2014;48:8881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dietrich KN, Eskenazi B, Schantz SL. Principles and practices of neurodevelopmental assessment in children: lessons learned from the Centers for Children's Environmental Health and Disease Prevention Research. Environ Health Perspect 2005;113:1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braun JM, Kalkbrenner AE, Calafat AM et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011;128:873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braun JM, Yolton K, Dietrich KN et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 2009;117:1945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romano ME, Webster GM, Vuong AM et al. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: The HOME Study. Environ Res 2015;138:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spanier AJ, Kahn RS, Kunselman AR et al. Prenatal exposure to bisphenol A and child wheeze from birth to 3 years of age. Environ Health Perspect 2012;120:916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spanier AJ, Kahn RS, Kunselman AR et al. Bisphenol A exposure and the development of wheeze and lung function in children through age 5 years. JAMA Pediatr 2014;168:1131–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braun JM, Lanphear BP, Calafat AM et al. Early-life bisphenol A exposure and child body mass index: a prospective cohort study. Environ Health Perspect 2014;122:1239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braun JM, Kalkbrenner AE, Just AC et al. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect 2014;122: 513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol 2011;33:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen A, Yolton K, Rauch SA et al. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: the HOME study. Environ Health Perspect 2014;122:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vuong AM, Webster GM, Romano ME et al. Maternal Polybrominated Diphenyl Ether (PBDE) Exposure and Thyroid Hormones in Maternal and Cord Sera: The HOME Study, Cincinnati, USA. Environ Health Perspect 2015;123: 1079–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donauer S, Chen A, Xu Y, Calafat AM, Sjodin A, Yolton K. Prenatal exposure to polybrominated diphenyl ethers and polyfluoroalkyl chemicals and infant neurobehavior. J Pediatr 2015;166:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Braun JM, Chen A, Romano ME et al. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring) 2016;24:231–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Braun JM, Daniels JL, Poole C et al. A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: the correlation between serum and meconium and their association with infant birth weight. Environ Health 2010;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yolton K, Khoury J, Xu Y et al. Low-level prenatal exposure to nicotine and infant neurobehavior. Neurotoxicol Teratol 2009;31:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braun JM, Daniels JL, Poole C et al. Prenatal environmental tobacco smoke exposure and early childhood body mass index. Paediatr Perinat Epidemiol 2010;24:524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spanier AJ, Kahn RS, Xu Y, Hornung R, Lanphear BP. Comparison of biomarkers and parent report of tobacco exposure to predict wheeze. J Pediatr 2011;159:776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phelan KJ, Khoury J, Xu Y, Liddy S, Hornung R, Lanphear BP. A randomized controlled trial of home injury hazard reduction: the HOME injury study. Arch Pediatr Adolesc Med 2011;165:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 2011;119:878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Research of Environmental Health Sciences. Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology Studies. Research Triangle Park, NC: NREHS, 2015. [Google Scholar]
- 31. Engel SM, Bradman A, Wolff MS et al. Prenatal organophosphorus pesticide exposure and child neurodevelopment at 24 months: an analysis of four birth cohorts. Environ Health Perspect 2015, Sep 29. PMID: 26418669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention. Vital Statistics Data Available Online. 2015. http://www.cdc.gov/nchs/data_access/vitalstatsonline.htm. (Accessed November 1, 2015).
- 33. Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 2009;169:236–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.