Abstract
Background. We recently published and validated the new serum creatinine (Scr)-based full-age-spectrum equation (FAScrea) for estimating the glomerular filtration rate (GFR) for healthy and kidney–diseased subjects of all ages. The equation was based on the concept of normalized Scr and shows equivalent to superior prediction performance to the currently recommended equations for children, adolescents, adults and older adults.
Methods. Based on an evaluation of the serum cystatin C (ScysC) distribution, we defined normalization constants for ScysC (QcysC = 0.82 mg/L for ages <70 years and QcysC = 0.95 mg/L for ages ≥70 years). By replacing Scr/Qcrea in the FAScrea equation with ScysC/QcysC, or with the average of both normalized biomarkers, we obtained new ScysC-based (FAScysC) and combined Scr-/ScysC-based FAS equations (FAScombi). To validate the new FAScysC and FAScombi we collected data on measured GFR, Scr, ScysC, age, gender, height and weight from 11 different cohorts including n = 6132 unique white subjects (368 children, aged ≤18 years, 4295 adults and 1469 older adults, aged ≥70 years).
Results. In children and adolescents, the new FAScysC equation showed significantly better performance [percentage of patients within 30% of mGFR (P30) = 86.1%] than the Caucasian Asian Paediatric Adult Cohort equation (P30 = 76.6%; P < 0.0001), or the ScysC-based Schwartz equation (P30 = 68.8%; P < 0.0001) and the FAScombi equation outperformed all equations with P30 = 92.1% (P < 0.0001). In adults, the FAScysC equation (P30 = 82.6%) performed equally as well as the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPIcysC) (P30 = 80.4%) and the FAScombi equation (P30 = 89.9%) was also equal to the combined CKD-EPI equation (P30 = 88.2%). In older adults, FAScysC was superior (P30 = 88.2%) to CKD-EPIcysC (P30 = 84.4%; P < 0.0001) and the FAScombi equation (P30 = 91.2%) showed significantly higher performance than the combined CKD-EPI equation (P30 = 85.6%) (P < 0.0001).
Conclusion. The FAS equation is not only applicable to all ages, but also for all recommended renal biomarkers and their combinations.
Keywords: all ages, all renal biomarkers, combined FAS equation, cystatin C, serum creatinine
INTRODUCTION
Serum creatinine (Scr)-based estimating glomerular filtration rate (eGFR) equations are commonly used and reported when Scr is measured. Despite the worldwide acceptance of isotope dilution mass spectrometry (IDMS)-standardized Scr assays, Scr-based eGFR equations are still relatively imprecise [1]. Also, different equations are proposed for children, adults and older adults as most equations lack continuity and accuracy across the full age spectrum.
We recently published a Scr-based full-age-spectrum (FAScrea) equation [2] that has been validated in a large number of healthy and kidney–diseased white individuals (n = 6870) including 735 children, 4371 adults and 1764 older adults against measured GFR (mGFR) and using IDMS-equivalent Scr. The FAScrea equation showed improved validity and continuity across the full age spectrum and was less biased and more accurate than the currently recommended Scr-based eGFR equations.
The FAS equation is based on three fundamental assumptions:
The average GFR for healthy populations (children, adolescents and young adults) is equal to a value of 107.3 mL/min/1.73 m2 after kidney function matures (around 2 years of age) until the age of 40 years. This assumption is also supported by the results of a recent meta-analysis in living kidney donors [3].
The age decline of GFR begins at around 40 years.
GFR and population-normalized Scr (Scr/Qcrea) are inversely related (Qcrea being the mean or median Scr concentration of the corresponding age-/sex-matched healthy population).
These three assumptions have led to the construction of the simple age-knotted FAScrea equation, which takes the form [2]:
The equation is simple and intuitive and can be easily explained: when Scr/Qcrea deviates from ‘1’, the eGFR will deviate from the average value of 107.3 mL/min/1.73 m2. Scr/Qcrea, for every healthy age-/sex-matched population, is normally distributed (Gaussian distribution) around the mean of ‘1’ (a consequence of the definition of Qcrea). It has been shown that the 2.5th percentile (Pct) = 0.67 and the 97.5th Pct = 1.33, or, equivalently, the standard deviation (SD) is 0.1683 [2].
Serum cystatin C (ScysC) is considered to be a potential alternative to Scr for estimating GFR [4], especially since a certified reference cystatin C material became available in 2010, allowing standardization of ScysC assays [5].
In this article, we demonstrate that the last assumption (that GFR is inversely related to the normalized Scr biomarker) also applies to ScysC, if properly normalized. We show that the FAScrea equation can be transformed into a ScysC-based FAS equation (FAScysC) and a combined Scr-/ScysC-based FAS equation (FAScombi), by simply replacing the normalized Scr (Scr/Qcrea) by ScysC/QcysC or by the combination of Scr/Qcrea and ScysC/QcysC [i.e. the (weighted) average of Scr/Qcrea and ScysC/QcysC], where QcysC is the normalization factor for ScysC.
In the first part of this study, we give a rationale for choosing the normalization factor QcysC for ScysC. Next, we validate the FAScysC and FAScombi equation against mGFR and compare the performance of these equations with the currently recommended and most used eGFR equations (SchwartzcysC [6], Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPIcysC) [4], Caucasian Asian Paediatric Adult Cohort equation (CAPA) [7], combined CKD-EPIcombi [4] and BIS2 [8]). Finally, we evaluate the performance of all FAS equations (by varying the weighting factors for the normalized biomarkers) in all age groups.
MATERIALS AND METHODS
Overview of study design and participants
We collected data from 11 cohorts, forming a representative sample of the general population and renal disease patients. For the same six cohorts (Saint-Etienne, Tromsø, Rochester and Minnesota for adults; Kent and Berlin for older adults [2]) that were used for the validation of the FAScrea equation, we additionally collected the ScysC results. The other cohorts used in the previous validation did not have ScysC data available, and, therefore, we collected data of new cohorts. For children, adolescents and young adults (<21 years), one cohort came from the University Hospital in Leuven (n = 114), and one from Lyon (n = 695). Both cohorts contained children and adolescents with established renal pathologies. The data from Leuven contained single-time point measurements per child and the data from Lyon (n = 695) were from 259 children with serial measurements over a period of several years, but we used the first measurement only. We further collected data from a cohort of healthy and renal disease adults from Paris (n = 603), from Lyon (n = 598) and from the Chronic Renal Insufficiency Cohort (CRIC; n = 3939) [9], which we restricted to whites only (n = 1824) and to the first visit where all required variables were available (n = 674). All datasets were centralized by the first author for data analysis. This retrospective non-interventional study was approved by the Institutional Ethical Board of the University Hospital of Leuven, Belgium.
In total, we collected data on mGFR, Scr, ScysC, age, gender, height and weight for n = 6132 participants (n = 368 children aged between 1 and 18 years; n = 4295 adults aged between 18 and 70 years and n = 1469 older adults aged ≥70 years).
We further used a separate cohort (n = 1333) from the Berlin Initiative Study [8] of apparently healthy older subjects to study the age dependency of the ScysC distribution. This cohort was obtained from 2069 subjects (2069 baseline samples and 1693 follow-up samples) aged >70 years (Berlin residents), which we reduced to a subset of 1333 individuals who were defined as apparently healthy; i.e. no history of myocardial infarction, no history of stroke, not on dialysis, not deceased between first and second follow-up study visit, no albuminuria (ACR <30 mg/g), arterial blood pressure <160/90 mmHg.
Methods
The new FAS equation(s)
The form of the FAScrea equation was maintained, but Scr/Qcrea is replaced by ScysC/QcysC:
By extending the same concept, we used the weighted average of the two normalized biomarkers Scr/Qcrea and ScysC/QcysC, leading to the general form of:
The coefficient ‘α’ in the denominator may be considered as a weighting factor for the normalized renal biomarkers. In case α = 1, the FAS equation depends entirely on Scr/Qcrea and equals the FAScrea equation; in case α = 0, the FAS equation becomes the ScysC-based FAScysC equation. In all other situations for 0 < α < 1, the equation is a combined Scr/ScysC equation. For α = 0.5, the denominator is equal to the average of both normalized biomarkers. We further discuss the influence of α in the ‘Results’ section.
mGFR, Scr and cystatin C assays
A summary of the methods used in the different collaborating centres is given in Tables 1 and 2. Direct GFR measurements were obtained with different reference methods as described previously [2, 10]. Scr was measured with an enzymatic assay, equivalent to IDMS, or directly with IDMS, or recalculated to the enzymatic assay, in all centres. ScysC was measured with the calibrated particle-enhanced nephelometric (PENIA) method of Siemens in Saint-Etienne, Berlin and partially in Lyon. The ScysC measurements for the CRIC Study were done with the non-calibrated PENIA assay of Siemens, but calculated back to the certified reference material, as previously described [4]. The non-calibrated PENIA assay of Siemens was also used in Rochester, Kent, and partially in Lyon, and the results were recalculated to the certified reference standard, using the multiplication factor in Rochester [11] and in Lyon and Kent [12], according to the manufacturer’s specifications. Tromsø used the non-calibrated (with back calculation) and Leuven used the calibrated particle-enhanced turbidimetric (PETIA, Tina quant®) assay of Roche (Tables 1 and 2).
Table 1.
Overview of the methods used in this study for mGFR and Scr
| Origin | mGFR | Scr |
|---|---|---|
| Leuven, Belgium | 51Cr-EDTA (4 points) | Creatinine Plus, Roche enzym. |
| Lyon, France | Inulina or Iohexol (3 points) | Creatinine Plus, Roche enzym. |
| Saint-Etienne, France | Iohexol (2 points) | Enzymatic, Orthoclinical Diagn. |
| Tromsø, Norway | Iohexol (1 point) | Creatinine Plus, Roche enzym. |
| Rochester, MN, USA | Iothalamatea | Creatinine Plus, Roche enzym. |
| Berlin, Germany | Iohexol (8 points) | Creatinine Plus, Roche enzym. |
| Kent, UK | Iohexol (3 points) | IDMS |
| Paris, France | 51Cr-EDTAa | Enzymatic, Siemens, standardized to IDMS |
| CRIC, USA | 125I-Iothalamatea | calculated back to Creatinine Plus, Roche enzym. |
For mGFR, arenal clearance, all other methods are plasma clearance methods. mGFR is indexed for body surface area using the Dubois formula.
Table 2.
Overview of the methods for ScysC
| Origin, time of measurement | ScysC assay | Calibration to reference (ERM®-DA471/IFCC) | Automate | CV (%) |
|---|---|---|---|---|
| Leuven, 2015 | Roche PETIA (Tina quant® Gen2) | Yes | Integra 400 Plus | 2.6, 1.2, 1.0 at 0.503, 2.98, 6.11 mg/L |
| Lyon, 2010–15 | Siemens N-Latex® PENIA | No, recalculation by MF = 1.11 before April 2011; yes after April 2011 | BN Prospec analyser | 3.5 at 2.3 mg/L |
| Saint-Etienne, 2012 | Siemens PENIA [13] | Yes | BN Prospec analyser | 2.9, 2.1 at 1.03, 1.93 mg/L |
| Tromsø, 2007–08 | Roche PETIA [14] (Tina quant® Gen1) | No, recalculation using −0.064 + ScysC × 0.998 | Gentian reagents, Modular P800 analyser | 3.2 |
| Rochester, 2001–11 | Siemens PENIA [11] | No, recalculation by MF = 1.14 | Dade Behring BN II Nephelometer | 3.5 |
| Berlin, 2011 | Siemens N-Latex® PENIA [8] | Yes | BN Prospec | 1.5, 3.5, 2.4 at 0.8, 2.3, 7.4 mg/L |
| Kent, 2008–11 | Siemens PENIA [12] | No, recalculation by MF = 1.11 | BN Prospec analyser | 3.5 at 2.3 mg/L |
| Paris, 2013 | Siemens PENIA | Yes | Dimension Vista | ≤3.5 |
| CRIC [9], 2003–08 | Siemens N-Latex® PENIA [4] | No, recalculation by authors = 1.12 × (0.083 + 0.789 × (0.039 + 1.061 × CRICcysC) | BN Prospec analyser | 4.9 |
MF, multiplication factor; CV, coefficient of variation.
eGFR equations
The new FAScysC equation and the FAScombi equation were compared and validated against mGFR and against the currently available and recommended eGFR equations listed in Table 3.
Table 3.
eGFR equations for the performance comparisons
| Scr-based equations | |
| Schwartzcrea [15] | eGFR = 0.413 × Ht/Scr |
| CKD-EPI [16] | eGFR = 141 × min(Scr/κ)α × max(Scr/κ)−1.209 × 0.993Age × (1.018 if female) κ = 0.7 for females and 0.9 for males; α = −0.329 for females and −0.411 for males |
| ScysC-based equations | |
| SchwartzcysC [6] | eGFR = 40.6 (1.8/ScysC)0.93 |
| CAPA [7] | eGFR = 130 × ScysC−1.069 × Age−0.117 – 7 |
| CKD-EPIcysc [4] | eGFR = 133 × min(ScysC/0.8,1)−0.499 × max(ScysC/0.8,1)−1.328 × 0.996Age × (0.932 if female) |
| Combined equations | |
| CKD-EPIcrea,cysc [4] | eGFR = 135 × min(Scr/κ,1)α × max(Scr/κ,1)−0.601 × min(ScysC/0.8,1)−0.375 × max(ScysC/0.8,1)−0.711 × 0.995Age × (0.969 if female) (κ = 0.7 for females, 0.9 for males, α = −0.248 for females and −0.207 for males) |
| BIS2 [8] | eGFR = 767 × ScysC−0.61 × Scr−0.40 × Age−0.57 × (0.87 if female) |
Scr, serum creatinine (mg/dL); ScysC, serum cystatin C (mg/L); Ht, height in cm.
Statistical analysis
The performance statistics are presented as constant bias (mean of eGFR–mGFR) and proportional bias (mean of eGFR/mGFR), root mean square error (RMSE) of prediction, Lin’s concordance correlation coefficient (Lin’s CCC, which is a measure of both correlation and agreement as it evaluates the degree to which pairs of observations fall on the identity line), P10 and P30 (the percentage of subjects within 10% and 30% of mGFR), for the different age groups, total and in subgroups according to mGFR <60 and ≥60 mL/min/1.73 m2. McNemar’s test is used to compare P30 among equations.
RESULTS
Description of the cohorts
Summary statistics for the patient characteristics of the 11 cohorts are given in Tables 4 and 5, and are described in Supplementary data.
Table 4.
Patient characteristics in the different cohorts from young age to old age (mean ± SD) (in years)
| Data origin | n | Age | mGFR | Scr | ScysC |
|---|---|---|---|---|---|
| Leuven (Belgium) | 114 | 8.8 ± 5.5 | 89.2 ± 21.5 | 0.58 ± 0.36 | 1.00 ± 0.35 |
| Lyon (France) | 259 | 11.1 ± 3.6 | 88.8 ± 33.5 | 0.68 ± 0.30 | 1.22 ± 0.43 |
| Saint-Etienne (France) | 203 | 48.7 ± 10.3 | 94.7 ± 24.4 | 0.87 ± 0.19 | 0.90 ± 0.26 |
| Paris (France) | 603 | 50.3 ± 13.1 | 67.1 ± 27.2 | 1.29 ± 0.74 | 1.41 ± 0.81 |
| Lyon (France) | 598 | 54.6 ± 13.7 | 74.9 ± 30.9 | 1.13 ± 0.57 | 1.24 ± 0.58 |
| CRIC (USA) | 674 | 56.9 ± 12.5 | 50.7 ± 21.8 | 1.60 ± 0.50 | 1.43 ± 0.51 |
| Tromsø (Norway) | 1627 | 58.1 ± 3.8 | 91.7 ± 14.4 | 0.76 ± 0.14 | 0.73 ± 0.12 |
| Rochester CKD (USA) | 687 | 64.8 ± 8.8 | 80.4 ± 21.3 | 0.85 ± 0.23 | 0.87 ± 0.24 |
| Rochester KFC (USA) | 406 | 65.9 ± 9.2 | 79.5 ± 20.7 | 0.84 ± 0.18 | 0.83 ± 0.18 |
| Berlin (Germany) | 567 | 78.5 ± 6.2 | 60.3 ± 16.4 | 0.99 ± 0.37 | 1.14 ± 0.38 |
| Kent (UK) | 394 | 80.4 ± 4.6 | 51.5 ± 18.8 | 1.30 ± 0.66 | 1.45 ± 0.61 |
| Total | 6132 | 58.2 ± 17.6 | 75.5 ± 26.5 | 1.01 ± 0.51 | 1.06 ± 0.52 |
n, number of patients; mGFR, measured glomerular filtration rate (mL/min/1.73 m2); Scr, serum creatinine (mg/dL); ScysC, serum cystatin C (mg/L).
Table 5.
Patient characteristics in the different age groups (mean ± SD)
| Group | n | No. of males | No. of females | mGFR | Scr | ScysC |
|---|---|---|---|---|---|---|
| Children ≤18 years | 368 | 193 | 175 | 89.2 ± 30.4 | 0.65 ± 0.31 | 1.15 ± 0.42 |
| Adults 18–70 years | 4295 | 2301 | 1994 | 80.2 ± 25.6 | 1.00 ± 0.50 | 0.99 ± 0.51 |
| Older adults ≥70 years | 1469 | 771 | 698 | 58.5 ± 20.0 | 1.13 ± 0.52 | 1.24 ± 0.51 |
| Total | 6132 | 3265 | 2867 |
n, number of patients; mGFR, measured glomerular filtration rate (mL/min/1.73 m2); Scr, serum creatinine (mg/dL); ScysC, serum cystatin C (mg/L).
Rationale for QcysC values for ScysC
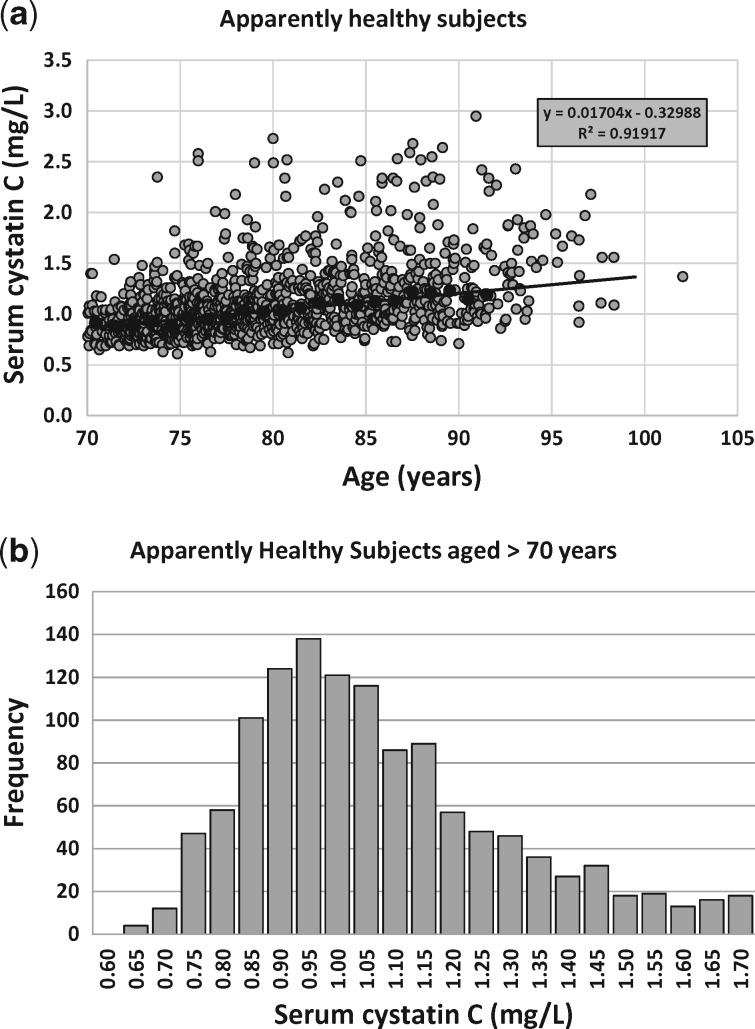
To define normalization factors for ScysC we searched the literature for normal reference ranges and we investigated whether these ranges depend on age or gender differences. We realized that the literature before the year 2010 was based on non-standardized cystatin C assays, but, in general, ScysC is independent of age (up to age 70 years) and gender in children, adolescents and adults [14–16], although there might be small differences between sexes and races [17]. We used the value of 0.82 mg/L as the normalization factor, as it is the middle of the normal reference interval for children, adolescents and adults up to ∼70 years (and in line with the manufacturer’s information on reference ranges) [8, 18]. The ScysC-based CKD-EPI equation normalized ScysC by 0.80 for both males and females [4], a value that is close to the proposed value of 0.82 in this study. The new CAPA equation does not have a gender factor in the equation, suggesting that the same QcysC normalization constant can be used for both sexes [7]. For older adults, we could not find normal reference ranges in the literature. In our dataset of 1333 apparently healthy older persons aged >70 years from the Berlin Initiative Study, we modelled QcysC as a linear function of age: QcysC = 0.01704 × Age – 0.3384 = 0.863 + 0.01704 × (Age – 70) (R2 = 0.919; see Figure 1). At the age of 67.5 years, the corresponding value of QcysC = 0.82. Therefore, we defined the QcysC normalization factor for cystatin C as 0.82 mg/L until the age of 70 years and then QcysC gradually (and linearly) increases (Figure 1). The mode of the ScysC distribution (Figure 2) of the n = 1333 apparently healthy subjects was 0.95 mg/L. Based on this analysis, and for the sake of simplicity, we fixed QcysC to 0.82 mg/L for all ages <70 years and to 0.95 mg/L for older ages.
FIGURE 1.
(a) The linear relationship between median (solid black circles) ScysC and age for the n = 1333 apparently healthy Berlin Initiative Study participants (grey circles). (b) Histogram for ScysC measurements of n = 1333 apparently healthy older adults.
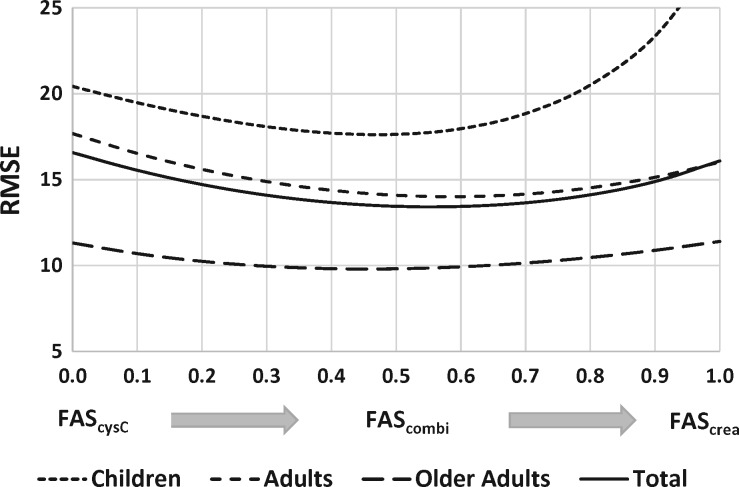
FIGURE 2.
RMSE as a function of the weighting factor α for children [based on Q(height)], adults and older adults. The total RMSE for all n = 6132 measurements is also shown. For children the FAScysC equation has smaller RMSE than the FAScrea equation. For adults there is a slightly smaller RMSE for the single biomarker FAScrea equation compared with the single marker FAScysC equation. For older adults there is no real preference for the value of α. For all age groups the RMSE is minimal for α ≈ 0.5 (= combined FAS equation).
Performance results of the different equations
The performance statistics for the three FAS equations for the different age groups are presented in Tables 6–8. The FAScrea equation [for children in two versions, based on Qcrea(age) and Qcrea(height)] is compared with the relevant Scr-based recommended equations (Schwartz for children, CKD-EPI for adults, BIS1 for older adults). The FAScysC equation is compared with the ScysC-based Schwartz equation (for children), the CAPA equation (for all ages) and ScysC-based CKD-EPI equation (for adults). Finally, the FAScombi equation is compared with the combined CKD-EPI equation (for adults and older adults) and the combined BIS2 equation for older adults. To our knowledge, there is no combined equation for children available yet (based on the certified reference material). Tables 6–8 are presented for all subjects within each age group, but also for subgroups according to the mGFR threshold of 60 mL/min/1.73 m2.
Table 6.
Children n = 368 (age ≤18 years)
| Scr-based eGFR | ScysC-based eGFR | Combined Scr-/ScysC-based eGFR |
||||||
|---|---|---|---|---|---|---|---|---|
| mGFR = 89.2 (n = 368) | FAScrea | FAScrea(Ht)* | Schwartzcrea | FAScysC* | CAPA | SchwartzcysC | FAScombi* | FAScombi(Ht)* |
| eGFR – mGFR | 12.3 (7.7; 17.0) | 3.8 (0.9; 6.6) | 11.1 (8.1; 14.1) | −5.1 (−7.2; −3.1) | 0.3 (−2.0; 2.6) | −21.6 (−23.7; −19.6) | 0.9 (−0.9; 2.7) | −2.2 (−4.0; −0.4) |
| eGFR/mGFR | 1.17 (1.12; 1.21) | 1.06 (1.04; 1.09) | 1.15 (1.12; 1.18) | 0.98 (0.96; 1.01) | 1.03 (1.00; 1.05) | 0.79 (0.78; 0.81) | 1.05 (1.03; 1.07) | 1.01 (0.99; 1.03) |
| RMSE | 47.0 (27.2; 67.6) | 28.3 (11.4; 39.2) | 31.3 (13.9; 42.9) | 20.4 (17.9; 22.5) | 22.3 (20.0; 24.3) | 29.6 (27.0; 32.1) | 17.5 (15.1; 19.7) | 17.6 (15.5; 19.7) |
| Lin’s CCC | 0.43 (0.36; 0.49) | 0.65 (0.59; 0.70) | 0.63 (0.57; 0.68) | 0.73 (0.68; 0.77) | 0.74 (0.68; 0.78) | 0.49 (0.44; 0.54) | 0.81 (0.77; 0.84) | 0.80 (0.77; 0.84) |
| P10 (%) | 32.3 (27.5; 37.1) | 42.7 (37.6; 47.7) | 40.5 (35.5; 45.5) | 40.5 (35.5; 45.5) | 36.4 (31.5; 41.4) | 16.0 (12.3; 19.8) | 44.6 (39.5; 49.7) | 43.2 (38.1; 48.3) |
| P30 (%) | 78.3 (74.0; 82.5) | 84.5 (80.8; 88.2) | 79.9 (75.8; 84.0) | 86.1 (82.6; 89.7) | 76.6 (72.3; 81.0) | 68.8 (64.4; 73.5) | 90.8 (87.8; 93.7) | 92.1 (89.4; 94.9) |
| mGFR <60 mL/min/1.73 m2 (n = 57) | ||||||||
| mGFR = 45.2 | FAScrea | FAScrea(Ht)* | Schwartzcrea | FAScysC | CAPA | SchwartzcysC* | FAScombi | FAScombi (Ht)* |
| eGFR – mGFR | 12.5 (10.0; 15.1) | 5.1 (3.0; 7.2) | 8.8 (6.5; 11.0) | 6.2 (3.1; 9.3) | 3.3 (−0.4; 7.1) | −2.4 (−5.0; 0.2) | 8.3 (6.2; 10.5) | 5.0 (2.9; 7.1) |
| eGFR/mGFR | 1.31 (1.24; 1.37) | 1.14 (1.08; 1.20) | 1.22 (1.16; 1.29) | 1.17 (1.09; 1.25) | 1.10 (1.01; 1.19) | 0.98 (0.91; 1.04) | 1.21 (1.15; 1.27) | 1.14 (1.08; 1.20) |
| RMSE | 15.8 (12.7; 18.4) | 9.4 (7.5; 10.9) | 12.2 (9.7; 14.2) | 13.1 (9.8; 15.7) | 14.3 (10.4; 17.3) | 10.0 (7.5; 12.0) | 11.6 (9.4; 13.5) | 9.3 (7.3; 11.0) |
| Lin’s CCC | 0.44 (0.29; 0.56) | 0.66 (0.50; 0.77) | 0.55 (0.40; 0.68) | 0.48 (0.29; 0.64) | 0.47 (0.28; 0.63) | 0.55 (0.35; 0.71) | 0.56 (0.41; 0.69) | 0.65 (0.49; 0.77) |
| P10 (%) | 10.5 (2.3; 18.7) | 45.6 (32.3; 58.9) | 36.8 (23.9; 49.8) | 24.6 (13.0; 36.1) | 29.8 (17.6; 42.1) | 36.8 (23.9; 49.8) | 28.1 (16.0; 40.1) | 38.6 (25.6; 51.6) |
| P30 (%) | 63.2 (50.2; 76.1) | 71.9 (59.9; 84.0) | 71.9 (59.9; 84.0) | 68.4 (56.0; 80.9) | 66.7 (54.0; 79.3) | 86.0 (76.7; 95.3) | 71.9 (59.9; 84.0) | 80.7 (70.1; 91.3) |
| mGFR ≥60 mL/min/1.73 m2 (n = 311) | ||||||||
| mGFR = 97.3 | FAScrea | FAScrea(Ht)* | Schwartzcrea | FAScysC* | CAPA | SchwartzcysC | FAScombi* | FAScombi (Ht)* |
| eGFR – mGFR | 12.3 (6.8; 17.8) | 3.5 (0.1; 6.9) | 11.5 (8.0; 15.1) | −7.2 (−9.5; −5.0) | −0.2 (−2.9; 2.4) | −25.2 (−27.4; −23.0) | −0.5 (−2.5; 1.6) | −3.5 (−5.6; −1.4) |
| eGFR/mGFR | 1.14 (1.09; 1.19) | 1.05 (1.02; 1.08) | 1.13 (1.10; 1.17) | 0.95 (0.93; 0.97) | 1.02 (0.99; 1.04) | 0.76 (0.74; 0.78) | 1.01 (0.99; 1.03) | 0.98 (0.96; 1.00) |
| RMSE | 50.7 (10.5; 77.5) | 30.5 (10.1; 42.0) | 33.7 (12.7; 45.9) | 21.5 (18.8; 23.8) | 23.5 (21.0; 25.8) | 32.0 (29.1; 34.5) | 18.4 (13.5; 20.8) | 18.8 (16.3; 21.0) |
| Lin’s CCC | 0.29 (0.22; 0.37) | 0.50 (0.41; 0.57) | 0.48 (0.40; 0.55) | 0.59 (0.52; 0.65) | 0.60 (0.52; 0.66) | 0.33 (0.27; 0.38) | 0.71 (0.65; 0.76) | 0.69 (0.62; 0.74) |
| P10 (%) | 36.3 (31.0; 41.7) | 42.1 (36.6; 47.6) | 41.2 (35.7; 46.7) | 43.4 (37.9; 48.9) | 37.6 (32.2; 43.0) | 12.2 (8.6; 15.9) | 47.6 (42.0; 53.2) | 44.1 (38.5; 49.6) |
| P30 (%) | 81.0 (76.6; 85.4) | 86.8 (83.0; 90.6) | 81.4 (77.0; 85.7) | 89.4 (85.9; 92.8) | 78.5 (73.9; 83.1) | 65.6 (60.3; 70.9) | 94.2 (91.6; 96.8) | 94.2 (91.6; 96.8) |
Asterisks indicate the best performing equation(s) [13] within the same biomarker category, across all performance statistics. The bold values are the best result(s) for each performance statistic, across all equations. FAS, full-age-spectrum eGFR equation, based on Q(age); FAS(Ht), FAS equation based on Q(height); Schwartz, Schwartz equation for children (Scr-based = 0.413 Ht/Scr; cystatin C-based = 70.1 ScysC − 0.93). FAScombi is calculated for α = 0.5.
Table 7.
Adults n = 4295 (age 18–70 years)
| Scr-based eGFR | ScysC-based eGFR | Combined Scr-/ScysC-based eGFR | |||||
|---|---|---|---|---|---|---|---|
| mGFR = 80.1 (n = 4295) | FAScrea* | CKD-EPIcrea* | FAScysC* | CKD-EPIcysC | CAPA | FAScombi* | CKD-EPIcombi |
| eGFR – mGFR | 1.4 (0.9; 1.9) | 2.4 (1.9; 2.8) | 4.2 (3.7; 4.8) | 8.0 (7.6; 8.5) | 8.9 (8.3; 9.5) | 1.9 (1.5; 2.4) | 6.2 (5.8; 6.6) |
| eGFR/mGFR | 1.05 (1.04; 1.06) | 1.06 (1.05; 1.07) | 1.08 (1.07; 1.09) | 1.11 (1.10; 1.12) | 1.12 (1.11; 1.13) | 1.05 (1.04; 1.06) | 1.09 (1.08; 1.10) |
| RMSE | 16.0 (15.4; 16.6) | 15.1 (14.6; 15.6) | 17.7 (17.2; 18.2) | 18.1 (17.6; 18.5) | 21.3 (20.8; 21.8) | 14.1 (13.6; 14.6) | 15.3 (14.9; 15.8) |
| Lin’s CCC | 0.80 (0.79; 0.81) | 0.82 (0.81; 0.83) | 0.78 (0.76; 0.79) | 0.78 (0.77; 0.80) | 0.73 (0.72; 0.74) | 0.84 (0.83; 0.85) | 0.83 (0.82; 0.84) |
| P10 (%) | 43.6 (42.1; 45.1) | 46.0 (44.5; 47.5) | 37.6 (36.2; 39.1) | 32.5 (31.1; 34.0) | 31.5 (30.1; 32.9) | 47.3 (45.8; 48.8) | 41.0 (39.6; 42.5) |
| P30 (%) | 87.6 (86.6; 88.6) | 88.1 (87.1; 89.0) | 82.6 (81.4; 83.7) | 80.4 (79.3; 81.6) | 75.6 (74.3; 76.9) | 89.9 (89.0; 90.8) | 88.2 (87.2; 89.1) |
| mGFR <60 mL/min/1.73 m2 (n = 925) | |||||||
| mGFR = 42.0 | FAScrea* | CKD-EPIcrea* | FAScysC | CKD-EPIcysC* | CAPA | FAScombi | CKD-EPIcombi* |
| eGFR – mGFR | 7.0 (6.2; 7.9) | 5.9 (5.0; 6.9) | 6.9 (6.1; 7.8) | 4.9 (3.9; 5.9) | 5.3 (4.3; 6.2) | 6.3 (5.5; 7.0) | 4.2 (3.4; 5.1) |
| eGFR/mGFR | 1.20 (1.18; 1.23) | 1.16 (1.13; 1.18) | 1.19 (1.17; 1.22) | 1.11 (1.09; 1.14) | 1.12 (1.10; 1.15) | 1.18 (1.16; 1.20) | 1.10 (1.08; 1.12) |
| RMSE | 15.4 (14.0; 16.6) | 16.1 (14.8; 17.2) | 14.8 (13.7; 15.8) | 16.2 (14.9; 17.5) | 16.0 (14.7; 17.3) | 13.2 (12.1; 14.2) | 14.4 (13.1; 15.6) |
| Lin’s CCC | 0.54 (0.50; 0.57) | 0.55 (0.51; 0.58) | 0.56 (0.52; 0.60) | 0.57 (0.53; 0.60) | 0.57 (0.53; 0.60) | 0.61 (0.58; 0.65) | 0.62 (0.58; 0.65) |
| P10 (%) | 31.5 (28.5; 34.5) | 31.7 (28.7; 34.7) | 27.9 (25.0; 30.8) | 29.0 (26.0; 31.9) | 28.5 (25.6; 31.5) | 33.8 (30.8; 36.9) | 34.4 (31.3; 37.4) |
| P30 (%) | 70.8 (67.9; 73.7) | 72.3 (69.4; 75.2) | 68.0 (65.0; 71.0) | 70.5 (67.5; 73.4) | 70.9 (68.0; 73.9) | 75.2 (72.5; 78.0) | 79.0 (76.4; 81.7) |
| mGFR ≥60 mL/min/1.73 m2 (n = 3370) | |||||||
| mGFR = 90.6 | FAScrea* | CKD-EPIcrea* | FAScysC* | CKD-EPIcysC | CAPA | FAScombi* | CKD-EPIcombi |
| eGFR – mGFR | −0.1 (−0.7; 0.4) | 1.4 (0.9; 1.9) | 3.5 (2.9; 4.1) | 8.9 (8.4; 9.5) | 9.9 (9.2; 10.6) | 0.8 (0.3; 1.2) | 6.7 (6.3; 7.2) |
| eGFR/mGFR | 1.01 (1.00; 1.01) | 1.03 (1.03; 1.04) | 1.05 (1.04; 1.06) | 1.11 (1.11; 1.12) | 1.12 (1.11; 1.13) | 1.02 (1.01; 1.02) | 1.09 (1.08; 1.09) |
| RMSE | 16.2 (15.5; 16.8) | 14.8 (14.2; 15.3) | 18.4 (17.8; 18.9) | 18.6 (18.1; 19.0) | 22.5 (21.9; 23.1) | 14.3 (13.8; 14.9) | 15.6 (15.1; 16.0) |
| Lin’s CCC | 0.59 (0.57; 0.61) | 0.57 (0.54; 0.59) | 0.51 (0.49; 0.54) | 0.48 (0.46; 0.50) | 0.42 (0.40; 0.45) | 0.64 (0.62; 0.66) | 0.58 (0.56; 0.60) |
| P10 (%) | 47.0 (45.3; 48.7) | 49.9 (48.2; 51.6) | 40.3 (38.6; 41.9) | 33.5 (31.9; 35.1) | 32.3 (30.7; 33.9) | 50.9 (49.3; 52.6) | 42.9 (41.2; 44.6) |
| P30 (%) | 92.2 (91.3; 93.1) | 92.4 (91.5; 93.3) | 86.6 (85.4; 87.7) | 83.2 (91.9; 84.4) | 76.9 (75.4; 78.3) | 93.9 (93.1; 94.8) | 90.7 (89.7; 91.7) |
Asterisks indicate the best performing equation(s) [13] within the same biomarker category, across all performance statistics. The bold values are the best result(s) for each performance statistic, across all equations. FAS, full-age-spectrum eGFR equation (Scr-based with Q = 0.70 mg/dL for females and Q = 0.90 mg/dL for males; SCysC-based with Q′ = 0.82 mg/L). FAScombi is calculated for α = 0.5.
Table 8.
Older people n = 1469 (age ≥70 years)
| Scr-based eGFR | ScysC-based eGFR | Combined Scr-/ScysC-based eGFR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| mGFR = 58.5 (n = 1469) | FAScrea* | CKD-EPIcrea | BIS1* | FAScysC* | CKD-EPIcysC | CAPA | FAScombi* | CKD-EPIcombi | BIS2* |
| eGFR – mGFR | −2.6 (−3.2; −2.0) | 4.6 (4.0; 5.3) | −2.9 (−3.5; −2.4) | 0.9 (0.3; 1.4) | 3.8 (3.1; 4.4) | 5.5 (4.9; 6.2) | −1.4 (−1.9; −0.9) | 4.5 (4.0; 5.1) | −1.2 (−1.7; −0.7) |
| eGFR/mGFR | 0.98 (0.97; 1.00) | 1.10 (1.09; 1.12) | 0.99 (0.98; 1.00) | 1.04 (1.03; 1.05) | 1.07 (1.05; 1.08) | 1.10 (1.09; 1.12) | 1.00 (0.99; 1.01) | 1.08 (1.07; 1.10) | 1.01 (1.00; 1.02) |
| RMSE | 11.4 (10.8; 12.0) | 12.8 (12.3; 13.3) | 11.3 (10.8; 11.9) | 11.3 (10.7; 11.9) | 12.9 (12.2; 13.5) | 13.8 (13.1; 14.5) | 9.8 (9.3; 10.3) | 11.8 (11.2; 12.3) | 9.6 (9.1; 10.2) |
| Lin’s CCC | 0.82 (0.81; 0.84) | 0.81 (0.79; 0.83) | 0.81 (0.79; 0.82) | 0.84 (0.82; 0.85) | 0.83 (0.81; 0.84) | 0.81 (0.79; 0.82) | 0.87 (0.86; 0.88) | 0.85 (0.84; 0.86) | 0.87 (0.86; 0.88) |
| P10 (%) | 42.1 (39.5; 44.6) | 36.3 (33.8; 38.7) | 42.1 (39.5; 44.6) | 43.0 (40.5; 45.6) | 38.1 (35.6; 40.6) | 36.0 (33.6; 38.5) | 50.5 (48.0; 53.1) | 40.0 (37.5; 42.5) | 52.3 (49.8; 54.9) |
| P30 (%) | 88.2 (86.6; 89.9) | 80.0 (77.9; 82.0) | 89.0 (87.4; 90.6) | 88.2 (86.6; 89.9) | 84.4 (82.6; 86.3) | 82.0 (80.1; 84.0) | 91.2 (89.8; 92.7) | 85.6 (83.8; 87.4) | 92.4 (91.0; 93.7) |
| mGFR <60 mL/min/1.73 m2 (n = 753) | |||||||||
| mGFR = 42.9 | FAScrea* | CKD-EPIcrea | BIS1* | FAScysC* | CKD-EPIcysC* | CAPA | FAScombi* | CKD-EPIcombi | BIS2* |
| eGFR – mGFR | 0.7 (0.0; 1.3) | 5.9 (5.1; 6.7) | 1.7 (1.1; 2.4) | 2.9 (2.2; 3.6) | 2.7 (1.9; 3.5) | 4.9 (4.1; 5.7) | 1.4 (0.8; 1.9) | 3.9 (3.1; 4.6) | 1.8 (1.2; 2.3) |
| eGFR/mGFR | 1.04 (1.02; 1.06) | 1.15 (1.12; 1.17) | 1.07 (1.05; 1.09) | 1.09 (1.07; 1.11) | 1.06 (1.04; 1.08) | 1.12 (1.10; 1.14) | 1.05 (1.03; 1.07) | 1.09 (1.07; 1.11) | 1.06 (1.05; 1.08) |
| RMSE | 9.2 (8.4; 9.9) | 13.0 (12.1; 13.8) | 8.7 (8.0; 9.3) | 9.8 (8.9; 10.6) | 11.4 (10.3; 12.3) | 12.1 (11.0; 13.2) | 8.1 (7.4; 8.7) | 11.0 (10.0; 11.8) | 7.9 (7.2; 8.6) |
| Lin’s CCC | 0.75 (0.71; 0.78) | 0.65 (0.62; 0.69) | 0.75 (0.72; 0.78) | 0.73 (0.70; 0.76) | 0.72 (0.69; 0.75) | 0.69 (0.66; 0.72) | 0.80 (0.77; 0.82) | 0.73 (0.70; 0.76) | 0.80 (0.78; 0.83) |
| P10 (%) | 41.2 (37.6; 44.7) | 31.2 (27.9; 34.5) | 42.5 (39.0; 46.0) | 38.5 (35.0; 42.0) | 35.5 (32.0; 38.9) | 32.9 (29.6; 36.3) | 47.9 (44.4; 51.5) | 35.5 (32.0; 38.9) | 48.3 (44.8; 51.9) |
| P30 (%) | 84.6 (82.0; 87.2) | 69.5 (66.2; 72.8) | 85.4 (82.9; 87.9) | 83.4 (80.7; 86.1) | 81.8 (79.0; 84.6) | 78.2 (75.3; 81.2) | 87.0 (84.6; 89.4) | 79.9 (77.1; 82.8) | 89.1 (86.9; 91.3) |
| mGFR ≥60 mL/min/1.73 m2 (n = 716) | |||||||||
| mGFR = 74.8 | FAScrea | CKD-EPIcrea* | BIS1 | FAScysC* | CKD-EPIcysC* | CAPA | FAScombi* | CKD-EPIcombi | BIS2* |
| eGFR – mGFR | −6.1 (−6.9; −5.2) | 3.3 (2.4; 4.2) | −7.9 (−8.7; −7.1) | −1.3 (−2.2; −0.4) | 4.9 (3.9; 5.9) | 6.2 (5.1; 7.2) | −4.3 (−5.1; −3.5) | 5.2 (4.3; 6.0) | −4.3 (−5.1; −3.6) |
| eGFR/mGFR | 0.93 (0.92; 0.94) | 1.06 (1.04; 1.07) | 0.90 (0.89; 0.91) | 0.99 (0.98; 1.00) | 1.07 (1.06; 1.09) | 1.09 (1.08; 1.10) | 0.95 (0.94; 0.96) | 1.08 (1.07; 1.09) | 0.95 (0.94; 0.96) |
| RMSE | 13.3 (12.5; 14.1) | 12.7 (12.1; 13.3) | 13.6 (12.7; 14.4) | 12.7 (11.8; 13.6) | 14.3 (13.5; 15.0) | 15.4 (14.4; 16.3) | 11.3 (10.5; 12.1) | 12.5 (11.9; 13.2) | 11.1 (10.3; 11.9) |
| Lin’s CCC | 0.49 (0.44; 0.54) | 0.46 (0.40; 0.51) | 0.44 (0.39; 0.49) | 0.55 (0.49; 0.60) | 0.50 (0.45; 0.55) | 0.49 (0.44; 0.54) | 0.59 (0.54; 0.63) | 0.55 (0.50; 0.59) | 0.59 (0.54; 0.63) |
| P10 (%) | 43.0 (39.4; 46.7) | 41.6 (38.0; 45.2) | 41.6 (38.0; 45.2) | 47.8 (44.1; 51.4) | 40.9 (37.3; 44.5) | 39.2 (35.7; 42.8) | 53.2 (49.5; 56.9) | 44.7 (41.0; 48.3) | 56.6 (52.9; 60.2) |
| P30 (%) | 92.0 (90.0; 94.0) | 91.1 (89.0; 93.2) | 92.9 (91.0; 94.8) | 93.3 (91.5; 95.1) | 87.2 (84.7; 89.6) | 86.0 (83.5; 88.6) | 95.7 (94.2; 97.2) | 91.5 (89.4; 93.5) | 95.8 (94.3; 97.3) |
Asterisks indicate the best performing equation(s) [13] within the same biomarker category, across all performance statistics. The bold values are the best result(s) for each performance statistic, across all equations. FAS, full-age-spectrum eGFR equation (Scr-based with Q = 0.70 mg/dL for females and Q = 0.90 mg/dL for males; SCysC-based with Q′ = 0.95 mg/L). FAScombi is calculated for α = 0.5.
For children, the FAScysC equation performs significantly better than the FAScrea equation based on Qcrea(age) and slightly better than or, in some cases, equivalent to the FAScrea equation based on Qcrea(height) (Table 6). We found that n = 7 children [for Qcrea(height)] and n = 20 children [for Qcrea(age)] with Scr/Qcrea <0.67 had FAScrea predictions that largely overestimate mGFR and were responsible for the large bias, RMSE and worse performance statistics. These children had spina bifida, Duchenne muscular dystrophy and severe growth retardation, explaining the very low Scr values and the poor match between Qcrea and age. The FAScysC equation has equivalent Lin’s CCC with CAPA but better RMSE. Also, P10 and P30 performance statistics were superior to CAPA. The SchwartzcysC equation shows the best performance in the mGFR <60 mL/min/1.73 m2 subgroup. However, although all children suffered from some underlying renal pathology, this subgroup was rather small (n = 57, 15%). The FAScombi equations [based on Qcrea(age) and Qcrea(height)] outperform all other paediatric equations and increase the precision for P10 to ≈45% and P30 to ≈90%, which is significantly higher (P < 0.0001) compared with single biomarker equations, including the single biomarker FAS equations.
For adults, the FAScysC equation performs worse than FAScrea, but better (overall and in the mGFR ≥60 mL/min/1.73 m2 subgroup) or equivalent (in the mGFR <60 mL/min/1.73 m2 subgroup) than the CKD-EPIcysC equation. The FAScysC equation is significantly better than the CAPA equation. The combined equations show higher precision, but the difference with the FAScrea equation is small. However, the FAScombi equation is overall the best prediction equation and performs better than the CKD-EPI combined equation, except for mGFR <60 mL/min/1.73 m2, where the performance is statistically equivalent.
In older adults, the FAScysC equation (with QcysC = 0.95) performs better than the CKD-EPIcysC equation and shows equivalent performance with the FAScrea equation. If we use the linear function QcysC = 0.863 + 0.01704 × (Age – 70) to normalize ScysC in the FAScysC and FAScombi equations, then the performance results (data not shown) are not significantly different than when QcysC = 0.95 is used to normalize ScysC in the FAS equation. The combined FAS equation is performing equivalent to the BIS2 equation, reaching P10 > 50% and P30 > 90%, and better than the combined CKD-EPI equation.
In Tables 6–8, we highlighted (the equations marked with asterisk) the best performing equation per biomarker category based on the scoring system previously used by Hoste et al. [13], which is based on bias, P10 and P30. We also highlighted (in bold) the best performance statistic.
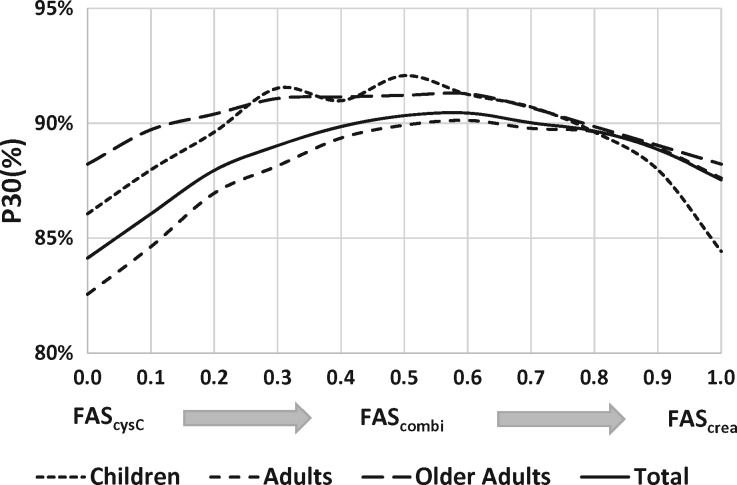
We also calculated the performance statistics (RMSE in Figure 2 and P30 in Figure 3) of the FAScombi equation as a function of the weighting parameter α. These figures show the performance statistics as a continuous function of α, evolving from the FAScysC equation (with α = 0) to the FAScrea equation (with α = 1), and in-between for the FAScombi for all values of α.
FIGURE 3.
P30 as a function of the weighting factor α for the different age groups.
DISCUSSION
Through the introduction of the international certified reference material ERM-DA471/IFCC for cystatin C [5] it has become possible to develop ScysC-based as well as combined Scr-/ScysC-based eGFR equations on the basis of normalized biomarkers. Despite the fact that manufacturers still need to improve the accuracy of cystatin C assays [19], we have shown here that the basic concept upon which the FAScrea equation was built [2] is not only applicable for normalized Scr, but can also be applied to normalized ScysC. By replacing normalized Scr with ScysC, or introducing the (weighted) average of both biomarkers, we can change from a Scr-based FAS equation to a ScysC-based FAS equation or a combined Scr-/ScysC-based FAS equation. These FAS equations show performance values that are equivalent or in some conditions superior to the currently recommended eGFR equations for children, adolescents, adults and older adults. Normalization of the biomarkers is a key in this construction. In the case of Scr, normalization is required to account for the difference in creatinine generation during childhood, the age/gender differences during adolescence and the difference between adult men and women. Normalization of ScysC is required to account for the age effect beyond the age of 70 years. For the healthy population, the normalized biomarkers show equivalent distributions with mean of ‘1’ and 2.5th and 97.5th Pct of 0.67 and 1.33, respectively. These similar characteristics of normalized biomarker concentration distributions lead to an interchangeable usage of both renal markers in the FAS equation(s).
The performance of the new FAScysC equation was better than the CAPA equation and better (in adults with mGFR ≥60 mL/min/1.73 m2 and in older adults) or equivalent (in adults with mGFR <60 mL/min/1.73 m2) to the CKD-EPIcysC equation. In children, the RMSE statistic is worst (highest) for the FAScrea equation due to a fraction of children with Scr/Qcrea <0.67. Therefore, we would recommend not to use FAScrea (or the combined FAS equations) when Scr/Qcrea <0.67 [2]. For adults, based on the performance statistics (RMSE and P30), there is still a slight preference for the single biomarker FAScrea equation over the single biomarker FAScysC equation. For older adults, both single biomarker FAS equations perform in a similar manner. However, for all age groups, the FAScombi equation with α ≈ 0.5 (corresponding to the average of both biomarkers) showed the smallest RMSE and the highest P30 and P10. Also the FAScombi equation outperformed all other combined equations, with the exception of the BIS2 equation, which showed an equivalent performance for older adults (but note that BIS data used to derive the BIS equations are part of the current validation dataset) and the CKD-EPI equation for adults with mGFR <60 mL/min/1.73 m2, where FAS showed equivalent performance results.
When the overall performance statistics for specific age groups was calculated, we found that α ≈ 0.5 corresponding to the average of normalized creatinine and cystatin C biomarker concentrations gave the best performance statistics for all age groups and demonstrated the smallest RMSE and highest P10 and P30 values. Although we calculated the average of both biomarkers and entered this into the FAS equation, it approximated the average of both single biomarker FAS equations (Scr and ScysC), a finding that has been observed by Björk et al. [20, 21] in a Swedish cohort, when combining the Scr-based Lund–Malmö and the ScysC-based CAPA equation. The choice to use a single or the mean of two biomarkers should be based on the clinical context, when conditions are disclosed that invalidate either Scr or ScysC as renal biomarker. The use of Scr may be discouraged in case of severe muscle wasting (anorexic patients, patients with muscle disorder, like Duchenne muscle dystrophy), immobile patients, or elderly cachectic patients with reduced muscle mass. Also, abnormal meat consumption, abnormal muscle development in athletes or weight lifters, or medication usage that affects creatinine generation may have an impact on the validity of creatinine as a renal biomarker. The use of ScysC-based equations may be discouraged when patients are treated with (high dose) glucocorticoids or other medication impacting on the biomarker’s serum concentration [22], in obese patients, tobacco users or patients with thyroid dysfunction or inflammation [11, 23–25]. The combination of both biomarkers has the advantage that it may cancel out the non-GFR-related factors influencing creatinine and cystatin C in different directions compared with mGFR [24–26]. The great advantage of our approach is that the same equation can be used, only the appropriate normalized biomarker has to be chosen (either Scr/Qcrea, or ScysC/QcysC or the average of both). However, the cost of cystatin C is relatively high and additional studies are needed to prove that measuring cystatin C is cost-effective. In the context of GFR estimation, the additional value of cystatin C could be defined by the clinical condition, knowing that non-GFR determinants influence both creatinine and cystatin C.
We also investigated the impact of the weighting factor α on the performance of the FAS equations by varying α (between 0 = FAScysC and 1 = FAScrea) and calculating the difference ‘FAS – mGFR’, on an individual basis. Due to the way the FAS equations are designed, FAScrea ≈ FAScysC ≈ FAScombi, in the case of the normalized biomarkers Scr/Qcrea ≈ ScysC/QcysC. When Scr/Qcrea strongly deviates from ScysC/QcysC, then FAScrea will strongly deviate from FAScysc and FAScombi will lie in-between both single biomarker FAS predictions. We found that, on an individual basis, in approximately one-third of the subjects, the FAScrea equation was closest to mGFR, in one-third of the subjects the FAScysC equation had the lowest individual bias and in one-third the FAScombi equation was the best choice for a specific value of α. In the latter, when mGFR lies between FAScrea and FAScysC predictions, there is always a value of α for which FAScombi = mGFR. We realize that this analysis is mainly speculative as we do not know the optimal value of α in actual clinical situations, but the intention of this analysis was to evaluate in which conditions a preference for single biomarker FAS predictions or for the combined biomarker FAS prediction might exist. Unfortunately, we could not identify specific conditions where one over the other equations was to be preferred (unless the situation where Scr/Qcrea <0.67).
The strength of this study is the large number of subjects (n = 6132) covering the complete age span from 2 to 100 years of age. This study partially used data from our previous study, where n = 6870 subjects were used to validate the FAScrea equation. Although both studies partially used the same subjects, the ScysC data was not part of the previous evaluation. All ScysC concentrations were analysed with cystatin C assays based on the international certified standard or were back-calculated using calibration curves developed for that purpose. The reference tests used in this study comprise all currently used direct measurement methods: 51Cr-EDTA (plasma/renal clearance), inulin (renal clearance), iohexol (plasma clearance in its different configurations) and iothalamate (renal clearance), illustrating the diversity of mGFR results and demonstrating the robustness of the FAS construction. Moreover, the cohorts used in this study were from different countries in Europe (Norway, Germany, France, Belgium) and the USA (Rochester, MN and the CRIC cohort), making the sample representative for the general Caucasian population and kidney disease population.
Our study has some limitations. First, we did not incorporate different ancestries, and, therefore, this validation study is limited to Caucasians only. Although it is known that creatinine generation (and thus Scr) is affected by ancestry, it is also known that ScysC is not influenced by differences in ancestry. We always have claimed that using appropriate ancestry-specific normalization factors for Scr may solve this problem and consequently the FAS concept remains applicable. Secondly, our goal was to validate the new FAS equations against mGFR and compare them with the existing and recommended equations, not to predict the risk of mortality. Whether the FAS equations are better predictors of mortality is another topic and requires further studies using a different statistical methodology [27].
CONCLUSIONS
The fundamental concept for the Scr-based FAS equation development, namely that mean GFR for healthy subjects evolves along an age-specific curve, and that deviation from that curve is related to the inverse of normalized Scr/Qcrea, also holds true for normalized ScysC/QcysC. The current work shows that the FAS equations display better or equivalent prediction performance than the currently recommended eGFR equations, across the full age spectrum, both in normal and reduced kidney function. The FAS equation is not only applicable to all ages, but also to all currently recommended renal biomarkers. The FAS concept may also be applicable to other renal biomarkers, if appropriately normalized, but this remains to be proven once standardized assays are in place.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
The Chronic Renal Insufficiency Cohort (CRIC) Study was conducted by the CRIC Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data from the CRIC Study reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with Investigators of the CRIC Study and does not necessarily reflect the opinions or views of the CRIC Study, the NIDDK Central Repositories, or the NIDDK. We would also like to thank all patients and researchers, service users, carers and lay people who contributed to the original datasets and who are not mentioned here as co-authors. No specific funding was obtained for this study. S.T.T. (1 R01 DK073537) was supported by research grants from the National Institutes of Health, US Public Health Service.
CONFLICT OF INTEREST STATEMENT
None declared.
(See related article by Agarwal. Glomerular filtration rate estimating equations: practical, yes, but can they replace measured glomerular filtration rate? Nephrol Dial Transplant 2017; 32: 405--407)
REFERENCES
- 1. Delanaye P, Pottel H, Botev R.. Con: should we abandon the use of the MDRD equation in favour of the CKD-EPI equation? Nephrol Dial Transplant 2013; 28: 1396–1403 [DOI] [PubMed] [Google Scholar]
- 2. Pottel H, Hoste L, Dubourg L. et al. An estimating glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 2016; 31: 798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pottel H, Hoste L, Yayo E. et al. Glomerular filtration rate in healthy living potential kidney donors: a meta-analysis supporting the construction of the full age spectrum (FAS) equation. Nephron Clin Pract 2016; Nephron 2016 Oct 21. [Epub ahead of print] DOI:10.1159/000450893 [DOI] [PubMed] [Google Scholar]
- 4. Inker LA, Schmid CH, Tighiouart H. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grubb A, Blirup-Jensen S, Lindström V. et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med 2010; 48: 1619–1621 [DOI] [PubMed] [Google Scholar]
- 6. Schwartz GJ, Schneider MF, Maier PS. et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 2012; 82: 445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grubb A, Horio M, Hansson L-O. et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem 2014; 60: 974–986 [DOI] [PubMed] [Google Scholar]
- 8. Schaeffner ES, Ebert N, Delanaye P. et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012; 157: 471–481 [DOI] [PubMed] [Google Scholar]
- 9. Feldman HI, Appel LJ, Chertow GM. et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol 2003; 14: S148–S153 [DOI] [PubMed] [Google Scholar]
- 10. Pottel H, Hoste L, De Waele L. et al. Measuring glomerular filtration rate using 51Cr-EDTA. Nucl Med Commun 2014; 35: 1150–1155 [DOI] [PubMed] [Google Scholar]
- 11. Rule AD, Bailey KR, Lieske JC. et al. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int 2013; 83: 1169–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kilbride HS, Stevens PE, Eaglestone G. et al. Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis 2013; 61: 57–66 [DOI] [PubMed] [Google Scholar]
- 13. Hoste L, Dubourg L, Selistre L. et al. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant 2013; 29: 944–947 [DOI] [PubMed] [Google Scholar]
- 14. Bökenkamp A, Domanetzki M, Zinck R. et al. Reference values for cystatin C serum concentrations in children. Pediatr Nephrol 1998; 12: 125–129 [DOI] [PubMed] [Google Scholar]
- 15. Filler G, Priem F, Lepage N. et al. β-trace protein, cystatin C, β2-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem 2002; 48: 729–736 [PubMed] [Google Scholar]
- 16. Finney H, Newman DJ, Price CP.. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem 2000; 37: 49–59 [DOI] [PubMed] [Google Scholar]
- 17. Groesbeck D, Köttgen A, Parekh R. et al. Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol 2008; 3: 1777–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Séronie-Vivien S, Delanaye P, Pieroni L. et al. SFBC “Biology of renal function and renal failure” working group. Clin Chem Lab Med 2008; 46: 1664–1686 [DOI] [PubMed] [Google Scholar]
- 19. Eckfeldt JH, Karger AB, Miller WG. et al. Performance in measurement of serum cystatin C by laboratories participating in the College of American Pathologists 2014 CYS Survey. Arch Pathol Lab Med 2015; 139: 888–893 [DOI] [PubMed] [Google Scholar]
- 20. Grubb A, Nyman U, Björk J.. Improved estimation of glomerular filtration rate (GFR) by comparison of eGFRcystatin C and eGFRcreatinine. Scand J Clin Lab Invest 2012; 72: 73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Björk J, Grubb A, Larsson A. et al. Accuracy of GFR estimating equations combining standardized cystatin C and creatinine assays: A cross-sectional study in Sweden. Clin Chem Lab Med 2015; 53: 403–414 [DOI] [PubMed] [Google Scholar]
- 22. Risch L, Herklotz R, Blumberg AHA.. Clin Chem effects of glucocorticoid immunosuppression on serum cystatin c concentrations in renal transplant patients. 2001; 47: 2055–2059 [PubMed] [Google Scholar]
- 23. Filler G, Bökenkamp A, Hofmann W. et al. Cystatin C as a marker of GFR - history, indications, and future research. Clin Biochem 2005; 38: 1–8 [DOI] [PubMed] [Google Scholar]
- 24. Schei J, Stefansson VTN, Mathisen UD. et al. Residual associations of inflammatory markers with eGFR after accounting for measured GFR in a community-based cohort without CKD. Clin J Am Soc Nephrol 2016; 11: 280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Delanaye P, Mariat C.. The applicability of eGFR equations to different populations. Nat Rev Nephrol 2013; 9: 513–522 [DOI] [PubMed] [Google Scholar]
- 26. Melsom T, Fuskevåg OM, Mathisen UD. et al. Estimated GFR is biased by non-traditional cardiovascular risk factors. Am J Nephrol 2015; 41: 7–15 [DOI] [PubMed] [Google Scholar]
- 27. Warnock DG. Estimated glomerular filtration rate: fit for what purpose? Nephron 2016; 134: 43–149 [DOI] [PubMed] [Google Scholar]
- 28. Gagneux-Brunon A, Delanaye P, Maillard N. et al. Performance of creatinine and cystatin C-based glomerular filtration rate estimating equations in a European HIV-positive cohort. AIDS 2013; 27: 1573–1581 [DOI] [PubMed] [Google Scholar]
- 29. Eriksen BO, Mathisen UD, Melsom T. et al. The role of cystatin C in improving GFR estimation in the general population. Am J Kidney Dis 2012; 59: 32–40 [DOI] [PubMed] [Google Scholar]
- 30. Schwartz GJ, Muñoz A, Schneider MF. et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20: 629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.