Abstract
Aims
Mechanical chest compression (CC) during cardiopulmonary resuscitation (CPR) with AutoPulse or LUCAS devices has not improved survival from cardiac arrest. Cohort studies suggest risk of excess damage. We studied safety of mechanical CC and determined possible excess damage compared with manual CC.
Methods and results
This is a randomized non-inferiority safety study. Randomization to AutoPulse, LUCAS, or manual CC with corrective depth and rate feedback was performed. We included patients with in-hospital cardiac arrest or with out-of-hospital cardiac arrest arriving with manual CPR at the emergency department. The primary outcome was serious or life-threatening visceral resuscitation-related damage, assessed blind by post-mortem computed tomography scan and/or autopsy or by clinical course until discharge. Non-inferiority hypothesis: mechanical CC compared with manual control does not increase the primary outcome by a risk difference of > 10% [upper 95% confidence interval (CI)]. We included 115 patients treated with AutoPulse, 122 with LUCAS, and 137 patients received manual CC. Safety outcome analysis was possible in 337 of 374 (90.1%) included patients. The primary outcome was observed in 12 of 103 AutoPulse patients (11.6%), 8 of 108 LUCAS patients (7.4%), and 8 of 126 controls (6.4%). Rate difference AutoPulse—control: +5.3% (95% CI − 2.2% to 12.8%), P = 0.15. Rate difference LUCAS—control +1.0% (95% CI − 5.5% to 7.6%), P = 0.75.
Conclusion
LUCAS does not cause significantly more serious or life-threatening visceral damage than manual CC. For AutoPulse, significantly more serious or life-threatening visceral damage than manual CC cannot be excluded.
Keywords: Chest compressions , Heart arrest , Mechanical chest compressions , Cardiopulmonary resuscitation , Damage , Safety
Introduction
American Heart Association (AHA) and European Resuscitation Council (ERC) Guidelines 2015 for cardiopulmonary resuscitation (CPR) specify that chest compressions should be delivered with a depth of at least 5 cm but not greater than 6 cm at a rate of 100–120/min.1,2 These specifications are rarely met: compression depth with manual CPR is frequently too shallow, rates are too high, and prolonged interruptions are frequent.3,4 Mechanical chest compression devices are designed to perform chest compressions at specified rate and depth and therefore were expected to improve outcome. There are at present two widely used and Food and Drug Administration-approved devices: the AutoPulse (Zoll Medical Corporation, Chelmsford, MA, USA), a load-distributed band device that rhythmically compresses and restricts the chest wall and the LUCAS (Physio-Control/Jolife AB, Lund, Sweden), a piston device with a cup that is placed in the centre of the chest and pushes the sternum down over a distance of 5.2 cm and pulls back to the neutral position. Significant improvement of aortic blood pressure and coronary perfusion pressure is documented in humans from the AutoPulse compared with manual chest compressions.5 Chest compression with LUCAS resulted in significantly higher end-tidal carbon dioxide in humans compared with manual chest compressions.6 For several years, only one randomized clinical trial with the AutoPulse was available (ASPIRE), which was terminated after interim analysis because of a trend to reduced survival to discharge compared with manual control CPR.7 None of the more recent randomized clinical trials demonstrated survival benefit of AutoPulse or LUCAS over manual controls.8–10 Anecdotal and possibly biased observations in our hospital and a published letter suggested increased damage caused by mechanical chest compression devices.11
Because of the perceived damage caused by mechanical chest compression devices, we performed a randomized and controlled study with the AutoPulse and the LUCAS in a non-inferiority design. The objective of our study was to investigate the hypothesis that mechanical chest compression devices do not cause an excess of severe or lethal visceral damage compared with manual chest compressions.
Methods
Setting and study design
We performed a prospective randomized clinical trial in the setting of a university hospital to study the safety of mechanical chest compression devices AutoPulse and LUCAS during CPR. The study was approved by the Medical Ethics Committee of our hospital with deferred consent because of the emergency situation, including the use of data from patients who did not survive out-of-hospital cardiac arrest (OHCA). Survivors of the arrest were approached for written consent, and patients refusing consent were withdrawn from the analysis. Clinical trial registration: ISRCTN14647429 (LUCAS) and ISRCTN75393297 (AutoPulse).
Patients
Patients were included between 3 November 2008 and 26 May 2014. Included were patients either with an in-hospital cardiac arrest (IHCA) or with an OHCA after arrival at the emergency department with ongoing CPR. All patients had a shorter (IHCA) or longer (OHCA) period of manual CPR before inclusion in the study. Excluded were patients with a traumatic cause of the arrest, patients with known or estimated age <18 years, patients who arrived at the emergency room with a mechanical chest compression device already applied by the ambulance crew, patients who had return of spontaneous circulation before application of the study device, and patients who had no cardiac arrest.
Randomization and masking
Randomization was done by computer-generated random numbers. Initially, we randomized patients to be treated either by a LUCAS chest compression device or control (LUCAS study) or by an AutoPulse chest compression device or control (AutoPulse study). Per-patient randomization with 1:1 allocation to either LUCAS study or AutoPulse study study was followed by per-patient randomization with 1:1 allocation to the study device or to manual control CPR, and the resulting treatment allocation concealed in opaque envelopes that were placed at the coronary care unit (CCU). Patients randomized to control treatment received manual chest compressions with corrective feedback of compression depth and rate from the sternal displacement transducer of a Philips Heartstart MRx defibrillator (Philips Healthcare, Eindhoven, The Netherlands). After randomization of 69 patients and observing a lower-than-expected inclusion rate, we decided to merge the patients allocated to the two control groups up to that moment into one control group for use in both comparisons and changed the allocation to 1:1:1 for AutoPulse:LUCAS:control for the remainder of the study (Figure 1). The control patients in both comparisons therefore included the same patients. This change in randomized treatment allocation was made before any blinded outcome assessment had been done. The data and safety monitoring committee approved this change in the protocol.
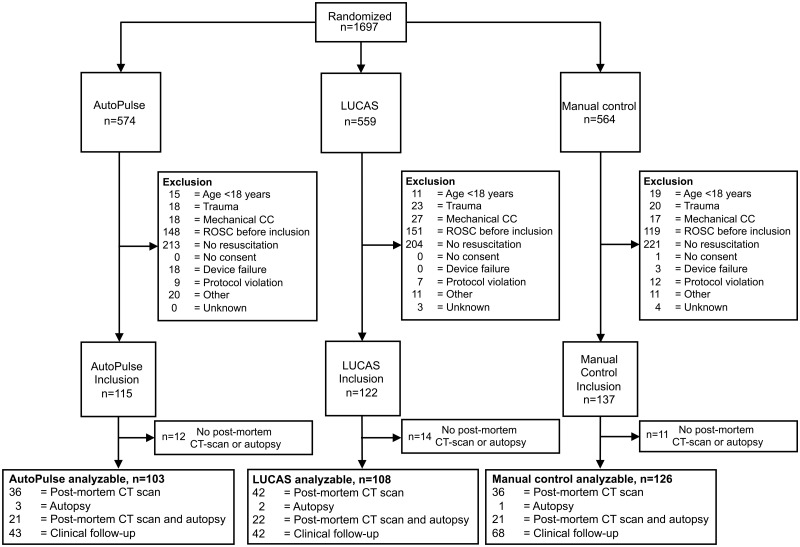
Figure 1.
Flowchart displaying randomization, exclusion, and inclusion. CC, chest compression; ROSC, return of spontaneous circulation; CT, computed tomography.
Procedures
One AutoPulse device, one LUCAS device, and one Philips MRx defibrillator were stationed at the CCU, together with the randomization envelopes. The devices were operated by the CCU nurses who participated in the regular hospital resuscitation team. All CCU nurses received training courses from the manufacturers of the compression devices in their correct and rapid application and handling and received refresher courses every 6 months. When an alert for a possible cardiac arrest was received at the CCU, one sealed randomization envelope was opened. The hospital resuscitation team then took the corresponding device to the site of the arrest, being the location of the in-hospital arrest or the emergency department where the patient arrived after OHCA with ongoing CPR. After arrival on-site, the resuscitation team confirmed that CPR was still ongoing, then evaluated the patients for possible exclusion criteria. If none of the exclusion criteria applied, the patients were treated with the designated device. Final inclusion and exclusion decisions therefore were done after randomization had taken place and was not blinded to the allocated treatment. If the mechanical chest compression device did not operate as intended because of battery failure or other malfuction, the device was removed from the patient, manual chest compression administered, and the patient was excluded from analysis.
Chest compression data from all patients were obtained from a LifePak 12 defibrillator (Physio-Control, Redmond, WA, USA) and analysed with specific software (CodeStat Suite V9, Physio-Control, Redmond, WA, USA) with automated time synchronization. The moment of initiation and termination of AutoPulse or LUCAS compressions was identified by the characteristic pattern of the impedance signal. From patients randomized to manual chest compressions, compression duration, rate, and depth were analysed with Q-CPR review software V 2.1 (Laerdal Medical, Stavanger, Norway). For patients with an OHCA, the duration of chest compressions before connection of the study device was estimated from the time interval between the moment of call to the ambulance dispatch centre for an OHCA and the initiation of study compressions in the emergency department. For patients with an IHCA, this interval was the time interval between the call for cardiac arrest to the hospital switchboard and the first study compression.
For patients not surviving resuscitation, autopsy was requested to the family. In addition, we requested permission to perform a post-mortem high-resolution multislice computed tomography (CT) scan to be performed within 1-2 h after death.12 For patients initially surviving resuscitation, all data from the subsequent clinical course in the intensive care unit (ICU) were obtained.
Outcomes
The primary outcome of the study was serious or life-threatening resuscitation-related damage to visceral organs, including large vessels and vertebrae as determined by post-mortem CT scan and/or autopsy or by clinical course (see Supplementary material online, Table S1). The secondary outcome was damage to bony structures of the chest wall (sternum and ribs). The study was analysed according to the actual treatment received, as acceptable for a safety study. The study was not intended nor powered to study survival for either of the two devices against manual controls, nor was the study intended to compare damage or survival between the two compression devices.
The post-mortem CT scans were evaluated by a panel of two experienced radiologists; autopsy was performed with a specific protocol. For patients surviving resuscitation, the subsequent clinical course until death or hospital discharge was evaluated for signs of visceral or chest cage damage by a panel of two radiologists, an intensivist and a cardiologist, using all available information. All assessors were blinded to the allocated treatment. During an autopsy, however, a characteristic round mark from the cup of the LUCAS or skin abrasions from the AutoPulse band could be visible and therefore the pathologist cannot be considered fully blinded.
The seriousness of the observed damage was distinguished in three levels of severity according to standard grading for patients in cardiac arrest13:
(i) life-threatening—reasonably expected to interfere with cardiovascular or respiratory function, exsanguination in excess of 800 mL; (ii) serious—demands therapy for repair or for alleviation of pain, expected to prolong hospitalization; and (iii) insignificant—requires no therapy (expected to heal spontaneously without complication) or may require limited one-time only therapy. This definition of the primary endpoint was clinically oriented and could not be assessed in full if the patient did not survive the resuscitation attempt. In these cases, the panel classified the damage with the anticipation of a clinical course as if the patient had initially survived.
The secondary endpoint damage to the ribcage was defined as serious if it included sternal fractures and/or involved fractures to >6 ribs if unilateral or >4 ribs if at least one rib fracture was bilateral. Otherwise rib fractures were classified as insignificant. Damage to the ribcage and sternum was never considered life-threatening by itself.
For those patients who had a post-mortem CT scan as well as an autopsy, the most severe reported damage was used in the analysis.
Statistical analysis
The study was designed as a non-inferiority study. The primary analysis tested whether mechanical chest compressions by either AutoPulse or LUCAS devices would not result in more life-threatening or serious visceral damage when compared with manual chest compressions. We assumed that the rate of serious or life-threatening visceral damage by manual chest compressions was 10%.13 To accept the non-inferiority hypothesis, the upper bound of the 95% confidence interval of an observed rate difference (RD) of damage between mechanical or manual chest compression-treated patients should not exceed +10%. To achieve a power of 80% and a one-sided α of 0.05, this required 2 × 112 patients in each comparison between experimental treatment and control.
Descriptive statistics were expressed as means with standard deviation or medians with interquartile range. Groups were compared with Student’s t-test for continuous variables, the χ2 statistic for proportions or the Mann–Whitney U test for non-normally distributed continuous variables. Calculations were done with IBM SPSS Statistics for Mac V.20 (IBM, Armonk, NY, USA). Rate differences were calculated with their 95% confidence intervals according to Miettinen and Nurminen.14
Results
Figure 1 shows the randomization, exclusion and inclusion of the patients, and number of patients available for outcome assessment. The hospital resuscitation team was alerted for 1697 patients for whom a randomization envelope was opened. An AutoPulse was brought on scene for 574 patients, a LUCAS for 559 patients, and a Philips MRx for manual chest compressions for 564 patients. After exclusion criteria were assessed, an AutoPulse was used in 115 patients, a LUCAS device was used in 122 patients, while 137 patients received manual chest compressions. For details of the reasons for exclusion, see Supplementary material online, Table S2. Location of the arrest for IHCA patients is available in Supplementary material online, Table S3. Permission for autopsy and post-mortem CT scanning was denied by the family for 12 AutoPulse patients, for 14 LUCAS-treated patients, and for 11 control patients, who did not survive resuscitation. Possible damage could therefore be assessed by autopsy and/or post-mortem CT scan or by clinical follow-up in 103 AutoPulse patients, in 108 LUCAS-treated patients, and in 126 controls (for details of clinical diagnostic information, see Supplementary material online, Table S1). One surviving control patient refused consent, and her data were removed from analysis.
Baseline data are presented in Table 1. Age and gender were not significantly different between patients allocated to either mechanical chest compression devices or to manual control. For patients with OHCA, the interval between estimated initiation of manual chest compressions and connection of the study device was approximately 60 min, not significantly different between the experimental and control groups. For patients with IHCA, this interval was much shorter and significantly shorter for control patients. In the control group, the mean compression depth was slightly below the recommended 50 mm. The compression rate in the control group was within the recommended range.1,2 For details of initial recorded rhythm and possible cause of arrest, see Supplementary material online, Tables S4 and S5.
Table 1.
Baseline and process data
| AutoPulse (n = 115) | LUCAS (n = 122) | Manual control (n = 137) | |
|---|---|---|---|
| Gender male, n (%) | 75 (65.2) | 82 (67.2) | 87 (63.5) |
| Age (years, mean ± SD) | 65 ± 15 | 63 ± 17 | 66 ± 13 |
| Location of arrest onset | |||
| Out-of-hospital, n (%) | 53 (46.1) | 53 (43.4) | 50 (36.5) |
| In-hospital, n (%) | 62 (53.9) | 69 (56.6) | 87 (63,5) |
| Interval call-start study device (min, median, IQR)a | |||
| Out-of-hospital | 60 (56–71) | 57 (48–62) | 57 (43–67) |
| In-hospital | 10 (5–20) | 8 (4–11) | 5 (3–8) |
| Duration of connection study device (min, median, IQR) | 21 (10–31) | 22 (7–39) | 16 (6–32) |
| Compression depth (mm, mean ± SD) | 48 ± 9 | ||
| Compression rate (per minute, mean ± SD) | 110 ± 14 | ||
For out-of-hospital cardiac arrest, the call was the call to the dispatch centre; for in-hospital cardiac arrest, it was the call to the hospital central switchboard.
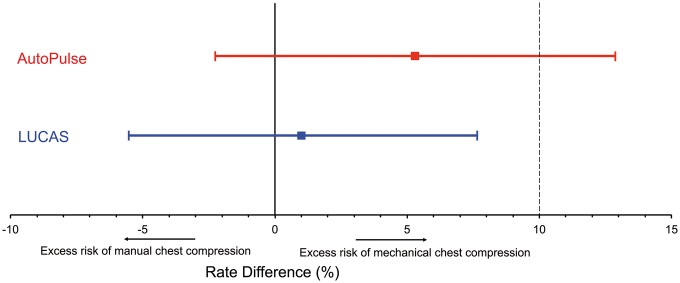
The primary outcome is presented in Table 2. Serious or life-threatening visceral resuscitation-related damage was seen in 12 of 103 AutoPulse patients (11.6%), in 8 of 108 LUCAS patients (7.4%), and in 8 of 126 controls (6.4%). The RD in occurrence of this primary endpoint between AutoPulse patients and control patients was +5.3% (95% CI −2.2% to 12.8%), P = 0.15 and between LUCAS patients and control patients +1.0% (95% CI −5.5% to 7.6%), P = 0.75 (Figure 2).
Table 2.
Primary and secondary outcomes in analysable patients
| AutoPulse (N = 103) | LUCAS (N = 108) | Manual control (N = 126) | Rate difference AutoPulse vs. control (%) (95% CI)a | Rate Difference LUCAS vs. Control (%) (95% CI)a | |
|---|---|---|---|---|---|
| Resuscitation-related structural visceral damage (primary endpoint) | |||||
| Serious or life-threatening damage, overall, n (%) | 12 (11.7) | 8 (7.4) | 8 (6.3) | 5.3 (−2.2 to 12.8) | 1.0 (-5.5 to 7.6) |
| Out-of-hospital arrest onset, n/N (%) | 6/44 (13.6) | 3/46 (6.5) | 2/48 (4.2) | 9.4 (−2.1 to 21.1) | 2.4 (-6.7 to 11.5) |
| In-hospital arrest onset, n/N (%) | 6/59 (10.2) | 5/62 (8.1) | 6/78 (7.7) | 2.5 (−7.2 to 12.2) | −0.23 (-8.9 to 8.4) |
| Insignificant damage, n (%) | 6 (5.8) | 11 (10.2) | 13 (10.3) | ||
| No damage, n (%) | 85 (82.5) | 89 (82.4) | 105 (83.3) | ||
| Serious or life-threatening resuscitation-related visceral damage—detailsb | |||||
| Pneumothorax, n | 6 | 2 | 4 | ||
| Tension pneumothorax, n | 1 | 1 | — | ||
| Pneumomediastinum/oesophagus haematoma, n | 4 | — | — | ||
| Pleural fluid/blood, n | 1 | 3 | 3 | ||
| Lung contusion/haematoma, n | 1 | — | 1 | ||
| Liver rupture, n | 1 | 2 | — | ||
| Intracranial air embolism, n | 1 | — | — | ||
| Pneumoperitoneum, n | — | 1 | — | ||
| Resuscitation-related rib and sternum damage (secondary endpoint) | |||||
| Serious, n (%) | 47 (45.6) | 43 (39.8) | 52 (41.3) | 4.4 (−8.5 to 17.3) | −1.5 (-14.1 to 11.2) |
| Insignificant damage, n (%) | 10 (9.7) | 9 (8.3) | 22 (17.5) | ||
| No damage, n (%) | 46 (44.7) | 56 (51.9) | 52 (41.3) | ||
| Mean number of fractured ribs, mean ± SDc | 8 ± 4 | 8 ± 4 | 7 ± 4 | n.s. | n.s. |
| Sternum fractures, n (%) | 3 (2.9) | 7 (6.5) | 5 (4.0) | −1.2 (−6.8 to 4.6) | 2.3 (-3.8 to 9.1) |
95% confidence interval according to Miettinen.14
Some patients had more than one kind of serious or life-threatening visceral damage.
Calculated for the patients with rib fractures.
Figure 2.
Rate differences of resuscitation-related serious or life-threatening damage between mechanical chest compressions and manual chest compressions. The dotted line at + 10% indicates the boundary of excess risk difference that should not be exceeded to accept the non-inferiority hypothesis.
The secondary outcome (severe rib and/or sternum fractures) was observed in 47 of 103 AutoPulse patients (45.6%), in 43 of 108 LUCAS patients (39.8%), and in 52 of 126 controls (41.3%). The RD between AutoPulse and controls was +4.4% (95% CI −8.5 to 17.3), P = 0.51 and between LUCAS and controls −1.5 (95% CI −14.1 to 11.2), P = 0.82 (Table 2).
Death of three patients was clearly and directly attributable to the resuscitation damage itself: two patients with LUCAS compressions had a liver rupture with massive bleeding (one died during resuscitation and one died in the ICU) and one AutoPulse-treated patient had a tension pneumothorax with air embolism involving the brain and died during resuscitation. The great majority of patients died in the ICU from neurologic ischaemic damage or from their pre-existing disease (Table 3).
Table 3.
In-hospital course after resuscitation
| AutoPulse (N = 115) | LUCAS (N = 122) | Manual control (N = 137) | ||
|---|---|---|---|---|
| Did not survive resuscitation, n (%) | 72 (62.6) | 80 (65.6) | 69 (50.4) | |
| Admitted to ICU after resuscitation, n (%) | 43 (37.4) | 42 (34.4) | 68 (49.6) | |
| Mode of death in ICU | ||||
| Neurologic, n | 18 | 11 | 26 | |
| Bleeding, n | 0 | 4 | 1 | |
| Sepsis, n | 1 | 1 | 4 | |
| Respiratory, n | 2 | 0 | 0 | |
| Pre-existing disease, n | 14 | 7 | 14 | |
| Complication from resuscitation, n | 0 | 1 | 0 | |
| Unknown, n | 1 | 1 | 2 | |
ICU, intensive care unit.
Discussion
In this randomized clinical non-inferiority trial on resuscitation-related damage, we found that excess serious and life-threatening visceral damage did not exceed the pre-determined upper 95% confidence interval of 10% during LUCAS use and the non-inferiority hypothesis could be accepted. Significant excess damage from the use of the AutoPulse was not excluded because the upper 10% confidence interval of 10% excess damage was exceeded, and the non-inferiority hypothesis for the AutoPulse was not accepted.
The first randomized clinical trial with the AutoPulse, the ASPIRE trial, was published in 2006. That trial was stopped prematurely because of a trend of excess mortality with the use of the AutoPulse.7 Recently, three clinical trials on survival benefit from mechanical chest compression devices were published. The CIRC study did not show survival benefit of the AutoPulse over control with manual CPR.8 Two studies employing the LUCAS device, the LINC study and the PARAMEDIC study, showed no survival benefit to discharge from use of the device over manual control.10,15 Nevertheless, these devices are used worldwide in large numbers. The Guidelines for Resuscitation from AHA and ERC 2015 consider mechanical chest compression acceptable for ongoing CPR during transportation or during coronary intervention.16,17 These conditions for use of mechanical chest compression have not yet proved to benefit patients and make it even more important that we are certain that the devices do not harm.
Why did all randomized trials fail to demonstrate overall benefit? Interruption of chest compressions during device positioning18,19, shifting device position during use to vulnerable places on the chest or abdomen, or too forceful compressions could be an explanation. Studies of potential damage from mechanical chest compression devices have been published and a variety of damage is reported, mostly from autopsy reports.20–22 The absolute difference in observed visceral damage between these studies is striking: in the study of Smekal et al.,20 visceral damage was observed in 28% and 19% in LUCAS-treated and manual-treated patients, respectively, with an odds ratio of 1.6 (95% CI 0.84–3.2). In the study of Lardi et al.,22 visceral damage in LUCAS-treated and manual-treated patients was 19% and 25%, respectively, with an odds ratio of 0.76 (95% CI 0.22–2.6), but in Pinto et al.’s21 study, this was only 3% and 1%, respectively, with an odds ratio of 3 (95% CI 0.3–30). A recent large retrospective cohort study using post-mortem CT scans compared AutoPulse treatment with controls.23 The odds ratio of abdominal injury in AutoPulse treated patients was 4.9 (95% CI 1.88–12.9). In all patients, both mechanical and manual chest compressions were given. Being a non-randomized study, the contribution of mechanical chest compression to damage cannot be clearly established. Our randomized study with damage assessment in >90% of all included patients allows a better assessment of the possible contribution of mechanical devices to damage from chest compressions, as the duration of initial manual CPR prior to device application was similar in all groups.
The numbers of various kinds of damage are too small to draw conclusions on their cause. The LUCAS concentrates its force on the sternum, and this may explain why more sternum fractures were observed in patients treated with LUCAS. The AutoPulse may generate high intra-thoracic pressure because of the force on the circumference of the chest. Severe rib and sternum damage did not occur significantly more in both mechanical chest compression devices. Rib and sternum damage was not considered life-threatening in itself, but it may have caused severe or life-threatening visceral pulmonary or mediastinal damage such as tension pneumothorax, mediastinal bleeding, or mediastinal emphysema.
Damage classified as life-threatening does not imply that it actually had caused death. In the setting of a cardiac arrest, the cause of the arrest, its consequences (e.g. aspiration or cerebral ischaemia), and the complications of chest compressions (e.g. pleural bleeding or pneumothorax) are a mix of conditions that each could contribute to death after resuscitation. In only three patients, the visceral damage could be classified as lethal in itself: liver rupture with massive bleeding and tension pneumothorax with cerebral air embolism.
Autopsy is not routinely performed after failed resuscitation, and most studies that report resuscitation-related damage only rely on autopsy performed in a minority of non-survivors. In a study specifically aimed to investigate damage from mechanical chest compressions, 222 of 691 non-surviving patients (32%) of a subset of the LINC study had an autopsy and patients with mechanical chest compressions were over-represented in the sample.20 In the study of Pinto et al.,21 it was unclear from what population of cardiac arrest the autopsy patients were originating. In our study, autopsy was permitted in only 70 of the 221 patients (32%) who did not survive the resuscitation. Because we added post-mortem CT scanning to our protocol, we could assess damage outcome in 90% of all patients in the study. Post-mortem CT scanning has initially been used in forensic medicine but also has been investigated for assessment after resuscitation. In general, there is a reasonable agreement between postmortem CT and autopsy. In some studies, rib and sternum damage was found more frequent in CT scans, but some visceral damage identified more often in autopsy.12,24,25 More control patients survived resuscitation (50%) than AutoPulse (37%) or LUCAS patients (34%) and therefore more often clinical diagnostic information was available (see Supplementary material online, Table S1). Many patients in the ICU had CT scans driven by suspicion of damage from chest compressions. We believe therefore that diagnostic bias was not relevant for this reason.
In our study, randomization took place before inclusion criteria and exclusion criteria could be assessed. This may raise concern of bias in treatment allocation. There was no significant difference in allocation to treatment groups, except for the exclusion criterium ROSC on arrival of the resuscitation team (Table 1). This can be explained by the fact that preparing and applying a mechanical chest compression device took on average 3–5 min longer than applying a sternal displacement transducer in the control group, a significant difference. Therefore, there was 3–5 min more time for ROSC to occur before the mechanical compression device was in place, while for those patients allocated to manual control, chest compressions had started earlier, before ROSC may have occurred. There was a notably higher number of device failures of the Autopulse caused by battery failure, resulting in some inbalance in active treatment. This did not affect the interpretation of the outcome of interest (damage) in patients who received active treatment.
There is an association between longer duration of CPR and more bone and visceral damage.26 Approximately half of the patients had the onset of cardiac arrest outside the hospital and had manual chest compressions during transport for refractory cardiac arrest before the study device was applied after hospital arrival. The duration of chest compressions before inclusion in the study was evenly distributed between mechanical and manual chest compression in the OHCA patients, the rate of severe damage even tended to be higher in the IHCA patients. Therefore, it is unlikely that the prolonged period of chest compressions in the OHCA patients confounded the comparison of damage rates in patients receiving mechanical or manual control chest compressions, but a specific contribution to possible severe or life-threatening damage during transportation was not assessed in this study. In the Resuscitation Guidelines of 2015 ambulance transport to the hospital with mechanical chest compression is reasonable for emergency coronary angiography of patients who failed to have return of organized rhythm before transport.16,17 In that context, longer duration of mechanical chest compression can be expected than studied in our patients.
Conclusions
The use of mechanical chest compressions with the LUCAS device does not cause more severe or life-threatening visceral damage than good quality manual chest compressions. For mechanical chest compressions with the AutoPulse, it cannot be excluded that more severe or life-threatening damage is caused, compared with good quality manual chest compressions.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The data and safety committee consisted of Jan G. Tijssen, clinical epidemiologist (chair); Anje M. Spijkerboer, radiologist; Robert Tepaske, intensivist; and Reinoud E. Knops, cardiologist, all from the Academic Medical Center, Amsterdam, The Netherlands.
We thank Stan de Rooij of the coronary care unit (CCU) for assistance during training and support with randomization and data collection and Rein Visser of the Department of Pathology for pathology data collection. We thank all CCU nurses, cardiologists, radiologists, residents, radiological technicians and emergency department personnel who assisted during the conduct of this study.
Funding
This work was supported financially and by supplying devices, disposables, and of device training by Physio-Control/Jolife AB, Lund, Sweden, and Zoll Medical, Chelmsford, MA, USA. The Philips MRx defibrillator was loaned from Grafimedics, Zeewolde, The Netherlands.
Conflict of interest: R.W.K. is the recipient of the funding from Physio-Control/Jolife and from Zoll Medical to conduct this study. He is also recipient of funding from Physio-Control (Redmond, WA, USA), Zoll Medical, Cardiac Science (Waukesha, WI, USA), Defibtech (Guilford, CONN, USA), and Philips Nederland B.V. (Eindhoven, the Netherlands) for cardiac arrest research, unrelated to the reported study. He is an unpaid advisor for Physio-Control and HeartSine. S.G.B. is supported by Physio-Control to conduct studies unrelated to this study. The other authors have no conflicts to report.
References
- 1. Kleinman ME, Brennan EE, Goldberger ZD, Swor RA, Terry M, Bobrow BJ, Gazmuri RJ, Travers AH, Rea T.. Part 5: adult basic life support and cardiopulmonary resuscitation quality: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S414–S435. [DOI] [PubMed] [Google Scholar]
- 2. Perkins GD, Handley AJ, Koster RW, Castrén M, Smyth MA, Olasveengen T, Monsieurs KG, Raffay V, Gräsner J-T, Wenzel V, Ristagno G, Soar J, Bossaert LL, Caballero A, Cassan P, Granja C, Sandroni C, Zideman DA, Nolan JP, Maconochie I, Greif R.. European Resuscitation Council Guidelines for Resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation 2015;95:81–99. [DOI] [PubMed] [Google Scholar]
- 3. Berg RA, Hemphill R, Abella BS, Aufderheide TP, Cave DM, Hazinski MF, Lerner EB, Rea TD, Sayre MR, Swor RA.. Part 5: adult basic life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010;122:S685–S705. [DOI] [PubMed] [Google Scholar]
- 4. Wik L, Kramer-Johansen J, Myklebust H, Sørebø H, Svensson L, Fellows B, Steen PA.. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA 2005;293:299–304. [DOI] [PubMed] [Google Scholar]
- 5. Timerman S, Cardoso LF, Ramires JAF, Halperin H.. Improved hemodynamic performance with a novel chest compression device during treatment of in-hospital cardiac arrest. Resuscitation 2004;61:273–280. [DOI] [PubMed] [Google Scholar]
- 6. Axelsson C, Karlsson T, Axelsson ÅB, Herlitz J.. Mechanical active compression–decompression cardiopulmonary resuscitation (ACD-CPR) versus manual CPR according to pressure of end tidal carbon dioxide (PETCO2) during CPR in out-of-hospital cardiac arrest (OHCA). Resuscitation 2009;80:1099–1103. [DOI] [PubMed] [Google Scholar]
- 7. Hallstrom A, Rea TD, Sayre MR, Christenson J, Anton AR, Mosesso VN, Van Ottingham L, Olsufka M, Pennington S, White LJ, Yahn S, Husar J, Morris MF, Cobb LA.. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: a randomized trial. JAMA 2006;295:2620–2628. [DOI] [PubMed] [Google Scholar]
- 8. Wik L, Olsen J-A, Persse D, Sterz F, Lozano M Jr, Brouwer MA, Westfall M, Souders CM, Malzer R, van Grunsven PM, Travis DT, Whitehead A, Herken UR, Lerner EB.. Manual vs. integrated automatic load-distributing band CPR with equal survival after out of hospital cardiac arrest. The randomized CIRC trial. Resuscitation 2014;85:741–748. [DOI] [PubMed] [Google Scholar]
- 9. Rubertsson S, Lindgren E, Smekal D, Östlund O, Silfverstolpe J, Lichtveld RA, Boomars R, Ahlstedt B, Skoog G, Kastberg R, Halliwell D, Box M, Herlitz J, Karlsten R.. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest. JAMA 2014;311:353.. [DOI] [PubMed] [Google Scholar]
- 10. Perkins GD, Lall R, Quinn T, Deakin CD, Cooke MW, Horton J, Lamb SE, Slowther A.. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2014;385:947–955. [DOI] [PubMed] [Google Scholar]
- 11. Englund E, Kongstad PC.. Active compression–decompression CPR necessitates follow-up post mortem. Resuscitation 2006;68:161–162. [DOI] [PubMed] [Google Scholar]
- 12. Roberts IS, Benamore RE, Benbow EW, Lee SH, Harris JN, Jackson A, Mallett S, Patankar T, Peebles C, Roobottom C, Traill ZC.. Post-mortem imaging as an alternative to autopsy in the diagnosis of adult deaths: a validation study. Lancet 2012;379:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krischer JP, Fine EG, Davis JH, Nagel EL.. Complications of cardiac resuscitation. Chest 1987;92:287–291. [DOI] [PubMed] [Google Scholar]
- 14. Miettinen O, Nurminen M.. Comparative analysis of two rates. Stat Med 1985;4:213–226. [DOI] [PubMed] [Google Scholar]
- 15. Rubertsson S, Lindgren E, Smekal D, Östlund O, Silfverstolpe J, Lichtveld RA, Boomars R, Ahlstedt B, Skoog G, Kastberg R, Halliwell D, Box M, Herlitz J, Karlsten R.. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest. JAMA 2013;311:53–61. [DOI] [PubMed] [Google Scholar]
- 16. Brooks SC, Anderson ML, Bruder E, Daya MR, Gaffney A, Otto CW, Singer AJ, Thiagarajan RR, Travers AH.. Part 6: alternative techniques and ancillary devices for cardiopulmonary resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S436–S443. [DOI] [PubMed] [Google Scholar]
- 17. Truhlář A, Deakin CD, Soar J, Khalifa GEA, Alfonzo A, Bierens JJLM, Brattebø G, Brugger H, Dunning J, Hunyadi-Antičević S, Koster Rudolph W, Lockey DJ, Lott C, Paal P, Perkins GD, Sandroni C, Thies K-C, Zideman DA, Nolan JP, Barelli A, Böttiger BW, Georgiou M, Handley AJ, Lindner T, Midwinter MJ, Monsieurs KG, Wetsch WA.. European Resuscitation Council Guidelines for Resuscitation 2015 Section 4. Cardiac arrest in special circumstances. Resuscitation 2015;95:148–201. [DOI] [PubMed] [Google Scholar]
- 18. Ong MEH, Annathurai A, Shahidah A, Leong BSH, Ong VYK, Tiah L, Ang SH, Yong KL, Sultana P.. Cardiopulmonary resuscitation interruptions with use of a load-distributing band device during Emergency Department Cardiac Arrest. Ann Emerg Med 2010;56:233–241. [DOI] [PubMed] [Google Scholar]
- 19. Yost D, Phillips RH, Gonzales L, Lick CJ, Satterlee P, Levy M, Barger J, Dodson P, Poggi S, Wojcik K, Niskanen RA, Chapman FW.. Assessment of CPR interruptions from transthoracic impedance during use of the LUCAS mechanical chest compression system. Resuscitation 2012;83:961–965. [DOI] [PubMed] [Google Scholar]
- 20. Smekal D, Lindgren E, Sandler H, Johansson J, Rubertsson S.. CPR-related injuries after manual or mechanical chest compressions with the LUCAS device: a multicentre study of victims after unsuccessful resuscitation. Resuscitation 2014;85:1708–1712. [DOI] [PubMed] [Google Scholar]
- 21. Pinto DC, Haden-Pinneri K, Love JC.. Manual and automated cardiopulmonary resuscitation (CPR): a comparison of associated injury patterns. J Forensic Sci 2013;58:904–909. [DOI] [PubMed] [Google Scholar]
- 22. Lardi C, Egger C, Larribau R, Niquille M, Mangin P, Fracasso T.. Traumatic injuries after mechanical cardiopulmonary resuscitation (LUCAS™2): a forensic autopsy study. Int J Legal Med 2015;129:1035–1042. [DOI] [PubMed] [Google Scholar]
- 23. Koga Y, Fujita M, Yagi T, Nakahara T, Miyauchi T, Kaneda K, Kawamura Y, Oda Y, Tsuruta R.. Effects of mechanical chest compression device with a load-distributing band on post-resuscitation injuries identified by post-mortem computed tomography. Resuscitation 2015;96:226–231. [DOI] [PubMed] [Google Scholar]
- 24. Smekal D, Hansen T, Sandler H, Rubertsson S.. Comparison of computed tomography and autopsy in detection of injuries after unsuccessful cardiopulmonary resuscitation. Resuscitation 2013;84:357–360. [DOI] [PubMed] [Google Scholar]
- 25. Wichmann D, Obbelode F, Vogel H, Hoepker WW, Nierhaus A, Braune S, Sauter G, Pueschel K, Kluge S.. Virtual autopsy as an alternative to traditional medical autopsy in the intensive care unit: a prospective cohort study. Ann Intern Med 2012;156:123–130. [DOI] [PubMed] [Google Scholar]
- 26. Boland LL, Satterlee PA, Hokanson JS, Strauss CE, Yost D.. Chest compression injuries detected via routine post-arrest care in patients who survive to admission after out-of-hospital cardiac arrest. Prehosp Emerg Care 2015;19:23–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.