Abstract
Aims
Impact of changes of treatments on outcomes in ST-elevation myocardial infarction (STEMI) patients in real-life health care has not been documented.
Methods and results
All STEMI cases (n = 105.674) registered in the nation-wide SWEDEHEART registry between 1995 and 2014 were included and followed for fatal and non-fatal outcomes for up to 20 years. Most changes in treatment and outcomes occurred from 1994 to 2008. Evidence-based treatments increased: reperfusion from 66.2 to 81.7%; primary percutaneous coronary intervention: 4.5 to 78.0%; dual antiplatelet therapy from 0 to 89.6%; statin: 14.1 to 93.6%; beta-blocker: 78.2 to 91.0%, and angiotensin-converting-enzyme/angiotensin-2-receptor inhibitors: 40.8 to 85.2% (P-value for-trend <0.001 for all). One-year mortality decreased from 22.1 to 14.1%. Standardized incidence ratio compared with the general population decreased from 5.54 to 3.74 (P < 0.001). Cardiovascular (CV) death decreased from 20.1 to 11.1%, myocardial infarction (MI) from 11.5 to 5.8%; stroke from 2.9 to 2.1%; heart failure from 7.1 to 6.2%. After standardization for differences in demography and baseline characteristics, the change of 1-year CV-death or MI corresponded to a linear trend of 0.915 (95% confidence interval: 0.906–0.923) per 2-year period which no longer was significant, 0.997 (0.984–1.009), after adjustment for changes in treatment. The changes in treatment and outcomes were most pronounced from 1994 to 2008.
Conclusion
Gradual implementation of new and established evidence-based treatments in STEMI patients during the last 20 years has been associated with prolonged survival and lower risk of recurrent ischaemic events, although a plateauing is seen since around 2008.
Keywords: Registry, Myocardial infarction, Time-trends, Outcomes
Introduction
During the last 30 years, a series of successful clinical trials have proven the improved survival and lower morbidity with several new treatments of ST-elevation myocardial infarction (STEMI). This has led to very consistent global treatment recommendations.1–4 However, the adoption of new treatments in clinical practice is variable between and within countries.5–8 Although clinical trial data demonstrate efficacy and safety in a selected target population, the real impact of new treatment modalities can only be certified by monitoring treatments and outcomes in entire unselected populations of patients with STEMI. Nation-wide clinical registries including all patients admitted for myocardial infarction are therefore the final loop of evaluating the effectiveness of new treatments in the real-life health care. Data from several registries have shown improved survival and reduced non-fatal complications in STEMI over time.9–14 However, these studies have been limited, including only selected populations or time-periods and often only short-term follow-up. Few studies have accounted for the changes in background risk characteristics and in the improved survival of the general population. Moreover, most previous studies reporting time-trends have been limited to mortality outcome and have not included the long-term risk of re-infarctions, stroke, and heart failure. Finally, very few studies have had continuous data on baseline characteristics, treatments, and outcomes in all individual patients allowing the possibility of relating changes in outcomes with alterations of treatments.
The aim of this nation-wide registry study was to describe temporal changes in treatments and long-term outcomes during the last 20 years from 1995 to 2014 in consecutive patients with STEMI admitted to any coronary care unit in an entire country. In addition, the study aimed to investigate if and how much the changes in patient characteristics, interventional procedures and medical treatments contributed to the changes in short- and long-term outcomes.
Methods
Study population
All cases admitted to a coronary care unit or other specialized facility with a suspected or definite acute coronary syndrome are continuously included in the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry.15 Currently, all Swedish hospitals (n = 72) that provide care for acute cardiac diseases participate. Patients are informed about their participation in the registry and are allowed to opt-out. Written consent is not required according to Swedish law. A negligible number of patients choose not to participate. Monitors regularly evaluate by random checks, the completeness and correctness of data entered into the registry with the medical records with an agreement of 96% on average. In the most recent monitoring performed between 2015 and 2016, the data entry for 67 variables in the SWEDEHEART registry were compared with the medical records for 30 patients at each of the 72 participating hospital (a total of 142 032 variables), with an agreement of 96.8%. In the study database, the baseline information on previously diagnosed concomitant diseases was enriched with information from the National Patient Registry, which includes the diagnoses of all hospital admissions in Sweden since 1987. Data regarding medication on admission, in-hospital treatments, and discharge medications are collected as part of the SWEDEHEART registry. This study included all cases in the SWEDEHEART registry admitted to a hospital with a STEMI between 1 January 1995 and 10 October 2014. The National Board of Health and Welfare approved the merging of these registries. The study protocol was approved by the regional ethics committee in Stockholm (nr 2011/60-31/2).
Outcome definitions: re-infarction, stroke, heart failure, and mortality
In-hospital re-infarction was obtained from the SWEDEHEART registry. Re-infarction within 30-days was defined as a new myocardial infarction recorded in SWEDEHEART registry if occurring more than 2 days after discharge. Re-infarction after 30 days was defined as readmission of a new myocardial infarction in the National Patient registry (see Supplementary material online). The definition of myocardial infarction has changed over time. Between 1996 and 2001, myocardial infarction was based on the World Health Organization definition16 from 1994 requiring a doubling of biomarkers (creatinine-kinase_MB, CK-MB) compared with the upper level of normal, in addition to typical electrocardiogram (ECG) changes and symptoms. From 2001 onwards, the definition for myocardial infarction has been based on the European Society of Cardiology/American College of Cardiologists/American Heart Association consensus document,17,18 consisting of troponin T/I or two CK-MB level above the 99th percentile, in addition to typical ECG changes and symptoms.
Hospitalization for stroke or heart failure (main diagnosis) was obtained from the National Patient registry. For re-infarction, stroke, and heart failure, the latest available follow-up was on the 31 December 2013. In-hospital, 30-day, 1-year mortality was obtained from the Swedish Population register with last follow-up on 11 November 2014, with no loss to follow-up. Only patients with complete 1-year follow-up in 2013 were included in the 1-year mortality analyses. Cardiovascular death was obtained from the National Cause of Death registry (see Supplementary material online).
Statistical analysis
The 20-year study period was divided into 2-year blocks. In the analysis, each patient was allowed to have only one STEMI during each 2-year block to allow the estimation of complications such as re-infarction, stroke, and heart failure hospitalization. However, the same individual could have a new STEMI in a different 2-year block. Therefore, since a patient could be analysed several times, we considered STEMI ‘cases’, and not ‘patients’. Baseline characteristics for cases are displayed as medians (interquartile range) for continuous variables or proportions for categorical variables. Treatments and outcomes were displayed over time as a percentage of patients during each 2-year block. Significant differences over time were tested by Jonckheere–Terpstra trend test for categorical variables and by linear-by-linear trend test for continuous data. Time-to-event was graphically displayed as Kaplan–Meier curves.
To assess the effect of time on outcome, assuming a linear association for each 2-year time-block and outcome, a logistic regression model (for in-hospital) and a Cox-regression model (for 1-year event) was fitted. The outcome explored by this analysis was the composite of cardiovascular death or re-infarction. These models explored the association for moving forward a 2-year-block at a time, adjusting stepwise for confounders as follows: (i) crude; (ii) age (three knots, restricted cubic spline) and gender; (iii) baseline characteristics [diabetes, hypertension, previous myocardial infarction, previous peripheral vascular disease, previous stroke, previous congestive heart failure, previous chronic obstructive pulmonary disease, cancer within 3 years, presence of pulmonary rales on admission, atrial fibrillation on admission, medication on admission (antiplatelet therapy, beta-blocker, angiotensin-converting-enzyme inhibitor/angiotensin-receptor-blocker (ACE/A2), statin)]; and (iv) in-hospital intervention for STEMI [reperfusion therapy and primary percutaneous coronary intervention (PCI)]. For hospital survivors, the models also adjusted for changes in the following discharge medications: antiplatelet therapy, beta-blocker, ACE/A2 inhibition and statin. In addition, in order to account for differences in patient characteristics and treatments throughout the observation period, standardization was performed using logistic regression models. A full explanation of these models and interpretation is given in the Supplementary material online.
In order to account for mortality changes in the underlying background population, standardized incidence ratios were also calculated, using an age, sex and calendar matched general population from Statistics Sweden (www.scb.se).
Missing data were handled using multiple imputations with the method of chained equations.19 Five imputed data sets were generated. As predictors, all variables in the model and events were used. All analyses were performed with R (version 3.1.0).
Results
Clinical characteristics
From 1995 to 2014, there were 105 674 STEMI cases fulfilling the inclusion criteria and with no exclusion criterion (see Supplementary material online). Several of the baseline characteristics showed only minor changes over time. The mean age was 70 years [interquartile range (IQR) 60–79], 34% were female, 20% had diabetes, and about 18% had a prior MI (Table 1). However, more patients had a history of PCI (12% vs. 2%) or coronary artery bypass grafting (4 to 2%), and over time more patients were treated with antihypertensive medication (54% vs. 32%), ACE/A2 inhibition (31% vs. 10%) and statins (21% vs. 4%) on admission. Fewer patients presented with signs of heart failure on admission in the later years (7% vs. 36%) and fewer were using diuretics (16% vs. 22%) and digitalis (1% vs. 7%).
Table 1.
Baseline characteristics over time
| Variable | 1995–96 | 1997–98 | 1999–2000 | 2001–02 | 2003–04 | 2005–06 | 2007–08 | 2009–10 | 2011–12 | 2013–14 | P-valuea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 5567 | 9450 | 11 473 | 11 657 | 11 483 | 11 259 | 11 628 | 11 663 | 11 745 | 9749 | |
| Demographics | |||||||||||
| Age (years) | 71 (61–78) | 71 (60–78) | 71 (60–79) | 71 (60–79) | 70 (59–79) | 70 (60–79) | 69 (60–79) | 69 (60–79) | 69 (60–79) | 69 (60–79) | 0.004 |
| Female | 1899 (34.1%) | 3226 (34.1%) | 4114 (35.9%) | 4189 (35.9%) | 3980 (34.7%) | 3847 (34.2%) | 3871 (33.3%) | 3720 (31.9%) | 3711 (31.6%) | 3070 (31.5%) | <0.001 |
| Risk factors | |||||||||||
| Diabetes mellitus | 1068 (19.2%) | 1821 (19.3%) | 2227 (19.4%) | 2259 (19.4%) | 2223 (19.4%) | 2228 (19.8%) | 2293 (19.7%) | 2300 (19.7%) | 2458 (20.9%) | 1995 (20.5%) | <0.001 |
| Hypertension | 1760 (31.6%) | 3036 (32.1%) | 3980 (34.7%) | 4300 (36.9%) | 4532 (39.5%) | 4990 (44.3%) | 5520 (47.5%) | 6004 (51.5%) | 6289 (53.5%) | 5258 (53.9%) | <0.001 |
| Current smoker | 1305 (25.2%) | 2324 (26.3%) | 2904 (27.3%) | 2962 (27.5%) | 2990 (28.2%) | 2996 (29.4%) | 3181 (29.5%) | 3160 (29.5%) | 3123 (28.8%) | 2448 (26.8%) | <0.001 |
| BMI (kg/m2) | 25.8 (23.6–28.4) | 25.8 (23.8–28.4) | 26.1 (23.8–29.0) | 26.0 (23.7–28.7) | 25.9 (23.8–28.4)] | 25.9 (23.7–28.5) | 26.0 (23.8–28.7) | 26.2 (23.9–29.0) | 26.2 (23.9–29.1)] | 26.3 (23.9–29.2) | <0.001 |
| Cardiovascular disease | |||||||||||
| Previous MI | 1120 (20.1%) | 1898 (20.1%) | 2099 (18.3%) | 2212 (19.0%) | 2134 (18.6%) | 1933 (17.2%) | 1934 (16.6%) | 2061 (17.7%) | 2005 (17.1%) | 1652 (16.9%) | <0.001 |
| Previous PCI | 89 (1.6%) | 247 (2.6%) | 361 (3.1%) | 450 (3.9%) | 592 (5.2%) | 761 (6.8%) | 953 (8.2%) | 1210 (10.4%) | 1264 (10.8%) | 1169 (12.0%) | <0.001 |
| Previous CABG | 133 (2.4%) | 256 (2.7%) | 362 (3.2%) | 447 (3.8%) | 441 (3.8%) | 434 (3.9%) | 468 (4.0%) | 498 (4.3%) | 504 (4.3%) | 409 (4.2%) | <0.001 |
| Previous CHF | 1139 (20.5%) | 1749 (18.5%) | 2130 (18.6%) | 2201 (18.9%) | 2016 (17.6%) | 1881 (16.7%) | 1772 (15.2%) | 1899 (16.3%) | 1970 (16.8%) | 1456 (14.9%) | <0.001 |
| Previous PVD | 192 (3.4%) | 383 (4.1%) | 418 (3.6%) | 465 (4.0%) | 465 (4.0%) | 431 (3.8%) | 399 (3.4%) | 430 (3.7%) | 472 (4.0%) | 402 (4.1%) | 0.45 |
| Previous ischaemic stroke | 421 (7.6%) | 730 (7.7%) | 941 (8.2%) | 1019 (8.7%) | 946 (8.2%) | 939 (8.3%) | 889 (7.6%) | 913 (7.8%) | 929 (7.9%) | 680 (7.0%) | 0.016 |
| Any previous stroke | 460 (8.3%) | 802 (8.5%) | 1020 (8.9%) | 1112 (9.5%) | 1045 (9.1%) | 1266 (11.2%) | 1221 (10.5%) | 1203 (10.3%) | 1204 (10.3%) | 905 (9.3%) | <0.001 |
| Atrial fibrillation on admission | 440 (8.6%) | 795 (8.4%) | 920 (8.1%) | 984 (8.5%) | 902 (7.9%) | 868 (7.7%) | 907 (7.8%) | 859 (7.4%) | 890 (7.6%) | 728 (7.6%) | <0.001 |
| Other disease | |||||||||||
| Previous COPD | 161 (2.9%) | 294 (3.1%) | 406 (3.5%) | 460 (3.9%) | 493 (4.3%) | 540 (4.8%) | 583 (5.0%) | 631 (5.4%) | 658 (5.6%) | 482 (4.9%) | <0.001 |
| Cancer within 3 years | 75 (1.3%) | 108 (1.1%) | 135 (1.2%) | 176 (1.5%) | 167 (1.5%) | 211 (1.9%) | 249 (2.1%) | 280 (2.4%) | 312 (2.7%) | 244 (2.5%) | <0.001 |
| Medication on admission | |||||||||||
| Aspirin | 1371 (24.9%) | 2576 (27.6%) | 3391 (29.8%) | 3657 (31.6%) | 3496 (30.7%) | 3265 (29.3%) | 3180 (27.7%) | 3220 (28.0%) | 3024 (26.1%) | 2403 (25.1%) | <0.001 |
| Dual antiplatelet | 0 (0.0%) | 4 (0.0%) | 30 (0.3%) | 86 (0.7%) | 240 (2.1%) | 316 (2.8%) | 337 (2.9%) | 337 (2.9%) | 304 (2.6%) | 216 (2.3%) | <0.001 |
| Warfarin | 174 (3.2%) | 232 (2.5%) | 314 (2.8%) | 357 (3.1%) | 329 (2.9%) | 310 (2.8%) | 364 (3.2%) | 370 (3.2%) | 378 (3.3%) | 353 (3.7%) | <0.001 |
| Betablocker | 1419 (25.8%) | 2396 (25.7%) | 3224 (28.3%) | 3494 (30.3%) | 3498 (30.8%) | 3365 (30.3%) | 3404 (29.7%) | 3318 (29.0%) | 3267 (28.4%) | 2724 (28.6%) | <0.001 |
| Calcium antagonist | 861 (15.6%) | 1290 (13.8%) | 1572 (13.8%) | 1584 (13.8%) | 1474 (13.0%) | 1442 (13.0%) | 1537 (13.4%) | 1785 (15.6%) | 1865 (16.2%) | 1651 (17.3%) | <0.001 |
| Digoxin | 382 (6.9%) | 514 (5.5%) | 567 (5.0%) | 513 (4.4%) | 370 (3.3%) | 320 (2.9%) | 240 (2.1%) | 188 (1.6%) | 151 (1.3%) | 94 (1.0%) | <0.001 |
| ACEi/ARB | 533 (9.7%) | 1032 (11.1%) | 1396 (12.3%) | 1710 (14.8%) | 2017 (17.7%) | 2244 (20.2%) | 2741 (23.9%) | 3092 (26.9%) | 3272 (28.4%) | 2952 (31.0%) | <0.001 |
| Diuretic | 1240 (22.5%) | 1954 (21.0%) | 2341 (20.6%) | 2408 (20.8%) | 2317 (20.4%) | 2224 (20.0%) | 2213 (19.3%) | 2114 (18.4%) | 1945 (16.9%) | 1500 (15.7%) | <0.001 |
| Statin | 203 (3.7%) | 528 (5.7%) | 956 (8.4%) | 1373 (11.9%) | 1674 (14.7%) | 1884 (16.9%) | 2209 (19.2%) | 2522 (21.9%) | 2509 (21.7%) | 2034 (21.2%) | <0.001 |
| Characteristics on presentation | |||||||||||
| Any pulmonary ralesb | 1951 (36.1%) | 2824 (30.8%) | 3079 (27.9%) | 2663 (24.4%) | 2045 (19.2%) | 1201 (11.6%) | 1069 (10.1%) | 1014 (9.2%) | 888 (7.8%) | 688 (7.3%) | <0.001 |
| Cardiogenic shock | Not available | Not available | Not available | Not available | Not available | 237 (2.2%) | 237 (2.2%) | 319 (2.8%) | 293 (2.5%) | 259 (2.7%) | 0.009 |
| Chest pain | 4796 (93.0%) | 8065 (93.5%) | 9663 (93.6%) | 9541 (92.9%) | 9545 (92.0%) | 10153 (90.8%) | 10468 (90.4%) | 10441 (90.2%) | 10280 (88.7%) | 8561 (88.7%) | <0.001 |
| In-hospital LVEF | |||||||||||
| ≥50% | Not available | 19 (28.4%) | 848 (30.6%) | 1638 (37.2%) | 2473 (40.6%) | 3238 (43.9%) | 3870 (45.1%) | 4208 (46.3%) | 4587 (47.5%) | 3900 (47.7%) | <0.001 |
| 40–49% | Not available | 23 (34.3%) | 1056 (38.1%) | 1522 (34.5%) | 1921 (31.6%) | 2003 (27.2%) | 2388 (27.9%) | 2521 (27.8%) | 2546 (26.4%) | 2142 (26.2%) | |
| 30–39% | Not available | 18 (26.9%) | 619 (22.3%) | 859 (19.5%) | 1163 (19.1%) | 1442 (19.6%) | 1652 (19.3%) | 1650 (18.2%) | 1763 (18.3%) | 1463 (17.9%) | |
| <30% | Not available | 7 (10.4%) | 252 (9.1%) | 388 (8.8%) | 529 (8.7%) | 688 (9.3%) | 66 (7.7%)2 | 704 (7.8%) | 762 (7.9%) | 673 (8.2%) | |
| Reperfusion strategyc | |||||||||||
| Thrombolysis for STEMI | 3426 (61.7%) | 5798 (61.5%) | 6774 (59.2%) | 6118 (53.0%) | 4153 (36.5%) | 1708 (15.2%) | 797 (6.9%) | 670 (5.7%) | 479 (4.1%) | 360 (3.7%) | <0.001 |
| Time onset of symptom to thrombolysis; median (IQR) | 200 (125–340) | 190 (120–325) | 180 (115–310) | 180 (110–310) | 180 (110–312) | (180 (110–347) | 162 (100–299) | 168 (101–293) | 150 (93–290) | 160 (100–300) | <0.001 |
| Primary PCI for STEMI | 251 (4.5%) | 541 (5.7%) | 932 (8.1%) | 1661 (14.4%) | 3446 (30.3%) | 6435 (57.2%) | 8010 (68.9%) | 8511 (73.0%) | 8900 (75.8%) | 7601 (78.0%) | <0.001 |
| Time from symptom to primary PCI; median (IQR) | Not applicable | Not applicable | Not applicable | Not applicable | 211 (140–376) | 207 (135–384) | 200 (128–370) | 193 (125–350) | 190 (121–340) | 190 (125–355) | <0.001 |
| In-hospital medication | |||||||||||
| Glycoprotein 2b-3a-inhibitor use | 61 (5.8%) | 179 (9.0%) | 323 (9.3%) | 788 (16.6%) | 3834 (59.3%) | 5685 (70.2%) | 5670 (63.3%) | 3808 (40.5%) | 1563 (15.9%) | 1126 (13.3%) | <0.001 |
Numbers presented are n (%), or median (IQR) as appropriate.
STEMI, ST-elevation myocardial infarction; PCI, Percutaneous coronary intervention; ACEi/ARB, Angiotensin-converting-enzyme inhibtor/angiotensin receptor blocker; IQR, interquartile range; COPD, chronic obstructive pulmonary disease; BMI, body mass index; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; CHF, congestive heart failure; MI, myocardial infarction.
P-values were tested with Jonckheere–Terpstra trend test for categorical variables and with linear-by-linear trend test for continuous data.
Pulmonary rales were assessed on admission, but logistics and timing of clinical examination among patients undergoing primary PCI may differ.
Further data is available in Supplementary material online, Table S2.
In-hospital course and treatment
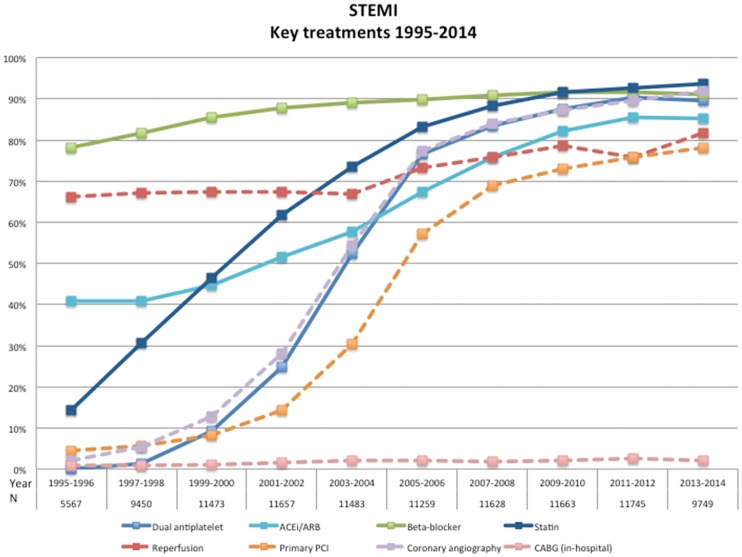
During these 20 years, there was a dramatic increase in the uptake and utilization of new and older evidence-based treatments. Reperfusion treatment increased from 1995/96 to 2013/2014 (Figure 1, Supplementary material online) from 66 to 82% and primary PCI from 5 to 78%. The time delays from symptom onset to reperfusion (Table1) have only shown slight improvements over the years. Over the 20 years medical treatment with aspirin increased from 82 to 94%, dual antiplatelet from 0 to 90%; beta-blocker from 78 to 91%; ACE-inhibor/ARB from 41 to 85%, and statins from 14 to 94%; (P-value for-trend <0.001 for all). Most of the changes in the use these treatments occurred between 1995 and 2006. Thereafter their utilization remained at the same high level, although changes within each treatment modality (choice of technology or pharmaceutical agent) could have occurred.
Figure 1.
Key treatments 1995–2014. STEMI, ST-elevation myocardial infarction.
Simultaneously there was a decreased use of in-hospital intravenous diuretics and inotropic drugs, from 44 to 20% and from 10 to 6%, respectively (see Supplementary material online). A reduced ejection fraction (EF < 50%) was present in 72% in 1997–98 and less common, 52%, in 2013–14. Correspondingly, there was a reduced prescription at discharge of digoxin from 9.6 to 1.3% and of diuretics from 39.4 to 21.5%.
Outcome
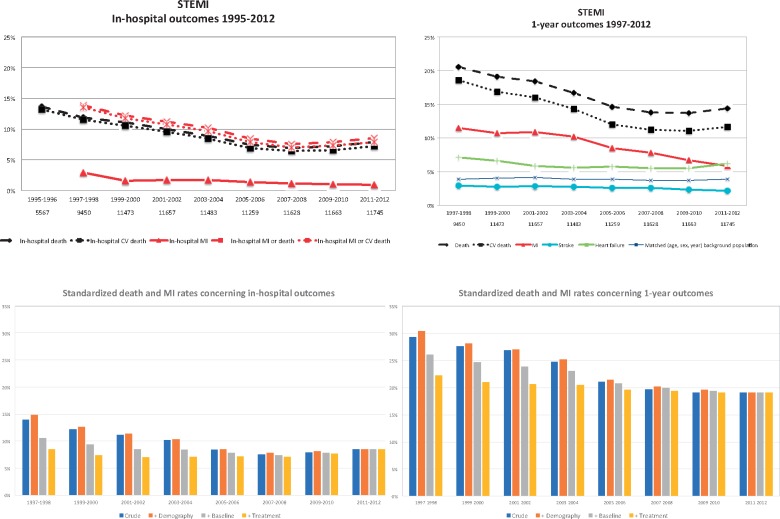
Over 20 years, the in-hospital, 30 day, and 1-year all-cause mortality decreased from 13.6, 15.8, and 22.1% in 1995/96 to 7.8, 9.2, and 14.1% in 2013/14 (Central IllustrationA, Supplementary material online and Figure S2). This was mainly driven by a reduction in CV-death where the corresponding mortalities decreased from 13.2, 15.2, and 20.1% to 7.2, 8.5, and 11.6%. When 1-year mortality was compared with an age-, gender-, and calendar-adjusted background population the standardized incidence ratio decreased from 5.54 [95% confidence interval (CI) 5.35–5.73] times higher in 1995/96 to 3.74 (3.47–4.03) times higher in 2013/14 (<0.001) (Central IllustrationA and Supplementary material online). After standardization and adjustments for changes in demography and baseline characteristics, there was still a reduction in 1-year mortality. If patients would have had the same demography and baseline characteristics as in 2013–14, the 1-year mortality would have decreased from 16.4% in 1995/96 to 14.1% in 2013/14 (see Supplementary material online).
Central illustration.
In-hospital and 1-year outcomes. (A) Absolute rates for in-hospital (1995–2012) and 1-year outcomes (1995–2012) in each 2-year block. (B) Standardized death and MI rates for in-hospital and 1-year outcomes. MI, myocardial infarction; STEMI, ST-elevation myocardial infarction.
The outcome improved also for non-fatal events. Between 1997/98 and 2011/12, the 1-year risk of re-infarction decreased from 11.5 to 5.8%, stroke from 2.9 to 2.1%, and heart failure from 7.1 to 6.2% (Central IllustrationA, Supplementary material online). The standardized 1-year risk (adjusting for changes in demography and baseline characteristics) for MI and the composite of death or MI decreased from 9.6 and 23.5% to 5.8 and 19.1% (see Supplementary material online).
Based on the continued registration of new events in all patients it could be established that the benefits in outcome by later years of entry was sustained and even further amplified during long-term follow-up for up to 20 years (Figure 2). The improvement in outcome was leveling out from 2007 to 2008 onward.
Figure 2.
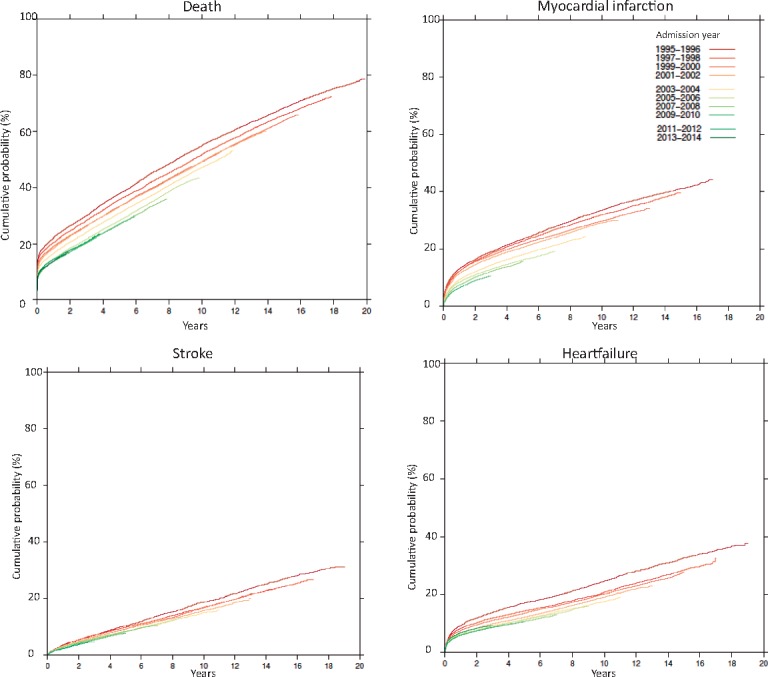
Kaplan–Meier curves for long-term outcomes in patients included from 1995–96 (dark red) to 2013–14 (dark green).
Association between time-period and outcome and the effect of treatment changes
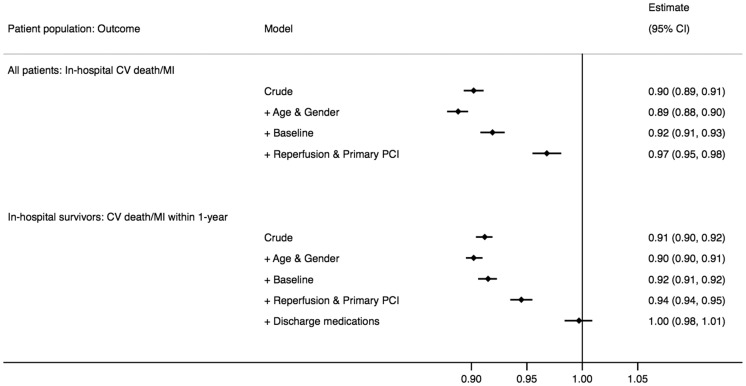
To examine the relative importance of changes in demography, baseline characteristics, and treatments for changes in outcomes, we analysed the association between change in time-period and the risk of cardiovascular death or MI using stepwise adjustments. Moving forward one time-period was significantly associated with improved in-hospital outcome in the crude analysis [odds ratio (OR) (95% CI): 0.90 (0.89–0.91)] and after adjustment for changes in baseline characteristics [0.92 (0.91–0.93)] but not after adjusting for changes in reperfusion and primary PCI treatment [0.97 (0.96–0.98)] (Figure 3). In hospital survivors, moving forward one time-period was significantly associated with the 1-year risk of cardiovascular death or MI in the crude analysis [0.91 (0.90–0.92)] and after adjustment for changes in demography and baseline characteristics [0.92 (0.91–0.92)]. The association was attenuated after adjusting for changes in reperfusion and primary PCI treatment [0.95 (0.94–0.96)] and was no longer significant after adjusting also for changes in discharge medications [1.00 (0.98–1.01)].
Figure 3.
Odds and hazard ratios for the association between 2 years change in time-period and outcome (cardiovascular death or MI)—unadjusted and after stepwise adjustment for differences in demography, baseline characteristics, and treatments over time. CV, cardiovascular; PCI, percutaneous coronary intervention; MI, myocardial infarction.
The results were consistent also when evaluating the changes over time as standardized event rates (Central IllustrationB. These data also illustrated that parallel to the leveling out of both changes in treatments outcomes (Figures 1 and Central IllustrationA), there were no further effects of changes in treatments on outcomes after the 2007–08 period.
Discussion
The present investigation was a unique evaluation of changes in baseline characteristics, treatments, and short- and long-term outcomes in almost all patients with STEMI in a whole country over the last 20 years. There were dramatic increases in treatment with reperfusion, primary PCI, aspirin, P2Y12 inhibition, beta-blockade, ACE/A2 inhibition, and statins. Simultaneously there was a continuous reduction in total and cardiovascular mortality over the years even when considering the lowering of mortality in the general population and when standardizing for changes in demography and patient characteristics. Compared to age- and gender-matched controls, a STEMI resulted in a 5.5 times higher 1-year mortality in 1995–96 and a 3.7 times higher 1-year mortality in 2013–14. Although the improved outcome could partly be explained by changes in baseline characteristics, there was still a substantial reduction in short- and long-term outcome, also when adjusting for those differences.
In addition, the study showed a similar continuous decrease in re-infarction, stroke, and hospitalization for heart failure over the years. These benefits were shown to begin in the hospital phase and continue over the first year. During these two decades, the 1-year risk of new MI was reduced by 50%, whereas the risk of readmission because stroke and heart failure were reduced by 30% and 20%, respectively. During long-term follow-up for up to 20 years, there was even a trend to further amplification of these effects.
The study showed that most benefits in short- and long-term outcomes in patients with STEMI were related to the uptake and increased use of new and, by time, established interventional and medical treatments.20 Thus, the data indicated that the improved in-hospital survival mainly was related to the increased use of reperfusion treatment including primary PCI. Concerning the 1-year outcomes, the results indicated that not only reperfusion and revascularization but also the broad uptake and prescription of aspirin, P2Y12-inhibition, beta-blockade, ACE/A2 inhibition, and statins contributed to the lower rates of events.
The changes in treatments were most dramatic from 1994 until around 2008 and then levelling out at a high level with use in 70–90% of all patients. Also, the lowering in event rates seemed to plateau at a new low level from around 2008 and onwards. These findings indicate that a further lowering of event rates might not be possible by further expansion of the current treatment modalities, which may already have been fully exploited. A more consistent and systematic application of evidence-based standard treatments to untreated subgroups, further improvement in the time to reperfusion treatment and modifications of established treatments (e.g. by new catheter based technologies and/or alternative medications within the same treatment class) is important, but will probably only have limited benefits on overall outcome. Thus, the plateauing of treatments and event rates during the last 5–10 years indicate a need to identify new effective treatment concepts for patients with STEMI who otherwise might remain at the current level of a 1-year mortality around 3.7 times higher than the general population and with around a 19% risk of death or MI within the next year.
The two phases with a rapid increase of several evidence based treatments from 1999–2000 to 2007–08 and the subsequent plateau are also in keeping with trends seen in other countries, including the USA, UK, New Zealand, France, and Germany, although the exact timing may differ between regions.10,21–25 The decline in the risk of recurrent MI has also been seen in other studies.24 The steady decrease in short-term mortality in STEMI patients until 2007–08 has been shown in other studies, but not all.10,25,26 Also, the lack of further reduction in mortality in recent years has been observed.23,27,28 Only one previous study with both STEMI and non-STEMI patients has shown a similar reduction in the 1-year risk of MI as in the present study.29
Our study has several strengths. It includes consecutive patients admitted to all hospitals taking care of acute cardiac patients in a nation-wide registry over a 20-year period which results in a high degree of generalizability and external validity. The inclusion of all STEMI cases in this registry over nearly two decades also resulted in a higher prevalence of previous MI and previous stroke. Our study also includes a complete long-term follow-up regarding not only mortality but also regarding the risk of new MI, stroke, and re-admission because of heart failure as the primary diagnosis, which makes it unique. Data have been prospectively collected and most variables have been mandatory for the last years, resulting in a high degree of completeness of data. The positive predictive value of a diagnosis of MI, stroke, and heart failure in the patient registry has been generally high in different validation studies.30
As other registry studies, the study has limitations. Although the validity is high, with an average 95–97% agreement between the register and the patient health records at monitoring, the quality of the individual data items are less good than in clinical trials or short-term well-conducted observational studies. As an observational study, causality between the changes in treatment strategies and improved outcomes cannot be fully proven by the adjustments in the statistical modelling as residual confounding can never be excluded. For the current study over 20 years, the analyses are limited to the variables that have been consistently and uniformly recorded since the start of registration. As the definition of cardiogenic shock was inconsistent the first 10 years and the routine use of echocardiograms and coronary angiography gradually increased over the years, these variables and variables related to these examinations (use of P2Y12-inhibitor or type of stent used) could not be included in the adjustments. Some angiography specific findings [e.g. degree of Thrombolysis in Myocardial Infarction (TIMI) flow] were not recorded. It should also be acknowledged that recording of baseline variables at hospital admissions might be different in patients undergoing primary PCI (usually bypassing the emergency department to reduce time to treatment), which might have affected the proportion of patients diagnosed with cardiogenic shock or pulmonary rales over the years.
In conclusion, the gradual implementation of new and established evidence-based treatments in patients with STEMI during the last 20 years has been associated with prolonged survival and lower risk of recurrent ischaemic events. However, the plateauing of changes in treatments and outcomes since around 2008 indicates need both for further optimizing current evidence-based treatments and the identification of new treatment concepts in order to further improve outcomes.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We are grateful to Professor Lars Wallentin and late Associate Professor Ulf Stenestrand who together initiated the national myocardial infarction registry RIKS-HIA, which later became the SWEDEHEART registry. We are also grateful to all participating patients and all SWEDEHEART co-workers who make the SWEDEHEART registry possible.
Funding
Swedish Foundation for Strategic Research; and Stockholm County Council (clinical research appointment) [to K.S.].
Conflict of interest: none declared.
References
- 1. Task Force on the management of STseamiotESoC, Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, DiMario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 2. Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S.. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 3. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ American College of C, American Heart Association Task Force on Practice G, Society for Cardiovascular A, Interventions, Society of Thoracic S, American Association for Clinical C . 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 4. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW American College of Cardiology Foundation/American Heart Association Task Force on Practice G . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 5. Chung S-C, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, Heuschmann P, Wallentin L, Deanfield J, Timmis A, Jernberg T, Hemingway H.. Acute myocardial infarction: a comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet 2014;383:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chung SC, Sundstrom J, Gale CP, James S, Deanfield J, Wallentin L, Timmis A, Jernberg T, Hemingway H Swedish Web-System for E, Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies/Register of I, Knowledge about Swedish Heart Intensive care A, National Institute for Cardiovascular Outcomes Research/Myocardial Ischaemia National Audit P, Studies CADRULB, Electronic Health R . Comparison of hospital variation in acute myocardial infarction care and outcome between Sweden and United Kingdom: population based cohort study using nationwide clinical registries. Br Med J 2015;351:h3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McNamara RL, Chung SC, Jernberg T, Holmes D, Roe M, Timmis A, James S, Deanfield J, Fonarow GC, Peterson ED, Jeppsson A, Hemingway H.. International comparisons of the management of patients with non-ST segment elevation acute myocardial infarction in the United Kingdom, Sweden, and the United States: the MINAP/NICOR, SWEDEHEART/RIKS-HIA, and ACTION Registry-GWTG/NCDR registries. Int J Cardiol 2014;175:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jernberg T, Johanson P, Held C, Svennblad B, Lindback J, Wallentin L Swedeheart/Riks HIA . Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011;305:1677–1684. [DOI] [PubMed] [Google Scholar]
- 9. Fox KAA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Granger CB, Flather MD, Budaj A, Quill A, Gore JM, Grace Investigators FT.. Decline in rates of death and heart failure in acute coronary syndromes, 1999-2006. JAMA 2007;297:1892–1900. [DOI] [PubMed] [Google Scholar]
- 10. Puymirat E, Simon T, Steg PG, Schiele F, Guéret P, Blanchard D, Khalife K, Goldstein P, Cattan S, Vaur L, Cambou J-P, Ferrières J, Danchin N.. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA 2012;308:998–1006. [DOI] [PubMed] [Google Scholar]
- 11. Gierlotka M, Gąsior M, Wilczek K, Wasilewski J, Hawranek M, Tajstra M, Osadnik T, Banasiak W, Poloński L.. Temporal trends in the treatment and outcomes of patients with non-ST-segment elevation myocardial infarction in Poland from 2004-2010 (from the Polish Registry of Acute Coronary Syndromes). Am J Cardiol 2012;109:779–786. [DOI] [PubMed] [Google Scholar]
- 12. Gale CP, Allan V, Cattle BA, Hall AS, West RM, Timmis A, Gray HH, Deanfield J, Fox KAA, Feltbower R.. Trends in hospital treatments, including revascularisation, following acute myocardial infarction, 2003-2010: a multilevel and relative survival analysis for the National Institute for Cardiovascular Outcomes Research (NICOR). Heart 2014;100:582–589. [DOI] [PubMed] [Google Scholar]
- 13. Yin W-H, Lu T-H, Chen K-C, Cheng C-F, Lee J-C, Liang F-W, Huang Y-T, Yang L-T.. The temporal trends of incidence, treatment, and in-hospital mortality of acute myocardial infarction over 15years in a Taiwanese population. Int J Cardiol 2016;209:103–113. [DOI] [PubMed] [Google Scholar]
- 14. Radovanovic D, Nallamothu BK, Seifert B, Bertel O, Eberli F, Urban P, Pedrazzini G, Rickli H, Stauffer J-C, Windecker S, Erne P.. Temporal trends in treatment of ST-elevation myocardial infarction among men and women in Switzerland between 1997 and 2011. Eur Heart J Acute Cardiovasc Care 2012;1:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L.. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 16. Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A.. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994;90:583–612. [DOI] [PubMed] [Google Scholar]
- 17. Thygesen K, Alpert JS, White HD.. Joint ESCAAHAWHFTFftRoMI. Universal definition of myocardial infarction. Eur Heart J 2007;28:2525–2538. [DOI] [PubMed] [Google Scholar]
- 18. Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502–1513. [DOI] [PubMed] [Google Scholar]
- 19. White IR, Royston P, Wood AM.. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 20. Authors/Task Force members, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 21. Hira RS, Bhatt DL, Fonarow GC, Heidenreich PA, Ju C, Virani SS, Bozkurt B, Petersen LA, Hernandez AF, Schwamm LH, Eapen ZJ, Albert MA, Liang L, Matsouaka RA, Peterson ED, Jneid H.. Temporal trends in care and outcomes of patients receiving fibrinolytic therapy compared to primary percutaneous coronary intervention: insights from the get with the guidelines coronary artery disease (GWTG-CAD) registry. J Am Heart Assoc 2016;5:e004113.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eagle KA, Nallamothu BK, Mehta RH, Granger CB, Steg PG, Van de Werf F, Lopez-Sendon J, Goodman SG, Quill A, Fox KAA.. Trends in acute reperfusion therapy for ST-segment elevation myocardial infarction from 1999 to 2006: we are getting better but we have got a long way to go. Eur Heart J 2008;29:609–617. [DOI] [PubMed] [Google Scholar]
- 23. Freisinger E, Fuerstenberg T, Malyar NM, Wellmann J, Keil U, Breithardt G, Reinecke H.. German nationwide data on current trends and management of acute myocardial infarction: discrepancies between trials and real-life. Eur Heart J 2014;35:979–988. [DOI] [PubMed] [Google Scholar]
- 24. Grey C, Jackson R, Wells S, Wu B, Poppe K, White H, Chan WC, Kerr AJ. First and recurrent ischaemic heart disease events continue to decline in New Zealand, 2005–2015. Heart 2017; doi: 10.1136/heartjnl-2017-311613. [DOI] [PubMed] [Google Scholar]
- 25. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M.. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016;37:3232–3245. [DOI] [PubMed] [Google Scholar]
- 26. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS.. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–2165. [DOI] [PubMed] [Google Scholar]
- 27. Menees DS, Peterson ED, Wang Y, Curtis JP, Messenger JC, Rumsfeld JS, Gurm HS.. Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med 2013;369:901–909. [DOI] [PubMed] [Google Scholar]
- 28. Nallamothu BK, Normand S-LT, Wang Y, Hofer TP, Brush JE, Messenger JC, Bradley EH, Rumsfeld JS, Krumholz HM.. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: a retrospective study. Lancet 2015;385:1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaudhry SI, Khan RF, Chen J, Dharmarajan K, Dodson JA, Masoudi FA, Wang Y, Krumholz HM.. National trends in recurrent AMI hospitalizations 1 year after acute myocardial infarction in medicare beneficiaries: 1999–2010. J Am Heart Assoc 2014;3:e001197.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J-L, Reuterwall C, Heurgren M, Olausson PO.. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.