Why was the cohort set up?
Worldwide, nutrition-related diseases have become a major health concern. The most apparent consequence of unhealthy diets and lack of physical activity is excess body weight and resulting cardiovascular and metabolic sequelae.1 Many of these unfavourable health outcomes have developmental origins and track into adulthood2 with unacceptable human, social and economic costs.1 Social inequalities create unequal pressures and opportunities, relating to a range of environmental, social and economic factors,3 some of which impinge on diet, addictive behaviours, physical activity, sedentariness, media exposure and parenting.4 Factors conducive to ill health cluster in certain segments of the society, e.g. those from lower socioeconomic position, those with poor mental health or poor cognitive abilities, or who are immigrants.5 These inequalities call for efforts of European policy to increase social cohesion and quality of life and to encourage sustainable healthy lifestyles for all citizens, especially children.6
There is an apparent lack of longitudinal studies that allow the investigation of biological markers and lifestyle behaviours combined with social, cultural and environmental factors and related to health and development across the early life course. There are some national birth and/ or child cohorts like ALSPAC7 in the UK, MoBa8 in Norway, the Aarhus Birth Cohort9 in Denmark, the Generation R Study10 in The Netherlands or KIGGS11 in Germany that may serve this aim. But to our knowledge there is no pan-European population-based cohort of children representing diverse European lifestyles and considering multiple exposures and outcomes.
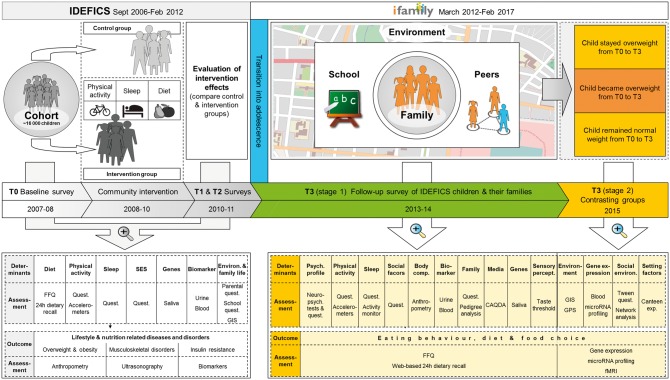
This gap is filled by the IDEFICS cohort. The first two examination waves of this cohort are from the IDEFICS (Identiflcation and prevention of dietary and lifestyle-induced health effects in children and infants) study. Dietary, behavioural and socioeconomic factors have been investigated in relation to non-communicable chronic diseases and disorders in this large sample of European children by means of a prospective cohort study, focusing on overweight and obesity.12 An extensive phenotyping in combination with genetic analyses (Figure 1, left section) allows us to disentangle the contributions of factors acting at various levels. Details of the objectives, the IDEFICS study design and the instruments foreseen for the examination waves have already been published before the study had started.13,14 Some study procedures had to be modified after completion of the pre-tests.15 The observational design of the IDEFICS study was complemented by a setting-based community-oriented intervention programme for primary prevention of obesity. It aimed to examine the feasibility, effectiveness and sustainability of a coherent set of intervention modules addressing diet, physical activity and stress.16
Figure 1.
Longitudinal design of the IDEFICS study, its concatenation with the I.Family study and overview of all examination modules. CAQDA, Computer-Assisted Qualitative Data Analysis; FFQ, Food Frequency Questionnaire; T0, baseline survey; T1, first follow-up examination; T2, mailed survey; T3, second follow-up examination.
An extension and a further follow-up (third examination wave) of the IDEFICS children’s cohort was performed in the framework of the EC FP7 project, I.Family, to create a longitudinal database of children and their families17 (Figure 1, right section). Given the limited knowledge about familial resemblance of dietary patterns rather than single food groups such as fruits and vegetables or fast food,18–21 I.Family investigates associations between children’s and parents’ dietary patterns and whether the family food environment mediates these associations, something that no other large study has done. The cohort provides repeated measurements of social and behavioural factors, individual characteristics and medical parameters to be related to health behaviours and health outcomes observed in later years in the same individuals. The data on health and nutrition are complemented with data on parenting style and family life, by including siblings and parents. It will be possible to determine the influence of families on children’s behaviour and to study the complex and dynamic transition from childhood to adolescence, when behaviours begin to be influenced by other social and environmental factors than familial habits.
Our research is conceptually based on the human ecological model.22 It provides an excellent framework for cross-cultural research, taking advantage of the diversity of genetic structures, physical environments, dietary habits, climate zones and socio-cultural contexts across Europe.
Who is in the cohort?
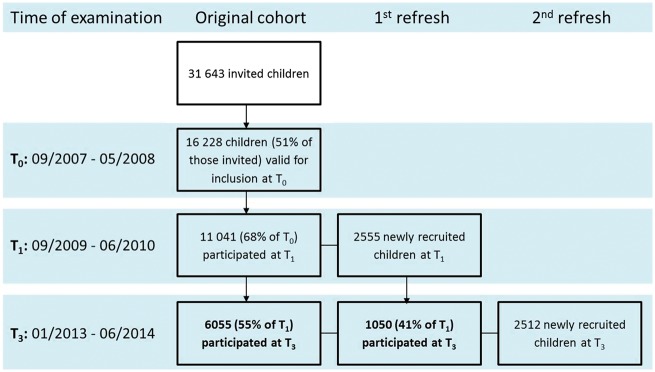
Figure 1 and Figure 2 illustrate the evolution of the study cohort. The baseline examination (T0) between September 2007 and June 2008 included 16 228 children aged 2 to 9.9 years from eight European countries: Belgium, Cyprus, Estonia, Germany, Hungary, Italy, Spain and Sweden. In each country we selected two or more communities whose socio-demographic profile and infrastructure were similar and typical for their region. Within each community all children attending kindergartens and primary schools were eligible. Parents were approached via these settings and asked for consent to examine their children. The main characteristics of the cohort at baseline have been described.23 Historical records of routine child visits were collected to extend the observation period from birth to enrolment into the study. We also collected maternity cards to obtain data on fetal growth. In Sweden, health archives were retrieved from non-participating as well as participating children in the study communities, yielding no evidence of under-representation of children with overweight at baseline; however, some biases with regard to familial socioeconomic factors were observed.24
Figure 2.
Flow chart of the baseline recruitment and subsequent follow-up examinations of the IDEFICS cohort and its extension by the I.Family studya T0, baseline survey; T1, first follow-up examination; T2, mailed survey; T3, second follow-up examination. aNot shown in the figure: process evaluation based on questionnaire mailed at T2 and selection of contrasting groups after T3 stage 1.
All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. Approval by the appropriate ethics committees was obtained by each of the centres doing the fieldwork. Study children did not undergo any procedure before both they and their parents had given consent for examinations, collection of samples, subsequent analysis and storage of personal data and collected samples. Study subjects and their parents could consent to single components of the study while abstaining from others.
Two years after baseline, 11 041 (68%) of all children participated in the first follow-up examination (T1) (Figure 2). Drop-outs between examinations were more likely to be overweight, to report low well-being scores and to come from less educated or single parent families. Moreover, attrition was positively associated with a high degree of item nonresponse at T0.25 Due to the setting-based recruitment, participation was offered to other schools and classmates of study participants. Thus 2555 children were newly recruited at T1. The same examination modules were deployed at T0 and T1. In addition, we assessed the penetration of the intervention messages by a mail survey (T2).
As the starting point of the I.Family study, another follow-up examination (T3) was conducted in 2013-2014, when the age range of index children, i.e. of children who had already participated at T0 or T1, was between 5 and 17 years. The mean age [standard deviation (SD)] was 6.0 (1.8) years at baseline, 7.9 (1.9) years at T1 and 10.9 (2.9) years at T3 with a nearly equal proportion of boys and girls. Since we aimed to investigate entire families, we invited all siblings in the same age range as the index children. The role of familial characteristics, family structure and family life in relation to the children’s development is a major focus of I.Family, and we thus strived for at least one parent of each index child to participate and to provide information on their household. In this way, 6167 families with on average 2 children and 4.1 members (including parents) per family provided the necessary data.
How often have they been followed up?
Figure 3 gives an overview of the sequence and timing of data collections. T0 denotes the baseline survey, i.e. the establishment of the cohort. All examinations performed at T0 were repeated at T1, both in index children volunteering to participate in the follow-up and in children newly recruited at T1. At T2 we only collected information on exposure to the intervention with a self-completion questionnaire mailed to the parents of index children in the intervention regions. At T3 we invited all children participating at T0 or T1 as well as their siblings and parents. The examination programme of this most recent follow-up covered the majority of the modules employed at T0 and T1. New modules on family life, peers and kinship structure were included at T3 (Table 1). The design of the study allowed for an additional, more extensive examination of ‘contrasting groups’ (see below).
Figure 3.
Timeline of the follow-up examinations of the IDEFICS cohort and its extension by the I.Family study T0, baseline survey; T1, first follow-up examination; T2, mailed survey; T3, second follow-up examination; CG, contrasting groups (extended examination in subgroups of the cohort).
Table 1.
Overview of measurements and variables collected at baseline examination (T0) and at two follow-up examinations (T1 and T3) in children and their parents
| Method/ instrument | Measure of interest | Time of measurement |
||
|---|---|---|---|---|
| T0 | T1 | T3 | ||
| Questionnaires | ||||
| Parental report for themselves, their children and their family | General information about the respondent/the family | X | X | X |
| Parenting style | ||||
| Information about pregnancy, breastfeeding and infancy for each child | ||||
| Attitudes towards TV advertisements | ||||
| Meal habits of the family | ||||
| Socio-demographic characteristics of parents | ||||
| Medical history (all children) | ||||
| Medications (all children) | ||||
| Physical activity | - | - | X | |
| Sleeping habits | ||||
| Dietary behaviour, dieting and food frequency | ||||
| Medical history | ||||
| Household structure and family kinship | ||||
| Web-based 24-h dietary recall | ||||
| Accelerometer diary | ||||
| Parental report for children aged < 12 years and self- report of adolescents aged ≥ 12 years | General information about the child/teenager | X | X | X |
| Well-being | ||||
| Children’s/teenagers’ spending | ||||
| Media consumption | ||||
| Physical activity | ||||
| Sleeping habitsa | ||||
| Dietary behaviour, dieting and food frequency | ||||
| Web-based/computer-assisted 24-h dietary recallb | ||||
| Accelerometer diary | ||||
| Self-report of parents and adolescents aged ≥ 12 years | Family life, family rules | X | X | X |
| Body image | - | - | X | |
| Impulsiveness | ||||
| Smoking/alcohol consumption | ||||
| School grades (adolescents only) | ||||
| Peer networks (adolescents only) | ||||
| Self-report of parents and children aged ≥ 6 years | Food and beverage preferences | – | – | X |
| Self-report of children aged ≥ 8 years | Tanner stage (drawing) | – | – | X |
| Examinations and testse | ||||
| Physical examination | Anthropometry (weight, height, waist circumference, skinfolds) | X | X | X |
| Bioelectrical impedance analysis (BIA) | ||||
| Calcaneal ultrasonography (bone stiffness)f | ||||
| Blood pressure | ||||
| Pulse ratef (only T0) | ||||
| Biological samples (non-invasive) | DNA from mouth mucosal cells in salivac | X | X | X |
| Biological markers in morning urine | ||||
| Biological samples (invasive)d | Biological markers in fasting venous or capillary blood | X | X | X |
| Accelerometry | Physical activity (T0-T1: 3 days; T3: 7 days) | X | X | X |
| Sleep duration and qualityf | – | – | X | |
| Accelerometry and GPS sensorsf | Location of physical activity using the global positioning system (GPS) | – | – | X |
| Physical fitness testsf | Handgrip strengthe | X | X | X |
| Coordination (flamingo balance, sit and reach), motor fitness (standing broad jump), cardiorespiratory fitness (shuttle-run-test, 40-m sprint) | X | – | – | |
| Sensory taste perception testsf | Taste thresholds (not T3), taste preference, taste intensity (only T3) | X | X | X |
| Neuropsychological tests in parents and children ≥ 8 years old | Self-administered computer-assisted tests on decision making (Hungry Donkey Test, Bechara Gambling Task), set shifting capacity (Wisconsin Card Sorting Test), inhibitory capacity (Stop Signal Test) | – | – | X |
| Functional magnetic resonance imaging (fMRI)f | Neurological response to visual food cues | – | – | X |
| Secondary data | ||||
| Geographic information systems (GIS)g | Linkage of characteristics of the built environment with GPS and accelerometer data | – | X | X |
| Maternity cards and records of routine child visits | Data on morbidity and growth of children during pregnancy and early childhood | X | X | X |
At T0 and T1 only sleep duration.
Self-report of children ≥ 8 years at T3.
Only newly recruited subjects.
If venepuncture was refused, children were asked for capillary blood (only T0 - T1).
Parents only at T3 and only optional.
Only in subsamples of school-aged children.
Only in three selected geographical regions.
The average observation period for children included in any of the follow-up examinations is 3.9 years (SD = 1.9), with the following distribution: 1 to <2 years: 1901 children; 2 to <3 years: 4068 children; 3 to <4 years: 670 children; 4 to <5 years: 413 children; 5 to <6 years: 3300 children; 6+ years: 2697 children. Overall, the cohort has accumulated 50 940 person-years.
What has been measured?
Table 1 gives an overview of the questionnaires and other examination modules employed at the various stages of recruitment and follow-up of the cohort. In the IDEFICS study we measured weight status and related health outcomes such as blood pressure and insulin resistance, proximal behavioural determinants such as physical activity, sedentary behaviours, sleep and diet and distal determinants such as social factors, electronic media exposure and the physical environment. Preference was given to established and/or validated instruments suitable for population-based studies in children. All instruments and measurement procedures were pre-tested and adapted for each survey centre. We also assessed the reliability of instruments and examinations. Results of pre-tests and reliability studies were published.26
The special focus of the follow-up study I.Family required the development of new instruments, e.g. a kinship questionnaire, and the use of additional measurement tools such as neuropsychological tests on decision making, set shifting capacity and inhibitory capacity as well as pictograms to assess maturation stages according to Tanner and a web-based 24-h dietary recall (24-HDR). Whereas the medical history, to be completed by parents for both their children and for themselves was obtained by interview, paper-and-pencil versions of the general questionnaire and the food frequency questionnaire were self-completed by almost all parents for their children below the age of 12 at all three time points. At T3, when the questionnaire on dietary habits and food consumption frequency was combined with the general questionnaire, teens completed a tailored version of it on a tablet PC and at least one parent completed it also for him/herself in 90% of the families.
At T0 and T1 parents were asked to complete at least one computer-based 24-HDR for their children at the study centre, with support from the study personnel. A web-based version was offered to all participants aged ≥ 8 years at T3 with the recommendation to complete the first one at the examination centre and another two 24-HDRs on non-consecutive days including one weekend day during the next 2 weeks. Parents were asked to assist smaller children (< 8 years old) in completing their 24-HDR. A second series of three 24-HDRs was requested 6 months after the T3 examination. IDEFICS instruments designed for small children and their proxies were adapted for use in adolescents and adults in order to yield comparable data for longitudinal analyses of repeated measurements. All instruments used in the second follow-up are listed in Supplementary Table 1, available as Supplementary data at IJE online.
Several specific tests and measurements were only performed in subgroups. At T0 and T1 approximately half of the children were asked to wear a uniaxial accelerometer (Actigraph) for 3 days and either a uniaxial or a three-axial accelerometer for a full week at T3. At this time, consistent with the I.Family focus, parents were also asked to wear an accelerometer. Most physical fitness tests were restricted to T0 and T1. Percentages of various modules completed by study participants differ because selected modules were only offered to subgroups, and subjects could opt out of single examination modules (Table 2).
Table 2.
Number of subjects who participated in the various examination modules at the three waves
| Examination modulesa | T0 N (%) | T1 N (%) | T3 (children) N (%) | T3 (adults) N (%) |
|---|---|---|---|---|
| General questionnaire (children, teenagers, parents) | 16117 (99.3%) | 13077 (96.2%) | 9018 (93.8%) | 7132 (89.8%) |
| Food frequency questionnaire | 15199 (93.7%) | 12047 (88.6%) | 8840 (91.9%) | 7088 (89.2%) |
| Medical history | 12418 (76.5%) | 10770 (79.2%) | 8304 (86.3%) | 6935 (87.3%) |
| 24-h dietary recall (24-HDR) (≥ 1 day) | 11671 (71.9%) | 6478 (47.6%) | 5117 (53.2%) | 3163 (39.8%) |
| 24-h dietary recall (24-HDR) (≥ 2 days) | 3193 (19.7%) | 1287 ( 9.5%) | 2947 (39.6%) | 2031 (29.8%) |
| Blood pressure | 14752 (90.9%) | 12785 (94.0%) | 8885 (92.4%) | 6169 (77.7%) |
| Heel ultrasonographyb | 7539 (46.5%) | 6886 (50.6%) | 2892 (30.3%) | 2460 (31.8%) |
| Bioelectrical impedance analysis (fasting state) | 15720 (96.9%) | 13118 (96.5%) | 9192 (95.6%) | 6259 (78.8%) |
| Skinfold thickness (subscapularis and triceps)c | 15160 (93.4%) | 12713 (93.5%) | 5967 (62.0%) | 1785 (22.5%) |
| Height | 16228 (100.0%) | 13596 (100.0%) | 9586 (99.7%) | 7663 (96.5%) |
| Weight | 16228 (100.0%) | 13596 (100.0%) | 9573 (99.5%) | 7642 (96.2%) |
| Waist circumference (fasting state) | 15746 (97.0%) | 13199 (97.1%) | 9242 (96.1%) | 6134 (77.2%) |
| Hip circumference | 15643 (96.4%) | 13124 (96.5%) | n.a. | n.a. |
| Venous blood (fasting state) | 9435 (58.1%) | 7516 (55.3%) | 6655 (69.2%) | 5486 (69.1%) |
| Capillary blood (fasting state)d | 3420 (21.1%) | 2599 (19.1%) | n.a. | n.a. |
| Morning urine | 13945 (85.9%) | 10590 (77.9%) | 6993 (72.7%) | n.a. |
| Salivae | 14273 (88.0%) | 714 (5.3%) | 2590 (26.9%) | 5174 (65.1%) |
| Accelerometer measurementf | 7447 (45.9%) | 5930 (43.6%) | 4288 (44.6%) | 1149 (14.5%) |
| Handgrip strength measurementg | 7444 ( 45.9%) | 8174 ( 60.1%) | 7631 ( 79.3%) | 4541 ( 57.2%) |
| Motor fitness testg | 6445 ( 39.7%) | 5855 ( 43.1%) | n.a. | n.a. |
| 40-m sprintg | 4968 ( 30.6%) | 3064 ( 22.5%) | n.a. | n.a. |
| Shuttle-run testg | 5657 ( 34.9%) | 5279 ( 38.8%) | n.a. | n.a. |
n.a., not available.
All physical examination modules were optional for parents (adults).
Optional examination module.
Optional at T3.
Capillary blood only asked from children who refused venepuncture.
Collection restricted to children for whom saliva was unavailable from previous examinations; 80% of children provided at least one saliva sample.
Module only offered to subgroups.
Module restricted to schoolchildren.
About 1 year after completion of T3 (stage 1), in-depth examinations of so-called contrasting groups (denoted as CG in Figure 1 and Figure 3), i.e. subsamples of children with divergent weight trajectories, were conducted (T3, stage 2). Three groups were defined based on weight status and change in body mass index (BMI) z-scores as follows: (i) children who retained normal weight, i.e. who showed a BMI z-score between -1 and +1 at baseline and follow-up and did not change more than ± 0.1 in BMI z-score per year; (ii) children who retained overweight or obesity, i.e. who had a BMI z-score of more than +1 at baseline and follow-up, respectively, and did not change more than ± 0.1 in BMI z-score per year; and (iii) children with excessive weight gain were those who started with a BMI z-score above -0.1 at baseline and who gained more than + 0.1 in BMI z-score per year during the follow-up period. Comparison of contrasting groups will facilitate the identification of determinants as well as consequences of different weight trajectories.
The additional examinations in these subgroups included objective measurements of sleep quality and sensory taste perception tests in both children and their parents. Tests on sensory taste thresholds and taste preferences were performed in a subsample of about 20% of school-aged children at T0 and T1. Preference tests were repeated in CGs and combined with taste intensity tests. In a subsample of a few hundred children, stool samples were collected at T1, at T3 and in CGs to analyse the gut microbiome in normal weight and overweight children longitudinally.
In selected countries, the measurement of physical activity using accelerometers was combined with global positioning system (GPS) sensors and information on the physical environment obtained from geographic information systems (GIS) was collected to determine the influence of the built environment on physical activity and health outcomes. The examination of contrasting groups also included functional magnetic resonance imaging of the brain (fMRI) in three countries to assess brain activation by visual food cues in a smaller subgroup of normal and overweight children and their parents.
Quality management was enforced by central training of field staff, detailed standard operating procedures, site visits during the field phase, central data management and processing of biological samples. A panel of statisticians supports state-of-the-art data analysis.
What has been found? Key findings and publications
Dietary behaviours
Dietary patterns rich in vegetables, wholemeal cereals and fruit and low in animal products were associated with lower risk of overweight/obesity and less 2-year weight gain.27,28 A cluster analysis to derive dietary patterns revealed that children from a lower socioeconomic background had persistently unhealthier dietary profiles over a 2-year period.29 Further, excess energy intake was longitudinally associated with increased BMI z-scores.30 In a subsample of primary school children, sensory preference for sugary/fatty foods was associated with overweight/obesity.31
Physical activity and the built environment
The proportion of children who meet physical activity (PA) guidelines of 60 min of moderate-to-vigorous physical activity (MVPA) per day ranged from 2.0% (Cyprus) to 14.7% (Sweden) in girls and from 9.5% (Italy) to 34.1% (Belgium) in boys.32 An additional 10 min per day of MVPA was related to an increased bone stiffness.33 To assess the impact of the built environment on PA in children, we applied a kernel density method to derive a moveability index from urban forms (based on geographic information systems). Regression analyses revealed a modest impact on the PA of 596 schoolchildren in the German study region.34 In particular, playground density and density of playgrounds and parks combined showed positive effects on MVPA.35
Sleep
Nocturnal sleep duration differed substantially between countries, with shorter durations in Southern Europe. A dose-dependent inverse association between sleep duration and overweight was observed where this association was stronger in school children than in preschool children.36 The inverse relationship between sleep duration and BMI is mainly explained by the association between sleep duration and body fat mass. Insulin may explain part of this association, in particular at the upper tail of the BMI distribution.37
Media consumption
One-third of children failed to meet current screen time recommendations (< 2h/day).38 Children who exceeded sedentary guidelines were at increased risk of developing high blood pressure.39 Also, watching television during meals, having a TV in the children’s bedroom and watching TV for more than 1 h/day were all associated with being overweight/obese.40 Higher exposure to TV was cross-sectionally associated with a preference for sugary/fatty foods40 and longitudinally with overweight/obesity and a higher consumption of sugar-sweetened beverages.41 Often asking for items advertised on TV was longitudinally associated with overweight/obesity and a preference for fatty foods. Parental resistance to these requests was inversely related to their child’s preference for sugary/fatty foods.42 Longitudinally, well-being was negatively affected by TV exposure and PC use as indicated by increased peer and emotional problems in girls and impaired family functioning in boys and girls.43
Metabolic health
The combined prevalence of overweight/obesity in 2-9.9-year-olds ranged from more than 40% in southern Europe to less than 10% in northern Europe. Overall, the prevalence was higher in girls (21.1%) as compared with boys (18.6%) and showed a negative gradient with education and income44.
Blood lipids, glucose and inflammatory markers as well as blood pressure and anthropometric measurements were used to derive age- and sex-specific reference values based on the Generalized Additive Models for Location, Scale and Shape (GAMLSS) method45 and to propose a novel metabolic syndrome (MetS) score for children.46 All reference values were published47 and have already received major attention. It is to be expected that they will have increasing utility within paediatric practice.
In order to identify sensitive periods of growth affecting health, linear-spline mixed-effects models were used to study the association between body mass index (BMI) trajectories during infancy/childhood and later metabolic risk.48 We observed that BMI at birth, rates of BMI change during infancy (0 to <9 months), early childhood (9 months to <6 years) and later childhood (≥ 6 years), as well as current BMI z-score, were associated with the MetS score at follow-up. Starting from birth, rapid BMI growth, especially in the time window of 9 months to < 6 years, increased later metabolic risk in children.
Genetic factors and gene expression patterns
We confirmed the positive association between the FTO rs9939609 and body mass and overweight/obesity.49 Over a 2-year period, a higher increase of body mass and central adiposity and a nearly doubled risk of developing overweight/obesity during growth were observed among A allele carriers. A multiple group structural equation model showed that children carrying the protective FTO genotype TT were more protected by a favourable social environment regarding the development of obesity than children carrying the AT or AA genotype.50
In a subsample, children with low-frequency consumption of sugary foods displayed higher TAS1R3 expression levels in peripheral blood cells (PBCs) compared with those with intermediate or high frequency. In turn, children with high-frequency consumption of fatty foods showed lower UCN2 expression levels compared with those with low or intermediate frequency. Thus, transcripts of TAS1R3 and UCN2 in PBCs may serve as potential biomarkers of consumption of sugary and fatty food.51
A genome-wide genotyping of children using the Affymetrix Axiom® chip has started. Once these data have become available for the full cohort, rigorous testing of causal hypotheses using Mendelian hypothesis-type approaches will be possible, using genetic risk scores for example on obesity risk or dietary behaviour. The cohort will also potentially contribute to gene discovery and epigenetic methylation studies.
Obesity prevention study
The community-oriented, setting-based IDEFICS intervention was developed using the intervention mapping protocol as a non-randomized controlled trial targeting physical activity, dietary behaviour and stress. Different modules at the community level, the (pre-) school level and the family level addressed six different target behavioural changes.16 Outcome and process evaluations assessed the impact and the sustainability of this multilevel intervention according to rigid scientific standards.52–54 Although the IDEFICS intervention was developed according to state-of-the-art knowledge, only weak effects were observed after 2 years of follow-up.55 However, beneficial effects after 2 years were seen in the subgroup of children who were already overweight at baseline.56 Moreover, 6 years after the intervention phase we observed that parents and children who were previously exposed to the IDEFICS intervention had lower propensities to consume sugar than control families.57
What are the main strengths and weaknesses?
Important strengths of this study include: detailed and repeated phenotyping of participants in this cohort; inclusion of thousands of children from diverse regions in Europe; the longitudinal approach across the key developmental period; and the inclusion of familial information. The harmonized protocol in all countries that is enforced by a central quality control, and data management ensures comparability of measurements across study centres. The study combines standardized questionnaires with innovative and objective examinations and tests. Biological samples stored in a central biorepository are used for the assessment of the genetic profile as well as several physiological parameters related to cardio-metabolic and other health outcomes. In the recent follow-up, parents and newly recruited siblings also underwent this protocol, to allow for the investigation of the role of genetic factors and the shared environment on children’s health. Together with the collection of maternity cards and records of routine child visits, these longitudinal data allow for a life-course approach that considers trajectories across key developmental periods.35 The assessment of social networks that become influential as children enter adolescence is a further asset. Additional examinations and the assessment of the physical and social environment in CGs are particularly informative because of their divergent growth trajectories.
There are also some limitations. The modular approach entailed the possibility to opt out of single examination modules, and some modules were only feasible in subgroups. This led to a varying number of subjects per examination module and sometimes small numbers for a given analysis. The study benefits from the diversity of lifestyles and environments across Europe but it was not feasible to implement a representative sampling frame for each country. Nevertheless, the primary scope of this study, i.e. the identification of factors shaping health-related behaviours in children and adolescents and the investigation of the interplay of various risk factors in their relation to future health outcomes, should not be invalidated by potential selection bias12,13 although external validity may be limited.
Future opportunities
The IDEFICS cohort has several features that make it a unique resource to identify early life factors affecting health outcomes that track into adulthood and that are already observable in childhood and adolescence. By covering the time from early childhood until adolescence, it allows the investigation of sensitive developmental periods such as the transitions from infancy into early childhood, pre-school to school ages and from childhood into adolescence in an early life-course approach. The inclusion of parents and siblings in the study and the assessment of peer networks enable us to move beyond the investigation of individual children towards the investigation of our study subjects as members of families and other social networks in a transgenerational approach. Repeated measurements in the same individuals allow the assessment of developmental trajectories. The broad spectrum of parameters measured, the inclusion of objective measurements and the collection of biosamples allow for a detailed phenotyping. The longitudinal perspective of the IDEFICS cohort allows identification of risk factors for metabolic disorders and other health outcomes. This will support the derivation of risk-based reference values and of risk scores for obesity or metabolic disorders, needed for paediatric practice and targeted prevention. Finally, the fact that approximately half of the children live in the intervention regions allows for the assessment of possible long-term intervention effects.
Can I get hold of the data? Where can I find out more?
Due to the prospective nature of this ongoing cohort study, the full anonymization of study data is ruled out and use of data requires a mutual agreement between our study consortium and interested third parties on a case-by-case basis. For corresponding requests, please contact the study coordinator (ahrens@leibniz-bips.de).
The IDEFICS cohort profile in a nutshell
The IDEFICS cohort addresses the impact of dietary, behavioural, biological, socioeconomic and environmental factors on non-communicable chronic diseases in a large diverse sample of European children during sensitive developmental periods. Inclusion of parents/siblings and assessment of peer networks enable investigation of the children as members of social networks in a transgenerational approach.
At baseline (2007-08), 16 228 children aged 2-9.9 years from Belgium, Cyprus, Estonia, Germany, Hungary, Italy, Spain and Sweden were examined.
Children were re-examined after 2 and 6 years, with more than 12 000 children having participated in at least two examination waves; 7105 index children and 2512 newly recruited siblings participated in the most recent wave.
Parents reported socio-demographic, behavioural, medical, nutritional and other lifestyle data for their small children and families and self-reports were collected from adolescents. Examinations of children included anthropometry, blood pressure, heel ultrasonography, physical fitness, accelerometry, DNA from saliva and physiological markers in blood and urine. The built environment, sensory taste perception, neuropsychological characteristics and other mechanisms of children’s food choices were studied in subgroups.
Use of data requires a mutual agreement between the study consortium and interested third parties on a case-by-case basis.
Supplementary Data
Supplementary data are available at IJE online.
Funding
The baseline data collection and the first follow-up work as part of the IDEFICS Study (www.idefics.eu) were financially supported by the European Commission within the Sixth RTD Framework Programme Contract No. 016181 (FOOD). The most recent follow-up was conducted in the framework of the I.Family study [www.ifamilystudy.eu] which was funded by the European Commission within the Seventh RTD Framework Programme Contract No. 266044 (KBBE 2010-14). Additional resources were invested by all participating partners. J.K. is supported by the Academy of Finland (grants #265240 & 263278). T.V. received the support of the Ministry of Education and Science, grant IUT 42-2. L.L. acknowledges the Swedish Research Councils (VR and Forte) for support of the IDEFICS and I.Family studies.
Conflict of interest: None declared.
Supplementary Material
Acknowledgements
We are grateful for the support of school boards, head teachers and communities. The authors wish to thank the IDEFICS children and their parents for participating in this extensive examination. We also express our gratitude to the entire IDEFICS-I.Family study teams, i.e. our study nurses and interviewers, intervention managers, student assistants, IT personnel, data managers, laboratory technicians, administrative staff, paediatricians and researchers. We are particularly grateful to our colleague Gianvincenzo Barba (Institute of Food Sciences, National Research Council, Avellino, Italy), who was one of the main investigators of the IDEFICS/ I.Family consortia. He played a prominent part in the development of our research tools and the IDEFICS intervention. He unexpectedly passed away in June 2014.
References
- 1. World Health Organization. Vienna Declaration on Nutrition and Noncommunicable Diseases in the Context of Health 2020.2013. http://www.euro.who.int/__data/assets/pdf_file/0003/234381/Vienna-Declaration-on-Nutrition-and-Noncommunicable-Diseases-in-the-Context-of-Health-2020-Eng.pdf (2 June 2016, date last accessed).
- 2. World Health Organization. The Minsk Declaration: The Life-course Approach in the Context of Health 2020.2015http://www.euro.who.int/__data/assets/pdf_file/0009/289962/The-Minsk-Declaration-en.pdf (2 June 2016, date last accessed).
- 3. Rowlingson K. Does Income Inequality Cause Health and Social Problems? York, UK: Rowntree Foundation, 2011. [Google Scholar]
- 4. Lynch JW, Davey Smith G, Kaplan GA, House JS.. Income inequality and mortality: importance to health of individual income, psychosocial environment, or material conditions. BMJ 2000;320:1200_04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solar O, Irwin A.. A Conceptual Framework for Action on the Social Determinants of Health. Geneva: World Health Organization, 2010. [Google Scholar]
- 6. World Health Organization. Report of the Commission on Ending Childhood Obesity. Geneva: WHO, 2016. [Google Scholar]
- 7. Boyd A, Golding J, Macleod J. et al. Cohort Profile: The ′children of the 90s′–the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42:111-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magnus P, Birke C, Vejrup K. et al. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2016;45:382-88. [DOI] [PubMed] [Google Scholar]
- 9. Mortensen LM, Bech BH. et al. Data Resource Profile: The Aarhus Birth Cohort biobank (ABC biobank). Int J Epidemiol 2013;42:1697-701. [DOI] [PubMed] [Google Scholar]
- 10. Jaddoe VW, Mackenbach JP, Moll HA. et al. The Generation R Study: Design and cohort profile. Eur J Epidemiol 2006;21:475-84. [DOI] [PubMed] [Google Scholar]
- 11. Wagner MO, Bös K, Jekauc D. et al. Cohort Profile: The Motorik-Modul Longitudinal Study: physical fitness and physical activity as determinants of health development in German children and adolescents. Int J Epidemiol 2014;43:1410-16. [DOI] [PubMed] [Google Scholar]
- 12. IDEFICS study: Identification and prevention of dietary- and lifestyle-induced health effects in children and infants. 2016. www.idefics.eu (2 June 2016, date last accessed). [DOI] [PubMed]
- 13. Ahrens W, Bammann K, De Henauw S. et al. ; European Consortium of the IDEFICS Project. Understanding and preventing childhood obesity and related disorders – IDEFICS: a European multilevel epidemiological approach. Nutr Metab Cardiovasc Dis 2006;16:302-08. [DOI] [PubMed] [Google Scholar]
- 14. Bammann K, Peplies J, Sjöström M. et al. ; IDEFICS Consortium. Assessment of diet, physical activity, biological, social and environmental factors in a multi-centre European project on diet- and lifestyle-related disorders in children (IDEFICS). J Public Health 2006;14:279-89. [Google Scholar]
- 15. Suling M, Hebestreit A, Peplies J. et al. ; IDEFICS Consortium. Design and results of the pretest of the IDEFICS study. Int J Obes (Lond) 2011;35(Suppl 1):30-44. [DOI] [PubMed] [Google Scholar]
- 16. De Henauw S, Verbestel V, Mårild S. et al. ; IDEFICS Consortium. The IDEFICS community-oriented intervention programme: a new model for childhood obesity prevention in Europe? Int J Obes. (Lond) 2011;35(Suppl 1):16-23. [DOI] [PubMed] [Google Scholar]
- 17. I.Family Project - Investigating the determinants of food choice, lifestyle and health in European children, adolescents and their parents. 2016. www.ifamilystudy.eu (2 June 2016, date last accessed).
- 18. Ray C, Roos E, Brug J. et al. Role of free school lunch in the associations between family-environmental factors and children's fruit and vegetable intake in four European countries. Public Health Nutr 2013;16:1109-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pearson N, Biddle SJ, Gorely T.. Family correlates of fruit and vegetable consumption in children and adolescents: a systematic review. Public Health Nutr 2009;12:267-83. [DOI] [PubMed] [Google Scholar]
- 20. Hannon PA, Bowen DJ, Moinpour CM, McLerran DF.. Correlations in perceived food use between the family food preparer and their spouses and children. Appetite 2003;40:77-83. [DOI] [PubMed] [Google Scholar]
- 21. Johnson L, van Jaarsveld CH, Wardle J.. Individual and family environment correlates differ for consumption of core and non-core foods in children. Br J Nutr 2011;105:950-59. [DOI] [PubMed] [Google Scholar]
- 22. Story M, Kaphingst K, Robinson-O’Brien R, Glanz K.. Creating healthy food and eating environments: policy and environmental approaches. Annu Rev Public Health 2008;289:253-72. [DOI] [PubMed] [Google Scholar]
- 23. Ahrens W, Bammann K, Siani A. et al. ; IDEFICS Consortium. The IDEFICS cohort: design, characteristics and participation in the baseline survey. Int J Obes (Lond) 2011;35(Suppl 1):S3-15. [DOI] [PubMed] [Google Scholar]
- 24. Regber S, Novak M, Eiben G. et al. Assessment of selection bias in a health survey of children and families — the IDEFICS Sweden-study. BMC Public Health 2013;13:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hense S, Pohlabeln H, Michels N. et al. Determinants of attrition to follow-up in a multicentre cohort study in children — Results from the IDEFICS study. Epidemiol Res Int 2013;2013:Article ID936365.. [Google Scholar]
- 26. Page AS, Winklhofer-Roob BM. . Identification and prevention of dietary- and lifestyle-induced health effects in children and infants: design, methodology and first results of the IDEFICS Study. Int J Obes (Lond) 2011;35(Suppl 1):16-23.21139560 [Google Scholar]
- 27. Pala V, Lissner L, Hebestreit A. et al. ; IDEFICS Consortium. Dietary patterns and longitudinal change in body mass in European children: a follow-up study on the IDEFICS multicenter cohort. Eur J Clin Nutr. 2013;67:1042-49. [DOI] [PubMed] [Google Scholar]
- 28. Tognon G, Hebestreit A, Lanfer A. et al. ; IDEFICS Consortium. Mediterranean diet, overweight and body composition in children from eight European countries: cross-sectional and prospective results from the IDEFICS study. Nutr Metab Cardiovasc Dis 2014;24:205—13. [DOI] [PubMed] [Google Scholar]
- 29. Fernández-Alvira JM, Börnhorst C, Bammann K. et al. Prospective associations between socioeconomic status and dietary patterns in European children: the Identification and Prevention of Dietary- and Lifestyle-induced Health Effects in Children and Infants (IDEFICS) Study. Br J Nutr 2015;113:517-25. [DOI] [PubMed] [Google Scholar]
- 30. Hebestreit A, Barba G, De Henauw S. et al. ; IDEFICS Consortium. Cross-sectional and longitudinal associations between energy intake and BMI z-score in European children. Int J Behav Nutr and Phys Act 2016;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lanfer A, Knof K, Barba G. et al. Taste preferences in association with dietary habits and weight status in European children: results from the IDEFICS study. Int J Obes (Lond) 2012;36:27-34. [DOI] [PubMed] [Google Scholar]
- 32. Konstabel K, Veidebaum T, Verbestel V. et al. ; IDEFICS Consortium. Objectively measured physical activity in European children: the IDEFICS study. Int J Obes (Lond) 2014;38(Suppl 2):135-43. [DOI] [PubMed] [Google Scholar]
- 33. Herrmann D, Buck C, Sioen I. et al. ; IDEFICS Consortium. Impact of physical activity, sedentary behaviour and muscle strength on bone stiffness in 2–10-year-old children: cross-sectional results from the IDEFICS study. Int J Behav Nutr Phys Act 2015;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buck C, Pohlabeln H, Huybrechts I. et al. Development and application of a moveability index to quantify possibilities for physical activity in the built environment of children. Health Place 2011;17:1191-201. [DOI] [PubMed] [Google Scholar]
- 35. Buck C, Tkaczick T, Pitsiladis Y. et al. Objective measures of the built environment and physical activity in children: from walkability to moveability. J Urban Health 2015;92:24-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hense S, Pohlabeln H, De Henauw S. et al. Sleep duration and overweight in European children: is the association modified by geographic region? Sleep 2011;34:885-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Börnhorst C, Hense S, Ahrens W. et al. ; IDEFICS Consortium. From sleep duration to childhood obesity - what are the pathways? Eur J Pediatr. 2012;171:1029-38. [DOI] [PubMed] [Google Scholar]
- 38. Santaliestra-Pasías AM, Mouratidou T, Verbestel V. et al. ; IDEFICS Consortium. Physical activity and sedentary behaviour in European children: the IDEFICS study. Public Health Nutr 2014;17:2295-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Moraes AC, Carvalho HB, Siani A. et al. ; IDEFICS consortium. Incidence of high blood pressure in children - effects of physical activity and sedentary behaviors: the IDEFICS study: high blood pressure, lifestyle and children. Int J Cardiol 2015;180:165-70. [DOI] [PubMed] [Google Scholar]
- 40. Lissner L, Lanfer A, Gwozdz W. et al. Television habits in relation to overweight, diet and taste preferences in European children: the IDEFICS study. Eur J Epidemiol 2012;27:705-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olafsdottir S, Berg C, Eiben G. et al. Young children's screen activities, sweet drink consumption and anthropometry: results from a prospective European study. Eur J Clin Nutr 2014;68:223-28. [DOI] [PubMed] [Google Scholar]
- 42. Huang CY, Reisch LA, Gwozdz W. et al. ; IDEFICS Consortium. Pester power and its consequences: Do European children’s food purchasing requests relate to diet and weight outcomes? Public Health Nutr 2016;19:2393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hinkley T, Verbestel V, Ahrens W. et al. ; IDEFICS Consortium. Early childhood electronic media use as a predictor of poorer well-being: a prospective cohort study. JAMA Pediatr 2014;168:485-92. [DOI] [PubMed] [Google Scholar]
- 44. Ahrens W, Pigeot I, Pohlabeln H. et al. ; IDEFICS Consortium. Prevalence of overweight and obesity in European children below the age of 10. Int J Obes (Lond) 2014;38(Suppl 2):99-107. [DOI] [PubMed] [Google Scholar]
- 45. Rigby RA, Stasinopoulos DM.. Generalized additive models for location, scale and shape. J R Stat Soc Ser C 2005;54:507-54. [Google Scholar]
- 46. Ahrens W, Moreno LA, Mårild S. et al. ; IDEFICS Consortium. Metabolic syndrome in young children: definitions and results of the IDEFICS study. Int J Obes. (Lond) 2014;38(Suppl 2):4-14. [DOI] [PubMed] [Google Scholar]
- 47. Ahrens W, Moreno LA, Pigeot I.. Obesity determinants and reference standards for health parameters in pre-adolescent European children: Results from the IDEFICS Study. Int J Obes. (Lond) 2014;38(Suppl 2):2-3. [Google Scholar]
- 48. Börnhorst C, Tilling K, Russo P. et al. Associations between early body mass index trajectories and later metabolic risk factors in European children: the IDEFICS study. Eur J Epidemiol 2016;31:513-25. [DOI] [PubMed] [Google Scholar]
- 49. Lauria F, Siani A, Bammann K. et al. ; IDEFICS Consortium. Prospective analysis of the association of a common variant of FTO (rs9939609) with adiposity in children: results of the IDEFICS study. PLoS One 2012;7:e48876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Foraita R, Günther F, Gwozdz W. et al. ; IDEFICS Consortium. Does the FTO gene interact with the socioeconomic status on the obesity development among young European children? Results from the IDEFICS study. Int J Obes (Lond) 2015;39:1-6. [DOI] [PubMed] [Google Scholar]
- 51. Priego T, Sánchez J, Picó C. et al. ; IDEFICS and I.Family consortia. TAS1R3 and UCN2 transcript levels in blood cells are associated with sugary and fatty food consumption in children. J Clin Endocrinol Metab 2015;100:3556-64. [DOI] [PubMed] [Google Scholar]
- 52. Pigeot I, Baranowski T, De, Henauw S; IDEFICS Intervention Study Group; IDEFICS Consortium. The IDEFICS intervention trial to prevent childhood obesity: design and study methods. Obes Rev 2015;16(Suppl 2):4-15. [DOI] [PubMed] [Google Scholar]
- 53. De Bourdeaudhuij I, Verbestel V, De Henauw S. et al. ; IDEFICS Consortium. Implementation of the IDEFICS intervention across European countries: perceptions of parents and relationship with BMI. Obes Rev 2015;16(Suppl 2):78-88. [DOI] [PubMed] [Google Scholar]
- 54. Nicholls SG, Pohlabeln H, De Bourdeaudhuij I. et al. Parents' evaluation of the IDEFICS intervention: an analysis focussing on socioeconomic factors, child's weight status and intervention exposure. Obes Rev 2015;16(Suppl 2):103-18. [DOI] [PubMed] [Google Scholar]
- 55. De Henauw S, Huybrechts I, De Bourdeaudhuij I. et al. ; IDEFICS Consortium. Effects of a community-oriented obesity prevention programme on indicators of body fatness in preschool and primary school children. Main results from the IDEFICS study. Obes Rev 2015;16(Suppl 2):16-29. [DOI] [PubMed] [Google Scholar]
- 56. Lissner L, De Bourdeaudhuij I, Konstabel K. et al. ; IDEFICS consortium. Differential outcome of the IDEFICS intervention in overweight versus non-overweight children: did we achieve ′primary′ or ′secondary′ prevention? Obes Rev 2015;16(Suppl 2):119-26. [DOI] [PubMed] [Google Scholar]
- 57. Arvidsson L, Bogl L, Eiben G. et al. ; IDEFICS Consortium. Fat, sugar and water intakes among families from the IDEFICS intervention and control groups: first observations from I.Family. Obes Rev. 2015;16(Suppl 2):127-37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.