Abstract
The liver is vital for xenobiotic and endobiotic metabolism. Previously, we demonstrated that a compromised liver worsened toxicity associated with exposure to polychlorinated biphenyls (PCBs), through disruption of energy homeostasis. However, the role of a compromised liver in defining dioxin-like PCB126 toxicity on the peripheral vasculature and associated inflammatory diseases is yet to be studied. This study investigated the effects of PCB126 on vascular inflammation linked to hepatic dysfunction utilizing a liver injury mouse model. Male C57Bl/6 mice were fed either an amino acid control diet (CD) or a methionine-choline deficient (MCD) diet in this 14-week study. Mice were exposed to PCB126 (0.5 mg/kg) and analyzed for inflammatory, calorimetric and metabolic parameters. MCD diet-fed mice demonstrated steatosis, indicative of a compromised liver. Mice fed the MCD-diet and subsequently exposed to PCB126 manifested lower body fat mass, increased liver to body weight ratio and alterations in hepatic gene expression related to lipid and carbohydrate metabolism, implicating metabolic disturbances. PCB126-induced steatosis irrespective of the diet type, but only the MCD + PCB126 group exhibited steatohepatitis and fibrosis. Furthermore, PCB126 exposure in MCD-fed mice led to increased plasma inflammatory markers such as Icam-1, plasminogen activator inhibitor-1 and proatherogenic trimethylamine-N-oxide, suggesting inflammation of the peripheral vasculature that is characteristic of atherosclerosis. Taken together, our data provide new evidence of a link between a compromised liver, PCB-mediated hepatic inflammation and vascular inflammatory markers, suggesting that environmental pollutants can promote crosstalk between different organ systems, leading to inflammatory disease pathologies.
Keywords: inflammation, liver, MCD; PCB126, steatohepatitis; toxicity
Persistent organic pollutants (POPs), namely polychlorinated biphenyls (PCBs), are a global environmental health concern because of positive associations between their exposures and multiple pathological conditions such as cardiovascular diseases (CVDs), liver diseases, diabetes, obesity,y and other metabolic-related disorders (Aminov et al., 2016; Cave et al., 2010; Crinnion, 2011; Park et al., 2016; Taylor et al., 2013). The production and use of PCBs was legally prohibited in the United States since the 1970s and worldwide in 2001 at the Stockholm Convention on POPs (Xu et al., 2013). However, the high chemical and thermodynamic stability of these chlorinated, organic compounds allows them to persist in the environment, consequently bioaccumulating in fat depots of living organisms including marine life, cattle and humans with ingestion of PCB contaminated food being a major route of exposure (Fernandez-Gonzalez et al., 2015; Schecter et al., 2001; Xue et al., 2014).
The effects of PCBs in exposed human cohorts and rodent models have been evaluated extensively and the nature of toxicity was defined by the dose and type of congener, among other factors. Depending on the number and position of chlorine atoms substituted in the 2 phenyl rings, there can be upto 209 PCB congeners. PCBs have been historically classified as either coplanar or noncoplanar congeners, and because they are xenobiotics, PCBs bind and activate hepatic receptors that regulate detoxification in the body (Luthe et al., 2008; Wahlang et al., 2014a). Coplanar congeners have 1- or 1-ortho chlorine substitutions in the phenyl ring and include PCBs 77 and 126 that predominantly activate the aryl hydrocarbon receptor (AhR), hence “dioxin-like” in nature (National Toxicology, 2006b; Safe, 1994). In contrast, noncoplanar PCBs constituting of more highly chlorinated PCBs that have more than 1-ortho substitutions such as 153 and 180 are primarily associated with the constitutive androstane receptor activation, hence “phenobarbital-like” (Al-Salman and Plant, 2012; Gahrs et al., 2013; National Toxicology, 2006a). In fact, traditional rodent studies have correlated exposures to “phenobarbital-like PCBs” with nonalcoholic fatty liver disease (NAFLD), obesity and related metabolic disorders while exposure to “dioxin-like” PCBs has been linked to endothelial inflammation, vascular injury, atherosclerosis and CVD (Lind et al., 2004; Wahlang et al., 2013, 2014b). However, outcomes of PCB exposure in humans correspond to a multitude of disorders because in all likelihood, human PCB exposure is not restricted to a single congener, but rather a mixture of congeners.
The liver is the principal site for PCB detoxification, as well as the first-line of PCB insult if exposure occurs through the oral route. The liver is pivotal for xenobiotic and endobiotic metabolism, and maintenance of energy homeostasis in the body, and therefore regulate numerous disease pathologies. Liver disorders such as NAFLD and its more severe form, nonalcoholic steatohepatitis (NASH) and hepatic fibrosis can be attributed to factors that are manifold and diverse such as diet, lifestyle, alcohol consumption, therapeutic drugs and chemical exposure. The occurrence of liver disease can predispose a person to other health complications such as diabetes, obesity, the metabolic syndrome and CVD (Lonardo et al., 2015). In fact, liver disease is considered a risk factor for CVD including atherosclerosis but studies showing mechanistic links or evidence of correspondence between the liver and the associated cardio-vasculature are few (Ballestri et al., 2014; Than and Newsome, 2015). Understanding the effects of environmental pollutants like POPs on liver and heart diseases is of utmost significance because, in the United States alone, approximately 20% of the population suffer from NAFLD while heart diseases kill about 610 000 people each year making it a leading cause of death for both men and women (Lazo et al., 2013; Writing Group et al., 2016).
Studies on AhR ligands have demonstrated links between AhR activation and occurrence of hepatic disorders as well as CVD including atherosclerosis, thereby illustrating that this could be a potential pathway linking liver disease with CVD (Oesterling et al., 2008; Pierre et al., 2014; Vogel et al., 2004; Xiao et al., 2014). Therefore, the objective of this study is to examine the effects of dioxin-like PCB126, an AhR activator on vascular dysfunction in the presence of a compromised liver. In order to test our hypothesis, hepatic fibrosis was induced in male C57Bl/6 mice by feeding them the methionine-choline deficient (MCD) diet and the toxicological outcomes of PCB126 exposure were evaluated on hepatic and extra-hepatic (peripheral) organ systems. Administration of the MCD diet results in diet-induced liver injury that better models human NAFLD (Machado et al., 2015). The MCD model was preferred over the high-fat diet approach that induces NAFLD and obesity, in order to minimize PCB accumulation in the adipose tissue and maximize PCB systemic levels. The data obtained from this study indicate the importance of the liver for energy metabolism and demonstrate how PCB126 exposure can alter liver function, exacerbate liver injury and aggravate peripheral inflammation which may predispose to associated vascular diseases.
MATERIALS AND METHODS
Animal experiments
The animal protocol was approved by the University of Kentucky Institutional Animal Care and Use Committee. Mice were housed in a temperature- and light-controlled room on a 12-h light/dark cycle with food and water ad libitum. Eight-week-old, wild type male C57Bl/6 mice were purchased form Taconic (Hudson, New York, USA). Upon arrival, mice were divided into 4 study groups (n = 10) based on diet type or PCB126 exposure during this 14-week study utilizing a 2 × 2 study design (Supplementary Figure 1). On week 1 all animals were fed the amino acid control diet (CD; TD.94149; Envigo, Madison, Wisconsin, USA). On week 2 mice were fed either CD or the MCD diet (TD.90262, Envigo). Mice were administered either corn oil as a vehicle control or PCB126 (purchased from AccuStandard, Connecticut, USA) in corn oil at 0.5 mg/kg by oral gavage on week 4. The dose of PCB126 chosen was considered a low dose, based on our previous study where PCB126 (4.5 mg/kg) resulted in premature mortality in the MCD-fed mice (Wahlang et al., 2017). Mice were monitored weekly for bodyweight and food intake measurements. Mice on the MCD diet were switched to CD- feeding on week 5–6 and resorted back to MCD diet for the rest of the study. This was done in order to prevent excess weight loss and avoid noncompliance with the IACUC protocol. A glucose tolerance test was performed at week 10. During week 13 and 14, 4 mice were randomly selected from each group and placed in metabolic chambers (PhenoMaster, TSE systems, Chesterfield, Missouri, USA) to assess food/water consumption and physical activity. For metabolic assessment, the oxygen consumption (vO2) and carbon dioxide production (vCO2) were monitored and used for computing the respiration exchange rate (RER) and total energy expenditure (EE) which is the number of calories burnt for energy. Physical activity (movement) was measured using infrared beams and sensors. The total movement (counts) which is the sum of ambulatory movement (mouse crosses 2 adjacent beams) and fine movement (mouse crosses the same beam twice) was calculated. Prior to euthanasia, the animals were analyzed for lean and body fat composition using the EchoMRI (EchoMRI LLC, Houston, Texas, USA). Animals were euthanized (ketamine/xylazine [obtained from Henry Schein, Melville, New York, USA], 100/20 mg/kg body weight [BW], i.p.) at the completion of week 14. Ethylenediaminetetraacetic acid was added to collected blood samples, briefly mixed, and centrifuged at 2000 g for 15 min at 4 °C to separate blood plasma. Plasma samples were frozen in liquid nitrogen and stored at −80 °C until processing. Tissues were harvested for mRNA or protein and stored at −80 °C prior to analysis. Thus, 4 different groups were evaluated; CD + vehicle, CD+ PCB126, MCD+ vehicle, MCD + PCB126.
Glucose tolerance test
Fasting blood glucose levels were measured with a hand-held glucometer (ACCU-CHECK Aviva, Roche, Basel, Switzerland) using 1–2 µl blood via tail snip after mice were fasted for 5 h. Glucose was then administered (1 mg glucose/g BW, sterile saline, i.p.), and blood glucose was measured at 5, 15, 30, 60, 90, and 120 min postinjection.
Histological studies
Sections from the liver and adipose tissue were fixed in 10% neutral buffered formalin and embedded in paraffin for routine histological examination. Tissue sections were stained with hematoxylin-eosin (H&E) or Sirius Red and examined by light microscopy. Reagents were obtained Sigma-Aldrich Corp. (St Louis, Missouri, USA). Photomicrographic images were captured using a high-resolution digital scanner at 10× and 40× magnification. Quantification of fibrosis was performed using ImageJ software (NIH, Bethesda, Maryland, USA) as previously described in Hadi et al. (2011).
Cytokine, adipokine, and lipoprotein measurements
The Milliplex Map Mouse Adipokine Magnetic Bead Panel (Millipore Corp, Billerica, Massachusetts, USA) was utilized to measure plasma cytokines (tumor necrosis factor alpha (Tnfα), interleukin-6 (IL-6), macrophage chemoattractant protein-1 [Mcp-1]), insulin, adipokines (leptin, resistin), and plasminogen activator inhibitor-1 (PAI-1); while the Milliplex Map Mouse CVD Magnetic Bead Panel 1 was used to measure plasma CVD markers on the Luminex Xmap MAGPIX system (Luminex Corp, Austin, Texas, USA), as per the manufacturer’s instructions. Plasma aspartate transaminase (AST) activity, alanine transaminase (ALT) activity, cholesterol, triglycerides and high-density lipoprotein (HDL) were measured with the Piccolo Xpress Chemistry Analyzer using Lipid Panel Plus reagent disks (CLIAwaived Inc, San Diego, California, USA). The concentration for low-density lipoprotein (LDL) was calculated automatically by the instrument using the directly determined values for total cholesterol, HDL, and triglycerides and the standard Friedewald equation while the concentration of very LDL (VLDL) was calculated using the standard triglycerides/5 (if units in mg/dl) equation. If lower than the measuring range of the instrument, a “<“appears with an asterisk, eg, CHOL < 20* mg/dl.
Measurement of trimethylamine-N-oxide
Trimethylamine-N-oxide (TMAO) was extracted from plasma/liver and analyzed by HPLC electrospray ionization tandem mass spectrometry as described previously with minor modifications (Petriello et al., 2016; Taesuwan et al., 2017). TMAO was extracted from plasma (10 μl) and liver tissue (∼50 mg) using methanol and acetonitrile, respectively. Analysis was carried out using Shimadzu HPLC coupled with an AB Sciex 6500-QTRAP hybrid linear ion trap triple quadrupole mass spectrometer in multiple reaction monitoring mode. Mass labeled choline (d4-choline) was used as an internal standard and for relative quantitation. TMAO was analyzed using a Primesep 100, 3 μm, 2.1 × 100 mm column (SIELC Technologies, Wheeling IL) and data were processed using ABSciex Multiquant software.
Real-time PCR
Mouse tissue samples were homogenized and total RNA was extracted using the TRIzol reagent (Thermo Fisher Scientific Inc, Waltham, Massachusetts, USA). RNA purity and quantity were assessed with the NanoDrop 2000/2000c (Thermo Fisher Scientific Inc) using the NanoDrop 2000 (Installation Version 1.6.198) software. cDNA was synthesized from total RNA using the QuantiTect Reverse Transcription Kit (Qiagen, Valencia, California, USA). Polymerase chain reaction (PCR) was performed on the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, California, USA) using the Taqman Fast Advanced Master Mix (Thermo Fisher Scientific Inc). Primers (Supplementary Table 1) were obtained from Taqman Gene Expression Assays (Thermo Fisher Scientific Inc). The levels of mRNA were normalized relative to the amount of Actb mRNA for liver, aorta and spleen, and expression levels in mice fed CD and administered vehicle were set at 1. Gene expression levels were calculated according to the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Statistical analysis
Graphs were plotted using GraphPad Prism version 7.02 for Windows (GraphPad Software Inc., La Jolla, California, USA). Data are expressed as mean ± SEM. Statistical analyses were also performed using the GraphPad Prism statistical software. Multiple group data were compared using 2-way ANOVA (column factor: diet and row factor: PCB126) followed by Tukey’s post-hoc test for multiple comparisons. p < .05 was considered statistically significant. All statistical analyses were overseen by biostatisticians at the University of Kentucky.
RESULTS
Rationale for the Exposure Dose of PCB126
In previous studies by our laboratory group, we utilized a higher dose of PCB126 to induce toxicity. However, the higher dose caused premature mortality in the MCD-fed mice and therefore a study using lower doses was necessary. Besides, using a lower dose will allow for comparisons of dose-dependency on physiological responses. This study employed a dose of 0.5 mg/kg which was ∼10 times lower than the dose used previously.
PCB126 Exposure Worsened Liver Injury Caused by MCD Feeding
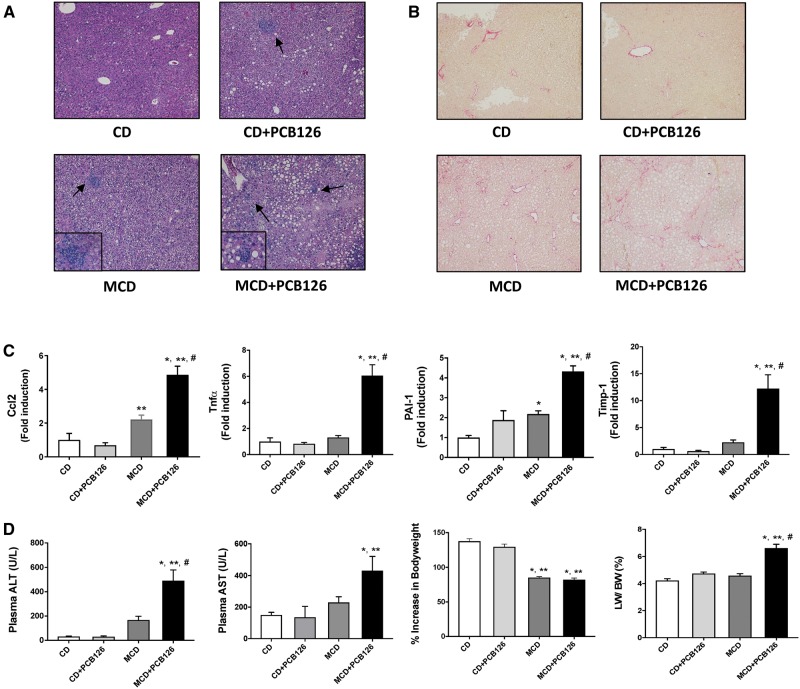
In order to examine if the presence of a compromised liver altered PCB126-mediated toxicity, hepatic endpoints including steatosis and inflammation were assessed. Histological analysis of tissue sections (H&E staining) demonstrated that the MCD-fed mice developed severe steatosis, seen by infiltration of fat globules in the hepatocytes, and this was accompanied by the presence of inflammatory foci, indicative of steatohepatitis (Figure 1A). The MCD groups also exhibited fibrosis as assessed representatively and quantitatively by picro-sirius red staining of liver sections (Figure 1B and Supplementary Figure 2). PCB126 exposure appeared to induce steatosis in the CD-fed mice while there was no observable fibrosis in this group. Because the amino acid CD has ∼20% kCal from fat, some of the hepatic sections (H&E staining) in the CD group showed some lipid accumulation, but the degree of steatosis was mild compared with the CD+ PCB126 or the MCD groups.
Figure 1.
Effects of PCB126 exposure on steatosis and BW. A, H&E staining of hepatic sections established the occurrence of hepatocellular hypertrophy and steatosis in the MCD-fed groups. Each illustration is representative per treatment group B, Picrosirius red staining of hepatic sections indicated presence of fibrosis in the MCD groups. C, Hepatic mRNA expression for Ccl2/Mcp-1, Tnfα, PAI-1 and Timp-1 were assessed. D, Plasma ALT and AST levels were measured using the Piccolo Xpress Chemistry Analyzer. Increase in BW with time for C57BL/6 (n = 10) taken weekly from week 1-14. The % increase in BW gain with time was calculated and the BW at week 1 was taken as 100%. Livers were weighed at euthanasia and the liver to bodyweight ratio was calculated. Values are mean ± SEM, *p < .05 versus CD-fed mice, **p < .05 versus CD-fed mice exposed to PCB126, #p < .05 versus MCD diet-fed mice, ##p < .05 versus MCD-fed mice exposed to PCB126.
Hepatic inflammation was assessed by measuring hepatic gene expression levels of inflammatory markers including Mcp-1 (Ccl2) and Tnfα, and fibrotic markers including PAI-1 and Timp-1 (Figure 1C). Interestingly, the MCD-fed mice exposed to PCB126 showed increased expression of hepatic Mcp-1, Tnfα, PAI-1, and Timp-1 compared with their CD counterparts and unexposed, MCD-fed mice. Liver injury, based on elevated plasma ALT and AST levels, was also strongly evident in the MCD + PCB126 group with a significant interaction between diet and PCB126 for elevated plasma ALT (p = .0005) (Figure 1D). Additional parameters such as BW and liver weight (LW) were also assessed. BW was measured throughout the 14-week study, and the MCD groups showed a decrease in BW versus the CD groups due to weight loss over time, as expected with MCD feeding. The LW to BW ratio was calculated and the MCD + PCB126 group had the highest LW/BW while PCB126 did not increase LW/BW in the CD group.
PCB126 Exposure Altered Hepatic Gene Expression in MCD-Fed Mice, Indicative of Impaired Liver Function
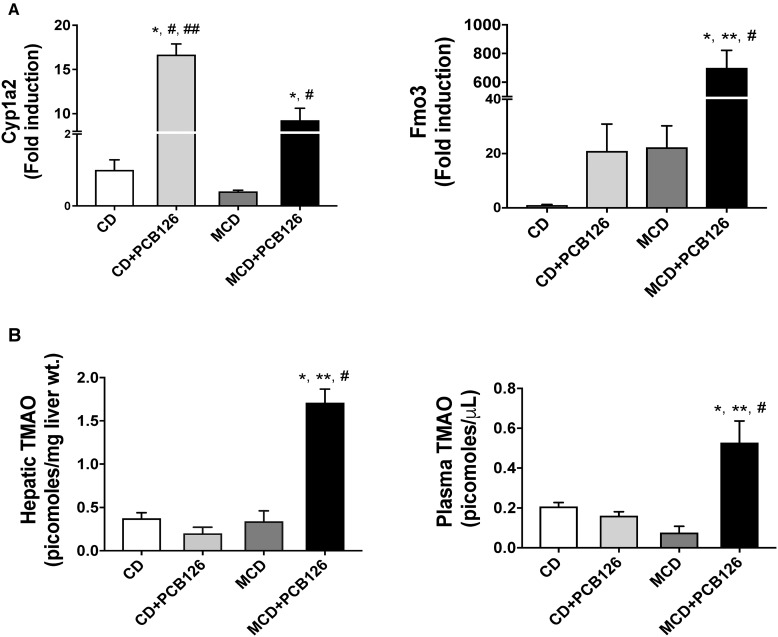
Hepatic expression of genes related to PCB126-mediated AhR activation as well as genes involved in lipid and carbohydrate metabolism were measured. As expected, the hepatic expression of the AhR target gene, Cyp1a2 was upregulated with PCB126 exposure in both diet groups (Figure 2A). Interestingly, the hepatic expression of another AhR target, Fmo3, was upregulated only in the MCD + PCB126 group. Increased hepatic Fmo3 expression has been reported for “dioxin-like” PCBs and correlated with an increased risk for CVD through TMAO synthesis. In this study, hepatic and plasma levels of TMAO were upregulated in the MCD + PCB126 group (Figure 2B) which was concordant with Fmo3 upregulation.
Figure 2.
Effects of PCB126 on AhR target gene expression and TMAO levels. A, Hepatic mRNA expression for AhR target genes Cyp1a2 and Fmo3 were measured. B, TMAO was extracted from liver tissue and plasma and was measured using an ion trap triple quadrupole mass spectrometer.
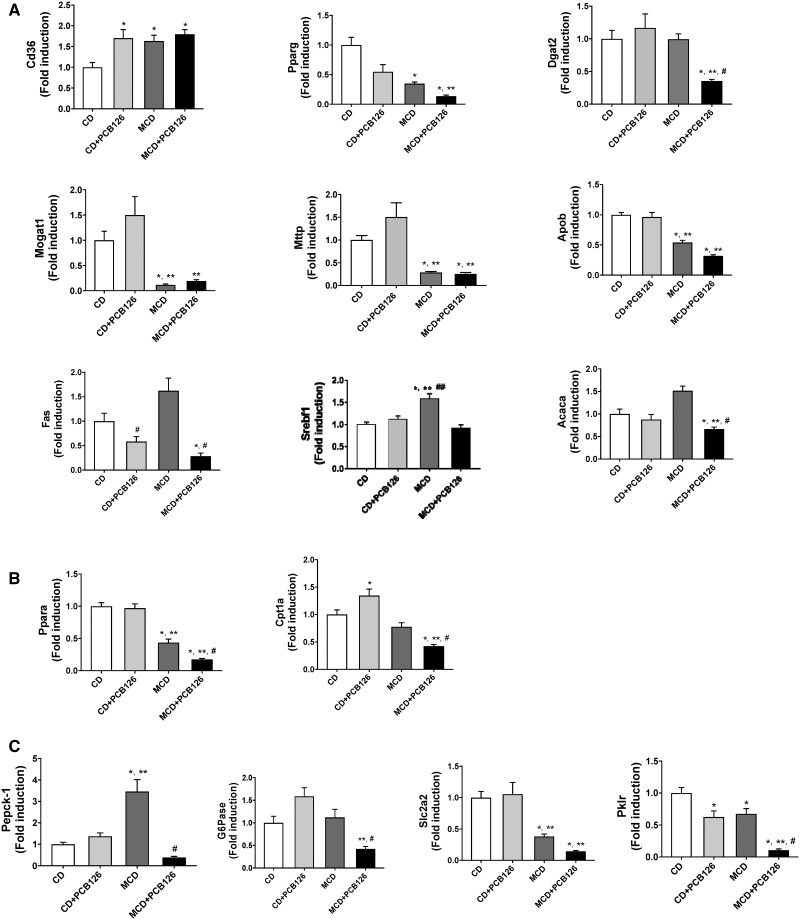
Markers of hepatic lipid accumulation such as the fatty acid transporter Cd36, were upregulated with PCB126 exposure as well as with MCD feeding but there were no synergistic or additive effects with both treatments (Figure 3A). Other markers of hepatic lipid accumulation and storage such as the nuclear receptor Pparg and major enzymes of triglyceride synthesis (Dgat2 and Mogat1) were downregulated in the MCD + PCB126 group. Moreover, the hepatic expression of Mttp, an enzyme required for LDL secretion as well as Apob, a major constituent of VLDLs, was downregulated in the MCD-fed mice with or without PCB126 exposure. In terms of lipogenic gene expression, PCB126 decreased Fas levels in both dietary groups while PCB126 counteracted the MCD diet-induced upregulation of Srebf1 and its target gene, Acaca involved in de novo fatty acid synthesis. In addition, hepatic genes involved in lipid breakdown and mitochondrial fatty acid oxidation (Ppara and Cpt1a) were also downregulated in the MCD + PCB126 group (Figure 3B).
Figure 3.
Effects of PCB126 on expression of hepatic genes involved in endobiotic metabolism. A, Hepatic mRNA expression for genes involved in hepatic lipid accumulation and synthesis were measured namely Cd36, Pparg, Dgat2, Mogat1, Mttp, Apob, Fas, Srebf1, and Acaca. B, Hepatic mRNA expression for genes involved in fatty acid breakdown including Pparα and Cpt1a were measured. C, Hepatic mRNA expression for genes involved in carbohydrate metabolism such as Pepck-1, G6Pase, Slc2a2 and Pklr were measured. Values are mean ± SEM, *p < .05 versus CD-fed mice, **p < .05 versus CD-fed mice exposed to PCB126, #p < .05 versus MCD diet-fed mice, ##p < .05 versus MCD-fed mice exposed to PCB126.
Furthermore, PCB126 exposure in MCD-fed mice drastically decreased the hepatic expression of major enzymes involved in gluconeogenesis, Pepck-1 and G6Pase (Figure 3C). The diminished hepatic expression of gluconeogenic genes was accompanied with decreased expression of Slc2a2/Glut2, a hepatic glucose transporter and Pklr which is a crucial enzyme of glycolysis.
Because a number of genes involved in maintaining energy homeostasis were affected, and with energy metabolism being regulated primarily by the liver, we also assessed parameters such as energy homeostasis and metabolic activity such as blood glucose and EE. This will allow us to determine if there were PCB126-related effects on behaviors such as movement and whole body energy metabolism. The glucose tolerance test showed that the MCD groups had a lower area under the curve compared with the CD groups (Supplementary Figure 3). Fasting blood glucose levels measured prior to the test showed that the MCD groups had lower blood glucose levels to begin with. Furthermore, fasting plasma insulin levels were also decreased in the MCD groups.
Effects on overall EE were assessed using calorimetric cages. PCB126 exposure did not affect metabolic parameters such as RER which is an estimate of the respiratory quotient (Supplementary Figure 4). RER estimates whether the fuel source/EE originated from carbohydrate or lipid metabolism. An RER of 0.70 suggests that fat is the predominant fuel source, whereas an RER of 0.85 indicates a mix of fat and carbohydrate utilization. The MCD feeding decreased RER versus CD feeding during the dark cycle, and this was indicative of lipid breakdown being a predominant fuel source. The MCD-fed mice also exhibited decreased EE which was the number of calories burnt for energy per hour versus their CD counterparts. In terms of physical activity, there was no difference between the groups when normalized to their lean mass, irrespective of a lower % lean mass composition in the MCD groups.
Plasma cholesterol, triglycerides, and lipoproteins were also measured to assess systemic effects of impaired liver function and dyslipidemia (Table 1). Interestingly, the MCD + PCB126 group portrayed plasma cholesterol levels that were <20 mg/dl while the MCD group also had lower cholesterol levels versus the CD groups. The MCD groups also showed a trend towards lower plasma triglyceride and VLDL levels while plasma HDL and LDL levels were not detected in the MCD + PCB126 group, indicating low levels of circulating lipoproteins in this group.
Table 1.
Serum Levels of Cholesterol, Triglycerides, HDLs, LDLs, and VLDLs
| (mg/dl) | CD | CD + PCB126 | MCD | MCD + PCB126 |
|---|---|---|---|---|
| Cholesterol | 73.2 ± 4.4 | 44.3 ± 6.2* | 20.8 ± 0.7* | <20* |
| Triglycerides | 33.5 ± 2.6 | 34.0 ± 5.1 | 25.8 ± 2.7 | 23.5 ± 1.6 |
| HDL | 63.7 ± 3.5 | 44.6 ± 4.5 | 16.0 ± 4.5 | n.a. |
| LDL | 2.5 ± 0.7 | 0.5 ± 0.7 | n.a. | n.a. |
| VLDL | 6.8 ± 0.5 | 7.0 ± 0.5 | 6.0 ± 0.4 | 6.0 ± 1.0 |
Values are mean ± SEM (mg/dl), *p < .05 versus CD-fed mice; n.a., not available.
PCB126 Exposure Upregulated Markers of Peripheral Vascular Inflammation in MCD-Fed Mice
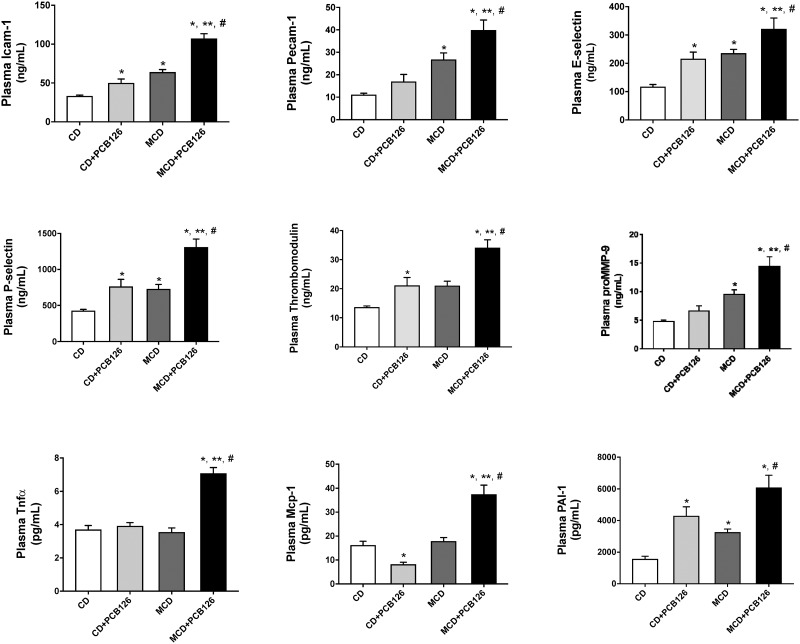
We hypothesized that PCB126 exposure could potentially affect other organ systems in the presence of a compromised liver. We therefore looked at the mouse plasma profile of adhesion molecules, cytokines and adipokines to assess PCB126 toxicity systematically (Figure 4). The MCD + PCB126 group displayed elevated levels of markers for early endothelial cell injury/vascular inflammation namely intercellular adhesion molecule 1 (Icam-1), platelet endothelial cell adhesion molecule (Pecam-1), E-selectin, P-selectin, thrombomodulin, and matrix metallopeptidase 9 (proMMP-9). Although PCB126 exposure did increase plasma levels of Icam-1, E-selectin, P-selectin and thrombomodulin in the CD-fed mice; the increase was subtle compared with the MCD group. In fact, there was a significant interaction between diet and PCB126 for Icam-1 (p = .002). Circulating levels of inflammatory cytokine, Tnfα and Mcp-1 as well as PAI-1 were also higher in the MCD + PCB126 group. There was a significant interaction between diet and PCB126 for both plasma Tnfα and Mcp-1 (p < .0001).
Figure 4.
PCB126 exposure increased circulating cytokine levels and markers of early vascular injury. Plasma levels of Icam-1, Pecam-1, P-Selectin, E-selectin, thrombomodulin, proMMP9, Tnfα, Mcp-1 and PAI-1 were measured using the MAGPIX system. Values are mean ± SEM, *p < .05 versus CD-fed mice, **p < .05 versus CD-fed mice exposed to PCB126, #p < .05 versus MCD diet-fed mice, ##p < .05 versus MCD-fed mice exposed to PCB126.
Effects of PCB126 and MCD Feeding on Extra-Hepatic Organ Systems
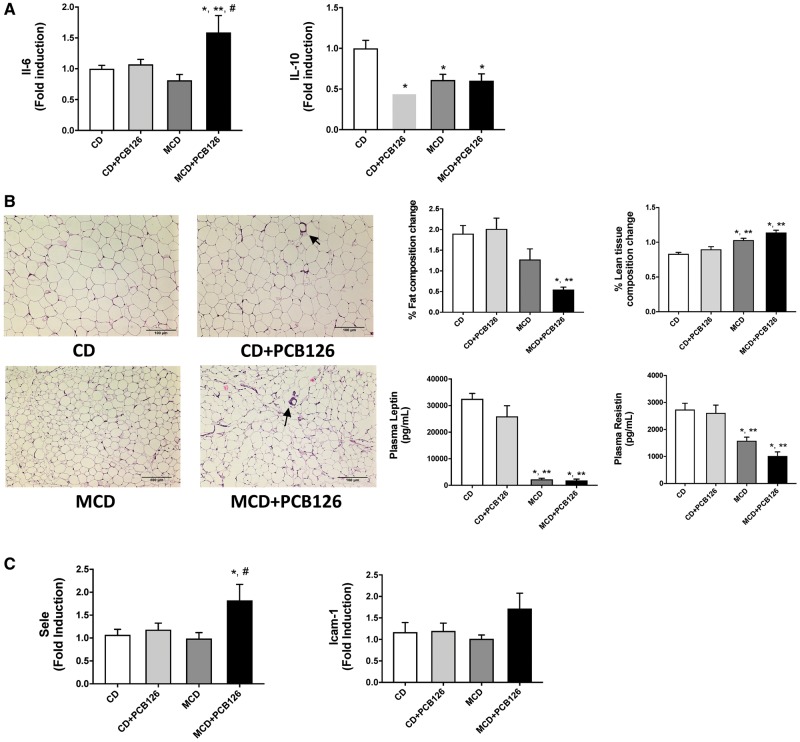
We hypothesized that the presence of an injured liver and subsequent environmental insults can damage other organ systems that are susceptible to inflammation such as spleen as well as tissues that PCBs are known to bioaccumulate such as the adipose tissue. Indeed, gene expression of the major inflammatory cytokine Il-6 was upregulated in the spleen with a concomitant decrease in the expression of anti-inflammatory cytokine Il-10 (Figure 5A). Furthermore, epididymal fat pads were removed from these mice and analyzed using histology. H&E staining revealed that the MCD-fed mice had smaller adipocytes compared withto their CD counterpart and this was accompanied by low levels of adipokines namely leptin and resistin in the MCD groups (Figure 5B). There also appeared to be crown like structures in the white adipose tissue sections of PCB126 exposed groups, indicating presence of inflammatory cells. Body composition analysis demonstrated that only the MCD + PCB126 group had a lower body fat composition versus the CD groups at the end of the study while both MCD groups exhibited higher lean mass composition. Moreover, plasma adipokines including leptin and resistin were decreased in the MCD groups. Additionally, aortic gene expression for adhesion molecules was measured and Sele (E-selectin) was upregulated in the MCD + PCB126 group while there also appeared to be a trend towards increased Icam-1 in this group (Figure 5C).
Figure 5.
Effects of PCB126 exposure on peripheral organ systems. A, The mRNA expression for Il-6 and Il-10 were measured in the spleen. B, Epididymal adipose tissue was stained with H&E. % fat composition and % lean tissue were measured at the end of the study using the EchoMRI. Plasma leptin and resistin were measured using the MAGPIX system. C, The mRNA expression for Sele and Icam-1 were measured in the aorta. Values are mean ± SEM, *p < .05 versus CD-fed mice, **p < .05 versus CD-fed mice exposed to PCB126, #p < .05 versus MCD diet-fed mice, ##p < .05 versus MCD-fed mice exposed to PCB126.
DISCUSSION
Exposure to POPs including PCBs is still a major health concern because multiple epidemiologic studies implicate POPs exposure to increased risk for various cardio-metabolic diseases. PCB levels in exposed populations as well as in the general public are still detected and measurable (Consonni et al., 2012; Pavuk et al., 2014), hence the importance of elucidating mechanism(s) of actions of these pollutants using rodent models. Measured PCB126 levels in humans are considerably lower compared with other congeners (Birnbaum et al., 2016; Cave et al., 2010), therefore using a lower dose was needed to better mimic human exposure patterns. Multiple studies on PCB126 have demonstrated its “dioxin-like” effects through AhR activation, consequently leading to oxidative stress, inflammation, hepatic steatosis, hepatocellular hypertrophy among other pathologies (Hennig et al., 2002; Lai et al., 2010, 2012; National Toxicology, 2006b). In fact, in terms of the toxic equivalency factor (TEF), a factor that expresses the toxicity of dioxin-like compounds based on the most potent dioxin (2,3,7,8-tetracholorodibenzo-p-dioxin), PCB126 has a TEF of 0.1 which is the highest of all PCB congeners. Recently a study demonstrated that the dose administered orally which can be considered as an intakeTEF does not necessarily reflect PCB126 systemic levels and this should be noted when describing PCB126 dose effects (van Ede et al., 2013). Notably, the dose employed in this study is in the same range as doses utilized in a number of rodent studies on PCB126 that look at AhR-driven endpoints (Boucher et al., 2015; Gadupudi et al., 2016; Lai et al., 2012; van Ede et al., 2013). Previously, we had utilized a higher dose of PCB126 to evaluate the aftermath of PCB exposure on a compromised liver and this higher dose induced mortality in the MCD mouse model during the study period (Wahlang et al., 2017), clearly indicating that there was an interaction between a compromised hepatic condition and pollutant exposure. Therefore, the primary objective of this study was to examine toxicity endpoints of a low dose PCB126 exposure in a mouse model that mimics hepatic injury.
This study proposed a “2-hit model” where the “first hit” was liver injury that may have resulted from factors such as hyper-caloric diet intake while the “second hit” was subsequent environmental insults including PCB exposure. Possible outcomes of such a 2-hit scenario takes into account the vital role that the hepatic organ system plays in both xenobiotic and endobiotic metabolism, thereby influencing PCB126 metabolism and systemic levels, while altering the endobiotic metabolic machinery simultaneously. In addition, studying the effects of PCB126 exposure in the presence of pre-existing diseased conditions such as NAFLD and NASH enable us to better comprehend the inter-organ communication such as the liver-peripheral vascular axis. It is pertinent to note that PCB126 by itself can elicit toxicity in different organs leading to different pathological outcomes which can further complicate organ-organ crosstalk. Indeed, the key findings from this study demonstrated that PCB126 exposure by itself induced a certain degree of toxicity on the liver and peripheral vasculature (Figure 6). PCB126-induced steatosis was evident in the histological hepatic sections in the CD group while upregulated circulating adhesion molecules such as Icam-1 and E-selection as well as PAI-1 indicated PCB126-mediated inflammation in the CD group. PCB126 exposure also mediated changes in hepatic gene expression such as increased Cyp1a2, the AhR target gene and Cd36, the fatty acid transporter which may have led to the observable hepatic fat accumulation. However, it was the combination of PCB126 exposure with an MCD diet that led to perturbations in key metabolic events and severe hepatic and extra-hepatic inflammation. Consistent with literature reports, MCD feeding induced loss in BW and steatohepatitis (H&E staining) (Dela Pena et al., 2005; Marcolin et al., 2011). PCB126 exposure in MCD-fed mice worsened steatohepatitis as seen with upregulated Mcp-1, Tnfα, PAI-1, and Timp-1 gene expression levels along with increased LW and decreased fat mass versus the MCD-fed mice without PCB126 exposure.
Figure 6.
A schematic diagram demonstrating possible molecular mechanisms involved in the observed exacerbated toxicity caused by PCB126 exposure in mice fed the MCD diet. Underlined text denotes a manifested outcome due to PCB126 effects. FA, fatty acids; TG, triglycerides.
With regards to PCB126-induced changes on hepatic molecular mechanisms, hepatic gene expression analysis suggested that the MCD + PCB126 group suffered from a hepatic metabolic breakdown due to downregulation of major genes involved in lipid and carbohydrate metabolism while the MCD group displayed changes for genes related to lipid accumulation. For instance, hepatic genes involved in lipid accumulation and triglyceride synthesis (Pparg, Dgat2, and Mogat1) were downregulated in the MCD and MCD + PCB126 group with the exception of Cd36, a target gene shared by AhR and other nuclear receptors. Additionally, major genes involved in VLDL secretion and lipid export from the liver (Mttp and Apob) were also downregulated in the MCD groups, implying that steatosis was due to lack of lipid mobility to extra-hepatic systems, a mechanism for MCD-induced liver injury (Rinella et al., 2008). However, hepatic genes involved in lipid synthesis (Fas and Srebf1) and fatty acid oxidation (Cpt1a and Acaca) were downregulated only in the MCD + PCB126 group, suggesting a lack of de novo fat synthesis in this group accompanied by an absence of fatty acid breakdown as well. The results suggested that the decrease in fatty acid synthesis was not a compensation of the excessive hepatic lipid accumulation because the hepatic expression of the involved genes (Fas, Srebf1, and Acaca) was not decreased in the unexposed, MCD diet. In terms of genes related to glucose handling, PCB126 in the presence of a compromised liver downregulated gluconeogenic genes (Pepck-1 and G6Pase) creating a hypoglycemic environment in these mice with a concomitant decrease in glucose uptake (Slc2a2) and glucose breakdown (Pklr) for energy production. Measured fasting blood glucose levels also confirmed the decreased blood glucose levels in both MCD groups albeit low insulin levels. Furthermore, deficiency in carbohydrate metabolism was reflected in the calorimetric results where RER was lower in the MCD groups suggesting lipid breakdown as a more prominent fuel source.
A compelling observation in this study was the drastic increase in circulating levels of inflammatory cytokines and early markers of vascular inflammation including Icam-1, Pecam-1, E-selectin and thrombomodulin indicative of endothelial cell dysfunction. We have demonstrated previously that coplanar, dioxin-like PCBs can induce endothelial cell inflammation leading to upregulation of adhesion molecules and inflammatory cytokines (Han et al., 2012). In this study, there was a significant interaction between the MCD diet and PCB126 for most markers including systemic PAI-1, implying that the combination resulted in a more severe response. PAI-1 is an inhibitor of the plasminogen-activation pathway, and its circulating levels is correlated with occurrences of proatherogenic plaque formation (Vaughan, 2005). Interestingly, the fold increase of circulating PAI-1 observed in the MCD + PCB126 group was much higher compared with that of hepatic expression, suggesting that PAI-1 may have been secreted by other nonhepatic tissues such as the endothelium. The severity of the toxic response to the MCD + PCB126 combination was further confirmed by upregulated Fmo3 levels and elevated plasma and hepatic TMAO levels. Importantly, Fmo3 falls in the AhR target gene battery, and a previous study from our laboratory group showed that upregulated Fmo3 by “dioxin-like” compounds could be a risk factor for CVDs based on its enzymatic role on TMAO conversion from TMA (Petriello et al., 2016). Epidemiologic studies have also reported increased circulating plasma TMAO, formed from choline and carnitine-rich foods, as a biomarker for atherosclerosis and CVD (Mente et al., 2015; Ufnal et al., 2015). However, the role of TMAO in CVD is not clear with another group reporting that high TMAO levels slowed aortic lesion formation (Collins et al., 2016). Therefore, the role of TMAO in CVD requires further studies and clarification in order to identify its causality in CVD. In this study, mice fed the MCD diet and exposed to PCB126 still exhibited upregulated TMAO levels in spite of choline deficiency in the diet, indicating that the mice used noncholine sources for TMAO formation. Even though evidence on the mechanistic role of TMAO in promoting CVD is still lacking, the results nonetheless established a link between AhR activation, Fmo3 upregulation and increased CVD biomarker plasma levels consistent with previous findings (Petriello et al., 2016; Schugar and Brown, 2015).
In terms of extra-hepatic toxicity related to other organ systems, the MCD + PB126 group showed upregulated pro-inflammatory Il-6 expression in the spleen with a concomitant decrease in anti-inflammatory Il-10 expression. PCB126 also appeared to induce inflammation in the white adipose tissue but PCB126-induced changes on leptin and resistin levels were not observed. Therefore, the results indicated that the MCD + PCB126 group of mice bore resemblance to a hypoglycemic phenotype (compromised hepatic glucose uptake and downregulated gluconeogenesis), while simultaneously exhibiting dysregulated lipid levels indicative of dyslipidemia caused by disrupted energy homeostasis and exacerbated inflammation on the peripheral vasculature that was absent in PCB126-exposed mice that did not suffer from a compromised liver.
Taken together, this study demonstrated that exposure to “dioxin-like” pollutants such as PCB126, in the presence of liver injury, aggravated systemic inflammation by inducing toxicity directly on the primary target as well as affecting other organ systems prone to inflammation such as the vascular endothelium. The study revealed key proteins and metabolites that may lead to crosstalk between fatty liver disease and peripheral vascular diseases indicative of atherosclerosis such as PAI-1 and TMAO. Although in the this model, we did not observe the occurrence of accelerated atherosclerotic lesion formation, future studies potentially utilizing atherosclerotic mouse models will provide more mechanistic insight on PCB126 effects on CVD. Furthermore, using even lower doses of PCB126 over longer study durations will also allow us to study effects of PCB exposures that closely resemble human exposure patterns. The findings of this study also shed some light on how disease pathology can unfold in NAFLD/NASH patients and cohorts who may be residing in hazardous areas susceptible to environmental pollutant exposure, and how improving hepatic health can be a positive mediator for attenuating cardiovascular complications. Furthermore, studying a dioxin-like PCB is applicable to human health because of its persistent, pro-oxidative and proinflammatory nature. Finally, human exposure encompasses multitudes of dioxin-like pollutants, which can exert AhR-like effects and have a mode of action similar to PCB126.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This study was supported by the National Institute of Environmental Health Sciences (P42ES007380, T32ES007266); and the National Institute of General Medical Sciences (8P20GM103527-06).
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr Wendy Katz (UK COBRE Research Core) for assisting with the metabolic cages and Dr Travis Sexton (UK Saha Cardiovascular Research Center) for assisting with the MAGPIX operation; and the University of Louisville for assistance with the Piccolo Xpress chemistry analyzer.
REFERENCES
- Al-Salman F., Plant N. (2012). Non-coplanar polychlorinated biphenyls (PCBs) are direct agonists for the human pregnane-X receptor and constitutive androstane receptor, and activate target gene expression in a tissue-specific manner. Toxicol. Appl. Pharmacol. 263, 7–13. [DOI] [PubMed] [Google Scholar]
- Aminov Z., Haase R., Rej R., Schymura M. J., Santiago-Rivera A., Morse G., DeCaprio A., Carpenter D. O. and Akwesasne Task Force on the, E. (2016). Diabetes Prevalence in Relation to Serum Concentrations of Polychlorinated Biphenyl (PCB) Congener Groups and Three Chlorinated Pesticides in a Native American Population. Environ. Health Perspect. 124, 1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestri S., Lonardo A., Bonapace S., Byrne C. D., Loria P., Targher G. (2014). Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J. Gastroenterol. 20, 1724–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum L. S., Dutton N. D., Cusack C., Mennemeyer S. T., Pavuk M. (2016). Anniston community health survey: Follow-up and dioxin analyses (ACHS-II)–methods. Environ. Sci. Pollut. Res. Int. 23, 2014–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher M. P., Lefebvre C., Chapados N. A. (2015). The effects of PCB126 on intra-hepatic mechanisms associated with non alcoholic fatty liver disease. J. Diabetes Metab. Disord. 14, 88.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave M., Appana S., Patel M., Falkner K. C., McClain C. J., Brock G. (2010). Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003-2004. Environ. Health Perspect. 118, 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins H. L., Drazul-Schrader D., Sulpizio A. C., Koster P. D., Williamson Y., Adelman S. J., Owen K., Sanli T., Bellamine A. (2016). L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis 244, 29–37. [DOI] [PubMed] [Google Scholar]
- Consonni D., Sindaco R., Bertazzi P. A. (2012). Blood levels of dioxins, furans, dioxin-like PCBs, and TEQs in general populations: A review, 1989-2010. Environ. Int. 44, 151–162. [DOI] [PubMed] [Google Scholar]
- Crinnion W. J. (2011). Polychlorinated biphenyls: persistent pollutants with immunological, neurological, and endocrinological consequences. Altern. Med. Rev. 16, 5–13. [PubMed] [Google Scholar]
- Dela Pena A., Leclercq I., Field J., George J., Jones B., Farrell G. (2005). NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology 129, 1663–1674. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R., Yebra-Pimentel I., Martinez-Carballo E., Simal-Gandara J. (2015). A Critical Review about Human Exposure to Polychlorinated Dibenzo-p-Dioxins (PCDDs), Polychlorinated Dibenzofurans (PCDFs) and Polychlorinated Biphenyls (PCBs) through Foods. Crit. Rev. Food Sci. Nutr. 55, 1590–1617. [DOI] [PubMed] [Google Scholar]
- Gadupudi G. S., Klaren W. D., Olivier A. K., Klingelhutz A. J., Robertson L. W. (2016). PCB126-Induced disruption in gluconeogenesis and fatty acid oxidation precedes fatty liver in male rats. Toxicol. Sci. 149, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahrs M., Roos R., Andersson P. L., Schrenk D. (2013). Role of the nuclear xenobiotic receptors CAR and PXR in induction of cytochromes P450 by non-dioxinlike polychlorinated biphenyls in cultured rat hepatocytes. Toxicol. Appl. Pharmacol. 272, 77–85. [DOI] [PubMed] [Google Scholar]
- Hadi A. M., Mouchaers K. T., Schalij I., Grunberg K., Meijer G. A., Vonk-Noordegraaf A., van der Laarse W. J., Belien J. A. (2011). Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol. (Dordr.) 34, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. G., Han S. S., Toborek M., Hennig B. (2012). EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol. Appl. Pharmacol. 261, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B., Meerarani P., Slim R., Toborek M., Daugherty A., Silverstone A. E., Robertson L. W. (2002). Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol. Appl. Pharmacol. 181, 174–183. [DOI] [PubMed] [Google Scholar]
- Lai I., Chai Y., Simmons D., Luthe G., Coleman M. C., Spitz D., Haschek W. M., Ludewig G., Robertson L. W. (2010). Acute toxicity of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status. Environ. Int. 36, 918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai I. K., Dhakal K., Gadupudi G. S., Li M., Ludewig G., Robertson L. W., Olivier A. K. (2012). N-acetylcysteine (NAC) diminishes the severity of PCB 126-induced fatty liver in male rodents. Toxicology 302, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo M., Hernaez R., Eberhardt M. S., Bonekamp S., Kamel I., Guallar E., Koteish A., Brancati F. L., Clark J. M. (2013). Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am. J. Epidemiol. 178, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind P. M., Orberg J., Edlund U. B., Sjoblom L., Lind L. (2004). The dioxin-like pollutant PCB 126 (3,3',4,4',5-pentachlorobiphenyl) affects risk factors for cardiovascular disease in female rats. Toxicol. Lett. 150, 293–299. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lonardo A., Ballestri S., Marchesini G., Angulo P., Loria P. (2015). Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig. Liver Dis. 47, 181–190. [DOI] [PubMed] [Google Scholar]
- Luthe G., Jacobus J. A., Robertson L. W. (2008). Receptor interactions by polybrominated diphenyl ethers versus polychlorinated biphenyls: a theoretical Structure-activity assessment. Environ. Toxicol. Pharmacol. 25, 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M. V., Michelotti G. A., Xie G., Almeida Pereira T., Boursier J., Bohnic B., Guy C. D., Diehl A. M. (2015). Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One 10, e0127991.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcolin E., Forgiarini L. F., Tieppo J., Dias A. S., Freitas L. A., Marroni N. P. (2011). Methionine- and choline-deficient diet induces hepatic changes characteristic of non-alcoholic steatohepatitis. Arq. Gastroenterol. 48, 72–79. [DOI] [PubMed] [Google Scholar]
- Mente A., Chalcraft K., Ak H., Davis A. D., Lonn E., Miller R., Potter M. A., Yusuf S., Anand S. S., McQueen M. J. (2015). The relationship between trimethylamine-N-oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can. J. Cardiol. 31, 1189–1194. [DOI] [PubMed] [Google Scholar]
- National Toxicology, P. (2006a). NTP technical report on the toxicology and carcinogenesis studies of 2,2',4,4',5,5'-hexachlorobiphenyl (PCB 153) (CAS No. 35065-27-1) in female Harlan Sprague-Dawley rats (Gavage studies). Natl Toxicol. Program Tech. Rep. Ser. 529, 4–168. [PubMed] [Google Scholar]
- National Toxicology, P. (2006b). NTP toxicology and carcinogenesis studies of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) in female Harlan Sprague-Dawley rats (Gavage Studies). Natl. Toxicol. Prog. Tech. Rep. Ser. 520, 4–246. [PubMed] [Google Scholar]
- Oesterling E., Toborek M., Hennig B. (2008). Benzo[a]pyrene induces intercellular adhesion molecule-1 through a caveolae and aryl hydrocarbon receptor mediated pathway. Toxicol. Appl. Pharmacol. 232, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. H., Lim J. E., Park H., Jee S. H. (2016). Body burden of persistent organic pollutants on hypertension: a meta-analysis. Environ. Sci. Pollut. Res. Int. 23, 14284–14293. [DOI] [PubMed] [Google Scholar]
- Pavuk M., Olson J. R., Sjodin A., Wolff P., Turner W. E., Shelton C., Dutton N. D., Bartell S. and Anniston Environmental Health Research, C. (2014). Serum concentrations of polychlorinated biphenyls (PCBs) in participants of the Anniston Community Health Survey. Sci. Total Environ. 473-474, 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petriello M. C., Hoffman J. B., Sunkara M., Wahlang B., Perkins J. T., Morris A. J., Hennig B. (2016). Dioxin-like pollutants increase hepatic flavin containing monooxygenase (FMO3) expression to promote synthesis of the pro-atherogenic nutrient biomarker trimethylamine N-oxide from dietary precursors. J. Nutr. Biochem. 33, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre S., Chevallier A., Teixeira-Clerc F., Ambolet-Camoit A., Bui L. C., Bats A. S., Fournet J. C., Fernandez-Salguero P., Aggerbeck M., Lotersztajn S., et al. (2014). Aryl hydrocarbon receptor-dependent induction of liver fibrosis by dioxin. Toxicol. Sci. 137, 114–124. [DOI] [PubMed] [Google Scholar]
- Rinella M. E., Elias M. S., Smolak R. R., Fu T., Borensztajn J., Green R. M. (2008). Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J. Lipid Res. 49, 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S. H. (1994). Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol. 24, 87–149. [DOI] [PubMed] [Google Scholar]
- Schecter A., Cramer P., Boggess K., Stanley J., Papke O., Olson J., Silver A., Schmitz M. (2001). Intake of dioxins and related compounds from food in the U.S. population. J. Toxicol. Environ. Health A 63, 1–18. [DOI] [PubMed] [Google Scholar]
- Schugar R. C., Brown J. M. (2015). Emerging roles of flavin monooxygenase 3 in cholesterol metabolism and atherosclerosis. Curr. Opin. Lipidol. 26, 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taesuwan S., Cho C. E., Malysheva O. V., Bender E., King J. H., Yan J., Thalacker-Mercer A. E., Caudill M. A. (2017). The metabolic fate of isotopically labeled trimethylamine-N-oxide (TMAO) in humans. J. Nutr. Biochem. 45, 77–82. [DOI] [PubMed] [Google Scholar]
- Taylor K. W., Novak R. F., Anderson H. A., Birnbaum L. S., Blystone C., Devito M., Jacobs D., Kohrle J., Lee D. H., Rylander L., et al. (2013). Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ. Health Perspect. 121, 774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than N. N., Newsome P. N. (2015). A concise review of non-alcoholic fatty liver disease. Atherosclerosis 239, 192–202. [DOI] [PubMed] [Google Scholar]
- Ufnal M., Zadlo A., Ostaszewski R. (2015). TMAO: A small molecule of great expectations. Nutrition 31, 1317–1323. [DOI] [PubMed] [Google Scholar]
- van Ede K. I., Andersson P. L., Gaisch K. P., van den Berg M., van Duursen M. B. (2013). Comparison of intake and systemic relative effect potencies of dioxin-like compounds in female mice after a single oral dose. Environ. Health Perspect. 121, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D. E. (2005). PAI-1 and atherothrombosis. J Thromb Haemost 3, 1879–1883. [DOI] [PubMed] [Google Scholar]
- Vogel C. F., Sciullo E., Matsumura F. (2004). Activation of inflammatory mediators and potential role of ah-receptor ligands in foam cell formation. Cardiovasc. Toxicol. 4, 363–373. [DOI] [PubMed] [Google Scholar]
- Wahlang B., Falkner K. C., Clair H. B., Al-Eryani L., Prough R. A., States J. C., Coslo D. M., Omiecinski C. J., Cave M. C. (2014a). Human receptor activation by aroclor 1260, a polychlorinated biphenyl mixture. Toxicol. Sci. 140, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B., Falkner K. C., Gregory B., Ansert D., Young D., Conklin D. J., Bhatnagar A., McClain C. J., Cave M. (2013). Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J. Nutr. Biochem. 24, 1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B., Perkins J. T., Petriello M. C., Hoffman J. B., Stromberg A. J., Hennig B. (2017). A compromised liver alters polychlorinated biphenyl-mediated toxicity. Toxicology 380, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B., Song M., Beier J. I., Cameron Falkner K., Al-Eryani L., Clair H. B., Prough R. A., Osborne T. S., Malarkey D. E., Christopher States J., et al. (2014b). Evaluation of Aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol. Appl. Pharmacol. 279, 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Group M., Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., Das S. R., de Ferranti S., Despres J. P., American Heart Association Statistics, C., and Stroke Statistics, S., et al. (2016). Executive Summary: Heart Disease and Stroke Statistics–2016 Update: A Report From the American Heart Association. Circulation 133, 447–454., [DOI] [PubMed] [Google Scholar]
- Xiao L., Zhang Z., Luo X. (2014). Roles of xenobiotic receptors in vascular pathophysiology. Circ. J. 78, 1520–1530. [DOI] [PubMed] [Google Scholar]
- Xu W., Wang X., Cai Z. (2013). Analytical chemistry of the persistent organic pollutants identified in the Stockholm Convention: A review. Anal. Chim. Acta 790, 1–13. [DOI] [PubMed] [Google Scholar]
- Xue J., Liu S. V., Zartarian V. G., Geller A. M., Schultz B. D. (2014). Analysis of NHANES measured blood PCBs in the general US population and application of SHEDS model to identify key exposure factors. J. Expo. Sci. Environ. Epidemiol. 24, 615–621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.