Abstract
Background. The evidence base regarding the safety of intravenous (IV) iron therapy in patients with chronic kidney disease (CKD) is incomplete and largely based on small studies of relatively short duration.
Methods. FIND-CKD (ClinicalTrials.gov number NCT00994318) was a 1-year, open-label, multicenter, prospective study of patients with nondialysis-dependent CKD, anemia and iron deficiency randomized (1:1:2) to IV ferric carboxymaltose (FCM), targeting higher (400–600 µg/L) or lower (100–200 µg/L) ferritin, or oral iron. A post hoc analysis of adverse event rates per 100 patient-years was performed to assess the safety of FCM versus oral iron over an extended period.
Results. The safety population included 616 patients. The incidence of one or more adverse events was 91.0, 100.0 and 105.0 per 100 patient-years in the high ferritin FCM, low ferritin FCM and oral iron groups, respectively. The incidence of adverse events with a suspected relation to study drug was 15.9, 17.8 and 36.7 per 100 patient-years in the three groups; for serious adverse events, the incidence was 28.2, 27.9 and 24.3 per 100 patient-years. The incidence of cardiac disorders and infections was similar between groups. At least one ferritin level ≥800 µg/L occurred in 26.6% of high ferritin FCM patients, with no associated increase in adverse events. No patient with ferritin ≥800 µg/L discontinued the study drug due to adverse events. Estimated glomerular filtration rate remained the stable in all groups.
Conclusions. These results further support the conclusion that correction of iron deficiency anemia with IV FCM is safe in patients with nondialysis-dependent CKD.
Keywords: anemia, cardiovascular disease, oxidative stress
INTRODUCTION
It is estimated that iron deficiency affects over half of all adults with chronic kidney disease (CKD) Stage 3 or 4 [1], with adverse consequences for both erythropoiesis and nonhematopoietic cellular functions for which iron is essential [2]. The Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Anemia in CKD suggests that clinicians consider iron therapy in patients with a serum ferritin concentration ≤500 µg/L, or a transferrin saturation (TSAT) level ≤30%, if they desire an increase in hemoglobin (Hb) or reduction of erythropoiesis-stimulating agent (ESA) dose [3].
Recent years have seen the introduction of new intravenous (IV) iron preparations that permit administration of larger single doses, expanding treatment opportunities [4]. However, if weakly bound ‘labile’ iron is released rapidly from IV iron complexes, the transport protein transferrin can become saturated. This can lead to significant amounts of nontransferrin-bound iron that can induce oxidative stress by catalyzing lipid peroxidation and reactive oxygen species formation [5], potentially contributing to endothelial injury with adverse cardiovascular or renal consequences [6]. There are also concerns that following IV iron therapy increased amounts of iron could become available to invading microbes [7], which, coupled with impaired immunological responses in the presence of excess iron [6], could increase the risk for infection.
Further evidence is required concerning the relative safety of different iron therapies in patients with CKD. However, trials of IV iron therapy in patients with nondialysis-dependent CKD have been relatively small and of short duration [8]. The evidence base regarding safety is incomplete, as discussed recently at a conference convened by the KDIGO initiative to identify gaps in knowledge so as to inform future clinical research [9].
FIND-CKD (Ferinject® assessment in patients with Iron deficiency anemia and NonDialysis-dependent Chronic Kidney Disease) was a randomized, multicenter trial of IV ferric carboxymaltose (FCM) versus oral iron in patients with nondialysis-dependent CKD and iron deficiency anemia who were not receiving treatment with an ESA [10]. The primary objective of the study was to test whether FCM reduces the need for alternative anemia therapy (e.g. blood transfusion or ESA therapy) compared with oral iron in this setting. Safety data were captured throughout the 1-year study. A post hoc analysis of adverse event rates per 100 patient-years was performed to assess the safety of FCM versus oral iron over an extended period.
MATERIALS AND METHODS
FIND-CKD was a 56-week, open-label, multicenter, prospective, randomized, three-arm study undertaken at 193 nephrology centers in 20 countries during 2009–12 (ClinicalTrials.gov number NCT00994318) [10].
Study population
Eligibility criteria have been described in full previously [10]. Adult patients with nondialysis-dependent CKD were eligible if (i) at least one Hb level was 9–11 g/dL; (ii) any ferritin level was <100 µg/L, or <200 µg/L with TSAT <20%; (iii) estimated glomerular filtration rate (eGFR) was ≤60 mL/min/1.73 m2 [Modification of Diet in Renal Disease-4 (MDRD-4) equation [11]] and eGFR loss was ≤12 mL/min/1.73 m²/year with predicted eGFR at 12 months ≥15 mL/min/1.73 m2; and (iv) no ESA therapy was received within 4 months of randomization. Exclusion criteria included a documented history of discontinuing oral iron products due to significant gastrointestinal distress and active malignancy.
Study treatment
Patients were randomized in 1:1:2 ratio to high ferritin FCM, low ferritin FCM or oral iron. FCM dose (Ferinject®, Vifor International, St Gallen, Switzerland) in the high ferritin and low ferritin FCM groups was adjusted to target a ferritin level of 400−600 and 100–200 µg/L, respectively [10]. Oral iron therapy consisted of commercially available ferrous sulfate at a dose of 304 mg (100 mg of iron) twice daily to Week 52.
Until Week 8 post-randomization, patients were not to receive ESAs, blood transfusion or any anemia therapy other than study drug unless there was an absolute requirement.
Safety data collection
All adverse events and serious adverse events were recorded at every study visit. A second or subsequent occurrence of the same adverse event in the same patient was documented.
An adverse event was classified as serious if it resulted in death, was life-threatening, required or prolonged hospitalization, led to persistent or significant disability/incapacity or was considered an important medical event. An event was classified as ‘related’ to study drug if a causal relationship between the event and the study drug was at least a reasonable possibility based on the investigator's medical judgement. In addition, adverse events that required hospitalization are described separately.
Ferritin and Hb were measured centrally. TSAT and C-reactive protein were measured locally. eGFR was calculated based on the MDRD-4 equation using locally measured creatinine values [11]. All laboratory values are reported for the intention-to-treat population (n = 616).
Statistical analysis
Adverse events and serious adverse events are reported per 100 patient-years of follow-up, both as (i) incidence, i.e. occurrence of one or more event and (ii) the cumulative number of events, i.e. every event regardless of whether it recurred in the same patient. The latter approach ensures that multiple occurrences of the same event in the same patient are recognized. Event rates were analyzed as only descriptive analyses, and no statistical inference was performed. Performing multiplicity testing and generating P-values for each category of adverse events for a nonsafety study was not deemed appropriate and would highly inflate global type-1 error.
Adverse events and serious adverse events are reported up to the point at which another anemia therapy was initiated and/or the randomized study medication was discontinued (‘the safety period’). For neoplasms, all events during the total study period to the final follow-up visit are reported. Additionally, the cumulative number of adverse events, serious adverse events, serious adverse events of special interest (events classified as cardiac disorders or as infections) and hospitalizations due to adverse events are reported during the total study period (i.e. to the final follow-up visit).
Safety analyses were based on all patients who received at least one dose of randomized treatment (the safety population).
All statistical analyses were performed at the 5% level using SAS Version 9.3® (SAS Institute Inc., SAS/STAT, Cary, NC, USA). Continuous outcomes (e.g. ferritin levels) were analyzed by ANCOVA. Binary outcomes such as onset of neoplasm were analyzed by logistic regression methodology. Time to event outcomes were analyzed by the log-rank statistic on Kaplan–Meier survival curves.
RESULTS
Patient population
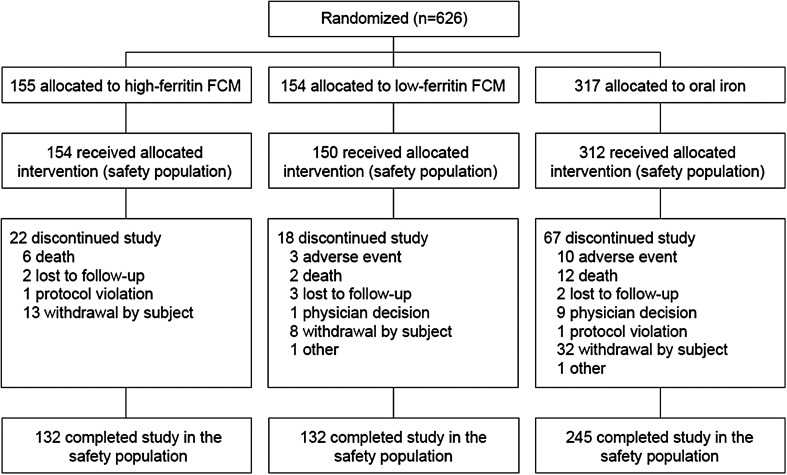
In total, 626 patients were randomized. Of these, 10 did not receive any study drug, such that the safety population included 616 patients (154 high ferritin FCM, 150 low ferritin FCM, 312 oral iron). The study was completed by 519 patients. The most frequent reasons for study discontinuation were patient withdrawal (n = 53), death (n = 20) and adverse events (n = 13) (Figure 1).
FIGURE 1.
Patient disposition (safety population). FCM, ferric carboxymaltose.
Demographic and baseline characteristics showed no marked differences between the three treatment groups (Table 1). The medical history was as expected for a population of patients with CKD and was similar between groups. Hypertension was present at the time of study entry in 31.2, 32.7 and 23.7% of patients in the high ferritin FCM, low ferritin FCM and oral iron groups. A history of neoplasm was reported in 20.1, 17.3 and 15.4% of patients, respectively.
Table 1.
Baseline characteristics and relevant medical history (safety population)
| High ferritin FCM (n = 154) | Low ferritin FCM (n = 150) | Oral iron (n = 312) | |
|---|---|---|---|
| Age, years | 69.5 (12.6) | 68.1 (13.3) | 69.3 (13.4) |
| Male gender, n (%) | 62 (40.3) | 54 (36.0) | 116 (37.2) |
| White, n (%) | 150 (97.4) | 142 (94.7) | 295 (94.6) |
| Body mass index, kg/m2 | 29.7 (6.6) | 29.9 (6.0) | 29.1 (5.9) |
| eGFR, mL/min/1.73 m2 | |||
| Mean (SD) | 32.9 (11.7) | 31.5 (10.7) | 32.2 (11.5) |
| Median (IQR) | 32 (24, 40) | 30 (23, 39) | 31 (24, 38) |
| Hemoglobin, g/dL | |||
| Mean (SD) | 10.3 (0.7) | 10.5 (0.8) | 10.4 (0.7) |
| Median (IQR) | 10.3 (10.0, 10.7) | 10.6 (10.1, 11.0) | 10.5 (7.3, 12.1) |
| Ferritin, µg/L | |||
| Mean (SD) | 58 (48) | 55 (48) | 57 (42) |
| Median (IQR) | 50 (16, 81) | 44 (17, 80) | 50 (26, 80) |
| Transferrin saturation, % | |||
| Mean (SD) | 16.2 (16.6) | 16.1 (8.3) | 15.5 (7.6) |
| Median (IQR) | 14.2 (9.2, 19.2) | 14.3 (10.0, 19.3) | 15.0 (9.4, 20.0) |
| C-reactive protein, mg/L | |||
| Mean (SD) | 6.8 (11.2) | 6.2 (9.1) | 5.2 (6.0) |
| Median (IQR) | 3.5 (2.0, 7.6) | 4.0 (1.7, 7.1) | 3.5 (1.6, 6.3) |
| Medical history | |||
| Hypertension, n (%) | |||
| History of hypertension | 134 (87.0) | 133 (88.7) | 268 (85.9) |
| Hypertension at study entrya | 48 (31.2) | 49 (32.7) | 74 (23.7) |
| Cardiac disorder, n (%) | 75 (48.7) | 72 (48.0) | 155 (49.7) |
| Atrial fibrillation | 22 (14.3) | 20 (13.3) | 31 (9.9) |
| Coronary artery disease | 20 (13.0) | 18 (12.0) | 41 (13.1) |
| Myocardial ischemia | 18 (11.7) | 13 (8.7) | 31 (9.9) |
| Myocardial infarction | 13 (8.4) | 11 (7.3) | 22 (7.1) |
| Cardiac failure | 8 (5.2) | 13 (8.7) | 30 (9.6) |
| Congestive heart failure | 9 (5.8) | 5 (3.3) | 6 (1.9) |
| Acute myocardial infarction | 5 (3.2) | 5 (3.3) | 1 (0.3) |
| Infection, n (%) | 19 (18.8) | 32 (21.3) | 57 (18.3) |
| Urinary tract infection | 9 (5.8) | 4 (2.7) | 15 (4.8) |
| Pneumonia | 3 (1.9) | 5 (3.3) | 9 (2.9) |
| History of diabetes, n (%) | 86 (57.1) | 96 (64.0) | 196 (62.8) |
| History of neoplasm, n (%) | 31 (20.1) | 26 (17.3) | 48 (15.4) |
Continuous variables are shown as mean (SD). The safety population included all patients who received one or more doses of randomized treatment.
FCM, ferric carboxymaltose; eGFR, estimated glomerular filtration rate; IQR, interquartile range; SD, standard deviation.
aDefined as systolic >145 mmHg or diastolic >95 mmHg.
Treatment
The mean [standard deviation (SD)] cumulative dose of FCM of the safety population was 2685 (978) mg iron and 1040 (618) mg iron in the high ferritin and low ferritin groups, respectively; the mean (SD) number of FCM injections was 4.2 (1.7) (range 1–10) and 5.2 (3.1) (range 1–14). The mean (SD) cumulative dose of oral iron was 52 435 (24 768) mg iron, and the mean (SD) total number of oral iron tablets was 524 (248) (range 2–800).
Observation time
The mean (SD) observation time from first dose of study drug to the end of the safety period was 10.8 (3.7) months in the high ferritin FCM group [median (interquartile range, IQR) 13.0 months (9.2, 13.0)], 10.3 (4.1) months in the low ferritin FCM group [median 12.8 months (7.4, 13.0)] and 9.4 (5.0) months in the oral iron group [median 12.8 months (4.0, 13.0)]. The total number of patient-years of follow-up was 138.5, 129.0 and 242.8 in the high ferritin FCM, low ferritin FCM and oral iron groups, respectively, and 267.8 years for both FCM groups combined.
Iron status
In the high ferritin FCM group, mean (SD) ferritin level increased from 58 (48) µg/L at baseline [median (IQR) 51 µg/L (15, 81)] to 403 (190) µg/L at Week 4 [median (IQR) 401 µg/L (255, 542)] and to 592 (195) µg/L at Week 24 [median (IQR) 583 µg/L (491, 670)], plateauing thereafter to Week 52, when the mean level was 585 (171) µg/L [median (IQR) 547 µg/L (476, 650)]. In the low ferritin FCM group, there was a gradual, smaller increase in ferritin which plateaued from Week 36 onwards; mean (SD) ferritin at Week 52 was 139 (57) µg/L [median (IQR) 132 µg/L (99, 169)]. Values in the oral iron group increased gradually throughout the study, reaching 199 (135) µg/L at Week 52 [median (IQR) 181 µg/L (123, 432)]. Ferritin level was significantly higher in the high FCM group versus the oral iron group from Week 4 onwards [all P < 0.001, analysis of covariance (ANCOVA) analysis].
All groups showed an increase in TSAT by Week 4. At Week 52, mean (SD) values for TSAT were higher in the high ferritin FCM group [31.4 (10.5)%] [median (IQR) 29.0% (26.0, 36.0)] and oral iron group [29.3 (16.5)%] [median (IQR) 26.0% (20.0, 33.0)] than in the low ferritin FCM group [23.8 (9.4)%] [median (IQR) 23.0% (18.0, 28.0)], but within KDIGO guidelines for all three groups [3].
High ferritin levels
Ferritin ≥800 µg/L occurred at least once in 41 patients (26.6%) in the high ferritin FCM group, 9 of whom had at least two consecutive measurements of ferritin ≥800 µg/L (5.8%). Ferritin ≥800 µg/L was not observed in any patient in the low ferritin FCM group, and in only one patient (0.3%) in the oral iron group. In the high ferritin FCM group, ferritin ≥1000 µg/L occurred at least once in 13 patients (8.4%), and ferritin ≥1200 µg/L occurred at least once in 5 patients (3.2%). One patient randomized to oral iron had ferritin ≥1000 µg/L (0.3%). The mean total dose of FCM in the high ferritin group was similar in patients with one or more ferritin values ≥800 µg/L, 2561 mg iron compared with the overall high ferritin FCM group (2685 mg iron). The incidences of adverse events and serious adverse events in patients with serum ferritin ≥800 µg/L (80.5 and 24.4%) were comparable to those seen in the overall safety population. No patient in the high ferritin FCM group with serum ferritin ≥800 µg/L at any time discontinued the study drug due to adverse events.
Low ferritin levels
The incidences of adverse events and serious adverse events in patients randomized to low ferritin FCM and oral iron in whom ferritin ≤200 µg/L was observed at least once were 88.1 and 23.1% for low ferritin FCM (n = 143) and 80.6 and 16.9% for oral iron (n = 278). For patients in whom the ferritin level was always ≤200 µg/L, the incidences of adverse and serious adverse events were 90.1 and 25.7% in the low ferritin FCM group (n = 101), and 82.6 and 21.0% in the oral iron group (n = 167).
FCM dose
Data from the high ferritin FCM and low ferritin FCM groups were pooled to assess adverse events according to quartiles of total FCM dose (<1000, 1000 to <1650, 1650–2500 and >2500 mg iron). The mean duration of follow-up for safety events was somewhat lower for patients in the lowest quartile of FCM dose (265 days) compared with patients receiving 1000 to <1650, 1650–2500 or >2500 mg iron (336, 337 and 346 days, respectively). From the lowest to the highest dose quartile, the incidence of any adverse event was 79.5% (58/73), 87.3% (69/79), 82.7% (67/81) and 85.9% (61/71); the cumulative number of events per 100 patient-years was 393, 435, 477 and 417. For serious adverse events, the incidence from the lowest to the highest quartile of FCM dose was 17.8, 27.8, 25.9 and 26.8%, with the cumulative number of events per 100 years being 36, 47, 41 and 40.
Adverse events
The incidence of one or more adverse events was 91.0, 100.0 and 105.0 per 100 patient-years in the high ferritin FCM, low ferritin FCM and oral iron groups, respectively (Table 2). The cumulative number of adverse events per 100 patient-years was similar across treatment groups both during the safety period (Table 3) and during the total study period to final follow-up (Supplementary data, Table S1). The adverse events that recurred (i.e. were reported more than once in the same patient) most frequently were peripheral edema (1.8% of patients overall), diarrhea (1.6%), nasopharyngitis (1.1%), hypertension (1.1%), urinary tract infection (1.0%) and hypoglycemia (1.0%).
Table 2.
Incidences of one or more adverse event or serious adverse event per 100 patient-years (PY) during the safety perioda (safety population)
| All events |
Events with suspected relation to study drug |
|||||
|---|---|---|---|---|---|---|
| High ferritin FCM (n = 154) (138.5 PY) |
Low ferritin FCM (n = 150) (129.0 PY) |
Oral iron (n = 312) (242.8 PY) |
High ferritin FCM (n = 154) (138.5 PY) |
Low ferritin FCM (n = 150) (129.0 PY) |
Oral iron (n = 312) (242.8 PY) |
|
| Adverse events (per 100 PY) | 91.0 | 100.0 | 105.0 | 15.9 | 17.8 | 36.7 |
| No. patients with any adverse event (%) | 126 (81.8) | 129 (86.0) | 255 (81.7) | 22 (14.3) | 23 (15.3) | 89 (28.5) |
| Median time (days) to first event | 46 | 57 | 35 | 148 | 111 | 29 |
| Gastrointestinal disorders (per 100 PY) | 23.1 | 29.5 | 52.7 | 3.6 | 4.7 | 26.8 |
| Diarrhea | 10.8 | 8.5 | 18.5 | 1.4 | – | 7.8 |
| Constipation | 1.4 | 3.9 | 15.2 | 0.7 | 0.8 | 11.5 |
| Nausea | 6.5 | 5.4 | 6.2 | 0.7 | 1.6 | 2.9 |
| Vomiting | 4.3 | 3.1 | 5.4 | – | – | 0.8 |
| Dyspepsia | 1.4 | 2.3 | 7.0 | – | 0.8 | 3.7 |
| Infections (per 100 PY) | 36.8 | 39.5 | 39.1 | 2.9 | 1.6 | 2.9 |
| Urinary tract infection | 13.0 | 7.8 | 7.0 | 0.7 | – | 0.4 |
| Nasopharyngitis | 9.4 | 7.8 | 6.6 | 0.7 | – | 0.4 |
| Influenza | 2.9 | 6.2 | 2.9 | 0.7 | – | 0.8 |
| General disorders and administrative site conditions (per 100 PY) | 26.0 | 27.1 | 27.6 | 2.9 | 2.3 | 1.2 |
| Peripheral edema | 15.2 | 16.3 | 11.9 | 0.7 | – | 0.8 |
| Fatigue | 0.7 | 4.7 | 5.8 | – | 1.6 | – |
| Musculoskeletal and connective tissue disorders (per 100 PY) | 25.3 | 32.6 | 23.1 | 3.6 | 2.3 | 2.9 |
| Back pain | 10.8 | 9.3 | 4.5 | – | 0.8 | 0.4 |
| Arthralgia | 7.2 | 5.4 | 6.2 | – | – | 0.4 |
| Pain in extremity | 1.4 | 6.2 | 6.2 | – | 0.8 | 0.8 |
| Muscle spasms | 0.7 | 5.4 | 1.2 | 0.7 | – | – |
| Metabolism and nutrition disorders (per 100 PY) | 22.4 | 24.8 | 25.5 | – | 0.8 | 2.9 |
| Hypoglycemia | 5.1 | 3.9 | 4.5 | – | – | – |
| Hyperkalemia | 3.6 | 5.4 | 2.9 | – | – | – |
| Hyperglycemia | 5.1 | 0 | 2.1 | – | – | 0.4 |
| Vascular disorders (per 100 PY) | 23.8 | 20.2 | 21.4 | 2.9 | 1.6 | 0.8 |
| Hypertension | 15.2 | 10.9 | 13.2 | 2.2 | 0.8 | 0.4 |
| Hypotension | 5.8 | 3.1 | 2.1 | 0.7 | – | – |
| Respiratory, thoracic and mediastinal disorders (per 100 PY) | 13.7 | 20.9 | 16.9 | 0.7 | 0.8 | 1.2 |
| Dyspnea | 5.1 | 8.5 | 4.5 | – | 0.8 | 0.4 |
| Nervous system disorders (per 100 PY) | 18.1 | 21.7 | 13.6 | 2.9 | 3.9 | 2.1 |
| Dizziness | 6.5 | 6.2 | 2.9 | 0.7 | 0.8 | 0.4 |
| Headache | 4.3 | 7.8 | 2.9 | – | 0.8 | 0.8 |
| Investigations (per 100 PY) | 15.9 | 15.5 | 17.7 | 2.9 | – | 2.1 |
| Injury, poisoning and procedural complications (per 100 PY) | 12.3 | 17.1 | 12.8 | 0.7 | 1.6 | – |
| Cardiac disorders (per 100 PY) | 15.2 | 10.9 | 11.9 | 1.4 | 0.8 | – |
| Renal and urinary disorders (per 100 PY) | 11.6 | 8.5 | 14.0 | – | 0.8 | – |
| Skin and subcutaneous tissue disorders (per 100 PY) | 11.6 | 9.3 | 13.2 | – | 0.8 | – |
| Blood and lymphatic system disorders | 5.8 | 8.5 | 5.4 | 0.7 | 1.6 | – |
| Anemia (per 100 PY) | 5.1 | 6.2 | 4.1 | 0.7 | 1.6 | – |
| Neoplasms benign, malignant and unspecified (including cysts and polyps)b (per 100 PY) | 8.7 | 3.9 | 3.3 | – | 0.8 | – |
| Serious adverse events (per 100 PY) | 28.2 | 27.9 | 24.3 | – | – | 0.4 |
| No. of patients with any serious adverse event (%) | 39 (25.3) | 36 (24.0) | 59 (18.9) | 0 | 0 | 1 (0.3) |
| Median time (days) to first event | 143 | 134 | 67 | – | – | 3 |
| Cardiac disorders (per 100 PY) | 7.2 | 5.4 | 5.8 | – | – | – |
| Acute myocardial infarction | 1.4 | – | 1.6 | |||
| Cardiac failure | 0.7 | – | 1.2 | |||
| Cardiac failure congestive | 1.4 | 0.8 | 0.4 | |||
| Acute coronary syndrome | 1.4 | – | 0.4 | |||
| Infections (per 100 PY) | 4.3 | 3.9 | 4.9 | – | – | – |
| Pneumonia | – | 0.8 | 1.6 | |||
| Renal and urinary disorders (per 100 PY) | 4.3 | 1.6 | 5.4 | – | – | – |
| Chronic renal failure | 0.7 | 0.8 | 2.5 | |||
| Musculoskeletal and connective tissue disorders (per 100 PY) | 1.4 | 2.3 | 1.2 | – | – | – |
| Back pain | 1.4 | 0.8 | – | |||
| Metabolism and nutrition disorders | 2.9 | 4.7 | 2.9 | – | – | – |
| Hypoglycemia (per 100 PY) | 0.7 | 0.8 | 2.1 | |||
| Diabetes mellitus | – | 2.3 | 0.4 | |||
| Nervous system disorders (per 100 PY) | 1.4 | 0.8 | 2.5 | – | – | – |
| Syncope | – | – | 1.2 | |||
The safety population included all patients who received one or more doses of randomized treatment.
aThe safety period included all events up to the point at which another anemia therapy was initiated and/or the randomized study medication was discontinued.
bAll events to final follow-up are included.
Table 3.
Cumulative number of adverse events and serious adverse events during the safety perioda (safety population)
| High ferritin FCM (n = 154) (138.5 PY) |
Low ferritin FCM (n = 150) (129.0 PY) |
Oral iron (n = 312) (242.8 PY) |
|
|---|---|---|---|
| Adverse events | |||
| No. of patients | 126 | 129 | 255 |
| No. of events | 607 | 553 | 1069 |
| No. of events per 100 PY | 438.3 | 428.7 | 440.3 |
| Adverse events with suspected relation to study drug | |||
| No. of patients | 22 | 23 | 89 |
| No. of events | 43 | 39 | 140 |
| No. of events per 100 PY | 31.0 | 30.2 | 57.7 |
| Serious adverse events | |||
| No. of patients | 39 | 36 | 59 |
| No. of events | 60 | 51 | 99 |
| No. of events per 100 PY | 43.3 | 39.5 | 40.8 |
| Serious adverse events with suspected relation to study drug | |||
| No. of patients | 0 | 0 | 1 |
| No. of events | 0 | 0 | 1 |
| No. of events per 100 PY | 0 | 0 | 0.4 |
| Adverse events with suspected relation to study drug leading to study drug discontinuation | |||
| No. of patients (%) | 1 (0.7) | 2 (1.3) | 23 (7.5) |
| No. of events | 1 | 2 | 26 |
| No. of events per 100 PY | 0.7 | 1.6 | 10.7 |
| Median time (days) to first event | 242 | 155 | 29 |
The safety population included all patients who received one or more doses dose of randomized treatment.
PY, patient-years.
aThe safety period included all events up to the point at which another anemia therapy was initiated and/or the randomized study medication was discontinued.
The most common adverse events were gastrointestinal, the incidence of which was 23.1, 29.5 and 52.7 per 100 patient-years in the high ferritin FCM, low ferritin FCM and oral iron groups, respectively (Table 2). The incidence of other frequently occurring adverse events, including infections and cardiac disorders, showed no clear differences between treatment groups (Table 2).
The incidence of one or more adverse events with a suspected relation to study drug was twice as high with oral iron compared with FCM (15.9, 17.8 and 36.7 per 100 patient-years in high ferritin FCM, low ferritin FCM and oral iron groups, respectively), due to the higher incidence of gastrointestinal events (Table 2). The cumulative number of adverse events with a suspected relation to study drug per 100 patient-years was 31.0, 30.2 and 57.7, respectively.
The time to first adverse event, and particularly to first event with a suspected relation to study drug, was shorter in the oral iron group than in either FCM group (Table 2).
The incidence of one or more serious adverse events was 28.2, 27.9 and 24.3 per 100 patient-years in the high ferritin FCM, low ferritin FCM and oral iron groups, respectively (Table 2). The cumulative number of serious adverse events per 100 patient-years showed no marked difference between treatment groups either during the safety period (Table 3) or during the total study period to final follow-up (Supplementary data, Table S1). The incidence of hospitalizations for adverse events, and the cumulative number of such events, was also similar between groups during the safety period (Table 4). The cumulative number of hospitalizations for adverse events during the total follow-up period was 42.5, 42.2 and 62.1 events per 100 patient-years in the high ferritin FCM, low ferritin FCM and oral iron groups, respectively (Supplementary data, Table S2). The first serious adverse event developed sooner in the oral iron group (median 67 days post-baseline compared with 143 and 134 in the high ferritin and low ferritin FCM groups, respectively). There were no serious adverse events with a suspected relation to FCM.
Table 4.
(a) Incidence of one or more hospitalizations related to adverse events; (b) cumulative number of hospitalizations related to adverse events, during the safety perioda (safety population)
| High ferritin FCM (n = 154) (138.5 PY) |
Low ferritin FCM (n = 150) (129.0 PY) |
Oral iron (n = 312) (242.8 PY) |
|
|---|---|---|---|
| (a) Incidence of events | |||
| Hospitalization related to an adverse event | |||
| No. of patients (%) | 33 (21.4) | 35 (23.3) | 57 (18.3) |
| No. of patients with events per 100 PY | 23.8 | 27.1 | 23.5 |
| Hospitalization related to an adverse event classified as a cardiac disorder | |||
| No. of patients (%) | 7 (4.5) | 6 (4.0) | 13 (4.2) |
| No. of patients with events per 100 PY | 5.1 | 4.7 | 5.4 |
| Hospitalization related to an adverse event classified as an infection | |||
| No. of patients (%) | 5 (3.2) | 4 (2.7) | 12 (3.8) |
| No. of patients with events per 100 PY | 3.6 | 3.1 | 4.9 |
| (b) Cumulative number of events | |||
| Hospitalization related to an adverse event | |||
| No. of events | 50 | 48 | 95 |
| No. of events per 100 PY | 36.1 | 37.2 | 39.1 |
| Hospitalization related to an adverse event classified as a cardiac disorder | |||
| No. of events | 9 | 6 | 16 |
| No. of events per 100 PY | 6.5 | 4.7 | 6.6 |
| Hospitalization related to an adverse event classified as an infection | |||
| No. of events | 5 | 4 | 20 |
| No. of events per 100 PY | 3.6 | 3.1 | 8.2 |
The safety population included all patients who received one or more doses of randomized treatment.
PY, patient-years.
aThe safety period included all events up to the point at which another anemia therapy was initiated and/or the randomized study medication was discontinued.
Adverse events leading to study drug discontinuation were rare under FCM [one and two in the high ferritin and low ferritin groups, respectively, experienced one such event each (0.7 and 1.6 events per 100 patient-years)]. In the oral iron group, 22 patients (7.1%) experienced 26 adverse events which led to study drug discontinuation (10.7 events per 100 patient-years), at a median of 29 days after start of study drug. Discontinuation of oral iron was overwhelmingly due to gastrointestinal adverse events (20 out of 26 events).
Laboratory data
eGFR remained stable in all three groups during the study. Mean (SD) values were 35.6 (13.8), 32.1 (12.7) and 33.4 (14.5) mL/min/1.73 m2 in the high ferritin FCM, low ferritin FCM and oral iron groups at Week 52, respectively, compared with 32.8 (11.7), 31.5 (10.7) and 32.3 (11.6) mL/min/1.73 m2 at baseline.
Hypersensitivity reactions
Two treatment-related hypersensitivity reactions were reported in the low ferritin FCM group. One was a nonserious allergic reaction that occurred 29 days after the last of four 200 mg doses of FCM; administration of FCM was not affected. The second event was a moderate allergic reaction in which the patient developed fever and chills on the day of the fourth FCM dose of 200 mg. FCM therapy was discontinued. Both events resolved without treatment and there were no sequelae. One patient in the oral iron group developed a rash, which the investigator categorized as a serious adverse event and considered to be a delayed hypersensitivity reaction related to study drug; this led to oral iron discontinuation after the first dose.
Infections
The incidence of one or more infections reported as adverse events per 100 patient-years was similar in all three treatment groups, both overall and for the most common types of infection (Table 2). Infections reported as serious adverse events were rare, occurring with an incidence of 4.3, 3.9 and 4.9 per 100 patient-years in the high ferritin FCM group, low ferritin FCM group and oral iron group, respectively. When assessed over the total study period to the final follow-up visit, the cumulative number of serious adverse events classified as infections was 5.1, 3.3 and 11.4 per 100 patient-years, respectively (Supplementary data, Table S1). The incidence of hospitalizations due to infection was similar between groups, but the cumulative number of such events was numerically higher under oral iron (Table 4; Supplementary data, Table S2).
Cardiovascular disorders
The incidence of one or more cardiac disorders was 15.2, 10.9 and 11.9 events per 100 patient-years in the high ferritin FCM, low ferritin FCM and oral iron groups, respectively, during the safety period (Table 2). Over the total study period to final follow-up, the cumulative number of serious adverse events classified as cardiac disorders was 9.7, 7.2 and 11.7 per 100 patient-years, respectively (Supplementary data, Table S1). Hospitalization for adverse events classified as cardiac disorders was similar in the three treatment groups (Table 4).
For major adverse cardiac events (MACE; acute myocardial infarction, acute coronary syndrome, coronary artery disease, unstable angina or myocardial ischemia) reported as serious adverse events, the incidence was 3.6 in the high FCM group, 2.3 in the low FCM group and 3.3 events in the oral iron group per 100 patient-years. There were no cases of stroke in any treatment group.
Neoplasms
A total of 33 (5.4%) neoplasms, as defined by the study investigator, were reported by the end of follow-up (mean observation period 359 days), occurring in 13 patients (8.4%) in the high ferritin FCM group, 7 patients (4.7%) in the low ferritin FCM and 13 patients (4.2%) in the oral iron group. No obvious pattern was seen for the type of neoplasms; the most frequent neoplasm was basal cell carcinoma. One death, in the high FCM group, was attributed to a neoplasm (recurrent laryngeal cancer). There were no statistically significant differences in the incidence and time to first neoplasm between treatment groups. Univariate and multivariate analyses showed that a previous history of malignancy (which was highest in the high ferritin group) was the only factor to exert a statistically significant (P < 0.05) impact on the development of neoplasms. None of the neoplasms was thought to be related to a study treatment by either the investigator or the Data Safety Monitoring Board.
Deaths
Twelve patients died during the safety period: five in the high ferritin FCM group (cardiac arrest, myocardial infarction, recurrent laryngeal cancer and respiratory failure), one in the low ferritin FCM group (acute cardiac failure) and six in the oral iron group (cardiac arrest, myocardial infarction, cerebrovascular accident, multiple myeloma, sepsis and multiple injuries secondary to trauma). None of the deaths was considered to be related to study drug by either the investigator or the Data Safety Monitoring Board.
DISCUSSION
FIND-CKD studied the safety and efficacy of IV versus oral iron in a randomized controlled trial of patients with nondialysis-dependent CKD over a 1-year period and represents the most substantial data set for FCM in this setting. While we previously reported the observed incidence in each treatment arm [10], the current study extends the safety analysis by reporting adverse event rates per 100 patient-years, thereby accounting for differences in exposure, as well as event rates according to applied FCM doses and iron status parameters, and examines the occurrence of adverse events requiring hospitalization. Results showed that the incidence of serious adverse events, and the incidence of events which required hospitalization, was similar for both FCM regimens versus oral iron therapy for the pre-specified safety period. No serious adverse event was considered to be related to FCM administration. FCM targeting either a higher or lower ferritin level showed half the incidence of adverse events with a suspected relation to study drug than oral iron, due to a lower incidence of gastrointestinal symptoms. Ferritin levels of 800 µg/L or above in the high ferritin FCM group were not associated with any increase in adverse events or serious adverse events. Higher FCM doses also showed no relation with increasing frequency of safety events.
Cardiac events, infections and renal function were of particular interest in light of concerns about a possible effect of IV iron on oxidative stress levels and the risk of an effect on the immune response. However, we found no differences. The incidence and cumulative frequency of hospitalization due to either cardiac disorders or infection was comparable between treatment groups. In particular, we observed no evidence for an increased risk of cardiac disorders or MACE with FCM compared with oral iron, although even in this high-risk population the number of patients could be insufficient to reveal a difference.
No substantive difference in adverse events was observed between the two FCM groups, despite an early profound rise in serum ferritin levels in the high ferritin cohort, as per protocol, which was sustained throughout the study. Elevated ferritin is atypical in patients with CKD who are not on dialysis unless they are acutely inflamed. Ferritin ≥800 µg/L occurred almost exclusively in the high ferritin FCM group but was not associated with a higher rate of adverse events and did not lead to study drug discontinuation. High ferritin concentrations were generally transient, with few patients in the high ferritin FCM group having more than one ferritin level of ≥800 µg/L (5.8%). Only 8.4% had one or more than one ferritin level ≥1000 µg/L.
Recent data from a single-center randomized study with 136 patients suggested a higher rate of cardiovascular events and infections reported as serious adverse events during 2 years' follow-up after an 8-week course of IV iron sucrose compared with oral iron [12]. In that study, which was discontinued before completion, there were 54.4 and 34.4 serious cardiovascular events per 100 patient-years in the IV and oral iron treatment groups. These findings are not consistent with FIND-CKD and other recent randomized trials in patients with nondialysis-dependent CKD [13, 14]. Notably, safety analyses from REVOKE were reported as multiple events (whereby every event is counted separately when a patient experiences repeated events) instead of the more standard way of reporting adverse events (whereby patients with repeated events are counted only once), and repeated events in the same patient were largely responsible for the observed difference. Despite using both approaches in the present analysis, we observed no difference in clinical adverse events between groups.
Although FIND-CKD was longer than previous completed trials and included a relatively large study population, the analysis had restricted power to detect small differences in the incidence of adverse events between treatment groups that could still be clinically meaningful. Moreover, follow-up time may still have been too short to detect long-term effects. Follow-up time differed between the three groups, being shorter in the oral iron group, but safety events were analyzed per 100 patient-years to account for this. Additionally, it should be borne in mind that safety reporting was censored at the point at which another anemia therapy (e.g. ESA or blood transfusion) was initiated and/or the patient discontinued study drug. This approach was taken in an attempt to obtain ‘clean’ data sets, but this advantage is counterbalanced by the risk that adverse events which first manifested after drug discontinuation would not be captured, and is thus a potential source of under-reporting. Inclusion of malignancies observed during the total follow-up period, and analyses for other key safety parameters over the same period, mitigates this limitation. Lastly, FIND-CKD also has the limitation of not including a placebo arm, so that no conclusions can be drawn on the effect of any iron therapy versus none.
In summary, these results further support the conclusion that correction of iron deficiency anemia with IV FCM is safe in patients with nondialysis-dependent CKD, consistent with results from a recent meta-analysis of FCM across multiple indications [15]. While this is encouraging, longer follow-up in larger populations is required to definitively ascertain the long-term safety profile of FCM and other IV iron therapies.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
The FIND-CKD Study Investigators are as follows: Australia: Simon D. Roger (Gosford), Alastair Gilles (Newcastle), Randall Faull (Adelaide), Nigel D. Toussaint (Parkville), Lawrence McMahon (Box Hill), Michael Suranyi (Liverpool), David Mudge (Brisbane), Brian Hutchison (Perth), Ashley Irish (Perth), Peter Kerr (Clayton), Hemant Kulkarni (Perth and Armadale), Grahame Elder (Westmead), Margaret Jardine (Concord); Austria: Karl Lhotta (Feldkirch), Gert Mayer (Innsbruck); Belgium: Raymond Vanholder (Gent), Bart Dirk Maes (Roeselare), Pieter Evenepoel (Leuven), Frédéric Debelle (Baudour), Michel Jadoul (Brussels), Max Dratwa (Brussels); Czech Republic: Igor Macel (Zdar nad Sazavou), Milan Dunaj (Litomysl), Milan Kvapil (Praha), Petr Bucek (Frydek-Mistek), Jitka Rehorova (Brno), Ales Hruby (Slavkov u Brna), Václava Honová (Pizen), Lada Malanova (Pizen), Martin Lucak (Prague), Dalibor Lecian (Praha), Martin Jirovec (Marianske Lazne), Jiri Vlasak (Sokolov), Ivan Rychlik (Sokolov), Stanislav Surel (Brno); Denmark: Anne-Lise Kamper (Kobehavn), Ove Ostergaard (Roskilde), Gudrun K Steffensen (Frederica); France: Leila Chenine (Montpellier), Gabrial Choukroun (Amiens), Philippe Zaoui (Grenoble); Germany: Christoph Wanner (Würzburg), Wolfgang Backs (Hamburg), Uwe Kraatz (Demmin), Frank Dellanna (Düsseldorf), Klaus Busch (Dortmund), Tobias Marsen (Köln), Wolfgang Seeger (Berlin), Rainer Woitas (Bonn), Nicholas Obermueller (Frankfurt/Main), Thomas Haak (Bad Mergentheim), Stephan Lueders (Cloppenburg), Frank Pistrosch (Hoyerswerda), Eckhard Mueller (Benkastel-Kues), Peter R. Mertens (Magdeburg), Werner Sutermer (Würzburg), Scott-Oliver Grebe (Wuppertal), Syrus Hafezi-Rachti (Mannheim-Käfertal), Silke Roeser (Eberswalde); Greece: Dimitrios Tsakiris (Thessaloniki), Dimitrios Memmos (Thessanloniki), Demetrios Vlachakos (Chaidari, Athens), Vassilis Vargemezis (Dragana, Alexandroupolis), Ioannis Stefanidis (Mezourlo, Larissa), Christos Syrganis (Volos), Polichronis Alivanis (Rhodes), Ioannis Papadakis (Athens), Nickolaos Papagalanis (Athens), Aimilios Andrikos (Joannina), Dimitrios Goumenos (Rios Patras), Kostas Siamopoulos (Ioannina), Charikelia Gouva (Arta), Gabriel Papadakis (Peireus), Ioannis Boletis (Athens), Myrsini Tsimnadi-Spanoudaki (Vestos), Dimitrios Stamatiades (Serres), Kyriaki Stamatelou (Athens), Spyridon Moutafis (Athens); Italy: Francesco Locatelli (Lecco), Antonio Santoro (Bologna), Francesco Quarello (Torino), Giuseppe Remuzzi (Bergamo), Salvatore Coppola (Piedmonte Matese), Rosella Ferraro Mortellaro (Dan Daniele del Friuli), Andrea Icardi (Arenzano), Giacomo Colussi (Milan), Franco Della Grotta (Anzio), Luigi Lombardi (Ctanzaro), Maurizio Gallieni (Milano), Giuseppe Villa (Pavia), Giuseppe Grandaliano (Foggia); The Netherlands: Carlo Gaillard (Amersfoort and Amsterdam), Sebastiaan Huisman (Delft), Jos Barendregt (Apeldoorn), Peter JH Smak Gregoor (Dordrecht); Norway: Cecilia Oien (Trondheim); Poland: Boleslaw Rutkowski (Gdansk), Robert Malecki (Warszawa), Michal Nowicki (Lodz), Przemyslaw Rutkowski (Starogard Gdanski), Kryzsztof Marczewski (Zamosc), Michal Mysliwiec (Bialystok), Antoni Sydor (Tarnow), Jacek Rysz (Lodz), Andrzej Rydzewski (Warszawa), Marian Klinger (Wroclaw), Rafal Wnuk (Dabrowa Gornicza), Piotr Kozminski (Mlawa), Anna Nocon (Wroclaw), Kazimierz Ciechanowski (Szczecin); Portugal: Pedro Correia (Amadora), Fernando Neves (Lisboa), José Barata (Carnaxide); Romania: Gabriel Mircescu (Bucuresti), Mihai Voiculescu (Bucuresti), Gheorghe Gluhovschi (Timisoara), Eugen Mota (Craiova); Spain: Angel Luís Martín De Francisco (Santander), Alberto Torre (Madrid), Alba Herreros (Barcelona), José Luño (Madrid), E. Gruss (Alcorcón), Judith Martins [Getafe (Madrid)], Marti Vallés (Girona), Julio Pascual (Barcelona); Sweden: Peter Bárány (Stockholm); Switzerland: Andreas H. Bock (Aarau), Patrice M. Ambuehl (Zürich); Turkey: Sehsuvar Erturk (Ankara), Mustafa Arici (Ankara), Saime Paydas (Adnana), Zeki Soypacaci (Izmir), Taner Camsari (Izmir), Sedat Ustundag (Edirne); UK: Iain C. Macdougall (London), Mark E. Thomas (Birmingham), Richard J. D'Souza (Exeter), Jo E. Taylor (Dorchester), Nicholas R. Pritchard (Cambridge), Robin Jeffery (Bradford), Stephen G. Riley (Cardiff), Deepak Bhatnagar (Oldham), Sunil Bhandari (Hull), David Reaich (Middlesborough), Paul E. Stevens (Canterbury), Mohsen El Kossi (Doncaster), Simon Roe (Nottingham), Brian Camilleri (Ipswich), Aimun Ahmed (Preston), Arif Khwaja (Sheffield), Barbara Thompson (Stevenage), Debasish Banerjee (London), Johann Nicholas (Wolverhampton), Alastair Hutchison (Manchester), Richard Borrows (Birmingham).
CONFLICT OF INTEREST STATEMENT
S.D.R. has received speaker's fees, honoraria and consultancy fees from several manufacturers of ESAs and IV iron, including Amgen, Hoffmann-La Roche, Janssen-Cilag, Novartis, Sandoz, Takeda and Vifor Pharma. C.A.G. has received speaker's fees, honoraria and consultancy fees from several manufacturers of ESAs and IV iron products, including Amgen, Pharmacosmos, Hoffmann-La Roche, Takeda and Vifor Pharma. A.H.B. has received speaker's honoraria and consultancy fees from Amgen, Hoffmann-La Roche and Vifor Pharma. F.C. has no conflicts of interest to declare. K.-U.E. has received speaker's and/or consultancy fees from several manufacturers of ESAs and IV iron, including Affymax, Amgen, Bayer, Johnson & Johnson, Hoffmann-La Roche and Vifor Pharma. D.B.V.W. is an employee and stockholder of DaVita Healthcare Partners, Inc. M.C., Y.M. and S.L. are employees of Vifor Pharma. I.C.M. has received speaker's fees, honoraria and consultancy fees from several manufacturers of ESAs and IV iron, including Affymax, AMAG, Amgen, Ortho Biotech, Pharmacosmos, Hoffmann-La Roche, Takeda and Vifor Pharma.
FUNDING
The FIND-CKD trial was supported by Vifor Pharma, Glattbrugg, Switzerland.
Contributor Information
The FIND-CKD Study Investigators:
Simon D. Roger, Alastair Gilles, Randall Faull, Nigel D. Toussaint, Lawrence McMahon, Michael Suranyi, David Mudge, Brian Hutchison, Ashley Irish, Peter Kerr, Hemant Kulkarni, Grahame Elder, Margaret Jardine, Karl Lhotta, Gert Mayer, Raymond Vanholder, Bart Dirk Maes, Pieter Evenepoel, Frédéric Debelle, Michel Jadoul, Max Dratwa, Igor Macel, Milan Dunaj, Milan Kvapil, Petr Bucek, Jitka Rehorova, Ales Hruby, Václava Honová, Lada Malanova, Martin Lucak, Dalibor Lecian, Martin Jirovec, Jiri Vlasak, Ivan Rychlik, Stanislav Surel, Anne-Lise Kamper, Ove Ostergaard, Gudrun K Steffensen, Leila Chenine, Gabrial Choukroun, Philippe Zaoui, Christoph Wanner, Wolfgang Backs, Uwe Kraatz, Frank Dellanna, Klaus Busch, Tobias Marsen, Wolfgang Seeger, Rainer Woitas, Nicholas Obermueller, Thomas Haak, Stephan Lueders, Frank Pistrosch, Eckhard Mueller, Peter R. Mertens, Werner Sutermer, Scott-Oliver Grebe, Syrus Hafezi-Rachti, Silke Roeser, Dimitrios Tsakiris, Dimitrios Memmos, Demetrios Vlachakos, Vassilis Vargemezis, Ioannis Stefanidis, Christos Syrganis, Polichronis Alivanis, Ioannis Papadakis, Nickolaos Papagalanis, Aimilios Andrikos, Dimitrios Goumenos, Kostas Siamopoulos, Charikelia Gouva, Gabriel Papadakis, Ioannis Boletis, Myrsini Tsimnadi-Spanoudaki, Dimitrios Stamatiades, Kyriaki Stamatelou, Spyridon Moutafis, Francesco Locatelli, Antonio Santoro, Francesco Quarello, Giuseppe Remuzzi, Salvatore Coppola, Rosella Ferraro Mortellaro, Andrea Icardi, Giacomo Colussi, Franco Della Grotta, Luigi Lombardi, Maurizio Gallieni, Giuseppe Villa, Giuseppe Grandaliano, Carlo Gaillard, Sebastiaan Huisman, Jos Barendregt, Peter JH Smak Gregoor, Cecilia Oien, Boleslaw Rutkowski, Robert Malecki, Michal Nowicki, Przemyslaw Rutkowski, Kryzsztof Marczewski, Michal Mysliwiec, Antoni Sydor, Jacek Rysz, Andrzej Rydzewski, Marian Klinger, Rafal Wnuk, Piotr Kozminski, Anna Nocon, Kazimierz Ciechanowski, Pedro Correia, Fernando Neves, José Barata, Gabriel Mircescu, Mihai Voiculescu, Gheorghe Gluhovschi, Eugen Mota, Angel Luís Martín De Francisco, Alberto Torre, Alba Herreros, José Luño, E. Gruss, Judith Martins, Marti Vallés, Julio Pascual, Peter Bárány, Andreas H. Bock, Patrice M. Ambuehl, Sehsuvar Erturk, Mustafa Arici, Saime Paydas, Zeki Soypacaci, Taner Camsari, Sedat Ustundag, Iain C. Macdougall, Mark E. Thomas, Richard J. D'Souza, Jo E. Taylor, Nicholas R. Pritchard, Robin Jeffery, Stephen G. Riley, Deepak Bhatnagar, Sunil Bhandari, David Reaich, Paul E. Stevens, Mohsen El Kossi, Simon Roe, Brian Camilleri, Aimun Ahmed, Arif Khwaja, Barbara Thompson, Debasish Banerjee, Johann Nicholas, Alastair Hutchison, and Richard Borrows
REFERENCES
- 1. Fishbane S, Pollack S, Feldman HI. et al. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol 2009; 4: 57–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Camaschella C, Strati P. Recent advances in iron metabolism and related disorders. Intern Emerg Med 2010; 5: 393–400 [DOI] [PubMed] [Google Scholar]
- 3. Kidney Disease Improving Global Outcomes (KDIGO). Clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012; 4: 279–335 [Google Scholar]
- 4. Macdougall IC. Intravenous iron therapy in non-dialysis CKD patients. Nephrol Dial Transplant 2014; 29: 717–720 [DOI] [PubMed] [Google Scholar]
- 5. Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 2011; 3: 12–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vaziri ND. Understanding iron: promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am J Kidney Dis 2013; 61: 992–1000 [DOI] [PubMed] [Google Scholar]
- 7. Parkkinen J, von Bonsdorff L, Peltonen S. et al. Catalytically active iron and bacterial growth in serum of haemodialysis patients after i.v. iron-saccharate administration. Nephrol Dial Transplant 2000; 15: 1827–1834 [DOI] [PubMed] [Google Scholar]
- 8. Rozen-Zvi B, Gafter-Gvili A, Paul M. et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: systematic review and meta-analysis. Am J Kidney Dis 2008; 52: 897–906 [DOI] [PubMed] [Google Scholar]
- 9. Macdougall IC, Bircher AJ, Eckardt KU. et al. Iron management in chronic kidney disease: conclusions from a ‘Kidney Disease: Improving Global Outcomes’ (KDIGO) controversies conference. Kidney Int 2016; 89: 23–39 [DOI] [PubMed] [Google Scholar]
- 10. Macdougall IC, Bock AH, Carrera F. et al. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in psatient with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 2014; 29: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levey AS, Bosch JP, Lewis JB. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 12. Agarwal R, Kusek JW, Pappas MK. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int 2015; 88: 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qunibi WY, Martinez C, Smith M. et al. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant 2011; 26: 1599–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalra PA, Bhandari S, Saxena S. et al. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney diseasepatients with anaemia. Nephrol Dial Translant 2015; 30: 1577–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore RA, Gaskell H, Rose P. et al. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disorders 2011; 11: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.