Abstract
Background
Interstitial fibrosis (IF), tubular atrophy (TA) and interstitial inflammation (II) are known determinants of progression of renal disease. Standardized quantification of these features could add value to current classification of glomerulopathies.
Methods
We studied 315 participants in the Nephrotic Syndrome Study Network (NEPTUNE) study, including biopsy-proven minimal change disease (MCD = 98), focal segmental glomerulosclerosis (FSGS = 121), membranous nephropathy (MN = 59) and IgA nephropathy (IgAN = 37). Cortical IF, TA and II were quantified (%) on digitized whole-slide biopsy images, by five pathologists with high inter-reader agreement (intra-class correlation coefficient >0.8). Tubulointerstitial messenger RNA expression was measured in a subset of patients. Multivariable Cox proportional hazards models were fit to assess association of IF with the composite of 40% decline in estimated glomerular filtration rate (eGFR) and end-stage renal disease (ESRD) and separately as well, and with complete remission (CR) of proteinuria.
Results
IF was highly correlated with TA (P < 0.001) and II (P < 0.001). Median IF varied by diagnosis: FSGS 17, IgAN 21, MN 7, MCD 1 (P < 0.001). IF was strongly correlated with baseline eGFR (P < 0.001) and proteinuria (P = 0.002). After adjusting for clinical pathologic diagnosis, age, race, global glomerulosclerosis, baseline proteinuria, eGFR and medications, each 10% increase in IF was associated with a hazard ratio of 1.29 (P < 0.03) for ESRD/40% eGFR decline, but was not significantly associated with CR. A total of 981 genes were significantly correlated with IF (|r| > 0.4, false discovery rate (FDR) < 0.01), including upstream regulators such as tumor necrosis factor, interferon gamma (IFN-gamma), and transforming growth factor beta 1 (TGF-B1), and signaling pathways for antigen presentation and hepatic fibrosis.
Conclusions
The degree of IF is associated with risk of eGFR decline across different types of proteinuric glomerulopathy, correlates with inflammatory and fibrotic gene expression, and may have predictive value in assessing risk of progression.
Keywords: gene expression, interstitial fibrosis, kidney biopsy, proteinuria
INTRODUCTION
Glomerulopathies represent a significant source of morbidity and a substantial cause of end-stage renal disease (ESRD). Disease course and progression depends not only on glomerular injury, but also on damage to tubules and interstitium. Earlier studies have emphasized the need for precise and reproducible definition of glomerular, interstitial and tubular histologic features to understand structural–functional correlations in kidney disease [1–3].
Systematic, standardized scoring of tubulointerstitial inflammation and scarring has long been a mainstay of the Banff classification for kidney allograft biopsies [4–7]. However, except for IgA nephropathy (IgAN) [8] and the National Institutes of Health chronicity score for lupus nephritis [9], most current classifications of glomerulopathies [10–13] are based on glomerular features, without need for incorporation of scoring for tubulointerstitial scarring or inflammation in diagnostic reports, which is a potential limitation of these conventional classification systems [14]. Recently published recommendations to include information on the tubulointerstitial compartment in diagnostic reports did not address the major proteinuric glomerulopathies: membranous nephropathy (MN) minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) [15].
In this study, tubulointerstitial features were examined in biopsies of patients with proteinuria and different types of glomerular lesions enrolled in the Nephrotic Syndrome Study Network (NEPTUNE), a multi-center prospective longitudinal cohort study of proteinuric adult and pediatric patients enrolled at the time of clinically indicated renal biopsy. The degree of interstitial fibrosis (IF), tubular atrophy (TA) and interstitial inflammation (II) was assessed on whole-slide images (WSI) according to the NEPTUNE digital pathology scoring system (NDPSS) [16]. The goals of this study were to assess whether a visual quantitative assessment of IF was correlated with clinical parameters of disease activity at the time of biopsy, disease progression and tissue messenger RNA (mRNA) expression levels, and to evaluate the potential of IF as a predictor of outcome in proteinuric glomerulopathies.
MATERIALS AND METHODS
Patients
The study was conducted on 315 NEPTUNE participants, assigned to one of the NEPTUNE cohorts (MCD/FSGS, MN and Others for IgAN) based on biopsy diagnosis, and meeting the inclusion criteria for this study: (i) children and adults with proteinuria of >500 mg/day, recruited at the time of first clinically indicated renal biopsy, as previously described (clinicaltrials.gov database under NCT1209000) [17]; and (ii) biopsy-proven MCD, FSGS, MN or IgAN; and (iii) availability of digital renal biopsies in the NEPTUNE digital pathology repository (DPR) comprehensive of de-identified WSI, immunofluorescence and electron microscopy images, as well as a pdf copy of the original pathology report [18]. Consent was obtained from individual patients at enrollment, and the study was approved by Institutional Review Boards of participating institutions.
Data collection
Visual assessment of WSI
WSI were obtained from glass slides stained with hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), trichrome and silver stain. Visual assessment was performed according to the NDPSS, on de-identified WSI of renal biopsies, in the NEPTUNE DPR according to the NEPTUNE digital pathology protocol (NDPP) [18]. Each glomerulus was annotated (enumerated) with an individual number that was maintained in all sections where the glomerulus was present as per NDPP [19]. Glomerular, tubular, interstitial and vascular features were evaluated by up to five participating renal pathologists (277 biopsies were evaluated by five pathologists, 38 biopsies by two pathologists), and the average score was used for each feature. Visual quantitative assessment of IF, TA and II, was reported as 0–100% of cortex involved. Additionally, post-scoring, the continuous variable for IF, TA and II were grouped into four categories (0 to <5%; 5–25%; 26–50%; and >50%) reflecting usual scoring schemes included in pathological classifications [4, 8]. A semiquantitative ordinary scale (0–3+) was used to assess arteriosclerosis [16]. Annotated glomeruli were counted in each biopsy across all sections. The number of annotated globally sclerotic and obsolescent glomeruli per total number of glomeruli was calculated in each digital biopsy. The presence or absence of global sclerosis/obsolescence was used to further stratify patients originally identified as MCD into (i) ‘MCD’ if neither segmental or global obliteration was present independently of the amount of foot process effacement, and (ii) ‘MCD-like’ if any percentage of global obliteration was present independently of the amount of foot process effacement [20]. The de-identified original biopsy reports stored in the NEPTUNE DPR were reviewed in a subset of patients to examine the concordance with the degree of IF/TA in the original report, if available, and IF/TA scores using WSI.
Clinical data
After enrollment, patients are assigned to one of the NEPTUNE cohorts (MCD/FSGS, MN and Others for IgAN) after the biopsy is reviewed by core study pathologists. Socio-demographic data, medical history, medication and local laboratory results are recorded, and blood and urine specimens are collected at baseline and in each follow-up visit (every 4 months for the first year, and then biannually thereafter for up to 5 years) for central measurement of serum creatinine and urine protein/creatinine ratio (UPCR). Obesity was defined as body mass index (BMI) ≥30 kg/m2 in adults or BMI percentile >95 for children. Hypertensive status was defined as blood pressure >140/90 in adults and >95th percentile for children.
Outcomes
Estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula for participants ≥18 years old and the modified CKiD-Schwartz formula for participants <18 years old. Progression of eGFR was evaluated with a composite of 40% decline in eGFR from baseline or ESRD [21]. ESRD was defined as the initiation of dialysis, receipt of kidney transplant or eGFR <15 mL/min/1.73 m2 at two visits [22]. Complete remission (CR) was defined as UPCR <0.3 mg/mg on either a single void specimen or 24-h urine collection
Molecular data
Genome-wide mRNA expression was available on a subset of 165 participants. Renal biopsy tissue was manually microdissected to separate the tubulointerstitial compartment from the glomerular compartment. Total RNA was isolated, reverse transcribed, linearly amplified and hybridized on an Affymetrix 2.1 ST platform as described previously [23, 24]. Gene expression was normalized, quantified and annotated at the Entrez Gene level. Only genes expressed 1 standard deviation (SD) above the negative control were considered to be expressed and included in the analysis.
Statistical analysis
Clinical and pathology data analysis
Descriptive statistics, including mean and SD for normally distributed variables, median and interquartile range (IQR) for skewed variables and proportions for categorical variables, were used to characterize baseline participant characteristics by severity of IF. Intra-class correlation coefficient (ICC) was used to assess the inter-rater reliability of IF/TA scores. Spearman’s rank correlation coefficient (rho) was used to assess the relationship between IF and baseline eGFR and UPCR. Separate Cox proportional hazards models were fit to estimate the hazard ratio (HR) for CR and the HR for the composite of 40% decline in eGFR or ESRD [21]. A multivariable linear generalized estimating equation (GEE) was used to further evaluate the association of IF with follow-up measures of eGFR. A GEE model was used rather than slope due to the concern for nonlinear trajectory in a subset of this population. Analyses were performed using both STATA, v12.1 (College Station, TX, USA) and SAS 9.3 (SAS Institute Inc., Cary, NC, USA) with two-sided tests of hypotheses and P < 0.05 as the criterion for statistical significance. Interactions of interest included IF severity with glomerulosclerosis (GS), medications, time and clinical-pathologic diagnosis cohort.
Molecular data analysis
A Pearson correlation coefficient was calculated between gene expression level and log-transformed IF score with adjustment for multiple testing using false discovery rate (FDR) of <0.01 as the criterion for statistical significance. IF-correlated transcripts with |r| >0.4 were analyzed for enrichment of canonical pathways and functional groupings using the Ingenuity Pathway Analysis Software Suite.
RESULTS
Patient characteristics
The study cohort included 315 NEPTUNE participants, with clinical pathologic diagnosis at time of enrollment of MCD (98), FSGS (121), MN (59) and IgAN (37). In the MCD group, a subset of 71 subjects fit the criteria for MCD-like category [20].
Table 1 shows demographic and baseline clinical characteristics, including baseline treatment, by severity of IF. Gender, race and ethnicity did not vary across IF groups. Those with lowest IF were younger, more likely to have received immunosuppression prior to renal biopsy and more likely to have a diagnosis of MCD. Those with highest IF had lower baseline eGFR and higher proteinuria (UPCR).
Table 1.
Baseline characteristics of IF/TA subcohort
| IF/TA subcohort | IF 0 to <5% | IF 5 to <25% | IF 25 to <50% | IF ≥50% | P-value | |
|---|---|---|---|---|---|---|
| N = 315 | N = 129 | N = 111 | N = 47 | N = 28 | ||
| Demographics | ||||||
| Age (years) | 34.4 (21.4) | 22.1 (19.9) | 41.4 (19.3) | 44.2 (18.4) | 44.0 (14.1) | <0.001 |
| Female | 125 (40) | 46 (36) | 44 (40) | 24 (51) | 11 (40) | 0.332 |
| Black | 87 (30) | 35 (30) | 25 (25) | 15 (33) | 12 (46) | 0.196 |
| Hispanic | 65 (22) | 23 (19) | 25 (23) | 8 (17) | 9 (33) | 0.661 |
| Disease characteristics | ||||||
| eGFR (mL/min/1.73 m2) | 81 (36) | 107 (27) | 76 (28) | 54 (23) | 30 (20) | <0.001 |
| UPCR (mg/mg) | 2.3 (0.7, 4.3) | 1.5 (0.2, 4.3) | 2.1 (0.8, 4.0) | 2.5 (1.2, 4.1) | 3.0 (2.0, 4.7) | 0.079 |
| Disease duration (months) | 5 (1, 24) | 4 (1, 15) | 5 (1, 20) | 4.5 (0, 35) | 5 (1, 72) | 0.998 |
| Cohort | <0.001 | |||||
| MCD | 27 (9) | 24 (19) | 3 (3) | 0 (0) | 0 (0) | |
| MCD-like | 71 (23) | 50 (39) | 16 (14) | 3 (6) | 2 (7) | |
| FSGS | 121 (38) | 28 (22) | 47 (42) | 25 (53) | 21 (75) | |
| MN | 59 (19) | 22 (17) | 30 (27) | 5 (11) | 2 (7) | |
| IgAN | 37 (12) | 5 (4) | 15 (14) | 14 (30) | 3 (11) | |
| Other pathology features | ||||||
| TA (% of interstitium) | 5 (1, 21) | 1 (0, 2) | 9 (5, 14) | 32 (25, 37) | 63 (53, 74) | <0.001 |
| Mononuclear cells (% of interstitium) | 1 (0, 15) | 0 (0, 0) | 3 (0, 10) | 20 (7, 30) | 46 (30, 54) | <0.001 |
| Global sclerosis (% of glomeruli) | 3 (0, 17) | 0 (0, 2) | 9 (0, 19) | 28 (11, 58) | 64 (47, 76) | <0.001 |
| Arteriosclerosis (% 3+) | 25 (8.3) | 1 (1) | 9 (9) | 6 (14) | 9 (33) | <0.001 |
| Physical exam | ||||||
| Obese | 119 (41) | 38 (33) | 46 (45) | 23 (53) | 12 (48) | 0.209 |
| Hypertensive | 88 (31) | 35 (31) | 31 (30) | 9 (20) | 13 (52) | 0.065 |
| Treatment prior to biopsy | ||||||
| Any IST | 111 (35) | 79 (61) | 23 (21) | 5 (11) | 4 (14) | <0.001 |
| Steroid | 105 (33) | 78 (60) | 21 (19) | 2 (4) | 4 (14) | <0.001 |
| CNI | 24 (8) | 18 (14) | 3 (3) | 2 (4) | 1 (4) | 0.006 |
| RAAS blockade | 158 (50) | 44 (34) | 67 (60) | 30 (64) | 17 (61) | <0.001 |
| Treatment at baseline | ||||||
| Any IST | 131 (45) | 78 (66) | 37 (36) | 12 (27) | 4 (15) | <0.001 |
| Steroid | 118 (40) | 74 (62) | 31 (30) | 9 (20) | 4 (15) | <0.001 |
| CNI | 29 (10) | 22 (18) | 5 (5) | 2 (5) | 0 (0) | <0.001 |
| RAAS blockade | 183 (62) | 53 (45) | 80 (77) | 31 (70) | 19 (73) | <0.001 |
| Clinical outcomes | ||||||
| Follow-up time (months) | 26 (16) | 25 (16) | 27 (16) | 29 (16) | 26 (15) | 0.503 |
| CR | 164 (52) | 88 (68) | 55 (50) | 15 (32) | 6 (21) | <0.001 |
| ESRD or 40% drop in eGFR | 84 (27) | 22 (17) | 26 (23) | 19 (40) | 17 (61) | <0.001 |
Continuous, normally distributed variables are presented as mean (SD); continuous, non-normally distributed variables are presented as median (IQR); and categorical variables are presented as number (%). CNI, calcineurin inhibitor; IST, immunosuppressive therapy.
Pathology
There was excellent reliability of IF/TA percentage estimates by different pathologists (ICC = 0.835) using WSI of all available biopsy cases, with no significant difference in the IF/TA estimates obtained using trichrome, PAS or silver-stained sections. These results confirm recently published observations on a smaller sample showing high reproducibility of the visual scoring of IF/TA by multiple observers using WSI [16]. A survey of 238 original local biopsy reports showed that 16 (7%) provided no IF/TA estimate, 47 (20%) described it as percentage of interstitial space involved, and 175 (73%) described it as none/absent, mild, moderate, diffuse or severe. When a continuous variable was provided in the local biopsy report, comparison of the reported score with WSI scores indicated good correlation (r = 0.75, r2 = 0.58, P < 0.0001, n = 47).
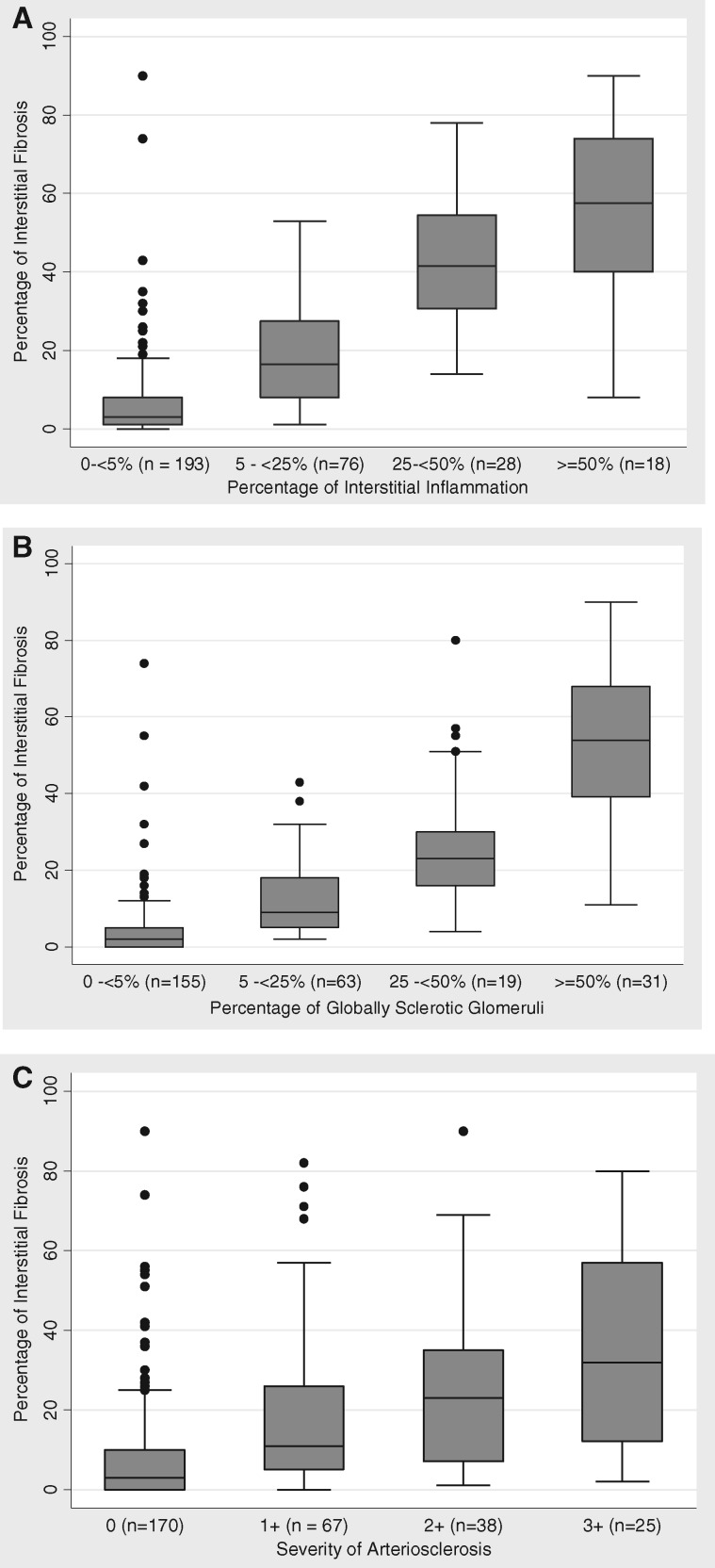
The majority of participants had a low amount of IF, TA or II, but wide ranges were observed. Median (IQR) for IF was 7 (2, 22)—range 0–90; median (IQR) for TA was 5 (1, 21)—range 0–93; and median (IQR) for II was 1 (0, 15)—range 0–85. IF and TA were tightly correlated (r = 0.99, r2 = 0.98, P < 0.0001), and for this reason IF was used as a reliable single parameter representative of interstitial scarring for statistical and correlative purposes. IF was also correlated with II (r = 0.78, r2 = 0.62, P < 0.0001) (Figure 1A). Although one goal of this study was to examine the predictive value of IF/TA in the progression of glomerular disease, its relation with global GS and arteriosclerosis, other major manifestations of renal parenchyma scarring, was also considered. IF was highly correlated with GS (r = 0.79, r2 = 0.63, P < 0.001) (Figure 1B). Patients with more severe arteriosclerosis had greater median IF (P < 0.001) (Figure 1C).
FIGURE 1.
Relationship between interstitial inflammation and (A) II, (B) global glomerulosclerosis and (C) arteriosclerosis.
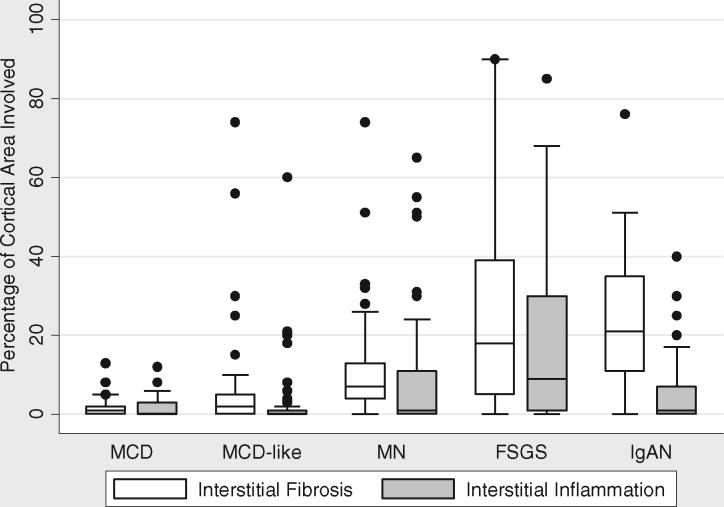
IF varied significantly among the four diagnosis groups (P < 0.001), being higher in the FSGS and IgAN groups and lowest in the MCD group (Table 1 and Figure 2). Median (IQR) for IF by diagnosis was FSGS: 18 (5, 39), IgAN: 21 (11, 35), MN: 7 (4, 13) and MCD: 1 (0, 3) (P < 0.001). The average inflammation (II) also differed significantly among the four diagnosis groups, being highest in the FSGS diagnosis group and lowest in the MCD group (Figure 2); 43% of the cohort had no II (0%). Median (IQR) for II was FSGS: 9 (1, 30), IgAN: 1 (0, 7), MN: 1 (0, 11) and MCD: 0 (0, 1) (P < 0.001). The subset of MCD-like cases showed higher IF and II than MCD (Figure 2).
FIGURE 2.
IF and II in biopsies with clinical pathologic diagnosis of MCD, MCD-like, MN, FSGS and IgAN.
Histologic features and disease activity and progression
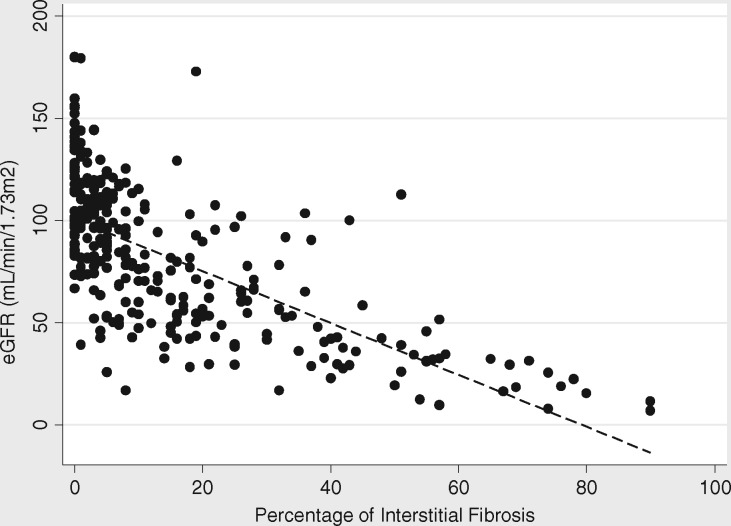
IF showed significant, inverse correlation with baseline eGFR (rho = −0.7, P < 0.001) (Figure 3). There was also a much lower, but still significant correlation between IF and baseline proteinuria (UPCR) (rho = 0.2, P < 0.001). The relationship with eGFR, but not UPCR, persisted even after adjustment for clinical pathologic diagnosis.
FIGURE 3.
Correlation between severity of IF and baseline eGFR (mL/min/1.73 m2, rho = −0.7, P < 0.001).
Mean follow-up among the participants was 26 months. Over that time, 52% of the cohort achieved CR, whereas 27% of participants had a 40% decline from baseline eGFR or reached ESRD. A greater proportion of patients with 0 to <5% IF achieved CR compared with those with >50% IF (68% versus 21%). Conversely, a smaller proportion of patients with 0 to <5% IF progressed to ESRD or 40% decline from baseline eGFR compared with those with >50% IF (17% versus 61%) (Table 1).
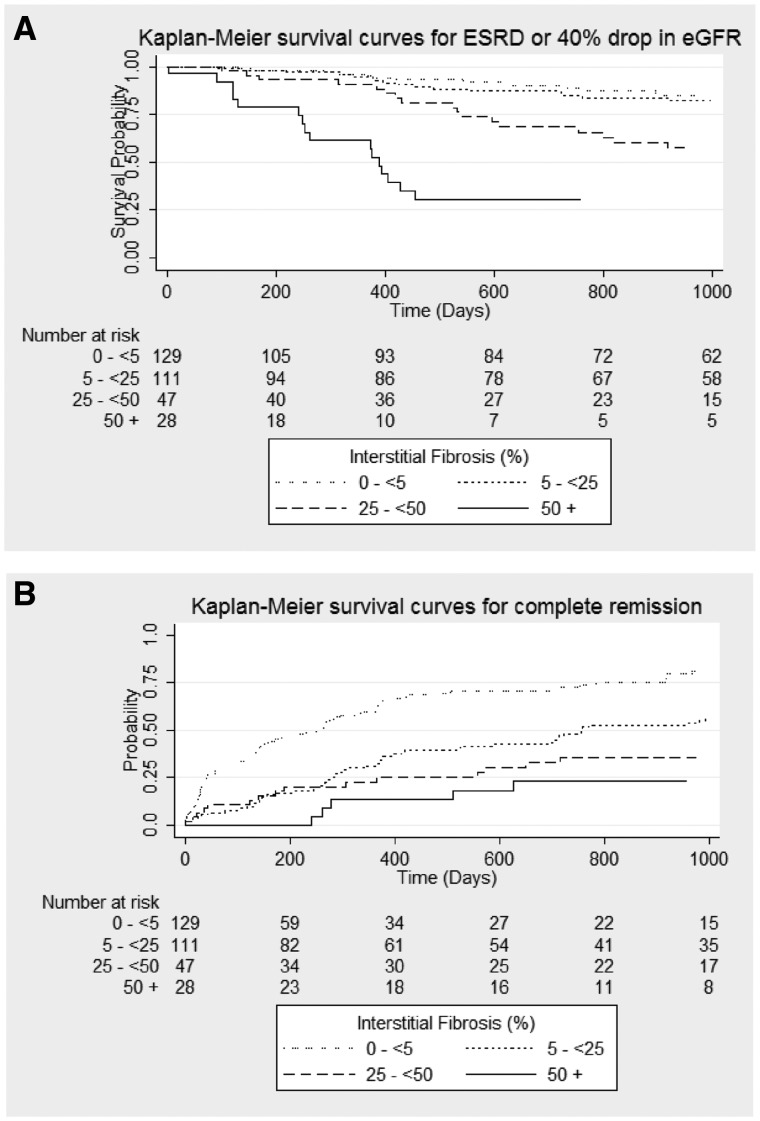
Two Cox proportional hazards models were fit separately to assess hazard of CR and composite of reaching ESRD or 40% decline from baseline eGFR with IF severity. Unadjusted Kaplan–Meier survival curves (Figure 4) demonstrate that greater IF was associated with an increased hazard of ESRD/40% decline from baseline eGFR and lower hazard of CR. In unadjusted models, each 10% increase in IF had an HR of 0.72 [95% confidence interval (95% CI) 0.63, 0.81, P ≤ 0.001) for CR and 1.31 (95% CI 1.20, 1.44, P < 0.001) for ESRD/40% eGFR decline from baseline. After adjusting for clinical pathologic diagnosis, age, race, GS, and baseline proteinuria, eGFR, renin–angiotensin–aldosterone blockade medications and immunosuppressive medications, the percentage of IF was still significantly associated with ESRD/40% eGFR decline, but its association with achieving CR lost statistical significance (Table 2). The C-index for the fully adjusted model of the composite ESRD/40% eGFR decline was 0.76. There was no significant interaction between IF and diagnosis or medication use.
Table 2.
Results of multivariable Cox proportional hazards models; HR for each 10% increase in IF
| Outcome | Model 1 (unadjusted) |
Model 2 (+ global sclerosis) |
Model 3 (+ all covariates)a |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | C-index | HR (95% CI) | P-value | C-index | HR (95% CI) | P-value | C-index | |
| CR (<1.0 less likely to remit) | 0.72 (0.63, 0.81) | <0.001 | 0.68 | 0.90 (0.77, 1.06) | 0.224 | 0.68 | 1.02 (0.83, 1.23) | 0.800 | 0.76 |
| ESRD or 40% drop in eGFR (>1.0 more likely to reach outcome) | 1.31 (1.20, 1.44) | <0.001 | 0.65 | 1.30 (1.10, 1.54) | 0.002 | 0.64 | 1.29 (1.03, 1.61) | 0.029 | 0.73 |
Covariates included: percentage of globally sclerotic glomeruli, cohort diagnosis, baseline UPCR (mg/mg), baseline eGFR (per 10 mL/min/1.73 m2), baseline immunosuppressive therapy (IST), baseline RAAS, Black race and age.
FIGURE 4.
Unadjusted Kaplan–Meier survival curves for (A) composite of ESRD/40% drop in eGFR from baseline and (B) CR of proteinuria.
To further confirm the association of IF with eGFR decline, a multivariable linear GEE model was fit to estimate the relationship between baseline IF and subsequent eGFR measurements. The model was adjusted for clinical pathologic diagnosis, time, age, race, GS, and baseline eGFR, UPCR, renin–angiotensin–aldosterone blockade medications and immunosuppressive medications. Each 10% increase in IF at baseline was associated with a 3.3 mL/min/1.73 m2 average decrease in eGFR at any time point (P < 0.004) (Table 3). No significant interaction between IF and type of pathologic diagnosis, medication or time was detected.
Table 3.
Results of multivariable linear GEE model of eGFR
| Variable | Coefficient | 95% CI | P-value |
|---|---|---|---|
| Percentage of IF (per 10% increase) | −3.3 | −5.5, 1.0 | 0.004 |
| Percentage of global glomerular sclerosis | −0.2 | −0.3, 0.0 | 0.011 |
| Cohort (ref: MCD) | |||
| MCD-like | −3.8 | −12.3, 4.8 | 0.387 |
| FSGS | −3.5 | −12.3, 5.3 | 0.435 |
| MN | −6.7 | −16.1, 2.7 | 0.163 |
| IgAN | −6.2 | −15.4, 3.0 | 0.185 |
| UPCR (mg/mg) | −1.0 | −1.6,0.5 | <0.001 |
| Baseline eGFR (per 10 mL/min/ 1.73 m2) | 5.3 | 4.0, 6.5 | <0.001 |
| Any IST at baseline (ref: no) | −1.5 | −6.9, 3.8 | 0.570 |
| RAAS blockade at baseline (ref: no) | −4.2 | −8.9, 0.4 | 0.076 |
| Black race (ref: all others) | −5.2 | −10.0, −0.4 | 0.034 |
| Age (per 10 years) | −1.5 | −2.9, −0.2 | 0.030 |
| Time (months) | −0.5 | −0.6, −0.4 | <0.001 |
IST, immunosuppressive therapy.
Tubulointerstitial gene expression
Genome-wide tissue mRNA expression from kidney biopsy tissue where the glomeruli had been removed by dissection was available on a subset of 165 participants: 4 (2%) MCD, 57 (35%) MCD-like, 47 (28%) FSGS, 39 (24%) MN and 18 (11%) IgAN. From a total of 25 583 genes expressed in the tubulointerstitial compartment, 981 genes were significantly correlated (|r| > 0.4) with percentage of IF at FDR <0.01. Top-upstream regulators (nodes predicted to be causally upstream of the associated gene expression changes) included tumor necrosis factor (TNF), TGF-B1, IFN-Gamma, IL-1, nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) (Table 4). The top canonical pathways (known as signal transduction pathways) affected by the degree of IF included the antigen presentation pathway, hepatic fibrosis and caveolar-mediated endocytosis signaling (Table 5).
Table 4.
Top 25 upstream regulators predicted to be causally upstream from 981 genes with correlation |r| >0.4 with percentage of IF
| Upstream regulators | P-value | |
|---|---|---|
| 1 | TNF | 5.59 × 10−38 |
| 2 | IFN-Gamma | 1.15 × 10−37 |
| 3 | TGF-B1 | 6.58 × 10−32 |
| 4 | IL-1B | 1.22 × 10−24 |
| 5 | NFkB (complex) | 3.54 × 10−24 |
| 6 | OSM | 6.68 × 10−22 |
| 7 | IL-4 | 1.07 × 10−21 |
| 8 | ERBB2 | 2.45 × 10−21 |
| 9 | STAT1 | 2.69 × 10-21 |
| 10 | HRAS | 2.64 × 10−20 |
| 11 | IL-6 | 1.65 × 10−19 |
| 12 | DYSF | 2.81 × 10−19 |
| 13 | CD40LG | 3.91 × 10−18 |
| 14 | STAT3 | 4.17 × 10−18 |
| 15 | RELA | 1.87 × 10−17 |
| 16 | SMARCA4 | 2.66 × 10−17 |
| 17 | TP53 | 4.23 × 10−17 |
| 18 | IL10RA | 1.23 × 10−16 |
| 19 | NFKBIA | 5.05 × 10−16 |
| 20 | SP1 | 5.47 × 10−16 |
| 21 | EGF | 6.49 × 10−15 |
| 22 | Interferon alpha (group) | 7.07 × 10−15 |
| 23 | Alpha catenin (group) | 1.45 × 10−14 |
| 24 | JUN | 1.73 × 10−14 |
| 25 | IL-5 | 1.99 × 10−14 |
The P-value refers to the probability that an upstream gene regulator is activated to produce the observed changes in the 981 genes of interest.
Table 5.
Top 25 canonical pathways enriched for the 981 genes with correlation |r| >0.4 with percentage of IF
| Canonical pathways | Overlap | P-value | |
|---|---|---|---|
| 1 | Antigen presentation pathway | 15/34 (44) | 2.35 × 10−11 |
| 2 | OX40 signaling pathway | 17/46 (37) | 3.36 × 10−11 |
| 3 | Cdc42 signaling | 25/121 (21) | 1.38 × 10−9 |
| 4 | Role of NFAT in regulation of the immune response | 28/155 (18) | 3.58 × 10−9 |
| 5 | Allograft Rejection Signaling | 14/40 (35) | 4.26 × 10−9 |
| 6 | Tryptophan degradation III (eucaryotic) | 10/20 (50) | 1.18 × 10−8 |
| 7 | iCOS-iCOSL signaling in T helper cells | 21/97 (22) | 1.19 × 10−8 |
| 8 | CD28 signaling in T helper cells | 22/107 (21) | 1.47 × 10−8 |
| 9 | B cell development | 11/26 (42) | 1.95 × 10−8 |
| 10 | Granulocyte adhesion and diapedesis | 27/157 (17) | 2.03 × 10−8 |
| 11 | Agranulocyte adhesion and diapedesis | 27/165 (16) | 5.95 × 10−8 |
| 12 | Hepatic fibrosis/hepatic stellate cell activation | 28/179 (16) | 9.15 × 10−8 |
| 13 | Phospholipase C signaling | 31/219 (14) | 1.96 × 10−7 |
| 14 | Caveolar-mediated endocytosis signaling | 16/69 (23) | 2.38 × 10−7 |
| 15 | Dendritic cell maturation | 25/158 (16) | 3.55 × 10−7 |
| 16 | Glutaryl co-A degradation | 7/12 (58) | 5.16 × 10−7 |
| 17 | Rac signaling | 19/101 (19) | 6.04 × 10−7 |
| 18 | Primary immunodeficiency signaling | 12/42 (29) | 6.87 × 10−7 |
| 19 | Leukocyte extravasation signaling | 27/188 (14) | 8.90 × 10−7 |
| 20 | Autoimmune thyroid disease signaling | 11/36 (31) | 9.53 × 10−7 |
| 21 | Complement system | 11/36 (3.1) | 9.53 × 10−7 |
| 22 | Valine degradation I | 8/18 (44) | 1.13 × 10−6 |
| 23 | Fatty acid beta-oxidation I | 10/30 (33) | 1.21 × 10−6 |
| 24 | PKC8 signaling in T lymphocytes | 19/107 (18) | 1.51 × 10−6 |
| 25 | Atherosclerosis signaling | 19/115 (17) | 4.61 × 10−6 |
The Overlap column shows the number (%) of genes that correlate with IF over the total number of genes in each pathway.
DISCUSSION
The main purpose of this study was to examine the value of visual quantitative estimation of tubulointerstitial scarring by WSI in four major proteinuric glomerulopathies: MCD (including the MCD-like subgroup), FSGS, MN and IgAN by assessing reliability and association with clinical and molecular parameters, and its potential value as a predictor of progression.
Controversy still exists over how to best estimate IF [25, 26], although visual assessment has been found to be comparable to morphometric measures of IF, for quantitation and for association with outcomes [25, 27]. We show that WSI quantitative visual assessment of the tubulointerstitial compartment of kidney biopsy samples had high reproducibility among five pathologists. In our study, high concordance was also found between visual scoring on WSI and IF estimate recorded in the original diagnostic pathology reports. A recent study on 83 biopsies from NEPTUNE patients showed strong concordance between the morphometric parameter fractional interstitial area (FIA) and the digital pathology descriptor IF [28]. Thus, visual assessment on WSI appears well suited for studying the association of tubulointerstitial morphology with clinical parameters and gene expression in kidney disease.
We show that IF correlates with other parameters of tubulointerstitial, glomerular and vascular damage and that severity of IF is highly correlated with worse baseline renal function, independent of distinct glomerular pathologic diagnosis in MCD, MCD-like, FSGS, MN and IgAN. While we did not find a significant interaction between time and IF in our GEE model (i.e. the effect of IF is consistent over time and slope is not different by degree of IF), the model was adjusted for baseline eGFR and clinical pathologic diagnosis. So, even among participants with the same baseline eGFR and diagnosis, those with higher IF on their biopsy have lower eGFR, on average, at any given time point. Additionally, greater baseline IF severity had a higher hazard of adverse clinical endpoint of ESRD/40% decline in eGFR, even after adjusting for diagnosis, demographics and initial treatment. These data suggest that even relatively modest interstitial scarring captures a more severe disease state across different types of pathologic diagnoses that are independent of the traditional clinical predictors (e.g. eGFR, UPCR and demographics). Our findings are consistent with previous studies correlating renal histology and function [1–3, 29], and corroborate more recent observations in a large IgAN cohort [30], and in 149 subjects with biopsy-proven diabetic nephropathy [31].
The association of IF with baseline proteinuria and CR was less strong. Although IF was associated with a greater hazard of failure to achieve CR in unadjusted analyses, after adjustment for key confounders, in particular, global GS, the association with failure to achieve CR was no longer significant. This is not surprising considering that glomerular injury is the primary target of treatment in proteinuric glomerulopathies, which may be less effective in reducing proteinuria when significant glomerular scarring is present [29, 32]. A reference limit for the number of globally sclerosed glomeruli accounting for age has recently been described in donor kidneys [33]. Devising a similar approach to distinguish between age-related and pathological GS in native kidney biopsies may be important to predict outcome in proteinuric glomerulopathies, particularly in subjects with MCD-like glomerular pathologic changes compared with classic MCD nephropathy, something that was beyond the scope of this study. Although these models were adjusted for diagnosis, specific characteristics of distinct types of glomerulopathies, such as higher scarring in FSGS, which includes 75% of patients with high IF, compared with MCD and MCD-like where most biopsies showed less than 25% IF, may affect responsiveness to therapy, thus accounting in part for the findings.
Analysis of the tissue mRNA gene expression data from the tubulointerstitial compartment revealed a robust gene signature correlated with the percentage of IF. When correlated genes were placed into their functional context, multiple upstream regulators such as TNF, TGF-B1, NFkB, IFN-Gamma, epidermal growth factor (EGF), interleukin 1B (IL-1B) and interleukin 4 (IL-4), and pathways known to be associated with inflammation and fibrosis were identified [34–38]. This finding lends further support to the biological validity of visual assessment of IF percentage by WSI because it correlates with underlying transcriptional programs linked to intra-renal inflammation and fibrosis. Recent studies have similarly highlighted the association of changes in the tubulointerstitial gene expression level of some of these mediators and IF in kidney tissue. For EGF, a gene with expression predominantly confined to kidney tubules and inversely associated with IF severity, significant correlation of interstitial EGF mRNA level with IF/TA, eGFR and eGFR loss was recently reported, and lower urinary EGF was shown to be an independent risk predictor of CKD progression [39]. Also, increased levels of circulating TNF receptors have been associated with increased IF in IgAN kidney biopsies [40].
While the impact of IF severity on response to different medications is of clinical interest, our sample size among subsets that were untreated or treated by specific immunosuppression classes were too small to adequately address this question, although the proportion of patients treated in the low IF group was larger compared with those with high IF. II and fibrosis were closely associated in the biopsies reviewed for this study and it is difficult to evaluate separately the impact of inflammation, which has been shown to be a potent predictor of graft failure in transplanted kidney [41, 42] and warrants further investigation in proteinuric glomerular diseases. These analyses were designed to determine if there was an association between IF and clinical outcomes, independent of classical clinical parameters, which we did show. Future studies with a larger sample size, including a derivation and validation cohort, are needed to quantitate the additive value of IF in addition to other parameters (e.g. genetic polymorphisms, urine biomarkers) in a predictive model.
In summary, the findings in this study support the importance of a visual quantitative assessment of the extent of IF in kidney biopsies as it is associated with disease progression in MCD, MCD-like, FSGS, MN and IgAN, independent of the type of glomerular lesion. Our data suggest that the degree of IF may have value as a predictor of progression of disease in proteinuric glomerulopathies, and provide an evidence-based rationale for routine inclusion of a precise estimate of the degree of IF/TA in the diagnostic report of native kidney biopsies of patients with proteinuria.
ACKNOWLEDGEMENTS
The Nephrotic Syndrome Study Network Consortium (NEPTUNE), U54-DK-083912, is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through a collaboration between the Office of Rare Diseases Research (ORDR), NCATS, and the National Institute of Diabetes, Digestive, and Kidney Diseases. Additional funding and/or programmatic support for this project has also been provided by the University of Michigan, the NephCure Kidney International and the Halpin Foundation.
CONFLICT OF INTEREST STATEMENT
Part of the data has been previously presented in a poster at the 2015 Renal Week meeting of the American Society of Nephrology in San Diego. None of the authors has any conflict of interest related to this manuscript.
REFERENCES
- 1. Striker GE, Schainuck LI, Cutler RE. et al. Structural-functional correlations in renal disease. I. A method for assaying and classifying histopathologic changes in renal disease. Hum Pathol 1970; 1: 615–630 [DOI] [PubMed] [Google Scholar]
- 2. Schainuck LI, Striker GE, Luther RE. et al. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol 1970; 1: 631–641 [DOI] [PubMed] [Google Scholar]
- 3. Bohle A, Mackensen-Haen S, von Giese H. et al. The consequences of tubulo-interstitial changes for renal function in glomerulopathies. A morphometric and cytological analysis. Pathol Res Pract 1990; 186: 135–144 [DOI] [PubMed] [Google Scholar]
- 4. Racusen LC, Solez K, Colvin RB. et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713–723 [DOI] [PubMed] [Google Scholar]
- 5. Racusen LC, Colvin RB, Solez K. et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 2003; 3: 708–714 [DOI] [PubMed] [Google Scholar]
- 6. Solez K, Axelsen R, Benedicktsson H. et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int 1993; 44: 411–422 [DOI] [PubMed] [Google Scholar]
- 7. Haas M, Sis B, Racusen LC. et al. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 2014; 14: 272–283 [DOI] [PubMed] [Google Scholar]
- 8. Cattran D, Coppo R, Cook H. et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76: 534–545 [DOI] [PubMed] [Google Scholar]
- 9. Austin III HA, Muenz LR, Joyce KM. et al. Prognostic factors in lupus nephritis: contribution of renal histologic data. Am J Med 1983; 75: 382–391 [DOI] [PubMed] [Google Scholar]
- 10. D'Agati VD, Fogo AB, Brujin JA. et al. Pathological classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis 2004; 43: 368–382 [DOI] [PubMed] [Google Scholar]
- 11. Weening JJ, D’Agati VD, Schwartz MM. et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004; 15: 241–250 [DOI] [PubMed] [Google Scholar]
- 12. Berden AE, Ferrario F, Hagen EC. et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010; 21:1628–1636 [DOI] [PubMed] [Google Scholar]
- 13. Tervaert TWC, Mooyaart AL, Amann K. et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010; 21: 556–563 [DOI] [PubMed] [Google Scholar]
- 14. Haas M, Rastaldi MP, Fervenza FC.. Histologic classification of glomerular diseases: clinicopathologic correlations, limitations exposed by validation studies, and suggestions for modification. Kidney Int 2014; 85: 779–793 [DOI] [PubMed] [Google Scholar]
- 15. Sethi S, Haas M, Markowitz GS. et al. Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol 2016; 29: 671–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barisoni L, Troost JP, Nast C. et al. Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gadegbeku CA, Gipson DS, Holzman LB. et al. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 2013; 83: 749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barisoni L, Nast CC, Jennette JC. et al. Digital pathology evaluation in the multicenter Nephrotic Syndrome Study Network (NEPTUNE). Clin J Am Soc Nephrol 2013; 8: 1449–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenberg AZ, Palmer M, Merlino L. et al. The application of digital pathology to improve accuracy in glomerular enumeration in renal biopsies. PLoS ONE 2016; 11: e0156441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nast CC, Lemley KV, Hodgin JB. et al. Morphology in the digital age: integrating high-resolution description of structural alterations with phenotypes and genotypes. Semin Nephrol 2015; 35: 266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Inker LA, Matsushita K. et al. GFR decline as an end point for clinical trials in ckd: a scientific workshop sponsored by the national kidney foundation and the US Food and drug administration. Am J Kidney Dis 2014; 64: 821–835 [DOI] [PubMed] [Google Scholar]
- 22. Gipson DS, Troost JP, Lafayette RA. et al. Complete remission in the Nephrotic Syndrome Study Network. Clin J Am Soc Nephrol 2016; 11: 81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lai JY, Luo J, O’Connor C. et al. MicroRNA-21 in glomerular injury. J Am Soc Nephrol 2015; 26: 805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmid H, Boucherot A, Yasuda Y. et al. Modular Activation of Nuclear Factor-κB Transcriptional Programs in Human Diabetic Nephropathy. Diabetes 2006; 55: 2993–3003 [DOI] [PubMed] [Google Scholar]
- 25. Farris AB, Adams CD, Brousaides N. et al. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol 2011; 22: 176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farris AB, Alpers CE.. What is the best way to measure renal fibrosis?: A pathologist’s perspective. Kidney Int Suppl 2014; 4: 9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sund S, Grimm P, Reisæter AV. et al. Computerized image analysis vs semiquantitative scoring in evaluation of kidney allograft fibrosis and prognosis. Nephrol Dial Transplant 2004; 19: 2838–2845 [DOI] [PubMed] [Google Scholar]
- 28. Lemley KV, Bagnasco SM, Nast CC. et al. Morphometry predicts early GFR change in primary proteinuric glomerulopathies: a longitudinal cohort study using generalized estimating equations. PLoS ONE 2016; 11: e0157148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis 2000; 36: 227–237 [DOI] [PubMed] [Google Scholar]
- 30. Barbour SJ, Espino-Hernandez G, Reich HN. et al. The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int 2016; 89: 167–175 [DOI] [PubMed] [Google Scholar]
- 31. Mise K, Hoshino J, Ueno T. et al. Prognostic value of tubulointerstitial lesions, urinary n-acetyl-β-d-glucosaminidase, and urinary β2-microglobulin in patients with type 2 diabetes and biopsy–proven diabetic nephropathy. Clin J Am Soc Nephrol 2016; 11: 593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zuo K, Wu Y, Li S-J. et al. Long-term outcome and prognostic factors of idiopathic membranous nephropathy in the Chinese population. Clin Nephrol 2013; 79: 445–453 [DOI] [PubMed] [Google Scholar]
- 33. Kremers WK, Denic A, Lieske JC. et al. Distinguishing age-related from disease-related glomerulosclerosis on kidney biopsy: the Aging Kidney Anatomy study. Nephrol Dial Transplant 2015; 30: 2034–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Henger A, Kretzler M, Doran P. et al. Gene expression fingerprints in human tubulointerstitial inflammation and fibrosis as prognostic markers of disease progression1. Kidney Int 2004; 65: 904–917 [DOI] [PubMed] [Google Scholar]
- 35. Hotchiss H, Chu T, Hancock W. et al. Differential expression of profibrotic and growth factors in chronic allograft nephropathy. Transplantation 2006; 81: 342–349 [DOI] [PubMed] [Google Scholar]
- 36. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 2006; 69: 213–217 [DOI] [PubMed] [Google Scholar]
- 37. Campanholle G, Ligresti G, Gharib SA. et al. Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol 2013; 304: C591–C603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 2014; 124: 2299–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ju W, Nair V, Smith S. et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 2015; 7: 316ra193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sonoda Y, Gohda T, Suzuki Y. et al. Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PLoS ONE 2015; 10: e0122212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solez K, Colvin RB, Racusen LC. et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008; 8: 753–760 [DOI] [PubMed] [Google Scholar]
- 42. Mannon RB, Matas AJ, Grande J. et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. Am J Transplant 2010; 10: 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]