INTRODUCTION
Due to the scarcity of randomized clinical trials (RCTs) in nephrology [1], particularly in dialysis, there has been uncertainty about optimal practices. Using standardized data collection, the Dialysis Outcomes and Practice Patterns Study (DOPPS) has shown very large between- and within-country differences in hemodialysis (HD) practices and patient outcomes. These observational data are especially useful in areas where results from clinical trials are not yet available or where a randomized design may not be feasible, e.g. for ethical reasons or high cost. Observational studies have influenced medical treatments for many decades, but can result in biased and misleading findings if appropriate statistical methodology is not applied, since treatments are often confounded by clinical indication. For example, we observe that HD patients treated with erythropoietin have lower—not higher—post-treatment hemoglobin levels than patients not treated with this agent because the indication for therapy is a low hemoglobin level [2]. In the DOPPS, a vast amount of information on potential confounders, as well as application of sophisticated statistical techniques, contributes to improve comparability among treatment groups with the intent to yield more robust results.
In the following sections, we review selected studies from the DOPPS that have clinical implications for practice at the dialysis facility, and we provide some comments about the reasons for the analytical approaches used.
The DOPPS
Launched in 1996, the DOPPS is an international prospective cohort study of HD patients and dialysis facility practices that is currently ongoing in more than 400 facilities in nearly 20 countries. Dialysis facilities are selected to be nationally representative, and patients within the selected facilities are sampled at random, to minimize selection bias [3–5]. Study objectives include description of differences in practice patterns and examination of their associations with patient outcomes while accounting for patient case mix and other potential confounders. Practice patterns may include preference of, or protocols for medication use or dose, clinical target values, average dialysis prescription, staffing levels, physician opinions, etc. Examples of outcomes include mortality, hospitalization, vascular access and patient-reported outcomes such as quality-of-life.
VASCULAR ACCESS
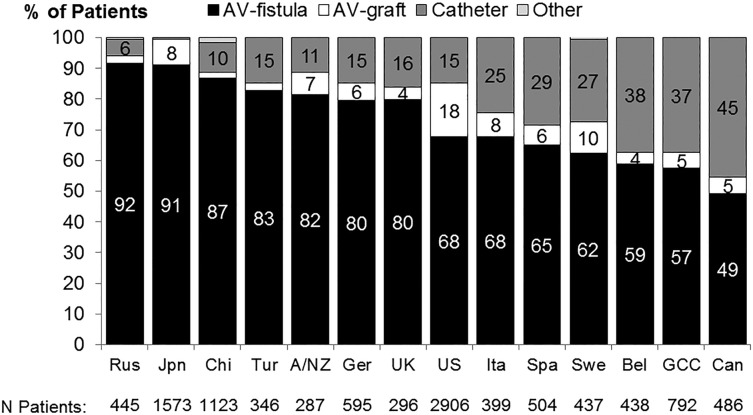
An HD patient's vascular access is often viewed as the lifeline for the delivery of HD therapy, with a native arteriovenous fistula (AVF) widely recognized as the vascular access with the best outcomes overall, compared with an AV graft (AVG) or central venous catheter (CVC). The DOPPS has documented large differences in vascular access use across countries and across dialysis units within countries. International differences in vascular access use among prevalent dialysis patients are depicted in Figure 1, based on DOPPS phase 5 (2012–14), showing AVF use varying from 49% in Canada to 92% in Russia, and CVC use ranging from <2% in Japan to 45% in Canada.
FIGURE 1.
Distribution of vascular access use in prevalent patients, by DOPPS country (2012–14). Data from Russia (Rus), China (Chi), Turkey (Tur), Sweden (Swe), Belgium (Bel) and Gulf Cooperation Council (GCC) countries are based on patient's vascular access at the initial cross section of DOPPS 5; data from Japan (Jpn), Australia/New Zealand (A/NZ), Germany (Ger), United Kingdom (UK), United States (US), Italy (Ita), Spain (Spa) and Canada (Can) are based on a cross section of HD patients in August 2013. Adapted from Pisoni et al. [6].
HD patients who receive dialysis through a CVC or an AVG tend to have greater comorbidities, are older and in poorer health, compared with patients using a native AVF. Over the last two decades, we and others have reported substantially higher mortality rates for patients using a CVC and somewhat higher mortality rates if using an AVG as a vascular access (versus AVF), even after extensive adjustment for these factors [6–8]. A concern with these analyses, however, was that if patient characteristics by vascular access were not balanced on measured covariates, then they were likely also imbalanced across unmeasured confounders. To address this limitation we applied a facility-level approach, assigning the adjusted proportion of patients prescribed a catheter in the facility as the exposure for each patient in the facility. The DOPPS has shown that vascular access type tends to be more strongly related to provider preference than to patient characteristics [4, 6], resulting in great variation in vascular access use across HD facilities. Before applying this approach, we compared the distribution of 20+ key patient characteristics across four levels of facility catheter use (i.e., the proportion of catheter users in each facility), revealing that the distributions of age and comorbidities were similar in facilities having lower versus higher percentage CVC use. Thus, variation in catheter use likely reflected differences in provider preference for CVC use. We then conducted two types of mortality analyses [6]. (i) The standard Cox regression with extensive adjustment for patient characteristics showed in the fully adjusted model a hazard ratio (HR) for catheter versus AVF of 1.32 [95% confidence interval (CI) 1.22, 1.42] and an HR for AVG versus AVF of 1.15 (95% CI 1.06, 1.25). (ii) The facility-level analysis with adjustment for case mix and other facility practices were qualitatively consistent in demonstrating the survival benefits of AVF: the mortality HR (95% CI) was 1.14 (1.06, 1.22) per 20% greater facility CVC use and 1.07 (1.01, 1.13) per 20% greater facility AVG use. Further more, the facility-level model was minimally affected by adjustment for additional covariates, an indicator of a more robust finding.
LENGTH OF HEMODIALYSIS SESSIONS
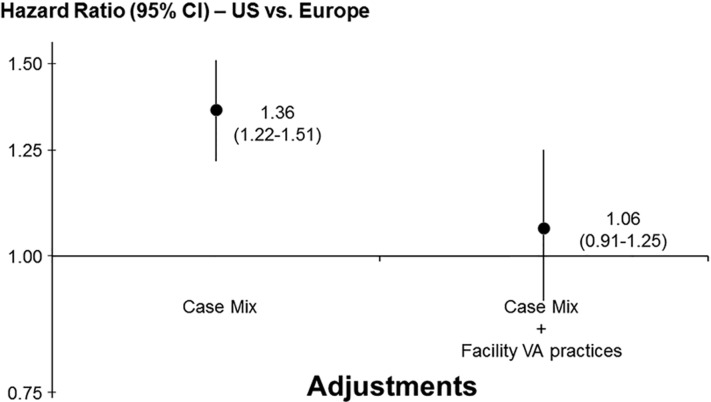
Moreover, prior findings of higher adjusted mortality in the US versus European DOPPS countries [9] was largely explained by differences in vascular access practices as shown in Figure 2 [6].
FIGURE 2.
HR of mortality in the US DOPPS versus Europe DOPPS, with and without adjustment for differences in facility vascular access (VA) use in DOPPS phases 1–2 (1996–2004). The HR of mortality for HD patients in the US versus Europe (n = 24 398) stratified by study phase is shown on the left, adjusted for patient age, sex, black race, number of years with end-stage renal disease, body weight, 14 summary comorbid conditions, whether treated in a hospital-based unit, facility median treatment time, facility % patients with serum phosphorus >5.5 mg/dL and facility % patients with serum calcium >10 mg/dL; and, on the right, additionally adjusted for % facility vascular access use. All models accounted for facility clustering effects. Europe refers to France, Germany, Italy, Spain and the United Kingdom. Adapted with permission from Pisoni et al. [6].
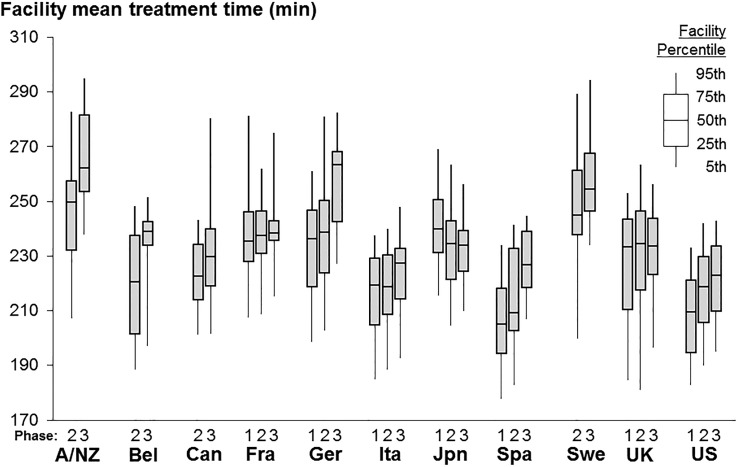
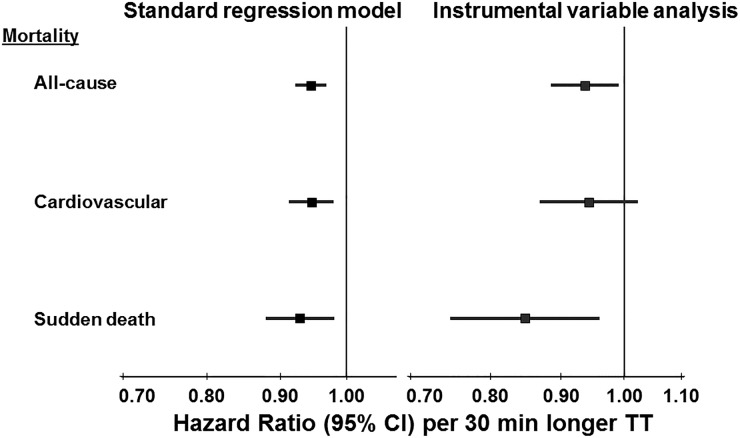
When HD treatment first became available in the 1960s, the thrice weekly session length was often 8–10 h. The complication of dialysis disequilibrium syndrome during and after HD sessions was common, particularly with shorter sessions that removed urea and fluid more rapidly. This complication could be avoided, however, by raising dialysate sodium [10]. Subsequently, in the 1970s and 1980s, dialysate sodium was increased from 132 to about 140 mEq/L, allowing for standard session lengths of only 4 h thrice weekly. The National Cooperative Dialysis Study, a large randomized trial in the USA, yielded results that were interpreted to mean no benefit from longer treatment times (P = 0.06, labeled ‘not significant’) [11] and dialysis time in the USA was shortened further, often to 3 h. Concurrently, blood flow rates were increased to achieve a similar dialysis dose for urea (Kt/V). To test the hypothesis that longer treatment time reduces mortality, we took advantage of the large variation in observed mean prescribed treatment times across facilities within each DOPPS country as shown in Figure 3 [12]. Among prevalent HD patients, those prescribed longer dialysis sessions were more likely to have hyperkalemia or large interdialytic weight gains, likely reflecting the practice of prescribing patients longer session times to remove excess potassium or fluid. In analyses adjusted extensively for case mix, we showed that a 30-min longer treatment time was associated with a 6% lower mortality rate (HR 0.94; 95% CI 0.92, 0.97). Recognizing the potential for treatment by indication however, we also applied an instrumental variable (IV) analysis that can minimize unmeasured confounding. The international DOPPS is well-suited for this type of analysis due to the large variation in practice patterns such as treatment time, making the dialysis facility a reasonable instrument candidate. We conducted a two-stage IV analysis using the dialysis facility as the instrument, adjusting for patient case-mix. Results of the IV analysis (Figure 4) were consistent with the standard Cox model, providing additional evidence for the benefit of longer treatment time [12].
FIGURE 3.
Facility mean prescribed treatment time by DOPPS country and DOPPS data collection phase (1996–2008). For country abbreviations see Figure 1. Adapted with permission from Tentori et al. [12].
FIGURE 4.
HR of mortality associated with prescribed treatment time (TT, per 30 min longer) in DOPPS phases 1–3 (1996–2008). Cox regression models stratified by country and phase, accounted for facility clustering, and adjusted for age, sex, black race, time on dialysis, body mass index, 13 summary comorbid conditions, residual kidney function, prescribed blood flow rate, and catheter use. Adapted with permission from Tentori et al. [12].
We also estimated the effect of treatment time on mortality independent of dialysis dose (Kt/V). Adjusting also for Kt/V, which is primarily based on pre- and post-dialysis urea or blood urea nitrogen (BUN) values and body size (with little contribution of treatment time in the Kt/V formula), we found that the HR was slightly attenuated, but a 4% lower mortality rate per 30 min longer treatment time remained (HR 0.96; 95% CI 0.93, 0.99) [12]. We were puzzled, however, by differences observed across DOPPS regions: the association (unadjusted for Kt/V) was strong in Europe and Japan but very weak in North America [12]. With this unexplained caveat, DOPPS findings provided strong evidence that longer treatment time is associated with lower mortality.
DIALYSATE SODIUM
Recent opinions have suggested that the dialysate sodium concentration (DNa) should be lowered to avoid sodium transfer to the patient during HD [13]. We investigated this issue with international DOPPS data and discovered that almost half the DOPPS facilities used a single DNa level for virtually all their patients (excluding ≤10% patients whose DNa deviated). In these ‘non-individualized’ facilities, confounding by indication was not an issue because the patient's DNa was not influenced by clinical indications. The other ‘individualized’ facilities allowed us to examine differences in patient characteristics by the DNa level used. In so doing, we found evidence that DNa was prescribed by indication in this latter group of facilities, and thus standard analyses could be subject to confounding by indication. For example, patients were more likely to be prescribed a lower DNa if their predialysis systolic blood pressure (SBP) was higher and a higher DNa if their intradialytic drop in SBP was greater [14].
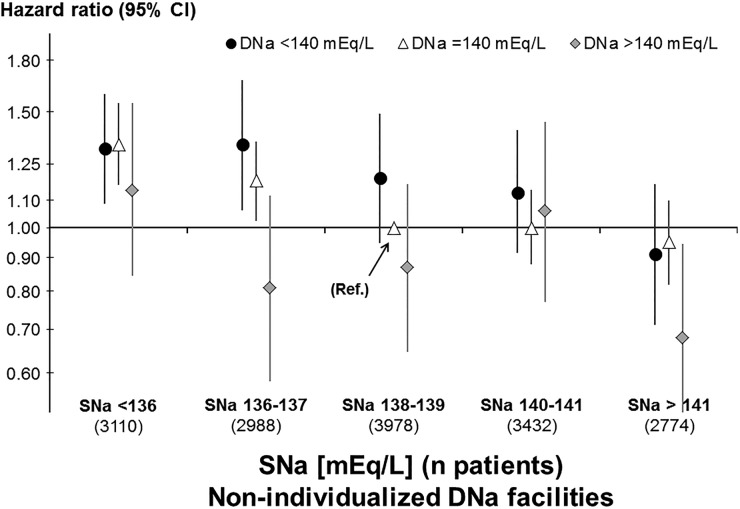
DNa ranged from 137 to 143 mEq/L in 97% of non-individualized facilities and we found that the mean interdialytic weight gain during 1 week was greater by 0.17% of body weight per 2 mEq/L higher DNa. This translates to about 120 g or mL for a patient with a weight of 70 kg, or about 60 mL/day. While this modest fluid gain may be of concern to some clinicians, mortality analyses showed that the adjusted HR for all-cause mortality, without adjusting for weight gain, was not elevated but reduced at higher DNa (HR 0.88 per 2 mEq/L higher DNa; 95% CI 0.83, 0.94) [15]. This observed mortality HR remained in sensitivity analysis adjusting for other practice patterns and excluding Japanese patients (HR 0.91; 95% CI 0.85, 0.97) [16]. We also found association between mortality and a larger dialysate-to-serum sodium difference (gradient), as reported by others; however, a further investigation by serum sodium revealed that this sodium gradient association was fully explained by low serum sodium and not by the DNa (Figure 5) [15, 17]. Despite confirmation of greater weight gain between dialysis sessions, our finding of lower mortality (and lower hospitalization rate) with higher DNa suggests that the prior recommendation for lowering DNa was at least partly based on analyses favoring lower DNa that may have been confounded by indication.
FIGURE 5.
HR of mortality by categories of dialysate sodium (DNa) and predialysis serum sodium (SNa), in 425 facilities that used a non-individualized DNa prescription (no confounding by indication) in DOPPS phases 1–4 (1996–2011). Reference category DNa = 140 mEq/L and SNa = 138–139 mEq/L. All Cox regression models were stratified by country and study phase, accounted for facility clustering, and adjusted for demographics, comorbidities and laboratory values. Analyses updated from Hecking et al. [15] and figure adapted with permission from Port [17].
In a subsequent DOPPS study of patient-reported time to recovery after HD sessions, Rayner et al. found that higher DNa was associated with shorter recovery time, suggesting a further benefit of higher DNa [18]. This finding requires confirmation in follow-up studies.
SERUM PHOSPHORUS
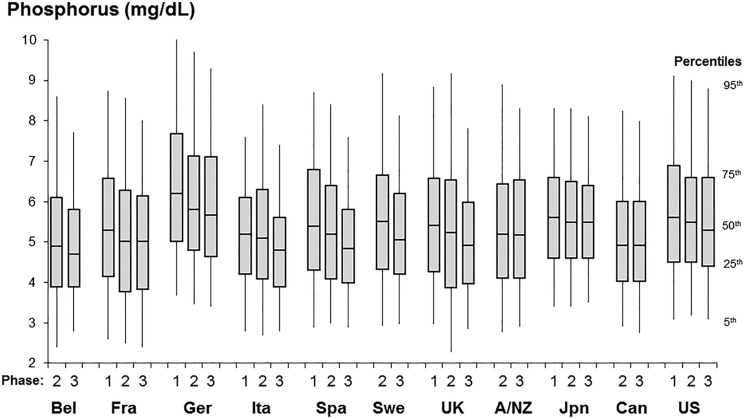
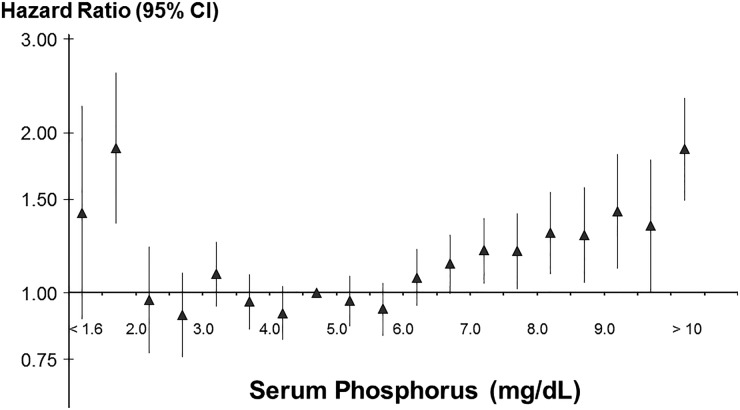
Control of phosphorus in dialysis patients is often difficult, as evidenced by the wide variation in phosphorus control across and within countries (Figure 6). One may speculate that this variation is due to differences in educational efforts by the dialysis staff, as well as patient compliance and dietary intake. We examined the association between serum phosphorus level at entry into the DOPPS and mortality. After extensive adjustment for case mix the mortality rate was clearly elevated for predialysis serum phosphorus above 6.5 mg/dL compared with patients whose level was 4.6–5.0 mg/dL. Additionally, very low serum phosphorus levels were also associated with adverse outcomes, which may reflect poor nutritional intake (Figure 7) [19]. In a further analysis we found that the cause-specific mortality due to cardiovascular causes (censoring patients who died of other causes) was much higher than the all-cause mortality HR for phosphorus levels >6.0 mg/dL [19]. Given the large differences in the average control of phosphorus at the dialysis facility level, we also studied whether poorer facility control of phosphorus was associated with higher mortality in case mix adjusted models. Tentori et al. found qualitatively consistent results of higher mortality for patients treated at facilities with higher percentages of patients having serum phosphorus >6.0 mg/dL [19]. We recognize that the association of phosphorus with mortality is more complex as additional DOPPS research has shed light on the potential additional relationship of phosphorus-binders and improved nutrition [20].
FIGURE 6.
Distribution of baseline serum phosphorus among patients on dialysis >180 days, by DOPPS country and DOPPS data collection phase (1996–2008). For country abbreviations see Figure 1. Adapted with permission from Tentori et al. [19].
FIGURE 7.
HR of mortality by categories of baseline serum phosphorus among patients on dialysis >180 days in DOPPS phases 1–3 (1996–2008; n = 25 529 patients, n = 5857 deaths). Adapted with permission from Tentori et al. [17].
HEALTH-RELATED QUALITY OF LIFE
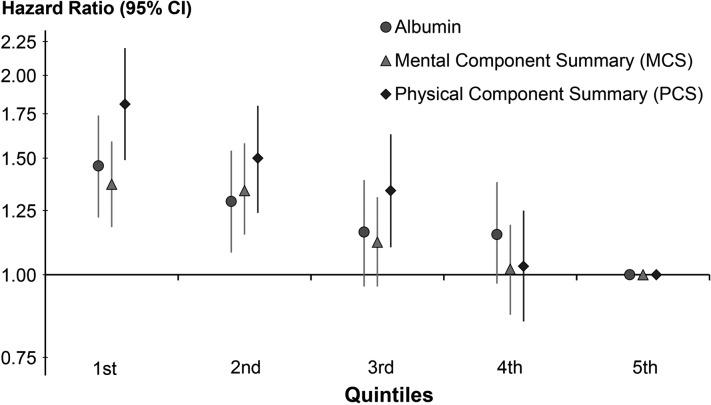
Since the beginning, the DOPPS has placed great emphasis on the importance of the patient's experience, collected through a patient questionnaire that is completed by most DOPPS participants close to their enrollment into the study and annually thereafter. In DOPPS analyses, the physical component summary (PCS) and mental component summary (MCS) score from the KDQOL 36 are low compared with the general population, indicating lower levels of functioning among HD patients in all countries. Not surprisingly, patients with lower PCS and MCS scores have higher mortality rates than those with higher scores, even after adjustment for potential confounders (Figure 8; association of serum albumin also shown) [21]. These findings suggest that patient-reported indicators deserve attention since they are prognostic markers. The DOPPS has noted strong associations of numerous patient-reported measures with outcomes including a strong association of mortality with depressive symptoms that may be treatable [22, 23].
FIGURE 8.
HR of mortality by quintile of two summary scores of health-related quality of life and for serum albumin in DOPPS phase 1 (1996–2001). Adapted with permission from Mapes et al. [21].
DISCUSSION
The findings presented here provide some perspectives on ‘real world’ practice differences between HD facilities and their association with clinical outcomes in the large international DOPPS.
It is important to recognize that in any type of comparative study, even RCTs, we observe associations, not (causal) effects. The main advantage of randomized studies (experiments), relative to observational studies, is the potential for mitigation of confounding (bias), even when we have not identified or measured the variables (confounders) responsible for the bias. Of course, RCTs also have methodological limitations (e.g. non-adherence to treatment protocols; lack of blinding; limited generalizability; and low power due to small samples, weak effects, or rare outcomes), and they are often impractical, costly and lengthy in duration, or judged to be unethical.
Consequently, observational research like the DOPPS has remained critical for advancing clinical practice in nephrology, but it requires more attention to certain types of bias such as confounding, selection, and reverse causation. To this end, the design of an observational study should be based on imagining what hypothetical experiment (RCT) could be done to test the primary hypothesis. This helps to determine the source population (eligibility), when randomization should be done (and follow-up should begin), what parameter should be estimated and how the statistical analysis could be done. For example, this strategy helps us understand why we cannot determine the best time [e.g. estimated glomerular filtration rate (eGFR)] to start dialysis by conducting a study in a population identified at the start of dialysis. The problem is that persons are not followed before they start dialysis; therefore, we cannot compare chronic kidney disease (CKD) patients with a given eGFR who start versus do not start dialysis at that time, as we would in an RCT.
It is sometimes tempting to conduct (ecologic) analyses at the facility or country level without taking into account patient-level data; but those associations may be severely biased and very misleading. For example, we found that blood flow rates during dialysis are lower in Japan than elsewhere in the DOPPS and mortality is lower in Japan, which taken together (ecologic evidence) could suggest that lower blood flow is beneficial for patient survival. However, we found that higher blood flow was associated with lower mortality in the USA, Europe, and even Japan [24]. Thus, the ecologic analysis was very misleading, and the problem—called ecologic bias—was not simply confounding [25].
The DOPPS study of outcomes by dialysis session length is relevant even though a large randomized trial, the National Cooperative Dialysis Study, compared outcomes for patients assigned to short versus long treatment time (2.5–3.5 h versus 4–4.5 h) and high versus low time-averaged BUN [11]. The hospitalization rate, as the primary outcome, was lower for patients randomized to the longer treatment-time arm, but the P-value of 0.06 was labeled ‘not significant’ and therefore was interpreted as showing no benefit of longer treatment times. That conclusion was a misinterpretation of the statistical findings because P < 0.05 is an arbitrary cutpoint and the P-value is influenced by the size of the study as well as the magnitude of the association; it does not reflect the probability that the null hypothesis is true or that the results were due to chance. This long-established limitation of significance testing for interpreting statistical findings was recently released as an official consensus statement by the American Statistical Association [26].
The large differences in practices across facilities observed in the DOPPS allow the use of facility-level or IV analyses. In recent years the DOPPS approach has evolved to using a two-stage IV analysis, with the dialysis facility as the instrument, to control for unmeasured confounders while adjusting for several measured confounders at both stages. These analyses control for unmeasured or inadequately measured confounders, since confounding by indication is usually present and adjustment for measured confounders may not be sufficient to remove the bias. To enhance the validity of this approach, we also adjust for indicators of other facility practices. In an extension of this principle, restricting analysis to facilities with uniform, but contrasting, practice patterns (e.g. concentration of a dialysate component) avoids confounding by indication when comparing those practices, but there still may be confounding due to unmeasured facility (contextual) factors [27].
Patient-reported indicators of health-related quality of life usually require lengthy questionnaires to be filled out. A caveat for most analyses of patient reporting is that the sickest patients often are not able or willing to provide information, e.g. due to an acute illness or mental status change. Our previous analyses of responders versus non-responders indicate that non-responders are sicker and likely at greater risk of dying [28]. To deal with this limitation, multiple imputation may assign missing exposure status in non-responders, or sensitivity analyses may be useful. Our analyses of outcomes associated with depressive symptoms or with recovery time after HD showed that a single question may provide prognostic information and allow for potential clinical intervention.
The outcomes of interest for the DOPPS and the clinician clearly go beyond mortality. Although not addressed in this article, we note that the results for each of the studies covered here did not only show strong associations with mortality but also with time to first hospitalization (see respective citations). Given the disruption to patients' lives caused by dialysis, validated patient-reported outcome instruments are also increasingly recognized as important outcomes capturing the experiences of dialysis patients.
There is a great need to study the transition from CKD to dialysis, particularly since there has been a trend to starting dialysis earlier with a higher eGFR and since we observed a particularly high mortality in the early months of HD. Outcomes for incident dialysis patients are influenced by factors outside the dialysis facility such as prior CKD therapy, rate of CKD progression and level of residual renal function.
The examples presented are based primarily on prevalent patients, i.e., patients with variable vintage on HD, in order to be useful to the clinician when making rounds in the dialysis facility. In addition, some findings from cohort studies may provide motivation for clinical trials, as well as data to inform design of such trials. IV analysis using the dialysis facility as the instrument is a promising method to control for unmeasured confounders. Finding consistent results with conventional analysis is reassuring when the instrument is strong; however, inconsistent results must be evaluated on the basis of a priori clinical knowledge that is not necessarily captured in the data.
The DOPPS reached its 20 year anniversary in 2016 and continues to bring new clinically useful information for the practicing nephrologist, not only for HD, but also recently for peritoneal dialysis (PDOPPS) and advanced CKD practices (CKDopps). The large observed variations in numerous treatment variables among dialysis facilities suggest uncertainties about optimal practice patterns. The DOPPS is designed to continue pursuing such questions from ‘real world’ practices and associated outcomes.
ACKNOWLEDGEMENTS
The DOPPS program is supported by Amgen, Kyowa Hakko Kirin, AbbVie, Sanofi Renal, Baxter Healthcare and Vifor Fresenius Medical Care Renal Pharma. Additional support for specific projects and countries is provided by Keryx Biopharmaceuticals, Merck Sharp & Dohme Corp., Proteon Therapeutics, Relypsa and F. Hoffmann-LaRoche; in Canada by Amgen, BHC Medical, Janssen, Takeda and the Kidney Foundation of Canada (for logistics support); in Germany by Hexal, DGfN, Shire and the WiNe Institute; and for PDOPPS in Japan by the Japanese Society for Peritoneal Dialysis (JSPD). All support is provided without restrictions on publications.
CONFLICT OF INTEREST STATEMENT
R.L.P. reports personal fees from Kyowa Hakko Kirin, non-financial support from National Kidney Foundation, outside the submitted work. B.M.R. received personal fees from University of Toronto, Rhode Island Hospital and Kyowa Hakko Kirin. H.M. is a consultant at Arbor Research Collaborative for Health. All other authors have nothing to declare.
REFERENCES
- 1. Palmer SC, Sciancalepore M, Strippoli GF. Trial quality in nephrology: how are we measuring up? Am J Kidney Dis 2011; 58: 335–337 [DOI] [PubMed] [Google Scholar]
- 2. Rayner HC. How can we learn from each other to improve outcomes for patients? Br J Renal Med 2012; 17: 12–14 [Google Scholar]
- 3. Robinson BM, Bieber B, Pisoni RL et al. Dialysis Outcomes and Practice Patterns Study (DOPPS): its strengths, limitations, and role in informing practices and policies. Clin J Am Soc Nephrol 2012; 7: 1897–1905 [DOI] [PubMed] [Google Scholar]
- 4. Young EW, Dykstra DM, Goodkin DA et al. Hemodialysis vascular access preferences and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 2002; 61: 2266–2271 [DOI] [PubMed] [Google Scholar]
- 5. Pisoni RL, Zepel L, Port FK et al. Trends in US vascular access use, patient preferences, and related practices: an update from the US DOPPS Practice Monitor with international comparisons. Am J Kidney Dis 2015; 65: 905–915 [DOI] [PubMed] [Google Scholar]
- 6. Pisoni RL, Arrington CJ, Albert JM et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 2009; 53: 475–491 [DOI] [PubMed] [Google Scholar]
- 7. Ethier J, Mendelssohn DC, Elder SJ et al. Vascular access use and outcomes: an international perspective: results from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 2008; 23: 3219–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhingra RK, Young EW, Hulbert-Shearon TE et al. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 2001; 60: 1443–1451 [DOI] [PubMed] [Google Scholar]
- 9. Goodkin DA, Young EW, Kurokawa K et al. Mortality among hemodialysis patients in Europe, Japan, and the United States: case-mix effects. Am J Kidney Dis 2004; 44(Suppl 2): S16–S21 [DOI] [PubMed] [Google Scholar]
- 10. Port FK, Johnson WJ, Klass DW. Prevention of dialysis disequilibrium syndrome by use of high sodium concentration in the dialysate. Kidney Int 1973; 3: 327–333 [DOI] [PubMed] [Google Scholar]
- 11. Lowrie EG, Laird NM, Parker TF et al. Effect of the hemodialysis prescription on patient morbidity—report from the National Cooperative Dialysis Study. N Engl J Med 1981; 305: 1176–1181 [DOI] [PubMed] [Google Scholar]
- 12. Tentori F, Zhang J, Li Y et al. Longer dialysis session length is associated with better intermediate outcomes and survival among patients on in-center three times per week hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2012; 27: 4180–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parker TF 3rd, Straube BM, Nissenson A et al. Dialysis at a crossroads—Part II: a call for action. Clin J Am Soc Nephrol 2012; 7: 1026–1032 [DOI] [PubMed] [Google Scholar]
- 14. Hecking M, Karaboyas A, Rayner H et al. Dialysate sodium prescription and blood pressure in hemodialysis patients. Am J Hypertens 2014; 27: 1160–1169 [DOI] [PubMed] [Google Scholar]
- 15. Hecking M, Karaboyas A, Saran R et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol 2012; 7: 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecking M, Karaboyas A, Port FK. Dialysate sodium and mortality. Letter to the Editor. Nephrol Dial Transplant. [Epub 1 October 2012, available at: http://ndt.oxfordjournals.org/content/27/4/1613/reply. ]
- 17. Port FK. Practice-based versus patient-level outcomes research in hemodialysis: the DOPPS (Dialysis Outcomes and Practice Patterns Study) experience. Am J Kidney Dis 2014; 64: 969–977 [DOI] [PubMed] [Google Scholar]
- 18. Rayner HC, Zepel L, Fuller DS et al. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2014; 64: 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tentori F, Blayney MJ, Albert JM et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530 [DOI] [PubMed] [Google Scholar]
- 20. Lopes AA, Tong L, Thumma J et al. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): evaluation of possible confounding by nutritional status. Am J Kidney Dis 2012; 60: 90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mapes DL, Lopes AA, Satayathum S et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 2003; 64: 339–349 [DOI] [PubMed] [Google Scholar]
- 22. Lopes AA, Bragg J, Young E et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int 2002; 62: 199–207 [DOI] [PubMed] [Google Scholar]
- 23. Lopes AA, Albert JM, Young EW et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int 2004; 66: 2047–2053 [DOI] [PubMed] [Google Scholar]
- 24. Kimata N, Karaboyas A, Bieber BA et al. Gender, low Kt/V, and mortality in Japanese hemodialysis patients: opportunities for improvement through modifiable practices. Hemodial Int 2014; 18: 596–606 [DOI] [PubMed] [Google Scholar]
- 25. Morgenstern H. Ecologic studies. In: Rothman KJ, Greenland S, Lash TL (eds). Modern Epidemiology, 3rd edn Philadelphia: Lippincott Williams & Wilkins, 2008, 511–531 [Google Scholar]
- 26. Wasserstein RL, Lazar NA. The ASA's statement on P-values: context, process, and purpose. Am Stat 2016; 70: 129–133 [Google Scholar]
- 27. Li Y, Lee Y, Wolfe RA et al. On a preference-based instrumental variable approach in reducing unmeasured confounding-by-indication. Stat Med 2015; 34: 11501168. [DOI] [PubMed] [Google Scholar]
- 28. Perl J, Karaboyas A, Morgenstern H et al. Association between changes in quality of life and mortality in hemodialysis patients: results from the DOPPS. Nephrol Dial Transplant 2016[Epub ahead of print 7 June 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]