Abstract
Background. In adults, glomerular hyperfiltration is associated with abnormalities related to metabolic syndrome (MetS). We investigated if glomerular hyperfiltration was associated with metabolic abnormalities in US adolescents without diabetes.
Methods. We analyzed data from the National Health and Nutrition Examination Survey, a nationally representative sample of US adolescents ages 12–17 years. Estimated glomerular filtration rate (eGFR) was determined using the bedside Schwartz equation; adolescents with hyperfiltration (eGFR >120 mL/min/1.73 m2) were compared to those with normal eGFR (90–120 mL/min/1.73 m2). We calculated mean levels of factors related to MetS, insulin resistance and diabetes risk, adjusting for age, race/ethnicity, sex, socioeconomic status, and BMI z-score.
Results. Overall, 11.8% of US adolescents had hyperfiltration [95% confidence interval (CI) 10.6–13.0]. Hyperfiltration prevalence varied by race (20.2% in Hispanics versus 9.8% non-Hispanic whites and 7.4% non-Hispanic blacks; P< 0.001). Compared to those with normal eGFR, adolescents with hyperfiltration had higher adjusted mean levels of triglyceride (83 versus 77 mg/dL; P = 0.05), fasting insulin (15.1 versus 12.9; P< 0.001) and homeostatic model assessment of insulin resistance (3.52 versus 3.01; P = 0.001). These differences persisted after adjusting for BMI z-score. Adolescents with hyperfiltration had increased odds for hypertriglyceridemia [odds ratio 1.58 (95% CI 1.11–2.23)]. These relationships varied by racial/ethnic group.
Conclusions. Glomerular hyperfiltration is associated with hypertriglyceridemia and increased insulin resistance independent of BMI z-score in a nationally representative sample of US adolescents. Hispanic adolescents are more likely to have hyperfiltration than other racial/ethnic groups. These findings could have significance in evaluations of renal function and MetS in adolescents to identify related risks and target interventions.
Keywords: dyslipidemia, ethnicity, insulin, metabolic syndrome, pediatrics
INTRODUCTION
An excessive glomerular filtration rate (GFR) may initiate glomerular damage secondary to increased glomerular pressure and hypertrophy and may be a harbinger for the development of chronic kidney disease (CKD) in individuals both with and without diabetes [1–4]. Epidemiological studies in adults have shown that glomerular hyperfiltration is associated with metabolic abnormalities related to cardiovascular disease [5] and stroke [6].
Multiple mechanisms have been postulated for the pathogenesis of glomerular hyperfiltration, which ultimately results from altered hemodynamics and/or increases in podocyte permeability [7]. Some of these mechanisms—including inappropriate activation of the renin–angiotensin system [8, 9], increased oxidative stress [10] and hyperinsulinemia [11]—also are operative in obesity [12], metabolic syndrome (MetS) [5, 13] and diabetes [7, 14, 15].
Among adults, hyperfiltration has been linked to both obesity [7, 12] and MetS [13] independent of diabetes and has been linked to albuminuria in disease states [16, 17]. However, in the pediatric population, the association between glomerular hyperfiltration with obesity and metabolic abnormalities in the absence of diabetes has not been well characterized [1]. Given the ongoing childhood obesity epidemic [18], and that hyperfiltration can be reversed in the setting of substantial weight loss [19], such associations may have substantial long-term public health implications.
Our goal in this study was to determine the association between glomerular hyperfiltration and MetS-related abnormalities in a large national sample of US adolescents without diabetes. We additionally sought to characterize potential racial/ethnic differences in hyperfiltration and evaluate whether any associations between MetS and hyperfiltration were related to weight status.
MATERIALS AND METHODS
Data were obtained from the Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey (NHANES) from 1999 to 2012. NHANES is a series of surveys conducted in 2-year waves that represents a cross-sectional random selection of the noninstitutionalized US civilian population using a stratified, multistage probability design that includes demographic, anthropometric, questionnaire, and clinical laboratory data. Detailed NHANES protocols have been previously published [20]. The National Center for Health Statistics ethics review board approved all study protocols and participants gave informed consent or assent.
We analyzed data from participants ages 12–17 years old with complete data for determining estimated glomerular filtration rate (eGFR). For assessment of the population prevalence of hyperfiltration, we excluded participants who were pregnant or had physician-diagnosed diabetes. For our analytic sample assessing metabolic differences between those with hyperfiltration or normal filtration, we additionally excluded participants with active hepatitis B infection or those using antihyperlipidemic and antidiabetic agents, as these may affect assessment of baseline MetS.
Three systolic blood pressure readings were obtained for each NHANES participant. If one or more of these readings were disrupted, a fourth reading was obtained. Mean systolic and diastolic blood pressure was calculated from the available readings for each participant. Elevated systolic blood pressure was defined as mean systolic and/or diastolic blood pressure ≥95th percentile for the participant's age, sex, and height according to the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents [21]. Participants using antihypertensive medications were classified as having elevated blood pressure, regardless of the blood pressure measurements obtained.
Laboratory measurements were obtained using controlled protocols and equipment specified by the CDC NHANES databook [22]. Clinical data regarding the individual MetS components were collected under fasting conditions. Serum creatinine was measured with an isotope dilution mass spectrometry (IDMS) standardized method starting in 2008 [23]. Before 2008, non-IDMS-standardized serum creatinine measurements were analyzed and adjusted using Deming regression [24]. Correction factors to pre-2008 serum creatinine measurements were applied as recommended by the CDC NHANES databook [22].
Before 2007, urine creatinine measurements were obtained via a Jaffe reaction. Starting in 2007, urine creatinine was measured with a creatininase endpoint reaction. Correction factors to pre-2007 urine creatinine measurements were applied as recommended by the CDC NHANES databook [22]. Urine albumin was measured with a solid-phase fluorescent immunoassay. Albuminuria was defined as a urine albumin:creatinine ratio (UACR) ≥30 but <300.
To assess the severity of MetS, we used the MetS z-score, a linear score correlated with metabolic abnormality [25, 26]. Childhood elevations in this MetS z-score [27] are associated with the development of cardiovascular disease and type 2 diabetes mellitus in adulthood [28–30]. Use of the MetS z-score allows for a quantitative analysis of the correlation between filtration status and overall metabolic abnormality. The presence of MetS was defined by the adolescent adaptation of the National Cholesterol Education Program's Adult Treatment Panel III (NCEP ATP-III) criteria [31]. Individual clinical criteria for MetS were defined as BMI z-score ≥1.645 (obesity), fasting glucose ≥100 mg/dL (hyperglycemia), fasting triglycerides ≥110 mg/dL (hypertriglyceridemia), HDL ≤40 mg/dL (low HDL), and systolic or diastolic blood pressure exceeding the 90th percentile for height, age and sex (elevated blood pressure).
The homeostasis model of insulin resistance (HOMA-IR) was calculated as [fasting insulin (mU/mL) × fasting glucose (mmol/L)/22.5]. Prediabetes was defined according to criteria set by the American Diabetes Association: hemoglobin A1c (HbA1c) >5.7% but <6.4% or fasting glucose >100 mg/dL but <125 mg/dL [32].
NHANES participants self-identified as non-Hispanic white, non-Hispanic black, Mexican American Hispanic and other Hispanic based on demographic survey question responses. For our analyses, we combined the Mexican American Hispanic and other Hispanic groups into a single Hispanic classification.
We used the bedside Schwartz equation derived from the Chronic Kidney Disease in Children (CKiD) study to calculate eGFR: eGFR (mL/min/1.73 m2) = 0.413 × height (cm)/serum creatinine (mg/dL) [33–35]. Participants were classified as either having hyperfiltration (eGFR ≥120 mL/min/1.73 m2) [36–40] or normal filtration (eGFR 90–120 mL/min/1.73 m2). Participants with eGFR <90 mL/min/1.73 m2 were excluded because of the potential for early CKD [4].
Statistical analysis was performed using SAS (version 9.4: SAS Institute, Cary, NC, USA). Statistical significance was designated as P < 0.05. Survey procedures were used to account for the complex sampling design of NHANES. All multivariable statistical models accounted for sex, age, race/ethnicity and household income:needs ratio. Race/ethnicity was dropped from multivariable statistical models when specific single racial/ethnic groups were being analyzed.
Because we sought to determine whether hyperfiltration was associated with metabolic abnormalities independent of body size, we created two multivariable statistical models, both with and without adjustment for BMI z-score. Differences between adolescents with glomerular hyperfiltration and normal filtration were assessed with survey regression adjusted means analyses. Odds of metabolic abnormalities were compared between adolescents with glomerular hyperfiltration and normal filtration via logistic regression, again adjusting for the aforementioned covariates.
RESULTS
Epidemiology, demographics and anthropomorphic values
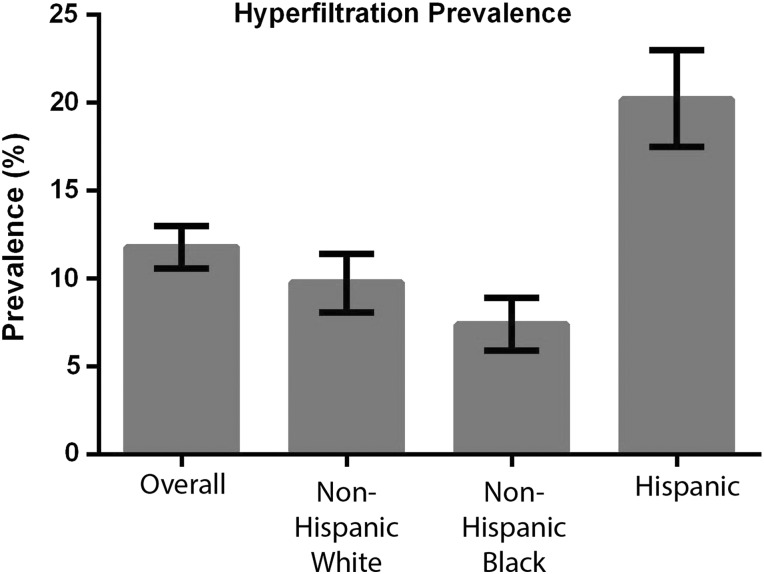
There were 8793 NHANES participants ages 12–17 years for whom serum creatinine was collected (Supplementary data, Figure S1). We next assessed the prevalence of hyperfiltration among US adolescents without diabetes. From the 8793 adolescents with creatinine, we sequentially excluded those who were missing height data (n = 60), were pregnant (n = 54) or were previously diagnosed with diabetes (n = 36), leaving 8643 participants in the sample (Supplementary data, Figure S1). The prevalence of hyperfiltration in this nationally representative sample was 11.8% [95% confidence interval (CI) 10.6–13.0]. The prevalence (with CI) varied by race/ethnicity: non-Hispanic white [9.8% (8.1–11.4)], non-Hispanic black [7.4% (5.9–8.9)] and Hispanic [20.2% (17.5–23.0)] (Figure 1).
FIGURE 1.
Prevalence of hyperfiltration in a nationally representative sample of US adolescents ages 12–17 years. This sample was restricted to participants who were nonpregnant and without diabetes. The error bars represent the 95% confidence interval.
For the remainder of the analyses of metabolic differences between adolescents with normofiltration and hyperfiltration, we excluded participants who were nonfasting (n = 317), lacked complete MetS-related data (n = 4512), were taking any antidiabetic or antihyperlipidemic agents (n = 6), had active hepatitis B infection (n = 3) or had eGFR <90 mL/min/1.73 m2 (n = 1221; n = 105 with eGFR <60 mL/min/1.73 m2). This left 2584 participants in the final analytic sample to compare metabolic differences between adolescents with hyperfiltration and those with normal filtration. Demographic characteristics of this sample are shown in Table 1. Compared with the 8643-participant sample used to assess national prevalence of hyperfiltration, the 2584-participant analytic sample did not differ with respect to composition of sex, race/ethnicity and socioeconomic status. However, participants in the larger sample were slightly older than those in the final analytic sample [14.5 (95% CI 14.5, 14.6) versus 14.1 (14.0–14.2) years].
Table 1.
Participant characteristics among the analytic cohort
| Overall | Hyperfiltration | Normal filtration | P-value | |
|---|---|---|---|---|
| Final analytic sample | 2584 | 564 | 2020 | – |
| % Male | 50.2 (47.7–52.8) | 40.2 (32.9–47.5) | 45.2 (41.9–48.5) | <0.001 |
| % Non-Hispanic White | 65.8 (62.5–69.1) | 54.0 (45.9–62.1) | 67.9 (64.1–71.7) | <0.001 |
| % Non-Hispanic Black | 15.3 (13.2–17.4) | 10.7 (7.6–13.8) | 14.1 (11.7–16.4) | <0.001 |
| % Hispanic | 18.9 (16.2–21.7) | 35.3 (27.9–42.8) | 18.1 (15.1–21.0) | <0.001 |
| % Household income: needs 1–3 | 38.6 (35.9–41.4) | 42.0 (36.1–47.9) | 38.8 (35.2–42.4) | <0.001 |
| % Household income: needs >3.0 | 41.7 (38.6–44.9) | 31.3 (25.5–37.0) | 42.4 (38.4–46.4) | <0.001 |
| % Household income: needs ≤1.0 | 19.6 (17.6–21.7) | 26.8 (21.4–32.1) | 18.7 (16.2–21.2) | <0.001 |
| % MetS (ATP-III) | 7.3 (6.0–8.6) | 9.7 (6.7–12.7) | 7.3 (5.5–9.2) | 0.180 |
| % Prediabetes | 17.1 (15.0–19.2) | 20.4 (15.8–24.9) | 16.3 (14.0–18.7) | 0.103 |
| eGFR | 109.5 (108.4–110.5) | 135.6 (131.7–139.6) | 103.3 (102.8–103.7) | <0.001 |
| Age | 14.1 (14.0–14.2) | 13.4 (13.2–13.5) | 14.3 (14.2–14.4) | <0.001 |
| BMI z-score | 0.58 (0.51–0.65) | 0.56 (0.40–0.72) | 0.58 (0.51–0.65) | 0.804 |
| Height-for-age z-score | 0.30 (0.22–0.38) | 0.30 (0.16–0.43) | 0.30 (0.21–0.39) | 0.991 |
Survey means are reported with 95% CIs in parentheses. Chi-squared tests were used to assess differences in the proportion of categorical variables between the hyperfiltration and normal groups. T-tests were used to assess differences in numerical measurements between the two groups. Significant differences are shown in bold (P < 0.05).
Our statistical models accounted for sex, age, race/ethnicity, socioeconomic status and body size. Of these, younger age, female sex and Hispanic race/ethnicity classification were associated with increased odds for hyperfiltration (Table 2). Socioeconomic status, assessed by the household income:needs ratio, was not associated with hyperfiltration. There were no significant differences in BMI z-score or height–age z-score between adolescents with normal eGFR and hyperfiltration (Table 1).
Table 2.
Factors influencing odds of being classified as having hyperfiltration
| Odds ratio of hyperfiltration (eGFR >120 mL/min/1.73 m2) | 95% CI | P-value | |
|---|---|---|---|
| Older age | 0.67 | (0.61–0.74) | <0.0001 |
| Male sex | 0.66 | (0.47–0.92) | 0.014 |
| BMI z-score | 0.96 | (0.82–1.12) | 0.578 |
| Race/ethnicity (versus non-Hispanic white) | <0.0001 | ||
| Non-Hispanic black | 0.83 | (0.54–1.27) | |
| Hispanic | 2.23 | (1.56–3.20) | |
| Household income: needs ratio (versus 1.0–3.0) | 0.092 | ||
| >3.0 | 0.74 | (0.51–1.06) | |
| ≤1.0 | 1.15 | (0.82–1.63) |
Data shown are from a multivariate logistic model. Significant differences are shown in bold (P < 0.05).
Metabolic measurements
Comparisons in metabolic measurements between adolescents with hyperfiltration and normal filtration are reported in Table 3. Adolescents with hyperfiltration had higher fasting insulin and HOMA-IR measurements regardless of adjustment for BMI z-score (all P < 0.05). After adjustment for BMI z-score, adolescents with hyperfiltration had higher fasting triglyceride measurements (P= 0.049) and higher MetS z-scores (P = 0.025). These associations were not seen when the statistical models did not include adjustment for BMI z-score. Using linear regression, eGFR was positively linked to triglycerides (P < 0.05), insulin (P < 0.0001) and HOMA-IR (P < 0.0001), both with and without adjustment for BMI z-score. There were no significant differences in mean systolic blood pressure, fasting glucose, HDL and HbA1c by hyperfiltration classification (Table 3). In a sensitivity analysis, we lowered the cutoff for normal filtration to 75 mL/min/1.73 m2. Fasting insulin and HOMA-IR were still significantly elevated among adolescents with hyperfiltration compared with normal filtration both with and without adjustment for BMI; however, differences in fasting triglyceride measurements between groups were not observed (data now shown).
Table 3.
Metabolic differences between adolescents with glomerular hyperfiltration and normal filtration, with and without adjustment for BMI z-score
| Without body size adjustment |
Adjustment for BMI z-score |
|||||
|---|---|---|---|---|---|---|
| Hyperfiltration | Normal | P-value | Hyperfiltration | Normal | P-value | |
| MetS z-score | −0.02 (−0.14–0.10) | −0.08 (−0.13 to −0.03) | 0.356 | 0.00 (−0.07–0.06) | −0.09 (−0.12 to −0.06) | 0.025 |
| Mean SBP | 108.1 (106.7–109.6) | 108.4 (107.7–109.0) | 0.765 | 108.3 (106.9–109.6) | 108.3 (107.7–108.9) | 0.925 |
| Fasting triglycerides | 83.2 (77.9–88.5) | 77.2 (74.7–79.8) | 0.053 | 83.6 (77.9–89.3) | 77.1 (74.6–79.6) | 0.049 |
| Fasting glucose | 93.6 (92.8–94.5) | 92.8 (92.2–93.4) | 0.114 | 93.6 (92.8–94.5) | 92.8 (92.2–93.4) | 0.105 |
| HDL | 52.0 (50.3–53.7) | 53.4 (52.7–54.1) | 0.132 | 51.8 (50.2–53.4) | 53.4 (52.7–54.1) | 0.077 |
| Fasting insulin | 14.9 (13.5–16.3) | 12.9 (12.2–13.6) | 0.010 | 15.1 (13.9–16.2) | 12.9 (12.3–13.4) | 0.001 |
| HOMA-IR | 3.5 (3.2–3.8) | 3.02 (2.8–3.2) | 0.012 | 3.5 (3.3–3.8) | 3.0 (2.9–3.2) | 0.001 |
| Hb A1C | 5.3 (5.2–5.3) | 5.3 (5.2–5.3) | 0.896 | 5.27 (5.2–5.3) | 5.3 (5.2–5.3) | 0.722 |
Estimated marginal means for metabolic risk factors were obtained from multivariable linear regression. Both statistical models adjust for age, sex, socioeconomic status and race/ethnicity. The second model additionally adjusted for body size using BMI z-score. Mean values are reported with 95% CIs reported in parentheses. SBP, systolic blood pressure. Significant differences are shown in bold (P < 0.05).
Survey regression analyses showed eGFR was linearly correlated with fasting triglycerides (P = 0.017), fasting insulin (P < 0.0001) and HOMA-IR (P < 0.0001) in our analytic sample. This was true regardless of if adjustment for BMI z-score was included.
The associations between hyperfiltration and hypertriglyceridemia, ATP-III MetS and prediabetes were assessed with logistic regression including adjustment for BMI z-score (Table 4). Adolescents with hyperfiltration had increased odds for hypertriglyceridemia. Not surprisingly, the reverse association was also true: adolescents with hypertriglyceridemia were significantly more likely to have hyperfiltration. There were no associations between hyperfiltration status and ATP-III MetS and prediabetes.
Table 4.
Associations between hyperfiltration and clinical abnormalities
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
| Odds of other clinical abnormalities in adolescents with hyperfiltration | |||
| Hypertriglyceridemiaa | 1.56 | (1.05, 2.31) | 0.027 |
| ATP-III MetS diagnosisb | 1.16 | (0.67, 1.99) | 0.592 |
| Prediabetesc | 1.36 | (0.89, 2.07) | 0.151 |
| Odds of hyperfiltration given other clinical abnormalities | |||
| Hypertriglyceridemiaa | 1.62 | (1.10, 2.40) | 0.015 |
| MetSb | 1.60 | (0.93, 2.76) | 0.093 |
| Prediabetesc | 1.35 | (0.90, 2.04) | 0.147 |
Survey logistic regression was performed with weighted data and a multivariate model accounting for age, sex, race/ethnicity, socioeconomic status, and BMI z-score. Significant differences are shown in bold (P < 0.05).
aTriglyceride level ≥110 mg/dL.
bATP-III MetS diagnosis was defined by a modified set of pediatric criteria: BMI z-score ≥1.645, fasting glucose ≥100 mg/dL, fasting triglycerides ≥110 mg/dL, HDL ≤40 mg/dL and systolic or diastolic blood pressure exceeding the 90th percentile for height, age and sex [31].
cHb A1c >5.7% to <6.4% or fasting glucose >100 mg/dL but <125 mg/dL [32].
Because hyperfiltration varied by race/ethnicity, we additionally evaluated race/ethnicity-specific subsamples in multivariable models accounting for BMI z-score. In non-Hispanic white adolescents, hyperfiltration was associated with elevated fasting insulin (13.9 versus 11.5 μU/mL; P= 0.03) and elevated HOMA-IR (3.3 versus 2.7; P = 0.03). There was no association in non-Hispanic black adolescents. In Hispanic adolescents, hyperfiltration was associated with elevated fasting insulin (16.4 versus 14.2 μU/mL; P= 0.003) and elevated HOMA-IR (3.9 versus 3.4; P = 0.006).
Albuminuria
The prevalence of albuminuria in our final analytic sample was 12.5% (95% CI 10.8–14.3). The prevalence of albuminuria (with 95% CI) in each racial/ethnic group was as follows: non-Hispanic white 12.7% (10.1–15.3), non-Hispanic black 13.5% (10.3–16.8), Hispanic 11.7 (9.5–14.0). There were no significant differences in albuminuria prevalence between racial/ethnic groups.
There was no significant association between hyperfiltration status and albuminuria [normal:nonhyperfiltration odds ratio (OR) 0.98 (95% CI 0.66–1.46)]. Log-transformed UACR was not associated with eGFR. We did not find significant associations between albuminuria and ATP-III MetS status, hypertriglyceridemia and prediabetes (data not shown). Log-transformed UACR was positively associated with MetS z-score (estimate = 0.03, P = 0.019, R2 = 0.67).
In additional analyses, we compared participants with both hyperfiltration and albuminuria (n = 72) to participants with neither (n = 1786). Several factors decreased the odds of having both hyperfiltration and albuminuria:increasing age [OR 0.65 (95% CI 0.50–0.84)], male sex [OR 0.17 (0.07–0.41)] and increasing BMI z-score [OR 0.48 (0.35–0.66)]. Being Hispanic (versus non-Hispanic white or black) significantly increased the odds of having both hyperfiltration and albuminuria [OR 4.0 (95% CI 2.1–7.4)].
In adjusted means analyses controlling for sex, race/ethnicity, age and income, participants with hyperfiltration and albuminuria (versus neither) had significantly lower BMI z-scores [mean = 0.04 (95% CI −0.30–0.38) versus mean = 0.79 (0.72–0.86), respectively]. Subsequent adjusted means analyses included adjustment for BMI z-score. The double risk factor group had significantly higher triglycerides (88.0 versus 77.3; P = 0.03), lower HDL (49.9 versus 53.3; P = 0.03), higher fasting insulin (16.8 versus 13.1; P = 0.007) and higher HOMA-IR (4.0 versus 3.1; P = 0.008). There were no significant differences in fasting glucose, mean systolic blood pressure, MetS z-score and HbA1C (data not shown).
DISCUSSION
There is increasing recognition of glomerular hyperfiltration as a precursor to CKD [7]. However, to date there has been limited study of hyperfiltration in pediatrics. Previous research has focused on specific subpopulations (such as those with diabetes or sickle cell disease) [1]. Here, we present population-based estimates of glomerular hyperfiltration in US adolescents without diabetes. Just as in adults, glomerular hyperfiltration was associated with MetS abnormalities: compared to those with normal eGFR, adolescents with glomerular hyperfiltration were more likely to have elevated fasting triglyceride, fasting insulin and HOMA-IR measurements, even after accounting for BMI z-score. In addition, BMI z-score did not vary between adolescents with hyperfiltration or normal filtration, demonstrating that the association between glomerular hyperfiltration and these abnormalities was not driven solely by obesity.
While adolescents with hyperfiltration had increased odds for hypertriglyceridemia, as expected, the reverse association was also true. This may have dual clinical implications in that adolescents with hypertriglyceridemia could be screened for hyperfiltration and adolescents with hyperfiltration could also be screened for lipid abnormalities. Given that weight loss has demonstrated efficacy in reducing both hyperfiltration [19] and hypertriglyceridemia [41], identification of these abnormalities could serve as an additional motivation toward lifestyle changes.
Even though there was no association between hyperfiltration and prediabetes as assessed by HbA1c level, adolescents with hyperfiltration did have laboratory measurements suggesting insulin resistance. Hyperinsulinemia can have deleterious effects on the kidneys even before the onset of type 2 diabetes mellitus [11, 42, 43], and elevations in insulin may be directly related to elevations in eGFR [11, 44]. Hyperinsulinemic monkeys have glomerular hypertrophy with preserved glomerular architecture when compared with normal monkeys [43]. If further validated, this link between hyperfiltration and insulin resistance could suggest a need to assess kidney function in adolescents with insulin resistance.
There is a growing understanding of the physiologic interplay between hypertriglyceridemia, insulin resistance and hyperfiltration. Leptin receptor is overexpressed in glomeruli of patients with obesity-related glomerulopathy [45], and experimental evidence suggests a central role for leptin dysregulation in the development of glomerular hyperfiltration [46]. Leptin stimulates cellular proliferation within glomeruli [46] and hyperleptinemia has been linked to hyperfiltration [47], hypertriglyceridemia and insulin resistance [48–51]. Although leptin measurements were not collected for NHANES, Tomaszewski et al. [13] found elevated leptin levels in a study of white, young adult, generally lean (median BMI 22.3) males with hyperfiltration.
Renal function alterations in the context of pediatric obesity are not well understood. We found no difference in BMI z-score between the hyperfiltration groups; however, previous studies have shown increased BMI z-score to be related to both abnormally increased and decreased eGFR, depending on the formula used to assess eGFR [52]. One group of investigators postulated that the relationship between BMI z-score and eGFR may be modified by age of obesity onset: children obese for a greater portion of their lives may be more likely to present with decreased eGFR, whereas children with more recent onset of obesity may be more likely to present with hyperfiltration [52]. This could be consistent with the idea that prolonged physiologic disturbances contribute to the development of glomerulopathy, such as in leptin dysregulation [13, 46–51]. Unfortunately, data regarding the duration of obesity status were unavailable for our sample, so we were unable to evaluate for this relationship in our analysis.
Previous population-level epidemiological studies of hyperfiltration have been done in predominantly white European countries [5, 13]. In the more racially diverse US adolescent population, we noted differences in hyperfiltration and its metabolic associations across racial/ethnic groups. While non-Hispanic white and Hispanic adolescents with hyperfiltration had increased odds for insulin resistance, this association was not found in non-Hispanic black adolescents. It is unclear whether this was due to a smaller subgroup sample size or to the variation of other factors that contribute to hyperfiltration and that vary by race/ethnicity, such as sickle cell trait, which has a prevalence of 5–10% among African Americans, compared with 1.5% in the general population [39]. Additionally, non-Hispanic black adolescents were found to have the lowest prevalence of hyperfiltration. This could be related to racial/ethnic differences in serum creatinine values secondary to differences in body muscle mass [53, 54]. The MDRD and Chronic Kidney Disease Epidemiology Collaboration equations for estimating adult GFR account for black race in their calculations, whereas the Schwartz equation does not. The serum creatinine value differences may not be apparent in younger children, but could start having a significant effect on eGFR calculations in adolescents as they progress through puberty.
A noteworthy and potentially concerning finding was the significantly greater proportion of Hispanic adolescents with hyperfiltration. Compared with their non-Hispanic white or black peers, Hispanic adolescents were approximately twice as likely to have hyperfiltration. Although there has been a rapid increase in the incidence and prevalence of end-stage renal disease (ESRD) in Hispanics over the last 2 decades [55], Hispanics have not been well represented in most large studies and clinical trials of CKD [56]. Further research is needed to determine whether the greater prevalence of hyperfiltration in Hispanic adolescents could be related to the increasing rates of CKD and ESRD in Hispanic adults.
We found that children with both hyperfiltration and albuminuria had findings of dyslipidemia and insulin resistance despite having lower BMI z-scores. However, albuminuria was not found to have associations with hyperfiltration, MetS and prediabetes in this study. In this study of adolescents without diabetes, albuminuria may have limited functionality as an assessment of hyperfiltration outcome due to a high prevalence of benign orthostatic proteinuria in this population [57]. More rigorous assessments, such as assessment of void before rising in the morning, remain necessary for a more definitive test of the relationship between hyperfiltration and albuminuria in this age range.
All studies of hyperfiltration are limited by the lack of a clear eGFR cutoff between glomerular hyperfiltration and normal kidney function. We designated eGFR >120 mL/min/1.73 m2 as hyperfiltration for several reasons. This definition has been previously used as the threshold for hyperfiltration in other studies [36–40, 58]. A recent study found glomerular hyperfiltration to be linked to cardiovascular risk factors in non-diabetic adults with the highest 20% of GFR [5]. Using 120 mL/min/1.73 m2 as the hyperfiltration threshold resulted in ∼13% of nondiabetic adolescent NHANES participants having glomerular hyperfiltration, suggesting potential for a significant number of adolescents at higher risk.
Additionally, the lower cutoff for defining normal filtration is unclear. For adolescents with eGFR values between 60 and 90 mL/min/1.73 m2, additional studies need to be performed to differentiate normal status versus early stage CKD [4]. A previous study estimated that ∼9% of adolescents had eGFRs <75, suggesting a significant number of NHANES adolescent participants with eGFRs between 75 and 90 for which CKD status was indeterminate [59]. In a sensitivity analysis, we lowered the cutoff for normal filtration to 75. While fasting insulin and HOMA-IR were still significantly elevated in the hyperfiltration group, the differences in fasting triglyceride measurements were not observed. The reason for this difference when assessing the expanded normal filtration group is unclear; however, this may reflect that a small number of participants with eGFRs between 75 and 90 may have CKD and exhibit CKD-associated elevations in fasting triglycerides—raising the mean value of the expanded normal filtration group. Further evaluation of differences in metabolic status between those with hyperfiltration, normal filtration and confirmed CKD are needed in this age range.
We were limited in our dependence on the Schwartz bedside formula to determine eGFR. The Schwartz bedside formula had been previously validated in epidemiological studies [33–35]. While the creatinine–Cystatin–C–based CKID equation has been shown to be more accurate for estimating GFR in the 15–75 range, this equation has not been validated outside of that range [60]. Another potential important advantage of future use of the creatinine-Cystatin-C-based equation is that studies suggest it is a more accurate estimation of GFR in more obese individuals [52]. We were unable to use the combined formula because cystatin C measurements were only available in a small subset of adolescent NHANES participants.
Because NHANES is a cross-sectional survey study, we were only able to assess correlation and not causation and were unable to obtain longitudinal follow-up. For participants deemed to have hyperfiltration, we were unable to follow renal function status over multiple time points. This presents an opportunity for future studies in longitudinal cohorts.
In conclusion, glomerular hyperfiltration is associated with hypertriglyceridemia and reduced insulin sensitivity in adolescents without diabetes, independent of BMI z-score. Hyperfiltration is particularly common among Hispanic adolescents. These findings may have significance for assessing for metabolic abnormalities among children who have hyperfiltration and potentially intervening with lifestyle modifications to improve their metabolic status.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by National Institutes of Health grant 1R01HL120960 (M.J.G. and M.D.D.).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Cachat F, Combescure C, Cauderay M. et al. A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol 2015; 10: 382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes 2012; 3: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Magee GM, Bilous RW, Cardwell CR. et al. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 2009; 52: 691–697 [DOI] [PubMed] [Google Scholar]
- 4. Hogg RJ, Furth S, Lemley KV. et al. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics 2003; 111(6 Pt 1): 1416–1421 [DOI] [PubMed] [Google Scholar]
- 5. Eriksen BO, Løchen ML, Arntzen KA. et al. Subclinical cardiovascular disease is associated with a high glomerular filtration rate in the nondiabetic general population. Kidney Int 2014; 86: 146–153 [DOI] [PubMed] [Google Scholar]
- 6. Nistrup Holmegaard S, Christoffersen H, Haase J. Albuminuria, intermittent hyperfiltration and salt wasting in patients with stroke: a pilot study. Scand J Clin Lab Invest 2006; 66: 437–449 [DOI] [PubMed] [Google Scholar]
- 7. Helal I, Fick-Brosnahan GM, Reed-Gitomer B. et al. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 2012; 8: 293–300 [DOI] [PubMed] [Google Scholar]
- 8. Ribstein J, Du Cailar G, Fesler P. et al. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol 2005; 16: 1320–1325 [DOI] [PubMed] [Google Scholar]
- 9. Cherney DZ, Lai V, Scholey JW. et al. Effect of direct renin inhibition on renal hemodynamic function, arterial stiffness, and endothelial function in humans with uncomplicated type 1 diabetes: a pilot study. Diabetes Care 2010; 33: 361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee HB, Yu MR, Yang Y. et al. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol 2003; 14(8 Suppl 3): S241–S245 [DOI] [PubMed] [Google Scholar]
- 11. Sarafidis PA, Whaley-Connell A, Sowers JR. et al. Cardiometabolic syndrome and chronic kidney disease: what is the link? J Cardiometab Syndr 2006; 1: 58–65 [DOI] [PubMed] [Google Scholar]
- 12. Henegar JR, Bigler SA, Henegar LK. et al. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 2001; 12: 1211–1217 [DOI] [PubMed] [Google Scholar]
- 13. Tomaszewski M, Charchar FJ, Maric C. et al. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int 2007; 71: 816–821 [DOI] [PubMed] [Google Scholar]
- 14. Keller CK, Bergis KH, Fliser D. et al. Renal findings in patients with short-term type 2 diabetes. J Am Soc Nephrol 1996; 7: 2627–2635 [DOI] [PubMed] [Google Scholar]
- 15. Vora JP, Dolben J, Dean JD. et al. Renal hemodynamics in newly presenting non-insulin dependent diabetes mellitus. Kidney Int 1992; 41: 829–835 [DOI] [PubMed] [Google Scholar]
- 16. Vazquez B, Shah B, Zhang X. et al. Hyperfiltration is associated with the development of microalbuminuria in patients with sickle cell anemia. Am J Hematol 2014; 89: 1156–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cachat F, Combescure C, Chehade H. et al. Microalbuminuria and hyperfiltration in subjects with nephro-urological disorders. Nephrol Dial Transplant 2013; 28: 386–391 [DOI] [PubMed] [Google Scholar]
- 18. Ogden CL, Carroll MD, Kit BK. et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014; 311: 806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Navarro-Díaz M, Serra A, Romero R. et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol 2006; 17(12 Suppl 3): S213–S217 [DOI] [PubMed] [Google Scholar]
- 20. Flegal KM, Carroll MD, Ogden CL. et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 303: 235–241 [DOI] [PubMed] [Google Scholar]
- 21. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004; 114(2 Suppl 4th Report): 555–576 [PubMed] [Google Scholar]
- 22. National Center for Health Statistics. National Health and Nutrition Examination Survey protocol Vol 2015 http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm
- 23. Myers GL, Miller WG, Coresh J. et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006; 52: 5–18 [DOI] [PubMed] [Google Scholar]
- 24. Selvin E, Manzi J, Stevens LA. et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 2007; 50: 918–926 [DOI] [PubMed] [Google Scholar]
- 25. Gurka MJ, Ice CL, Sun SS. et al. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol 2012; 11: 128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee AM, Gurka MJ, DeBoer MD. A metabolic syndrome severity score to estimate risk in adolescents and adults: current evidence and future potential. Expert Rev Cardiovasc Ther 2016; 14: 411–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee AM, Gurka MJ, DeBoer MD. Trends in metabolic syndrome severity and lifestyle factors among adolescents. Pediatrics 2016; 137: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeBoer MD, Gurka MJ, Woo JG. et al. Severity of metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: The Princeton Lipid Research Cohort Study. Diabetologia 2015; 58: 2745–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DeBoer MD, Gurka MJ, Woo JG. et al. Severity of metabolic syndrome as a predictor of cardiovascular disease between childhood and adulthood: the Princeton Lipid Research Cohort Study. J Am Coll Cardiol 2015; 66: 755–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DeBoer MD, Gurka MJ, Morrison JA. et al. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes (Lond) 2016. doi:10.1038/ijo.2016.81doi:10.1038/ijo.2016.81 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ford ES, Li C, Cook S. et al. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007; 115: 2526–2532 [DOI] [PubMed] [Google Scholar]
- 32. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37(Suppl 1): S14–S80 [DOI] [PubMed] [Google Scholar]
- 33. Schwartz GJ, Muñoz A, Schneider MF. et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20: 629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Staples AO, Greenbaum LA, Smith JM. et al. Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol 2010; 5: 2172–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Staples A, LeBlond R, Watkins S. et al. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol 2010; 25: 2321–2326 [DOI] [PubMed] [Google Scholar]
- 36. Bodas P, Huang A, O'Riordan MA. et al. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease—a cross sectional review. BMC Nephrol 2013; 14: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumar M, Kedar A, Neiberger RE. Kidney function in long-term pediatric survivors of acute lymphoblastic leukemia following allogeneic bone marrow transplantation. Pediatr Hematol Oncol 1996; 13: 375–379 [DOI] [PubMed] [Google Scholar]
- 38. Silva Junior GB, Libório AB, Vieira AP. et al. Evaluation of renal function in sickle cell disease patients in Brazil. Braz J Med Biol Res 2012; 45: 652–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wigfall DR, Ware RE, Burchinal MR. et al. Prevalence and clinical correlates of glomerulopathy in children with sickle cell disease. J Pediatr 2000; 136: 749–753 [PubMed] [Google Scholar]
- 40. Luaces M, Martínez-Martínez E, Medina M. et al. The impact of bariatric surgery on renal and cardiac functions in morbidly obese patients. Nephrol Dial Transplant 2012; 27(Suppl 4): iv53–iv57 [DOI] [PubMed] [Google Scholar]
- 41. Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factor in overweight adolescents. J Pediatr 2003; 142: 253–258 [DOI] [PubMed] [Google Scholar]
- 42. McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab 2001; 86: 713–718 [DOI] [PubMed] [Google Scholar]
- 43. Cusumano AM, Bodkin NL, Hansen BC. et al. Glomerular hypertrophy is associated with hyperinsulinemia and precedes overt diabetes in aging rhesus monkeys. Am J Kidney Dis 2002; 40: 1075–1085 [DOI] [PubMed] [Google Scholar]
- 44. Hall JE, Brands MW, Mizelle HL. et al. Chronic intrarenal hyperinsulinemia does not cause hypertension. Am J Physiol 1991; 260(5 Pt 2): F663–F669 [DOI] [PubMed] [Google Scholar]
- 45. Wu Y, Liu Z, Xiang Z. et al. Obesity-related glomerulopathy: insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology 2006; 147: 44–50 [DOI] [PubMed] [Google Scholar]
- 46. Wolf G, Chen S, Han DC. et al. Leptin and renal disease. Am J Kidney Dis 2002; 39: 1–11 [DOI] [PubMed] [Google Scholar]
- 47. Wei P, Lane PH, Lane JT. et al. Glomerular structural and functional changes in a high-fat diet mouse model of early-stage type 2 diabetes. Diabetologia 2004; 47: 1541–1549 [DOI] [PubMed] [Google Scholar]
- 48. Ren J. Leptin and hyperleptinemia—from friend to foe for cardiovascular function. J Endocrinol 2004; 181: 1–10 [DOI] [PubMed] [Google Scholar]
- 49. Naderali EK, Brown MJ, Pickavance LC. et al. Dietary obesity in the rat induces endothelial dysfunction without causing insulin resistance: a possible role for triacylglycerols. Clin Sci (Lond) 2001; 101: 499–506 [DOI] [PubMed] [Google Scholar]
- 50. Hintz KK, Aberle NS, Ren J. Insulin resistance induces hyperleptinemia, cardiac contractile dysfunction but not cardiac leptin resistance in ventricular myocytes. Int J Obes Relat Metab Disord 2003; 27: 1196–1203 [DOI] [PubMed] [Google Scholar]
- 51. Anderson CM, Ren J. Leptin, leptin resistance and endothelial dysfunction in pre-eclampsia. Cell Mol Biol (Noisy-le-grand) 2002; 48 Online Pub: OL323-329 [PubMed] [Google Scholar]
- 52. Correia-Costa L, Afonso AC, Schaefer F. et al. Decreased renal function in overweight and obese prepubertal children. Pediatr Res 2015; 78: 436–444 [DOI] [PubMed] [Google Scholar]
- 53. Jones CA, McQuillan GM, Kusek JW. et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis 1998; 32: 992–999 [DOI] [PubMed] [Google Scholar]
- 54. Rule AD, Bailey KR, Schwartz GL. et al. For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int 2009; 75: 1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. US Renal Data System. USRDS 2009 Annual Data Report: Atlas of end-stage renal disease in the United States. Bethesda, MD: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2009 [Google Scholar]
- 56. Fischer MJ, Go AS, Lora CM. et al. CKD in Hispanics: baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC studies. Am J Kidney Dis 2011; 58: 214–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brandt JR, Jacobs A, Raissy HH. et al. Orthostatic proteinuria and the spectrum of diurnal variability of urinary protein excretion in healthy children. Pediatr Nephrol 2010; 25: 1131–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Magri CJ, Fava S. Albuminuria and glomerular filtration rate in type 2 diabetes mellitus. Minerva Urol Nefrol 2011; 63: 273–280 [PubMed] [Google Scholar]
- 59. Fadrowski JJ, Neu AM, Schwartz GJ. et al. Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol 2011; 6: 1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schwartz GJ, Schneider MF, Maier PS. et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 2012; 82: 445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.