Persistent stuttering reflects atypical brain activity and connectivity, most notably within the left hemisphere speech production networks. Neef et al. link right frontal overactivations common to stuttering to structural networks that support right hemisphere specialisations. In particular, severe stuttering reflects increased activity in a global motor response inhibition network.
Keywords: persistent developmental stuttering, diffusion tractography, connection strength, right frontal networks, speech motor control
Abstract
A neuronal sign of persistent developmental stuttering is the magnified coactivation of right frontal brain regions during speech production. Whether and how stuttering severity relates to the connection strength of these hyperactive right frontal areas to other brain areas is an open question. Scrutinizing such brain–behaviour and structure–function relationships aims at disentangling suspected underlying neuronal mechanisms of stuttering. Here, we acquired diffusion-weighted and functional images from 31 adults who stutter and 34 matched control participants. Using a newly developed structural connectivity measure, we calculated voxel-wise correlations between connection strength and stuttering severity within tract volumes that originated from functionally hyperactive right frontal regions. Correlation analyses revealed that with increasing speech motor deficits the connection strength increased in the right frontal aslant tract, the right anterior thalamic radiation, and in U-shaped projections underneath the right precentral sulcus. In contrast, with decreasing speech motor deficits connection strength increased in the right uncinate fasciculus. Additional group comparisons of whole-brain white matter skeletons replicated the previously reported reduction of fractional anisotropy in the left and right superior longitudinal fasciculus as well as at the junction of right frontal aslant tract and right superior longitudinal fasciculus in adults who stutter compared to control participants. Overall, our investigation suggests that right fronto-temporal networks play a compensatory role as a fluency enhancing mechanism. In contrast, the increased connection strength within subcortical-cortical pathways may be implied in an overly active global response suppression mechanism in stuttering. Altogether, this combined functional MRI–diffusion tensor imaging study disentangles different networks involved in the neuronal underpinnings of the speech motor deficit in persistent developmental stuttering.
Introduction
Persistent stuttering is a fluency disorder that occurs during early childhood without obvious reason and persists in ∼1% of the adult population, predominantly in males (Yairi and Ambrose, 1999, 2013). Characteristic signs are involuntary sound and syllable repetitions, sound prolongations, and speech blocks (Bloodstein and Ratner, 2008). Dysfluencies are frequently accompanied by facial grimacing, head and limb movements. Furthermore, negative emotions and avoidance behaviour accompany stuttering (Iverach and Rapee, 2014). Depending on severity, persistent stuttering seriously compromises quality of life (Koedoot et al., 2011).
Fluent speech production relies on the dynamic organization of large-scale brain networks that coordinate cognitive, sensorimotor, and emotional systems. Reliable connectivity and effective signal transfer are essential to converge and convey speech, but involved brain regions show atypical activity in persistent developmental stuttering (Fox et al., 1996; De Nil et al., 2000; Salmelin et al., 2000; Neumann et al., 2003; Lu et al., 2010; Toyomura et al., 2011; Craig-McQuaide et al., 2014; Etchell et al., 2014). Atypical activation patterns vary with imaging method and paradigm (Ingham et al., 2012) as well as with the neural signatures left by lifelong experience of stuttering and diverse therapies (Wymbs et al., 2013). One of the most robust neural signatures of stuttering is an excessive recruitment of right frontal cortical areas while speaking. Right primary motor cortex, premotor cortex, supplementary motor area (SMA), pre-SMA, inferior frontal gyrus (IFG), insula, frontal and the rolandic operculum show amplified speech-related activity (Budde et al., 2014; Belyk et al., 2015), with most robust evidence for right rolandic operculum and precentral gyrus [Brodmann area (BA) 4/6, Belyk et al., 2017]. Previous studies discuss whether right frontal hyperactivations are related to compensatory, causal, or maladaptive mechanisms (Fox et al., 2000; Neumann et al., 2003; Preibisch et al., 2003; Watkins et al., 2008; Chang et al., 2009; Kell et al., 2009; Neef et al., 2011, 2016). To separate these mechanisms several studies correlated local brain activity with stuttering severity. Negative correlations have been taken to suggest a compensatory role of the right frontal operculum (Preibisch et al., 2003; Kell et al., 2009). Positive correlations were reported for activity in the right primary motor cortex (Fox et al., 2000; Chang et al., 2009), right SMA, and right anterior insula (Fox et al., 2000). However, cross-sectional studies of the adult brain do not allow a distinction between causal factors that are associated with the risk of developing stuttering, and maladaptation, which is a result of life-long stuttering. Thus, right frontal regions show both signs of compensatory activity and signs of causal or maladaptive activity.
We still lack a clear understanding of supposed compensatory or causal mechanisms. Our previous functional MRI study, in which we propose a potential neuropathological principle, showed that right IFG activity relates to the inhibition of speech responses (Neef et al., 2016). We propose that stuttering might be caused by an overly active global response suppression mechanism mediated via the subthalamic nucleus-right IFG-basal ganglia hyperdirect pathway. An amplified involvement of the hyperdirect pathway might increase the system’s tendency to globally inhibit motor responses. Fast inhibition via the hyperdirect pathway is unspecific and induces a global reduction of the thalamo-cortical drive (Nambu et al., 2002; Aron, 2011; Aron et al., 2014). If this global inhibition is too strong or imbalanced, as proposed in stuttering, the stopping of an ongoing speech motor programme and/or the selection of a succeeding speech motor programme might fail. Such a failure would lead to sound prolongations, sound repetitions and blocking of speech as characteristic for stuttering. An overly involved hyperdirect pathway might exhibit an increased structural connectivity within the larger network. Connections between right hemisphere IFG, pre-SMA, subthalamic nucleus, and striatum are the structures of interest already suggested by neuroimaging evidence (Aron and Poldrack, 2006; Aron et al., 2007; Jahanshahi et al., 2015). Here, we investigate white matter structures that connect right frontal hyperactive regions with the rest of the brain, in order to test whether connection properties support our proposed hypothesis, specifically that stuttering might be related to an irregular global response suppression mediated via the hyperdirect pathway.
Diffusion-weighted MRI quantifies properties of white matter structures and, thus, helps to scrutinize structural connectivity. In the context of stuttering, a number of diffusion-weighted MRI studies report a reduced fractional anisotropy in speech-related fibre pathways, such as the left superior longitudinal fasciculus (SLF; Sommer et al., 2002; Chang et al., 2008; Watkins et al., 2008; Kell et al., 2009; Cykowski et al., 2010; Cai et al., 2014a; Kronfeld-Duenias et al., 2016b), a prominent dorsal fibre pathway that includes the arcuate fasciculus (Makris et al., 2005) and that connects fronto-parieto-temporal regions (Catani et al., 2005) of the speech network (Hickok and Poeppel, 2007). More recently, structural differences were also shown in the left frontal aslant tract (FAT; Kronfeld-Duenias et al., 2016a), a fibre pathway that connects the posterior region of the IFG with the SMA and pre-SMA (Catani et al., 2012), relevant for fluent speech production (Guenther, 2016; Kemerdere et al., 2016). However, the picture that emerges from previous diffusion-weighted MRI studies on stuttering is diffuse (Neef et al., 2015). Although only a few findings have been replicated, these jointly highlight the implication of left hemisphere speech-related fibre paths. Reports on right hemisphere white matter differences are less robust (Cai et al., 2014a; Neef et al., 2015). For the frontal lobe, structural differences are reported in the white matter underneath the IFG, premotor cortex, and middle frontal gyrus (MFG; Watkins et al., 2008; Connally et al., 2014; Chang et al., 2015), in the anterior segment of the right anterior fasciculus (Kronfeld-Duenias et al., 2016b), and in the right FAT (Kronfeld-Duenias et al., 2016a).
Inconsistency among white matter findings can partly be explained by the variety of analysis approaches used. White matter differences in stuttering resulted from voxel-based statistics (Sommer et al., 2002), region of interest-based analysis (Chang et al., 2008), or tract-based spatial statistics (TBSS; Watkins et al., 2008; Kell et al., 2009; Cykowski et al., 2010; Cai et al., 2014a; Connally et al., 2014; Chang et al., 2015; Civier et al., 2015). Fewer studies used fibre tracking methods to scrutinize structural connectivity (Chang et al., 2011; Cai et al., 2014a; Connally et al., 2014; Cieslak et al., 2015). The former methods locate microstructural changes at specific points in the white matter, while the latter mainly characterize the probability and strength of connection between any two points in the brain (Morris et al., 2008). Hybrid approaches locate microstructural white matter differences within such a connecting volume (Kronfeld-Duenias et al., 2016a, b).
As a major difference to previous diffusion tensor imaging (DTI) studies on stuttering, we used a different approach to test structural connectivity. To enable comparison with most previous studies we also calculated TBSS. This first step helped with locating microstructural differences in the white matter skeleton, when comparing adults who stutter and fluent control speakers. In a second step we fed TBSS seeds into probabilistic tractographies to generate connection probability density maps (Behrens et al., 2003, 2007) and to reconstruct involved fibre pathways within and between both hemispheres. This procedure allowed us to determine fibre pathways that are affected in stuttering. Furthermore, a larger brain network that is involved in stuttering was derived by this procedure. In a third step, we combined DTI-based probabilistic fibre tracking and functional MRI to gain probability density maps of right frontal hyperactive cortical regions in adults who stutter. Eventually, in a fourth step, we used these probability density maps to determine space and volume for correlation analyses between the connection probability density of each voxel and stuttering severity. In this context, a significant cluster reflects the likelihood and the relative connection density, a seed region shows with this white matter region when related to stuttering severity. For the sake of simplicity, we use the term connection strength to refer to connection probability density.
In this study, we combined TBSS, DTI-based probabilistic fibre tracking and functional MRI to determine white matter differences between adults who stutter and matched control participants, and to scrutinize how the structural connectivity of right frontal hyperactive areas relates to stuttering severity. Only a few studies combined both functional MRI and DTI to study the neuronal basis of stuttering (Watkins et al., 2008; Kell et al., 2009; Chang et al., 2011), and only one study combined structural and functional connectivity analyses in the same subjects (Chang et al., 2011). This latter study showed that both functional and structural connectivity of the left and right IFG pars opercularis with their ipsilateral premotor and motor regions were decreased in the left hemisphere, but tended to be increased in the right hemisphere in adults who stutter compared to fluent speakers. Until today, no study investigated the link between right frontal hyperactivations, white matter connectivity, and stuttering severity within the right frontal lobe, examined here. In the context of our hypothesis that stuttering might be caused by an irregular global response suppression, such knowledge would make a good starting point for the rational design of brain stimulation approaches (Chesters et al., 2017), because it would advise the appropriate site and direction (inhibitory or excitatory) of potential stimulation protocols.
Materials and methods
Participants
Participants were recruited for an MRI study that tested sex differences in persistent developmental stuttering (Bütfering, 2015). Here we included only participants that successfully finished functional MRI scanning and diffusion-weighted MRI scanning, of which 31 participants were adults who stutter (15 females, aged 19–63 years, median age 36.0 years, SD = 12.3) and 34 participants were matched controls (17 females, 20–62 years, median age 35.5 years, SD = 12.3). Participants reported no neurological impairment, drug use, or medical history that might affect their brain structure or function. Twenty-two adults who stutter reported a family history of stuttering. None of the fluent speakers reported a family history of speech or language disorders. Groups were matched for age, sex, handedness (Oldfield, 1971), and years of formal education. Table 1 summarizes the demographic information of the participants. Individual characteristics of all participants are provided in Supplementary Tables 1 and 2. Approval from the local ethics committee at the University Medical Center, Göttingen, Germany, and written informed consent was obtained prior to the study. Each participant was paid €20 for participating.
Table 1.
Participants, demographic information, and behavioural results
| AWS | Control | P-value | |
|---|---|---|---|
| n | 31 | 34 | n/a |
| Age in years (mean) | 36.7 (12.3) | 37.1 (12.7) | 0.90a |
| Sex (male) (%) | 16 (52) | 17 (50) | 0.90b |
| Handedness (mean LQ) | 92.6 (11.5) | 95.2 (8.5) | 0.31a |
| Education (median) | 5 | 4 | 0.21c |
| Age of stuttering onset (years) | 4.3 (1.7) | n/a | n/a |
| SSI-4 total score (mean) | 16.6 (11.5) | n/a | n/a |
| OASES total score (mean) | 46.2 (12.0) | n/a | n/a |
aT-test.
bχ2-test.
cMann-Whitney U-test.
AWS = adults who stutter; Education (1 = school; 2 = high school; 3 = <2 years university; 4 = 2 years university; 5 = 4 years university; 6 = postgraduate); n/a = not applicable.
According to the OASES (German version of the Overall Assessment of the Speakers Experience of Stuttering; Yaruss and Quesal, 2006), two adults who stutter estimated the total impact of stuttering on their life as mild, 10 as mild-to-moderate, 16 as moderate, one as moderate-to-severe, and one as severe. One adult who stutters refused to fill out the OASES from section 2 to 4, thus no total impact was reported. The stuttering severity instrument (SSI-4) (Riley, 2009) captures the frequency and duration of stuttered syllables as well as physical concomitants of stuttering. According to the SSI-4, eight participants showed very mild stuttering, seven were mild, two were moderate, two were severe, and two were very severe. Eight participants had an SSI score lower than 10, but were included in the analysis because they perceived themselves as persons who stutter as indicated by the OASES. There are two possible reasons for the underscores. First, all participants who stutter reported that they participated in stuttering therapy at least once in their life. Although, nobody was under treatment while participating in the current study, treatment experience made it possible that fluency-inducing techniques were used during the course of the interview. We tried to minimize this effect by explicitly asking our participants to allow stuttering. Second, all participants were recruited by the author C.B., who stutters himself and who knew the participants who stutter from various annual meetings organized across Germany by the German Stuttering Association. He did not participate in the current study because his handedness is left. All interviews were conducted by C.B. Because of the familiarity between the interviewer and the participants who stutter and because the interviewer is a person who stutters himself, speech samples were acquired in a relaxed situation. It is highly likely that these circumstances led to an enhanced fluency and thus to an underestimation of stuttering severity.
Image acquisition
MRI was conducted in a 3-T scanner (Tim Trio, Siemens Healthineers) using an 8-channel head coil for signal reception. Initially, structural whole-brain T1-weighted MRI were recorded using a non-selective inversion-recovery 3D turbo FLASH sequence (repetition time = 2250 ms, echo time = 3.26 ms, flip angle = 9°, inversion time = 900 ms) at 1 mm3 isotropic spatial resolution. All functional MRI measures were based on a gradient-echo echo planar imaging sequence (repetition time = 2000 ms, echo time = 30 ms, flip angle 70°) at 3 mm3 isotropic spatial resolution. We acquired 33 consecutive slices in an axial-to-coronal orientation roughly parallel to the intercommissural plane, covering the whole brain (64 × 64 × 33). All images were corrected for motion in k-space as supplied by the manufacturer (Siemens Healthineers). Diffusion-weighted MRI was performed using a spin-echo echo planar imaging technique at 1.9 mm isotropic resolution (repetition time = 10 100 ms, echo time = 93 ms, parallel acquisition factor 2; acquisition matrix: 128 × 128, 74 sections), acquiring 64 image volumes with diffusion weighting (along 64 diffusion directions, b = 1000 s/mm2) and one reference image without diffusion weighting.
TBSS analysis
All diffusion-weighted MRI images were visually inspected for artefacts, which resulted in the exclusion of diffusion-weighted volumes (maximal four volumes) in three adults who stutter. Images were processed with tools from the FMRIB Software Library [FSL, http://www.fmrbi.ox.ac.uk/fsl/ (Jenkinson et al., 2012)]. Images were corrected for eddy currents and head motion by using affine registration to the non-diffusion volumes. For each voxel the diffusion tensor was calculated, and fractional anisotropy of the tensor as well as mean diffusivity, axial diffusivity (Behrens et al., 2003, 2007), and radial diffusivity were calculated. White matter skeleton-based voxel-wise statistical analysis of the fractional anisotropy data was carried out using the FSL tool TBSS (Smith et al., 2006). Therefore, the fractional anisotropy images of all participants were non-linearly registered to the image of the most typical brain. All images were then averaged, and a common white matter skeleton was created. For each brain, the local maximum close to the computed skeleton was projected to the skeleton for analysis. The data for the two groups were compared at each voxel location in the skeleton using randomization statistics. Reported clusters on the skeleton were considered significant at P < 0.002 at voxel level and exceeded a cluster size significance at P < 0.05 based on global smoothness estimation on the skeleton, computed with AFNI (version 16.1.28, http://ADD_URL, 3dClusterSim and 3dFWHMx, (Cox, 1996). Bi-sided thresholding resulted in a cluster size threshold of k ≥ 11. Subsequently, significant clusters were projected to the native space of each participant to extract further diffusion properties (mean, axial, and radial diffusivity).
TBSS-based tractography
To reconstruct the fibre pathways that run through the significant TBSS clusters, first, a two-fibre probabilistic diffusion model was computed in every voxel using bedpostx, implemented in FSL (Behrens et al., 2003, 2007). Individual TBSS clusters were used as seed masks to compute fibre pathways with FSL probtrackx (Behrens et al., 2003, 2007) using the default parameters (5000 sample per voxel, curvature threshold 0.2, maximum number of steps 2000, step length 0.5 mm, loopcheck enabled). Resulting connection probability maps were scaled by calculating the logarithm of the number of computed connections in each voxel divided by the logarithm of the total number of streamlines initialized (5000 × number of voxels). This resulted in images with normalized connection strength (connection probability distribution maps) between 0 and 1 in each voxel and allowed the following processing steps. Normalized connection maps were warped to the Montreal Neurological Institute (MNI) space and an average connectivity map was created and thresholded at 0.2 to exclude spurious connections.
Crossing-fibre measures
The diffusion tensor-based measures such as fractional anisotropy and radial diffusivity can only explain the underlying white matter microstructure to a limited extent in regions with crossing fibres. To analyse the origin of fractional anisotropy differences in areas with two fibre populations, the probability of the major fibre direction (F1) and of the secondary fibre direction (F2) were extracted from the clusters, which showed significant differences in the TBSS analysis.
Functional MRI
Task
To reveal the crucial functional brain region a task was adopted from a previous publication (Riecker et al., 2000) and was described in more detail in a previous study from our lab (Neef et al., 2016). Three functional MRI scanning runs were carried out. Each run consisted of 12 repetitions of a speech condition and 12 repetitions of a humming condition, in a random order. During the speech condition participants imagined themselves reciting the months of the year in a continuous, fluid manner. During the humming condition participants imagined humming the non-lyrical tune of a serenade (W.A. Mozart’s, Eine kleine Nachtmusik, KV 525). A trial was initiated by a visual cue. After 6 s, a plus symbol signalled participants to stop imagining and rest for the following 18 s. A run lasted 10 min. Two adults who stutter only finished two runs. Prior to the experiment, participants listened to the melody and performed the tasks outside the scanner to become familiarized with the test materials.
Analysis
Functional MRI data processing was carried out with FEAT, version 6.0, a tool from the FMRIB Software library (FSL; http://fsl.fmrib.ox.ac.uk). Preprocessing involved motion correction, smoothing with a Gaussian kernel of 8 mm full-width at half-maximum. Non-brain tissue was removed and all volumes were intensity-normalized. Temporal high-pass filtering was achieved by Gaussian-weighted least-squares straight line fitting, with a high-pass filter cut-off at 100 s. Functional images were spatially aligned to their respective anatomical image by affine registration (Jenkinson and Smith, 2001). A further non-linear registration of the anatomical images to the standard MNI152 template brain (Andersson et al., 2007) served to normalize the functional images. Boxcar models were convolved with a Gamma function. Model fit was determined by statistical time-series analysis in the framework of the general linear model. A fraction of the temporal derivative of the blurred original waveform was added to achieve a slightly better fit to the data. Across the three runs we calculated within-subject contrasts of imagining speaking > imagining melody humming and imagining melody humming > imagining speaking with a fixed-effect analysis. Across participants, mixed-effects group analyses were calculated. Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a corrected cluster significance threshold of P < 0.05 (Worsley et al., 1996).
Tractography
To reconstruct the fibre pathways that originate in hyperactive right frontal brain regions in adults who stutter, significant clusters from the functional MRI analysis were used as seed masks for tractography. Probtrackx parameters, normalization, and alignment with standard space were kept constant. Connection probability maps were then correlated for every voxel with stuttering severity scores (SSI-4, OASES) using FSL randomise (Anderson and Robinson, 2001; Winkler et al., 2014). Reported clusters in the tract volumes were significant at P < 0.005 at voxel-level and exceeded a cluster size significant at P < 0.01 based on global smoothness estimation of the connectivity maps in the thresholded tractography volume with AFNI (version 16.1.28, 3dClusterSim and 3dFWHMx) (Cox, 1996). Bi-sided thresholding resulted in the following cluster size thresholds, for the tract volume of the IFG k ≥ 169, for the tract volume of the frontal pole (FP) k ≥ 145, and for the tract volume of the MFG k ≥ 101.
Results
Group comparison with whole-brain TBSS
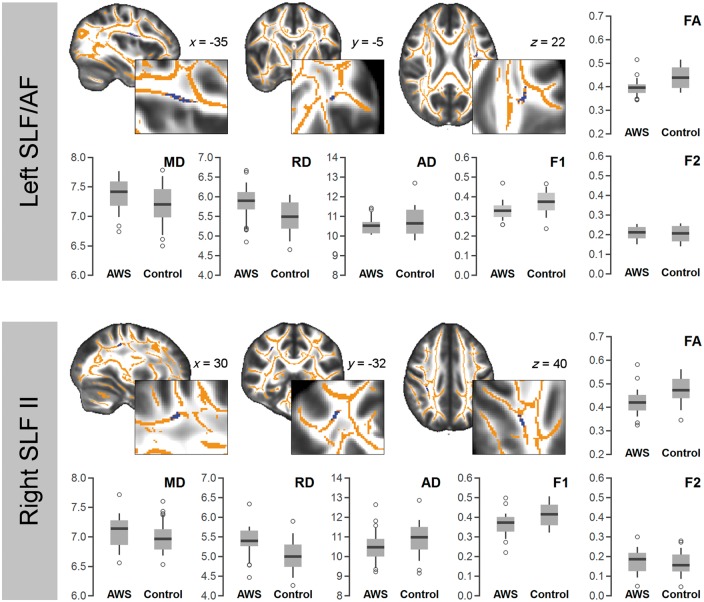
TBSS results are illustrated in Fig. 1. Compared to control participants, adults who stutter showed a reduced fractional anisotropy in the left SLF/arcuate fasciculus (MNI: −35, −5, 22; k = 51), in the parietal part of the right SLF II (MNI: 30, −32, 40; k = 40), and in the right FAT (MNI: 31, 7, 28; k = 27) close to the precentral sulcus.
Figure 1.
Diffusion properties of the three regions with a reduced fractional anisotropy in adults who stutter, determined by whole brain TBSS. AD = axial diffusivity; AF = arcuate fasciculus; F1 = major fibre direction; F2 = secondary fibre direction; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity.
Diffusion properties and crossing fibre measures
All clusters with a reduced fractional anisotropy in adults who stutter showed an increased radial diffusivity and an increased mean diffusivity, whereas the major fibre direction (F1) was reduced in adults who stutter compared to controls (Fig. 1). This more fine-grained analysis characterizes the significant differences in the fractional anisotropy analysis, but cannot be tested statistically.
TBSS-based tractography
With the probabilistic tractography we reconstructed fibre paths that link the significant TBSS clusters with cortical and subcortical regions (Fig. 2). Specifically, the cluster in the left SLF/arcuate fasciculus is most likely connected with the left IFG pars opercularis and pars triangularis, precentral and postcentral cortex, insula, posterior superior temporal gyrus, planum temporale, and posterior middle temporal gyrus. Subcortically, fibres run through the anterior limb of the internal capsule as well as through the external capsule, thereby reaching and passing the putamen, caudate, and thalamus towards the brainstem, where the tractogram involves fibres of the anterior thalamic radiation, cortical spinal/pontine tract, and cerebellar peduncle.
Figure 2.
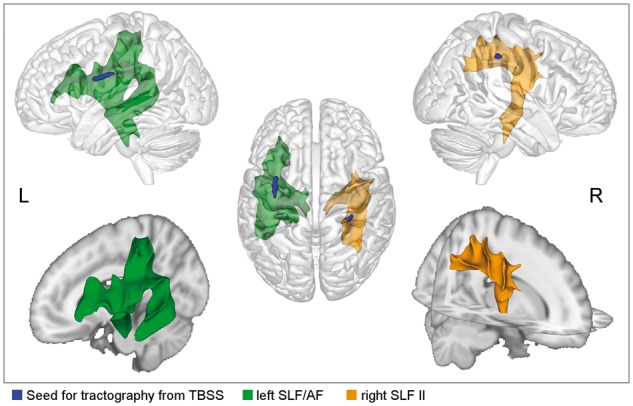
TBSS-based tractography indicate involved fibre tracts. The left tract connects the IFG with postcentral, superior parietal, and superior and middle temporal regions. On the right, precentral regions are connected to postcentral and inferior parietal regions (yellow).
The cluster in the right SLF II is most likely connected to the right IFG pars opercularis, the MFG, precentral, postcentral, superior parietal, inferior parietal regions of the right hemisphere. Subcortically, fibres run through the posterior limb of the internal capsule as well as through the external capsule, thereby passing the putamen, caudate, and thalamus towards the brainstem where the tractogram involves fibres of the superior thalamic radiation and the cortical spinal/pontine tract.
The cluster in the right FAT is most likely connected to the right IFG pars opercularis, SMA, MFG, and precentral gyrus. Fibre paths involve the right SLF, right anterior thalamic radiation, and transcallosal fibres through the body of the corpus callosum, which reach the superior corona radiata in the left hemisphere.
All TBSS-based tract volumes are provided as a 3D animation at: http://openscience.cbs.mpg.de/1/tbss_seeds.webm.
Functional MRI results
Group comparisons of imagining speaking > imagining melody humming revealed an increased activation in the right frontal pole, right posterior IFG pars opercularis adjacent to the precentral gyrus, and right medial frontal gyrus (Table 2 and Fig. 3). No other differences occurred. The Supplementary material summarizes the results of imagining speaking > imagining melody humming and imagining melody humming > imagining speaking across all participants (Supplementary Tables 3 and 4) and for each group separately (Supplementary Tables 5–8).
Table 2.
Brain activation in adults who stutter > control in speaking > humming (Z = 2.3, P = 0.05)
| Brain area | MNI-coordinates | Extent (voxels) | Z-score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Right frontal pole | 22 | 50 | −2 | 254 | 3.95 |
| Right inferior frontal gyrus (BA 44) | 58 | 8 | 16 | 179 | 3.17 |
| Right middle frontal gyrus | 36 | 4 | 52 | 127 | 3.5 |
Figure 3.
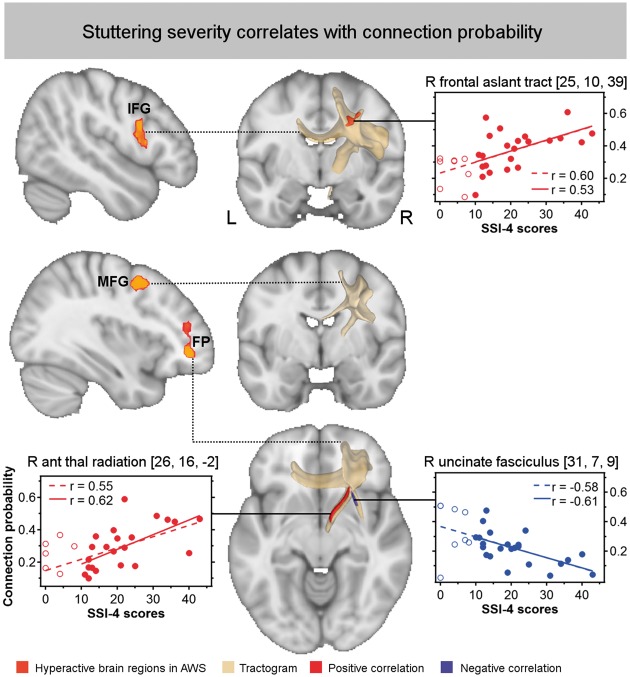
Probabilistic fibre tracking seeding in right frontal regions with increased blood oxygen level-dependent activity in adults who stutter. The connection probability was correlated with stuttering severity. Correlation coefficients are given for all adults who stutter in the upper line (n = 31, dotted line) and for those with SSI-4 > 10 in the lower line (n = 23, solid line). Positive correlations are displayed in red, negative correlation in blue, cluster-size corrected, P < 0.005, α < 0.01, two-sided.
Functional MRI-based tractography
To show which fibre pathways originate from the hyperactive right frontal regions in adults who stutter and whether adults who stutter have altered connectivity profiles, we computed probabilistic tractography of the involved fibre tracts. Resulting tractograms are shown in the middle column in Fig. 3.
The tractogram of the cluster in the right frontal pole involves connections to the superior frontal gyrus, MFG, frontal orbital cortex, anterior insular cortex. Fibre tracts included are (i) the anterior thalamic radiation towards the medial dorsal nucleus and the anterior nucleus of the right thalamus; (ii) transcallosal fibres through the forceps minor towards contralateral homologue regions of the frontal pole; and (iii) the uncinate fasciculus towards the posterior insular cortex and the superior temporal gyrus (see also http://openscience.cbs.mpg.de/1/seed_rightFP.webm).
The tractogram of the cluster in the right posterior IFG (pars opercularis) involves connections to the adjacent IFG pars triangularis, precentral gyrus, and MFG. Fibre tracts included are (i) the FAT towards the preSMA and SMA; (ii) the SLF towards the anterior intraparietal sulcus; (iii) transcollosal fibres through the callosal body towards the superior corona radiate of the contralateral hemisphere; and (iv) corticospinal tract passing through the posterior limb of the internal capsule, passing the putamen, the ventral lateral nucleus of the right thalamus, subthalamic nucleus, and cerebellar peduncle. The tractography ended in the brainstem at the level of the pons (see also http://openscience.cbs.mpg.de/1/seed_rightIFG.webm).
The tractogram of the cluster in the right posterior MFG involves U-shaped connections to the adjacent inferior frontal sulcus, precentral gyrus, and fundus of the central sulcus. Fibre tracts included are (i) the SLF towards the superior parietal lobule; (ii) transcallosal fibres through the callosal body; and (iii) fibres through the internal capsule ending at the level of the subthalamic nucleus as suggested by the tractography (see also http://openscience.cbs.mpg.de/1/seed_rightMFG.webm).
Connection probability and stuttering severity
We calculated voxel-wise correlation analyses across connection probability maps of adults who stutter (n = 31) to test the relationship between connection strength within each tractogram and stuttering severity as measured by the SSI-4 and the OASES. Only functional MRI-based tractograms revealed significant correlations and only with SSI-4 scores (Fig. 3). Stuttering severity was positively correlated with connection strength (i) in the right FAT when seeding in the right IFG (x = 25, y = 10, z = 39; k = 246); (ii) underneath the right precentral gyrus when seeding in the right MFG (x = 30, y = −4, z = 43; k = 102); and (iii) in the right anterior thalamic radiation when seeding in the right frontal pole (x = 10, y = 3, z = 7; k = 436; x = 26, y = 16, z = −2; k = 121). Stuttering severity was negatively correlated with connection strength in the right uncinate fasciculus/extreme capsule fibre system when seeding in the frontal pole (x = 31, y = 7, z = 43; k = 327).
Discussion
In this study, we analysed white matter brain structures in adults who stutter and those who do not. The outcome of this study is 2-fold. First, we have shown that stuttering severity is linked to the strength of white matter connections of hyperactive right frontal brain regions. This brain structure–behaviour relationship incorporates affected right frontal spatially separated cortical regions into disparate networks, thereby not only pinpointing areas of pathology, but advancing circuit-based interpretations of the neuronal basis of this disorder. The second valuable achievement of this study constitutes the findings of reduced white matter integrity in the bilateral SLF and the right FAT of adults who stutter. We calculated TBSS to enable comparison with previous DTI studies. Our large sample sizes and the stringent correction for multiple comparisons validated these findings that were inconsistently reported among previous studies.
Affected right frontal areas are not primarily linked to speech behaviour
In adults who stutter, right frontal hyperactive areas resulted from a functional MRI contrast between two motor imagery tasks, imagining speaking versus imagining humming. The three hyperactive regions, the posterior IFG, MFG, and frontal pole engage different networks and different cognitive resources, which are all relevant for carrying out the task. However, these areas are not primarily linked to speech behaviour.
The functional specialization in the right posterior IFG, the pars opercularis (BA 44), is manifold. Neuroimaging studies associate this region with motor imagery (Lacourse et al., 2005; Guillot et al., 2008), imitation and action observation (Heiser et al., 2003; Molnar-Szakacs et al., 2005; Kilner et al., 2009), proactive and reactive working memory control (Marklund and Persson, 2012), task switching, cognitive flexibility and go/no-go control (Buchsbaum et al., 2005; Hirose et al., 2009). Part of the right posterior IFG implements the control of response inhibition, which comprises cognitive and motor inhibition (Aron and Poldrack, 2006; Chambers et al., 2006; Li et al., 2006, 2008; Aron et al., 2014; Cai et al., 2014b). The MFG is likewise involved in various operations that are associated with higher-level executive functions and decision-related processes (Talati and Hirsch, 2005). Similar to the right IFG, the right MFG is active during imagery tasks, stop-signal and go/no-go tasks (Rubia et al., 2001; Yoo et al., 2001; Zheng et al., 2008; Sebastian et al., 2013). Given a large body of literature that associates these two cortical regions with aspects of imagery and inhibition, it is intriguing that an overactivation of these structures is a neural sign of stuttering.
The functional MRI task in the current study involved reactive inhibition because participants were asked to stop the speech motor imagery. In our previous study we conducted an independent component analysis thereby showing that the activation of the right posterior IFG, the pars opercularis, was related to both aspects of the task. Speech motor imagery is necessary to realize the task and reactive inhibition to stop this realization (Neef et al., 2016). Taking this line of thinking one step further, the imaginary task itself involves continuous inhibitory control in order to prevent overt motor responses. Accordingly, the involvement of the right IFG, the pars opercularis, during imagining speaking might be primarily associated with an executive control over action, concretely, the suppression of overt speech. This view would be consistent with the observation that imagining speaking recruits the right IFG (Tian et al., 2016), whereas overt speaking does not involve the right posterior IFG but the left hemisphere homologue (Turkeltaub et al., 2002; Ghosh et al., 2008; Guenther, 2016). It is tempting to speculate that the stronger recruitment of the right posterior IFG in stuttering is related to imagery and inhibitory control mechanisms rather than to processes that support compensation. And indeed, a recent behavioural study supports the idea that the motor control deficit in stuttering involves impaired motor inhibition (Markett et al., 2016). Thus, behavioural and neuroimaging studies accumulate evidence for an affected response inhibition mechanism in stuttering.
The third region that showed hyperactivity in adults who stutter was the right frontal pole. The function of the right frontal pole is poorly understood. Its involvement could be related to domain-general functions, such as task monitoring (Koechlin and Hyafil, 2007) or attentional gating (Burgess et al., 2007). The observation of an amplified involvement of regions that support imagery and attentional allocation to the self-generated representations suggests that speaking imagery was more challenging for a system that repeatedly fails to produce fluent speech. This view would be compatible with possible compensation mechanisms. An alternative view would be that these hyperactivations reflect an activation pattern inherent to the disorder and thus would signal maladaptive or causal activity even in the state of covert speech behaviour.
In summary, it is intriguing that none of the right frontal hyperactive regions is primarily involved in speech production in fluent speakers (Turkeltaub et al., 2002; Ghosh et al., 2008; Guenther, 2016). Rather, right IFG and MFG are involved in the ability to apply executive control over actions, while the frontal pole contributes to the ability to stay focused on a given task.
Severity of stuttering is related to increased structural connectivity of the motor response inhibition network
Right posterior IFG and MFG, however, are not solely responsible for response inhibition. Rather, they are part of large-scale networks that also include the right SMG, preSMA, subthalamic nucleus, and putamen. These cortical and subcortical structures are known to function together during the processing of go/no-go and stop signal tasks (Aron and Poldrack, 2006; Cai et al., 2014c). This observed pattern of functional connectivity is in agreement with anatomical tracer analyses of corticocortical connections in non-human primates (Schmahmann and Pandya, 2009) and diffusion-weighted imaging studies in humans (Aron et al., 2007; Neubert et al., 2010). Putamen and subthalamic nucleus receive input from various brain sites and thus constitute a part of the cortico-thalamocortical loops that are regulated by cortex, basal ganglia, and cerebellum (Alexander et al., 1986; Alexander and Crutcher, 1990). The tract volume of the right posterior IFG tractography reflects this connectivity at a macroanatomical scale, and hence, proves the network aspect. Moreover, the tract volume contains key nodes of the response inhibition network, suggesting that it includes white matter structures involved in this function.
The implication of these white matter structures in the context of stuttering has already been suggested (Aron et al., 2007). And indeed, our correlation analysis indicated a positive correlation between stuttering severity and the connection strength within the IFG tract volume. Our data related more severe motor signs of stuttering to stronger anatomical connections of the FAT linking the posterior IFG with SMA and preSMA. This finding indicates that the right FAT plays a crucial role in the motor aspect of the disorder, which might reflect cause or maladaptation.
The functional role of the right IFG versus the right preSMA in inhibitory control is not entirely clear yet (Aron et al., 2014). However, both structures function together with the subthalamic nucleus in a network that mediates fast global inhibition. The characteristic motor signs of stuttering are silent speech blocks, sound prolongations, and sound and syllable repetitions. Common to all these symptoms is the unsuccessful control of stop and go. While speaking, the control of these processes happens rather automatically. Accordingly, an overly active global response suppression mechanism that induces an unspecific broad inhibition is a most likely pathomechanism because it would hinder the smooth successive execution of appropriate motor actions. The stronger connectivity of the right FAT might be a neuroanatomical correlate of such an overly implicated global response suppression mechanism.
Severity of stuttering is related to increased connection strength between right frontal pole and anterior thalamic nuclei
The dorsolateral prefrontal cortex is part of the circuits constituting the non-motor basal ganglia prefrontal loop. Within these circuits, the frontal pole (BA 10) interacts with anterior thalamic nuclei (Alexander et al., 1986). These circuits might regulate the initiation and termination of cognitive processes such as planning, working memory, and attention (Graybiel, 1997). While the right IFG might function together with the preSMA, subthalamic nucleus and basal ganglia circuit to regulate reactive inhibition, the right dorsolateral prefrontal cortex might function together with the caudate and the thalamus to regulate proactive inhibition (Jahanshahi et al., 2015). Proactive inhibition is prospective and assumed to regulate thoughts, impulses, emotion, mood, and behaviour. In the context of stuttering this network might be implicated in response to the anticipation of stuttering. An overwhelming majority of adults who stutter often experience anticipation of stuttering (Jackson et al., 2015). There are two types of reactions, avoidance strategies such as word substitution and circumlocuting, adding meaningless speech, making non-speech movement, and stalling, and self-management strategies such as pause, change of speech rate, use of fluency-shaping or stutter-modification. Avoidance behaviour as well as self-management strategies very likely implicate circuits that regulate proactive inhibition. Until today, no neuroimaging study on stuttering scrutinized neuronal correlates of stuttering anticipation and respective behaviour. We are the first to bring such a brain–behaviour relationship into awareness. Future studies are necessary to test this hypothesis.
In stuttering, right frontal structural connectivity might support compensatory mechanisms
When seeding in the frontal pole, the probabilistic fibre tracking suggests connections of this region with further higher-order prefrontal areas and thalamo-cortical networks, but no connections with the primary motor or sensory cortex. Instead, reconstructed fibre pathways through the extreme capsule linked the frontal pole to higher-order auditory and multisensory areas of the superior temporal gyrus via the uncinated fasciculus. The negative correlation between stuttering severity and connection strength within the right uncinated fasciculus, suggests a compensatory role of this structure. The correlation was only evident when considering the motor aspects of stuttering (SSI-4), but not when considering emotional and social-cognitive aspects (OASES). Therefore, we can exclude that the brain structure–function relationship observed here results from the psychological strain the disorder entails. Rather, the observed correlation underscores a close link between the severity level of the motor aspect of the disorder and the right-hemispheric fronto-temporal connection strength of the ventrally located uncinate fasciculus. This finding is consistent with a previous diffusion-weighted MRI study with children who stutter; fractional anisotropy in the right uncinated fasciculus correlated negatively with stuttering severity as measured by the SSI (Chang et al., 2015). The fact that children who stutter are already displaying such a brain–behaviour relationship, suggests that implicated networks support fluent speech very early on.
The view of a compensatory role of this connection finds support in a previous interpretation (Neef et al., 2015) of recent functional MRI activation likelihood estimate (ALE)-metal analysis (Budde et al., 2014; Belyk et al., 2015), which, however, needs to be considered with caution because previous ALE meta-analyses are based on a liberal statistical thresholds and thus might involve false positive results (Belyk et al., 2017; Eickhoff et al., 2017). Nevertheless, when comparing functional MRI contrasts of dysfluent state and fluent state within affected individuals, it becomes evident that an additional recruitment of right superior temporal regions supports the fluent state. Presupposed, enhanced fluency requires additional cognitive efforts, such as the allocation of attention to the task and the multisensory monitoring of speech motor acts, in which a compensatory role of the uncinate fasciculus and the solid link between right fronto-temporal regions becomes highly plausible.
In stuttering, left and right dorsal pathways show signs of disconnection
A further important outcome of the present study is the consolidation of white matter differences in fibre pathways that are suspected of alterations in persistent stuttering. TBSS located a reduction of fractional anisotropy bilaterally in the SLF, a massive intrahemispheric fibre system that connects postrolandic regions with the frontal lobe (Catani et al., 2005; Makris et al., 2005). Previous TBSS studies on persistent stuttering have reported white matter differences throughout the brain (Chang et al., 2008, 2015; Watkins et al., 2008; Kell et al., 2009; Cykowski et al., 2010; Cai et al., 2014a; Connally et al., 2014; Civier et al., 2015). Our recent meta-analysis, however, assigned most robust reductions of fractional anisotropy to the left SLF/arcuate fasciculus (Neef et al., 2015), which is consistent with current observations. Effects in the left SLF are also reported in another study based the TBSS analyses with a comparably large population (Connally et al., 2014). According to this study a cluster in the left SLF had an extent of 27 voxels at (x = −37, y = −25, z = 30), which is close to the centre of gravity in the left SLF/arcuate fasciculus reported here. Our meta-analysis yielded no consistent cluster in the right hemisphere. Findings considering TBSS clusters of reduced fractional anisotropy in the right SLF are indeed less straight forward. A few TBSS studies report fractional anisotropy reductions along the right SLF but in different fractions (Cai et al., 2014a; Chang et al., 2015) when compared to the clusters found here.
Considering functions of bilateral SLF, it is important to separate two dorsal streams. It has been postulated that dorsal fibre tracts linking left BA44 and left posterior temporal cortex subserve syntactic processing (Catani et al., 2005; Friederici et al., 2006; Anwander et al., 2007), while a different segment of the SLF (SLF III) that connects left BA 44 and BA 6 with the left supramarginal gyrus supports articulation and repetition (Makris et al., 2005; Gierhan, 2013). A speech production model maps the processing of feedback control to fronto-parietal and fronto-temporal networks (Guenther et al., 1998; Bohland et al., 2009). Strikingly, left hemisphere feedback control is postulated to play a particular role during language acquisition; mapping sound to articulation is a crucial aspect in this period (Guenther, 1995; Perani et al., 2011). In contrast, online control of perturbed auditory feedback or perturbed somatosensory feedback during speaking is primarily mediated by right hemisphere fronto-parieto-temporal networks (Tourville et al., 2008; Golfinopoulos et al., 2011). Accordingly, our findings of bilateral white matter disorganization within dorsal fibre tracts in adults who persist to stutter, suggest a potential importance of feedback mechanisms in this disorder throughout life span.
Complementarily, in adults who stutter, previous fibre tracking studies report a reduced fractional anisotropy in the right anterior segment of the arcuate fasciculus (Kronfeld-Duenias et al., 2016b), reduced mean fractional anisotropy across the whole tract volume (Connally et al., 2014), and an anomalous white matter morphology of the right posterior segment of the right arcuate fasciculus (Cieslak et al., 2015). Here, fractional anisotropy was reduced in the SLF II and in the anterior segment of the SLF, a region that also contains fibres of the FAT, previously reported to be associated with stuttering (Kemerdere et al., 2016; Kronfeld-Duenias et al., 2016a). Thus, previous and current observations strengthen the view of a compromised intrahemispheric transfer of neural signals in stuttering that link the posterior IFG and the premotor cortex to parietal and temporal regions.
Probability maps help to assign labels to affected structures (Mori et al., 2005; Wakana et al., 2007; Hua et al., 2008), making it possible to draw inferences about disconnected brain regions. Here, we used TBSS-based fibre tracking additionally (Fig. 2) to pinpoint affected fibre tracts. Tractograms from both hemispheres involve parts of the SLF that connect the IFG with parietal areas. Furthermore, resulting tractograms indicate possible involvement of ascending thalamo-cortical projection fibres, and descending projections to the pons, cerebellar penduncle, and the corticospinal tract, common structures of suspect (Watkins et al., 2008; Connally et al., 2014), as they belong to the speech-production networks implicated in stuttering (Guenther, 2016).
Two other TBSS clusters of a recent ALE meta-analysis (Neef et al., 2015) have not been found in the current dataset, a cluster in the left inferior parietal lobule adjacent to the angular gyrus and the posterior division of the supramarginal gyrus, and a cluster in the posterior midbody of the corpus callosum. This recent meta-analysis was based on seven existing DTI studies that reported foci of reduced fractional anisotropy in participants older than 14 years. Overall, the pooled set considered 60 reported foci from 121 adults who stutter and 126 fluent speakers. The quantitative GingerALE analysis (Eickhoff et al., 2009) estimates the spatial uncertainty associated with each reported coordinate, thereby identifying regions which show a consistent reduction of fractional anisotropy across studies. This approach is one way of overcoming the problem of small sample sizes and low reliability inherent to previous DTI studies. Critically, seven studies have insufficient statistical power and only a few of the previous studies reported TBSS findings that were corrected for multiple comparisons. Hence, TBSS studies in the field of stuttering are susceptible to false positive findings. The advantage of the current study is that the high sample size and the applied cluster thresholding reduce this susceptibility.
The interpretation of an altered fractional anisotropy is complicated. This is particularly important in white matter regions that contain fibre crossings and fanning. In particular, fibre crossings should be considered when interpreting current TBSS findings. For the cluster in the right FAT, for example, we cannot exclude an influence of crossing fibres of the SLF. Thus, it is unclear whether fractional anisotropy reductions result, for example, from demyelination, reduced axonal packing, or increased diameter of axon calibres of the FAT tract itself, or from a complex geometry of involved fibres. One way of disentangling this complexity is to consider further diffusion properties such as mean, radial, and axial diffusivity, and to separate the major and second major direction of streamlines passing through certain voxels (F1 and F2). Strikingly, along with a reduced fractional anisotropy, adults who stutter showed an increased radial diffusivity, an increased mean diffusivity together with a reduced F1, and no differences for F2 when compared with controls. This combination of diffusion properties was evident across all three TBSS clusters, the cluster in the left arcuate fasciculus/SLF, in the right SLF, and in the right FAT. Thus, adults who stutter seem to exhibit a weakened connectivity of fibre tracts along the major diffusion direction, which favours the view that atypical structures are insufficiently myelinated or that the axonal packing is reduced therein. It is unlikely that crossing fibres of the secondary fibre direction exhibit a stronger integrity, because in such a case F2 would be increased. Consequently, our data reinforce previous findings of a white matter deficit of the left and right SLF and the right FAT. For the additional diffusion properties, group differences were not accessed statistically to avoid circularity.
Conclusion
Previous neuroimaging studies on persistent developmental stuttering related the hyperactivity of right frontal areas to compensatory or causal mechanisms of the disorder. This view was based on the idea that involved hyperactive regions are homologue areas of the left frontal network involved in speech production (Turkeltaub et al., 2002; Ghosh et al., 2008), and that its recruitment relates to interhemispheric interactions that might cause or compensate for a left hemisphere structural and functional deficit (Neumann et al., 2005; Watkins et al., 2008; Kell et al., 2009; Chang et al., 2011; Lu et al., 2012; Beal et al., 2015). The current study works out some details of these opposing views, thereby considering the special role of right hemisphere frontal regions in response inhibition and task monitoring. Here, we show that an increased right hemisphere structural connectivity between the posterior IFG pars opercularis, pre-SMA, and subthalamic nucleus was evident in participants with more severe stuttering, which might reflect an amplified activity of the hyperactive pathway that controls global response suppression and the ability to stop an ongoing motor response. In contrast, an increased structural connectivity of right fronto-temporal regions supports fluency possibly by a strengthened ability to allocate attention to the multisensory monitoring of speech motor responses. Accordingly, our combined functional MRI-DTI analysis presents a new view on pathophysiological principals of stuttering and focuses on networks that specifically engage the right hemisphere.
Supplementary Material
Acknowledgements
We are thankful to Kristina Anders for the analysis of the speech samples, Harald Euler for the provision of the German Version of the OASES, Peter Dechent for his recommendations regarding the MR sequences, and Ilona Pfahlert and Britta Perl for their help in MRI data recording.
Funding
This work was funded by the Dorothea Schlözer Fellowship Programme of the University of Göttingen (to N.E.N.), and by the Deutsche Forschungsgemeinschaft (NE 1841/1-1 to N.E.N; and SO 429/4-1 to M.S.).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- DTI
diffusion tensor imaging
- FAT
frontal aslant tract
- IFG
inferior frontal gyrus
- MFG
middle frontal gyrus
- OASES
overall assessment of stuttering the speakers experience of stuttering
- SLF
superior longitudinal fasciculus
- SMA
supplementary motor area
- SSI-4
stuttering severity instrument
- TBSS
tract-based spatial statistics
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990; 13: 266–71. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9: 357–81. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Robinson J. Permutation tests for linear models. Aust N Z J Stat 2001; 43: 75–88. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S TR07JA2: Non-linear registration, aka spatial normalisation. FMRIB Analysis Group Technical Reports. 2007. Available from: http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf. [Google Scholar]
- Anwander A, Tittgemeyer M, von Cramon D, Friederici AD, Knösche TR. Connectivity-based parcellation of Broca’s area. Cereb Cortex 2007; 17: 816–25. [DOI] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry 2011; 69: e55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 2007; 27: 3743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci 2006; 26: 2424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 2014; 18: 177–85. [DOI] [PubMed] [Google Scholar]
- Beal DS, Lerch JP, Cameron B, Henderson R, Gracco VL, De Nil LF. The trajectory of gray matter development in Broca’s area is abnormal in people who stutter. Front Hum Neurosci 2015; 9: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 2007; 34: 144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003; 50: 1077–88. [DOI] [PubMed] [Google Scholar]
- Belyk M, Kraft SJ, Brown S. Stuttering as a trait or state—an ALE meta-analysis of neuroimaging studies. Eur J Neurosci 2015; 41: 275–84. [DOI] [PubMed] [Google Scholar]
- Belyk M, Kraft SJ, Brown S. Stuttering as a trait or a state revisited: motor system involvement in persistent developmental stuttering. Eur J Neurosci 2017; 45: 622–4. [DOI] [PubMed] [Google Scholar]
- Bloodstein O, Ratner NB. A Handbook on stuttering. 6th edn Clifton Park, NY: Delmar Learning; 2008. [Google Scholar]
- Bohland JW, Bullock D, Guenther FH. Neural representations and mechanisms for the performance of simple speech sequences. J Cogn Neurosci 2009; 22: 1504–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang W-L, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting task and component processes. Hum Brain Mapp 2005; 25: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde KS, Barron DS, Fox PT. Stuttering, induced fluency, and natural fluency: a hierarchical series of activation likelihood estimation meta-analyses. Brain Lang 2014; 139: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci 2007; 11: 290–8. [DOI] [PubMed] [Google Scholar]
- Bütfering C. Geschlechtsspezifische Unterschiede sprechassoziierter Gehirnaktivität bei stotternden. Menschen: Eine klinische Studie mittels funktioneller Magnetresonanztomographie; 2015. [Google Scholar]
- Cai S, Tourville JA, Beal DS, Perkell JS, Guenther FH, Ghosh SS. Diffusion imaging of cerebral white matter in persons who stutter: evidence for network-level anomalies. Front Hum Neurosci 2014a; 8: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Cannistraci CJ, Gore JC, Leung H-C. Sensorimotor-independent prefrontal activity during response inhibition. Hum Brain Mapp 2014b; 35: 2119–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Ryali S, Chen T, Li C-SR, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci 2014c; 34: 14652–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Vergani F, Malik F, Hodge H, Roy P, et al. Short frontal lobe connections of the human brain. Cortex 2012; 48: 273–91. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol 2005; 57: 8–16. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, et al. Executive ‘brake failure’ following deactivation of human frontal lobe. J Cogn Neurosci 2006; 18: 444–55. [DOI] [PubMed] [Google Scholar]
- Chang SE, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage 2008; 39: 1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Horwitz B, Ostuni J, Reynolds R, Ludlow CL. Evidence of left inferior frontal–premotor structural and functional connectivity deficits in adults who stutter. Cereb Cortex 2011; 21: 2507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Kenney MK, Loucks TMJ, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. Neuroimage 2009; 46: 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Zhu DC, Choo AL, Angstadt M. White matter neuroanatomical differences in young children who stutter. Brain 2015; 138: 694–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesters J, Watkins KE, Möttönen R. Investigating the feasibility of using transcranial direct current stimulation to enhance fluency in people who stutter. Brain Lang 2017; 164: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslak M, Ingham RJ, Ingham JC, Grafton ST. Anomalous white matter morphology in adults who stutter. J Speech Lang Hear Res 2015; 58: 268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civier O, Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Ben-Shachar M. Reduced fractional anisotropy in the anterior corpus callosum is associated with reduced speech fluency in persistent developmental stuttering. Brain Lang 2015; 143: 20–31. [DOI] [PubMed] [Google Scholar]
- Connally EL, Ward D, Howell P, Watkins KE. Disrupted white matter in language and motor tracts in developmental stuttering. Brain Lang 2014; 131: 25–35. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res Int J 1996; 29: 162–73. [DOI] [PubMed] [Google Scholar]
- Craig-McQuaide A, Akram H, Zrinzo L, Tripoliti E. A review of brain circuitries involved in stuttering. Front Hum Neurosci 2014; 8: 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski MD, Fox PT, Ingham RJ, Ingham JC, Robin DA. A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: a potential role for impaired myelination. Neuroimage 2010; 52: 1495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nil LF, Kroll RM, Kapur S, Houle S. A positron emission tomography study of silent and oral single word reading in stuttering and nonstuttering adults. J Speech Lang Hear Res 2000; 43: 1038–53. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PM, Lancaster JL, Fox PT. Implementation errors in the GingerALE software: description and recommendations. Hum Brain Mapp 2017; 38: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. HumBrain Mapp 2009; 30: 2907–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchell AC, Johnson BW, Sowman PF. Behavioral and multimodal neuroimaging evidence for a deficit in brain timing networks in stuttering: a hypothesis and theory. Front Hum Neurosci 2014; 8: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C, et al. A PET study of the neural systems of stuttering. Nature 1996; 382: 158–62. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong J-H, Lancaster JL. Brain correlates of stuttering and syllable production A PET performance-correlation analysis. Brain 2000; 123: 1985–2004. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Bahlmann J, Heim S, Schubotz RI, Anwander A. The brain differentiates human and non-human grammars: Functional localization and structural connectivity. Proc Natl Acad Sci USA 2006; 103: 2458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH. A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. J Speech Lang Hear Res 2008; 51: 1183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierhan SME. Connections for auditory language in the human brain. Brain Lang 2013; 127: 205–21. [DOI] [PubMed] [Google Scholar]
- Golfinopoulos E, Tourville JA, Bohland JW, Ghosh SS, Nieto-Castanon A, Guenther FH. fMRI investigation of unexpected somatosensory feedback perturbation during speech. Neuroimage 2011; 55: 1324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and cognitive pattern generators. Schizophr Bull 1997; 23: 459–69. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Speech sound acquisition, coarticulation, and rate effects in a neural network model of speech production. Psychol Rev 1995; 102: 594–21. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Neural control of speech [Internet]. Cambridge, MA: MIT Press; 2016. Available from: https://mitpress.mit.edu/books/neural-control-speech (9 September 2016, date last accessed). [Google Scholar]
- Guenther FH, Hampson M, Johnson D. A theoretical investigation of reference frames for the planning of speech movements. Psychol Rev 1998; 105: 611–33. [DOI] [PubMed] [Google Scholar]
- Guillot A, Collet C, Nguyen VA, Malouin F, Richards C, Doyon J. Functional neuroanatomical networks associated with expertise in motor imagery. Neuroimage 2008; 41: 1471–83. [DOI] [PubMed] [Google Scholar]
- Heiser M, Iacoboni M, Maeda F, Marcus J, Mazziotta JC. The essential role of Broca’s area in imitation. Eur J Neurosci 2003; 17: 1123–8. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci 2007; 8: 393–402. [DOI] [PubMed] [Google Scholar]
- Hirose S, Chikazoe J, Jimura K, Yamashita K, Miyashita Y, Konishi S. Sub-centimeter scale functional organization in human inferior frontal gyrus. Neuroimage 2009; 47: 442–50. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008; 39: 336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham RJ, Grafton ST, Bothe AK, Ingham JC. Brain activity in adults who stutter: Similarities across speaking tasks and correlations with stuttering frequency and speaking rate. Brain Lang 2012; 122: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverach L, Rapee RM. Social anxiety disorder and stuttering: current status and future directions. J Fluen Disord 2014; 40: 69–82. [DOI] [PubMed] [Google Scholar]
- Jackson ES, Yaruss JS, Quesal RW, Terranova V, Whalen DH. Responses of adults who stutter to the anticipation of stuttering. J Fluen Disord 2015; 45: 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci 2015; 16: 719–32. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012; 62: 782–90. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001; 5: 143–56. [DOI] [PubMed] [Google Scholar]
- Kell CA, Neumann K, von Kriegstein K, Posenenske C, von Gudenberg AW, Euler H, et al. How the brain repairs stuttering. Brain 2009; 132: 2747–60. [DOI] [PubMed] [Google Scholar]
- Kemerdere R, de Champfleur NM, Deverdun J, Cochereau J, Moritz-Gasser S, Herbet G, et al. Role of the left frontal aslant tract in stuttering: a brain stimulation and tractographic study. J Neurol 2016; 263: 157–67. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD. Evidence of mirror neurons in human inferior frontal gyrus. J Neurosci 2009; 29: 10153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science 2007; 318: 594–8. [DOI] [PubMed] [Google Scholar]
- Koedoot C, Bouwmans C, Franken M-C, Stolk E. Quality of life in adults who stutter. J Commun Disord 2011; 44: 429–43. [DOI] [PubMed] [Google Scholar]
- Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Civier O, Ben-Shachar M. The frontal aslant tract underlies speech fluency in persistent developmental stuttering. Brain Struct Funct 2016a; 221: 365–81. [DOI] [PubMed] [Google Scholar]
- Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Civier O, Ben-Shachar M. Dorsal and ventral language pathways in persistent developmental stuttering. Cortex 2016b; 81: 79–92. [DOI] [PubMed] [Google Scholar]
- Lacourse MG, Orr ELR, Cramer SC, Cohen MJ. Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage 2005; 27: 505–19. [DOI] [PubMed] [Google Scholar]
- Li CR, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci 2006; 26: 186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Sinha R, Lee T-W. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage 2008; 41: 1352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Chen C, Ning N, Ding G, Guo T, Peng D, et al. The neural substrates for atypical planning and execution of word production in stuttering. Exp Neurol 2010; 221: 146–56. [DOI] [PubMed] [Google Scholar]
- Lu C, Chen C, Peng D, You W, Zhang X, Ding G, et al. Neural anomaly and reorganization in speakers who stutter: a short-term intervention study. Neurology 2012; 79: 625–32. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 2005; 15: 854–69. [DOI] [PubMed] [Google Scholar]
- Markett S, Bleek B, Reuter M, Prüss H, Richardt K, Müller T, et al. Impaired motor inhibition in adults who stutter—evidence from speech-free stop-signal reaction time tasks. Neuropsychologia 2016; 91: 444–50. [DOI] [PubMed] [Google Scholar]
- Marklund P, Persson J. Context-dependent switching between proactive and reactive working memory control mechanisms in the right inferior frontal gyrus. Neuroimage 2012; 63: 1552–60. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cereb Cortex 2005; 15: 986–94. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Zijl PCM van, Nagae-Poetscher LM. MRI atlas of human white matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Morris DM, Embleton KV, Parker GJM. Probabilistic fibre tracking: differentiation of connections from chance events. Neuroimage 2008; 42: 1329–39. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico–subthalamo–pallidal ‘hyperdirect’ pathway. Neurosci Res 2002; 43: 111–17. [DOI] [PubMed] [Google Scholar]
- Neef NE, Anwander A, Friederici AD. The neurobiological grounding of persistent stuttering: from structure to function. Curr Neurol Neurosci Rep 2015; 15: 1–11. [DOI] [PubMed] [Google Scholar]
- Neef NE, Jung K, Rothkegel H, Pollok B, von Gudenberg AW, Paulus W, et al. Right-shift for non-speech motor processing in adults who stutter. Cortex 2011; 47: 945–54. [DOI] [PubMed] [Google Scholar]
- Neef NE, Bütfering C, Anwander A, Friederici AD, Paulus W, Sommer M. Left posterior-dorsal area 44 couples with parietal areas to promote speech fluency, while right area 44 activity promotes the stopping of motor responses. Neuroimage 2016; 142: 628–44. [DOI] [PubMed] [Google Scholar]
- Neubert F-X, Mars RB, Buch ER, Olivier E, Rushworth MFS. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci USA 2010; 107: 13240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann K, Euler HA, von Gudenberg AW, Giraud A-L, Lanfermann H, Gall V, et al. The nature and treatment of stuttering as revealed by fMRI: a within- and between-group comparison. J Fluen Disord 2003; 28: 381–410. [DOI] [PubMed] [Google Scholar]
- Neumann K, Preibisch C, Euler HA, von Gudenberg AW, Lanfermann H, Gall V, et al. Cortical plasticity associated with stuttering therapy. J Fluen Disord 2005; 30: 23–39. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Anwander A, Spada D, Baldoli C, et al. Neural language networks at birth. Proc Natl Acad Sci USA 2011; 108: 16056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch C, Neumann K, Raab P, Euler HA, von Gudenberg AW, Lanfermann H, et al. Evidence for compensation for stuttering by the right frontal operculum. Neuroimage 2003; 20: 1356–64. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. [Miscellaneous Article]. Neuroreport 2000; 11: 1997–2000. [DOI] [PubMed] [Google Scholar]
- Riley GD. Stuttering severity instrument, Fourth Edition (SSI-4). 4th edn. Austin: Pro-Ed; 2009. [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: conjunctive brain activations across different versions of Go/No-Go and stop tasks. Neuroimage 2001; 13: 250–61. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Freund H-J. Single word reading in developmental stutterers and fluent speakers. Brain 2000; 123: 1184–202. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya D. Fiber pathways of the brain. Oxford: Oxford University Press; 2009. [Google Scholar]
- Sebastian A, Pohl MF, Klöppel S, Feige B, Lange T, Stahl C, et al. Disentangling common and specific neural subprocesses of response inhibition. Neuroimage 2013; 64: 601–15. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487–505. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Büchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet 2002; 360: 380–3. [DOI] [PubMed] [Google Scholar]
- Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual Go/No-Go decisions based on ‘what,’ ‘when,’ and ‘where’ related information: an fMRI study. J Cogn Neurosci 2005; 17: 981–93. [DOI] [PubMed] [Google Scholar]
- Tian X, Zarate JM, Poeppel D. Mental imagery of speech implicates two mechanisms of perceptual reactivation. Cortex 2016; 77: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage 2008; 39: 1429–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomura A, Fujii T, Kuriki S. Effect of external auditory pacing on the neural activity of stuttering speakers. Neuroimage 2011; 57: 1507–16. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 2002; 16: 765–80. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007; 36: 630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain 2008; 131: 50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014; 92: 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 1996; 12: 900–18. [DOI] [PubMed] [Google Scholar]
- Wymbs NF, Ingham RJ, Ingham JC, Paolini KE, Grafton ST. Individual differences in neural regions functionally related to real and imagined stuttering. Brain Lang 2013; 124: 153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yairi E, Ambrose N. Epidemiology of stuttering: 21st century advances. J Fluen Disord 2013; 38: 66–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yairi E, Ambrose NG. Early childhood stuttering I: persistency and recovery rates. J Speech Lang Hear Res 1999; 42: 1097–12. [DOI] [PubMed] [Google Scholar]
- Yaruss JS, Quesal RW. Overall assessment of the speaker’s experience of stuttering (OASES): documenting multiple outcomes in stuttering treatment. J Fluen Disord 2006; 31: 90–115. [DOI] [PubMed] [Google Scholar]
- Yoo S-S, Lee CU, Choi BG. Human brain mapping of auditory imagery: event-related functional MRI study [Miscellaneous Article]. Neuroreport 2001; 12: 3045–9. [DOI] [PubMed] [Google Scholar]
- Zheng D, Oka T, Bokura H, Yamaguchi S. The key locus of common response inhibition network for no-go and stop signals. J Cogn Neurosci 2008; 20: 1434–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.