Abstract
Aims
We evaluated for the first time the effects of angiogenic and lymphangiogenic AdVEGF-DΔNΔC gene therapy in patients with refractory angina.
Methods and results
Thirty patients were randomized to AdVEGF-DΔNΔC (AdVEGF-D) or placebo (control) groups. Electromechanical NOGA mapping and radiowater PET were used to identify hibernating viable myocardium where treatment was targeted. Safety, severity of symptoms, quality of life, lipoprotein(a) [Lp(a)] and routine clinical chemistry were measured. Myocardial perfusion reserve (MPR) was assessed with radiowater PET at baseline and after 3- and 12-months follow-up. Treatment was well tolerated. Myocardial perfusion reserve increased significantly in the treated area in the AdVEGF-D group compared with baseline (1.00 ± 0.36) at 3 months (1.31 ± 0.46, P = 0.045) and 12 months (1.44 ± 0.48, P = 0.009) whereas MPR in the reference area tended to decrease (2.05 ± 0.69, 1.76 ± 0.62, and 1.87 ± 0.69; baseline, 3 and 12 months, respectively, P = 0.551). Myocardial perfusion reserve in the control group showed no significant change from baseline to 3 and 12 months (1.26 ± 0.37, 1.57 ± 0.55, and 1.48 ± 0.48; respectively, P = 0.690). No major changes were found in clinical chemistry but anti-adenovirus antibodies increased in 54% of the treated patients compared with baseline. AdVEGF-D patients in the highest Lp(a) tertile at baseline showed the best response to therapy (MPR 0.94 ± 0.32 and 1.76 ± 0.41 baseline and 12 months, respectively, P = 0.023).
Conclusion
AdVEGF-DΔNΔC gene therapy was safe, feasible, and well tolerated. Myocardial perfusion increased at 1 year in the treated areas with impaired MPR at baseline. Plasma Lp(a) may be a potential biomarker to identify patients that may have the greatest benefit with this therapy.
Keywords: Gene therapy, Angiogenesis, Lymphangiogenesis, Therapeutic angiogenesis, PET, Safety
Introduction
Angina pectoris is the most common symptom of coronary artery disease (CAD). In spite of improved medical and revascularization therapies, 5–10% of patients undergoing coronary angiography have refractory angina (RA), i.e. they are severely symptomatic while on optimal medical therapy and prior revascularization and not amenable to further revascularization procedures.1,2 In the EU and USA, there are more than 200 000 new RA patients/year.2 Thus, an unmet clinical need exists for new therapies for this group of patients.1,2
Some patients with CAD develop collateral arteries, which can rescue ischaemic myocardium in spite of significant occlusions in coronary arteries and alleviate ischaemic symptoms. Therapeutic vascular growth stimulates this natural process and offers a potential new treatment for RA.3–6 However, most previous cardiovascular proangiogenic trials have been unsuccessful.7–11 This is likely due to (i) poor gene transfer efficiency in the myocardium, (ii) tested growth factors may not have been the most optimal ones, and (iii) inability to target therapy into ischaemic, but viable myocardium.3,4
To address these challenges, we used PET perfusion imaging and an electromechanical catheter system for gene transfer to identify ischaemic, hibernating myocardium with the lowest perfusion reserve for the targeted therapy. For the first time, we also used VEGF-DΔNΔC, a new member of the VEGF family that stimulates both angiogenesis and lymphangiogenesis.12,13 In addition, because Lp(a) is associated with pro-atherogenic, pro-inflammatory, and pro-thrombotic effects, elevated plasma levels were tested as a potential new biomarker to identify patients who might benefit from the induced therapeutic vascular growth.14
Methods
KAT301 is a randomized, blinded, controlled phase I/IIa trial which assessed the safety and feasibility (primary end points) of targeted intramyocardial gene therapy in RA patients using adenoviruses (Ad) expressing human VEGF-DΔNΔC. In addition, we assessed effects on myocardial perfusion reserve (MPR), improvement in symptoms [Canadian Cardiac Society Class (CCS Class)], and quality of life (QoL) at 3 and 12 months (secondary end points). Institutional review board and Finnish authorities approved the protocol. Patients gave written informed consent. Trial design is presented in Supplementary material online. Trial was registered at Clinical Trials Gov NCT01002430 and EudraCT 003295-22.
Patients and endocardial mapping
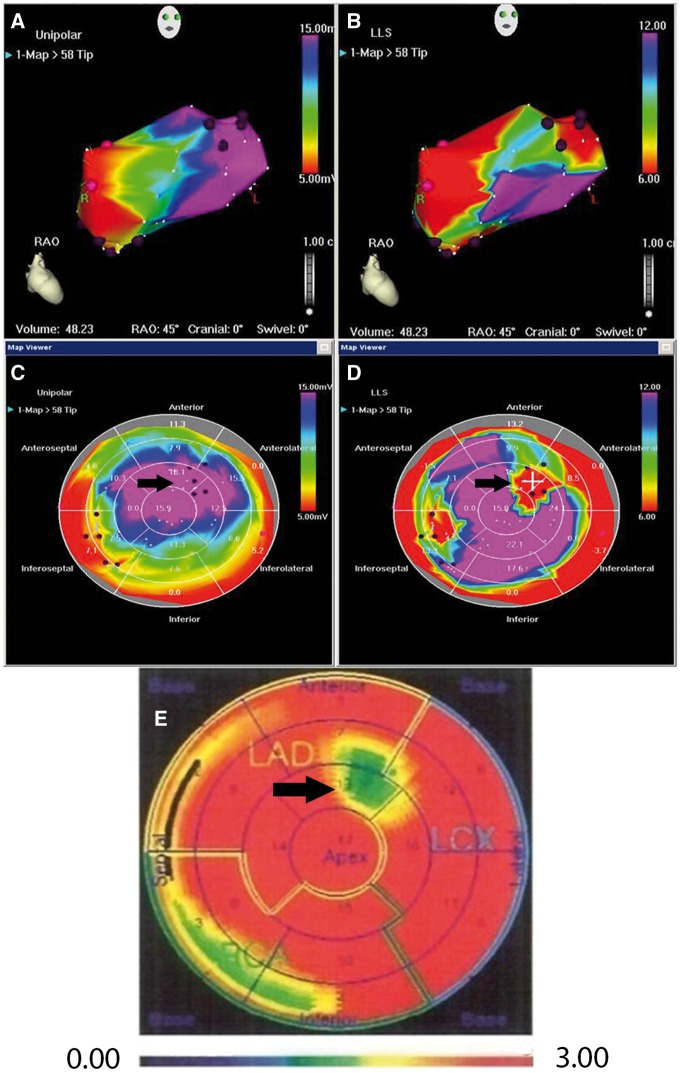
Thirty patients with severe RA were randomized 4:1 to VEGF-DΔNΔC therapy (AdVEGF-D group) and placebo (controls) in blocks of five patients. After transseptal puncture, an 8.5Fr introducer catheter (AgilisTM NxT St Jude Medical, USA) and an electroanatomical mapping and injection catheter (NOGA©, Johnson & Johnson, USA) were introduced into left ventricle. To select optimal sites for gene injections, the left ventricle was mapped to detect areas of viable myocardium with reduced contraction (Figure 1). Coronary angiography and baseline radiowater PET imaging15 were used to confirm viable myocardial segments with impaired MPR (Figure 1).
Figure 1.
NOGA (A–D) and PET radiowater images (E). Panels A–B are three-dimensional NOGA maps of the left ventricle and Panels C–D represent A-B converted to two-dimensional bull’s eye views. Panels A and C show myocardial viability (unipolar voltage maps). Purple colour in A and C indicates normal viability (black arrow). Panels B and D show myocardial contractility (local linear shortening maps). Red colour indicates areas with reduced contractility (black arrow). Panel E shows PET data in bull’s eye view. Black arrow indicates an area with reduced blood flow (green) which is the same area as seen in Panel C with normal viability and in Panel D with poor contractility. This area was treated with gene therapy. Black dots indicate injection sites in C–D. Colour scale in NOGA maps: purple (viable myocardium or normal contractility)-blue/green/yellow (reduced/low viability or contractility)-red (non-viable myocardium or poor contractility); Colour scale in PET map: red (best perfusion)-yellow/green (reduced/low perfusion)-blue (poor/no perfusion).
Gene transfer
Following mapping, the randomization code was opened in hospital pharmacy. NOGA© catheters were used to inject AdVEGF-DΔNΔC to 10 different sites (200 µl each) in the target myocardium. The control group underwent the same mapping procedure. Ten 200 μl injections of 0.9% NaCl were given into the selected sites with the needle withdrawn. Only the operator and hospital pharmacy were open to the randomization code. Other personnel and patients were blinded throughout the study.
Adenoviral vector and VEGF-DΔNΔC
Replication-deficient E1-E3-deleted serotype 5 adenoviruses were produced in 293 cells by FinVector Therapies Oy (Kuopio, Finland).16 AdVEGF-DΔNΔC is an angiogenic and lymphangiogenic growth factor which contains a VEGF homology domain but lacks the N- and C-terminal propeptides.17,18 Details of the biological effects and signalling of AdVEGF-DΔNΔC have been described.12,17,18
Perfusion imaging
Quantitative myocardial perfusion was determined with PET at baseline and at 3 and 12 months.15 A dynamic PET scan was performed (15O-H2O; 900–1100 MBq) at rest and during adenosine stress. Regional myocardial blood flow (MBF) was measured in 17 segments as an average of three repeated analyses (Carimas software 2.5; www.turkupetcentre.net/carimasturku) blinded to the treatment and clinical data.19 Myocardial perfusion reserve was calculated for each segment as the ratio of MBF during adenosine stress and at rest. Two areas of interest were defined: (i) low MPR defined as the myocardial area with the lowest MPR and (ii) reference MPR as the myocardial segment with the highest MPR at baseline.19
Quality of life and angina symptoms
Quality of life was assessed with a standardized 15 dimensions (15D) questionnaire at baseline and 3 months. The single index (15D score) on a 0–1 scale represents overall QoL.20 The maximum score is 1 (no problems on any dimensions) and the minimum is 0. A chance of ≥0.015 in the 15D score is clinically significant.21 CCS Class was evaluated at baseline and during the follow-up using standard methods.
Statistical analysis
Repeated measurements were analysed with linear mixed effect model and posthoc analyses were performed by the least significance method. Mann–Whitney U test was used to compare differences between the groups for continuous variables. Fisher’s exact test was used to calculate dichotomous variables. Results are expressed as means ± standard deviation for continuous variables, and as absolute and relative frequencies for categorical variables. Odds ratios and 95% confidence intervals were used to evaluate associations. Results were considered significant at P < 0.05 (SPSS Statistics version 21.0).
Results
Baseline characteristics and safety
The study groups were well balanced for baseline characteristics (Table 1). No significant differences were found between the AdVEGF-D and the control groups in early procedure-related (<14 days) or long-term (<360 days) adverse events (Table 2). No significant difference was found in MACE between the groups (Table 2).
Table 1.
Baseline characteristics
| Control | AdVEGF-D | P-value | |
|---|---|---|---|
| n = 6 | n = 24 | ||
| Demographics | |||
| Sex (male/female) | 5/1 (83%/17%) | 23/1 (96%/4%) | 0.67 |
| Age (years) | 70 ± 6 | 71 ± 6 | 0.40 |
| CCS-class | 2.67 ± 0.52 | 2.83 ± 0.38 | 0.56 |
| Medical history | |||
| Previous MI | 4 (67) | 17 (71) | 0.60 |
| Previous CABG | 6 (100) | 23 (96) | 0.80 |
| Previous PCI | 3 (50) | 15 (63) | 0.46 |
| Family history of CAD | 5 (83) | 19 (79) | 0.66 |
| Hypertension | 6 (100) | 22 (92) | 0.63 |
| Hypercholesterolaemia | 6 (100) | 23 (96) | 0.80 |
| Smoker (current/ex) | 0/4 (0/67) | 0/17 (0/71) | 0.90 |
| Diabetes | 3 (50) | 12 (50) | 1.00 |
| Drug therapy | |||
| Aspirin | 5 (83) | 22 (92) | 0.51 |
| Clopidogrel | 3 (50) | 12 (50) | 1.00 |
| Warfarin | 2 (33) | 8 (33) | 1.00 |
| B-blockers | 6 (100) | 24 (100) | 1.00 |
| ACEI/ARB | 6 (100) | 21 (88) | 0.49 |
| Statins | 5 (83) | 24 (100) | 0.20 |
| Long-acting nitrates | 5 (83) | 23 (96) | 0.37 |
Mean ± SD or n (%).
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; CAD, coronary artery disease; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation.
Table 2.
Adverse events
| Control | AdVEGF-D | OR (95% CI) | P-value | ||
|---|---|---|---|---|---|
| n = 6 | n = 24 | ||||
| Major complications | |||||
| Death | 0 (0) | 3 (13) | 2.12 (0.10–46.53) | 0.50 | |
| During 14-day follow-up | 0 (0) | 1 (4) | 0.83 (0.03–22.87) | 0.80 | |
| ACS or MI | 1 (17) | 3 (13) | 0.65 (0.06–7.64) | 0.61 | |
| During 14-day follow-up | 0 (0) | 0 (0) | 0.27 (0.00–14.69) | 1.00 | |
| Stroke | 0 (0) | 2 (8) | 1.47 (0.06–34.50) | 0.63 | |
| During 14-day follow-up | 0 (0) | 1 (4) | 0.83 (0.03–22.87) | 0.80 | |
| MACE | 1 (17) | 8 (33) | 2.50 (0.25–25.25) | 0.40 | |
| Other complications | |||||
| Minor bleeding | 2 (33) | 9 (38) | 1.20 (0.18, 7.93) | 0.62 | |
| Procedural complications | 1 (17) | 1 (4) | 0.22 (0.01, 4.09) | 0.37 | |
| New atrial fibrillation | 0 (0) | 1 (4) | 0.83 (0.03, 22.87) | 0.80 | |
| Pericardial effusion | 1 (17) | 7 (29) | 2.06 (0.20, 20.96) | 0.48 | |
| During 14-day follow-up | 0 (0) | 5 (21) | 3.67 (0.18–75.75) | 0.30 | |
Values are n (%). MACE = combined end point of death, ACS, MI, or stroke.
ACS, acute coronary syndrome; CI, confidence interval; MACE, major adverse cardiovascular event; MI, myocardial infarction; OR, odds ratio.
Laboratory analyses and haemodynamics
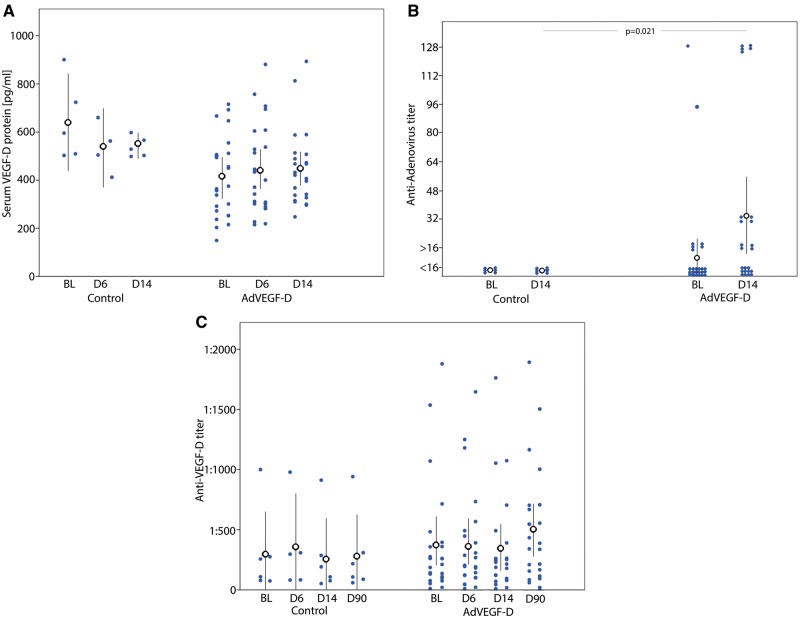
Plasma Tnt increased in both AdVEGF-D and control groups on the first post-operative day (P = 0.001 and 0.004, respectively). Small decreases were also detected in plasma haemoglobin and platelet concentrations on Day 1 in the AdVEGF-D group (P = 0.011 and 0.001, respectively). C-reactive protein increased in the AdVEGF-D group on Day 6 (P < 0.002) (Table 3). No changes were found in plasma VEGF-D protein concentration (Figure 2A) whereas elevated anti-adenovirus antibody titer (≥16) was found in 54% of the AdVEGF-D patients 14 days after the gene transfer (P = 0.021) but in none of the controls (Figure 2B). An elevated anti-VEGF-D antibody titer (increase ≥four-fold) was detected in two AdVEGF-D patients at 3 months but in none of the controls (Figure 2C).
Table 3.
Follow-up measurements
| Baseline |
1 day |
6 days |
14 days |
3 months |
12 months |
P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | AdVEGF-D | Control | AdVEGF-D | Control | AdVEGF-D | Control | AdVEGF-D | Control | AdVEGF-D | Control | AdVEGF-D | ||
| n = 6 | n = 6 | n = 6 | n = 6 | n = 6 | |||||||||
| n = 24 | |||||||||||||
| n = 24 | |||||||||||||
| n = 6 | n = 24 | ||||||||||||
| n = 24 | n = 24 | ||||||||||||
| n = 24 | |||||||||||||
| B-Hb (g/L) | 143 ± 15 | 141 ± 16 | 138 ± 14 | 136 ± 16 | 137 ± 19 | 138 ± 14 | 135 ± 18 | 137 ± 15 | 142 ± 17 | 141 ± 15 | 145 ± 18 | 142 ± 18 | 0.011 |
| B-Leuc (×109/L) | 8.4 ± 2.6 | 6.6 ± 1.4 | 7.8 ± 1.7 | 7.0 ± 1.5 | 7.2 ± 1.5 | 7.6 ± 4.0 | 6.9 ± 1.2 | 6.1 ± 1.1 | 7.9 ± 2.1 | 6.9 ± 1.2 | 8.6 ± 2.8 | 7.0 ± 1.2 | 0.569 |
| B-Thromb (×109/L) | 274 ± 70 | 214 ± 63 | 247 ± 53 | 186 ± 61 | 289 ± 38 | 218 ± 75 | 269 ± 29 | 229 ± 72 | 247 ± 61 | 201 ± 59 | 261 ± 58 | 205 ± 86 | 0.001 |
| P-Alt (U/L) | 34 ± 17 | 32 ± 28 | NA | NA | 39 ± 20 | 30 ± 17 | 31 ± 13 | 30 ± 15 | 32 ± 24 | 29 ± 15 | 34 ± 16 | 26 ± 11 | 0.277 |
| P-CRP (mg/l) | 4 ± 2 | 4 ± 2 | 6 ± 3 | 9 ± 8 | 5 ± 3 | 13 ± 29 | 3 ± 0 | 4 ± 3 | 4 ± 2 | 3 ± 0 | 5 ± 3 | 3 ± 0 | 0.022 |
| P-Tnt (ng/L) | 10 ± 4 | 14 ± 6 | 95 ± 56 | 93 ± 39 | 12 ± 2 | 21 ± 17 | 11 ± 5 | 16 ± 8 | 14 ± 4 | 16 ± 9 | 14 ± 6 | 14 ± 9 | 0.001 |
| Systolic BP (mmHg) | 158 ± 16 | 141 ± 20 | 134 ± 25 | 131 ± 16 | NA | NA | NA | NA | 151 ± 21 | 141 ± 22 | 148 ± 21 | 140 ± 20 | 0.886 |
| Diastolic BP (mmHg) | 88 ± 10 | 80 ± 13 | 74 ± 12 | 71 ± 15 | NA | NA | NA | NA | 83 ± 11 | 76 ± 13 | 82 ± 21 | 76 ± 13 | 0.018 |
| CCS class | 2.67 ± 0.52 | 2.83 ± 0.38 | NA | NA | NA | NA | NA | NA | 2.17 ± 0.75 | 2.43 ± 0.59 | 2.00 ± 0.71 | 2.11 ± 0.47 | 0.001 |
Mean ± SD. P-value for interaction time x group. P-values for all comparisons in Supplementary material online, Table S1.
TnT, Troponine-T, BNP, N-terminal pro-brain natriuretic peptide; CCS, Canadian Cardiovascular Society class.
Figure 2.
Serum VEGF-D protein levels (A), anti-adenoviral titers (B), and anti-VEGF-D titers (C) at baseline (BL) and 6 (D6), 14 (D14) and 90 (D90) days after gene transfer in controls (control) and AdVEGF-DΔNΔC treated patients (AdVEGF-D). Black dots and lines represent mean ± standard deviation. Blue dots represent values of individual patients.
Myocardial perfusion
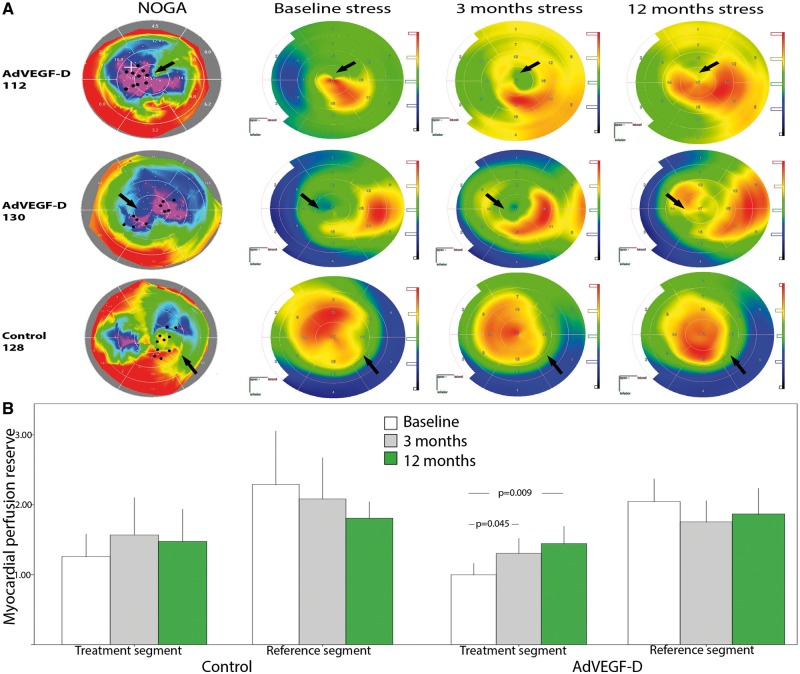
In the AdVEGF-D group, MPR of the treated area increased from 1.00 ± 0.36 at baseline to 1.31 ± 0.46 at 3 months (P = 0.045) and to 1.44 ± 0.48 at 12 months (P = 0.009) (Figure 3AandB). Myocardial perfusion reserve of the reference area (myocardium with the highest MPR at baseline) showed no significant change (2.05 ± 0.69, 1.76 ± 0.62, and 1.87 ± 0.69 at baseline, 3, and 12 months, respectively, P = 0.551). On the contrary, it tended to decrease by 10.7% and 8.8%, respectively (Figure 3B). Myocardial perfusion reserve in the control group showed no significant change from baseline to 3 and 12 months (1.26 ± 0.37, 1.57 ± 0.55, and 1.48 ± 0.48; respectively, P = 0.690) (Figure 3B).
Figure 3.
(A) Representative images of combined NOGA and stress PET radiowater images of two AdVEGF-D treated patients and one control patient. Black dots and arrows indicate sites for gene injections in viable but poorly perfused myocardium. Myocardial blood flow improved in the AdVEGF-D patients visualized as increases in red colour during the follow-up. Perfusion did not increase in the control patient. (B) Myocardial perfusion reserve in the treated and reference segments of the control and AdVEGF-DΔNΔC-treated patients. Colour scales in NOGA and PET maps as in Figure 1. Values are mean ± standard deviation.
No significant changes were found in the MPR of the reference area at baseline (2.29 ± 0.94), 3 months (2.08 ± 0.61, P = 0.550), or 12 months (1.81 ± 0.25, P = 0.647). In an exploratory analysis, AdVEGF-D patients belonging to the highest baseline Lp(a) tertile had the best response to the therapy (MPR 0.94 ± 0.32 and 1.76 ± 0.41, baseline and 12 months, respectively, P = 0.023, Table 4).
Table 4.
Myocardial perfusion reserve and Lp(a) levels of the AdVEGF-DdNdC treated patients
| Baseline | 3 months | 12 months | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lp (a) tertiles | 3rd | 2nd | 1st | 3rd | 2nd | 1st | 3rd | 2nd | 1st | |
| n = 5 | n = 5 | n = 5 | n = 5 | n = 5 | n = 4 | n = 5 | n = 5 | n = 5 | ||
| Lp(a) mg/dL | 52.7 ± 13.3 | 11.8 ± 6.4 | 3.8 ± 2.0 | 53.9 ± 20.5 | 11.0 ± 5.0 | 3.2 ± 1.3 | 59.1 ± 19.6 | 13.8 ± 4.1 | 3.3 ± 0.7 | 0.023 |
| Treated area MPR | 0.94 ± 0.32 | 1.16 ± 0.45 | 1.03 ± 0.34 | 1.43 ± 0.45 | 1.39 ± 0.57 | 1.17 ± 0.38 | 1.76 ± 0.41 | 1.42 ± 0.53 | 1.22 ± 0. | 0.089 |
Mean ± SD. Tertiles refer to Lp(a) at baseline. P-value for interaction time x tertiles. P-values for all comparisons in Supplementary material online, Table S2.
MPR, myocardial perfusion reserve; SD, standard deviation; Lp(a), lipoprotein(a).
Angina pectoris
There was a significant improvement in angina pectoris symptoms (CCS class) in the AdVEGF-D group at 12 months (2.83 ± 0.38 vs. 2.11 ± 0.47, P = 0.001) (Table 3). CCS class tended to improve also in the controls but it did not reach statistical significance (2.67 ± 0.52 vs. 2.00 ± 0.71, P = 0.279).
Quality of life
In the AdVEGF-D group, the 15D analysis score increased from 0.787 ± 0.108 at baseline to 0.803 ± 0.101 at 3 months. Thus, a clinically meaningful change (change ≥ +0.015) in the mean 15D score was observed at 3 months (+0.016). 15D scores in the controls (baseline 0.788 ± 0.129 and 3 months 0.790 ± 0.123) showed no statistical or clinically meaningful changes.
Discussion
Refractory angina refers to patients with CAD who suffer from chest pain and disability despite optimal medical and revascularization therapy and who are not eligible for additional coronary interventions.1,2 Therapeutic vascular growth (i.e. stimulation of both angiogenesis and lymphangiogenesis) is a new concept for the treatment of RA.4 The main finding in our study was that intramyocardial AdVEGF-DΔNΔC gene therapy was safe, feasible, and well tolerated. However, it is an invasive procedure and resulted in transient increases in plasma troponin, which were most likely due to the transseptal approach, mapping of the left ventricle, and intramyocardial injections. An increase in anti-adenovirus antibodies in 54% of the treated patients was an expected finding6 with no clinical consequences. However, if readministration of the AdVEGF-DΔNΔC would be required, the increased antibody levels might reduce the efficacy of the second administration. A modest decrease in diastolic blood pressure in the AdVEGF-D group on the first post-operative day was likely caused by an increased NO production induced by VEGF-DΔNΔC.12 A potential impact of elevated Lp(a) was also noted in the response of the RA patients to this therapy, with the most benefit in patients with the highest Lp(a) levels. This is consistent with a recent report that 50% of patients with RA have elevated Lp(a) (>50 mg/dL), and in whom Lp(a) lowering achieved by lipid apheresis was associated with objective evidence of myocardial blood flow improvement by MRI and significant relief of RA symptoms.22
There were no significant differences between the AdVEGF-D and control groups with respect to major complications. However, because of the small number of patients, these results should be interpreted cautiously. Three AdVEGF-D patients died during the follow-up. One death was due to myocardial infarction (MI) and two were sudden deaths. Patients suffering from sudden death underwent 24 h electrocardiogram recordings without any major arrhythmias (data not shown). In the AdVEGF-D group, three patients and one patient in the control group developed acute coronary syndrome (ACS) or MI during the follow-up. Coronary angiogram was available from three of these patients and in all of them a new stenosis in vein graft was responsible for the event. Thus, they were unlikely to be related to gene therapy. Mild pericardial effusion was found in 29% of the AdVEGF-D patients and 17% of the controls. VEGF-DdNdC is known to increase not only perfusion but also vascular permeability, albeit much less than VEGF-A.12,18,23 Thus, it is likely that pericardial effusion in the gene-treated patients mirrors the biological effect of VEGF-DΔNΔC. However, in all cases the mild effusions recovered spontaneously without sequelae and required no therapy.
Although the primary target of our study was the safety and feasibility it is of interest that myocardial perfusion increased in the treated myocardial segments at 3 and 12 months. To our knowledge, this is the first time that treatment aimed to cause vascular growth shows an objective long-term improvement in myocardial perfusion in the treated areas. Myocardial perfusion in the reference segments of the same patients as well as in the control group tended to decrease, which suggests progression of the underlying CAD.
In comparison to earlier studies,7–11 an important advancement in this study was that we selected VEGF-DΔNΔC as the therapeutic gene. It has several advantages over the previously used growth factors, such as VEGF-A: (i) AdVEGF-DΔNΔC has slower but more long-lasting signalling kinetics than VEGF-A through VEGF Receptor-2 thus providing a more sustained angiogenic stimulus12; (ii) It does not bind to matrix proteoglycans and diffuses better than VEGF-A in the transduced tissues12,13; (iii) AdVEGF-DΔNΔC binds to Neuropilin-1 and -2 which strengthens angiogenic responses12; (iv) AdVEGF-DΔNΔC stimulates lymphatic vessel growth via VEGF Receptor-3 which improves fluid drainage from the treated myocardium23; (v) AdVEGF-DΔNΔC induces less changes in vascular permeability than VEGF-A and reduces the risk of pericardial fluid accumulation23; (vi) AdVEGF-DΔNΔC does not bind to VEGF Receptor-1 on monocytes and therefore does not directly stimulate inflammatory mechanisms as compared with VEGF-A24; and (vii) Unlike some other VEGFs, AdVEGF-DΔNΔC does not induce ventricular arrhythmias in the compromised heart muscle.24
Another advancement in our study was that we used combined electromechanical mapping and PET perfusion imaging for the selection of the treatment area (Figure 1) as well as for the assessment of changes in MBF during the follow-up (Figure 3). SPECT has been used in previous gene therapy trials7,10,11 but it can only be analysed semiquantitatively using differences in relative counts between rest and stress as compared with areas with normal perfusion.25 Therefore, SPECT has limited sensitivity for assessing regional perfusion in patients with severe CAD and cannot be used to assess quantitative changes in MPR. PET permits the measurement of absolute myocardial blood flow and MPR from rest to stress states, providing an objective, validated surrogate end point.26 Reproducibility of quantitative MBF and MPR measurements are sufficient for the detection of significant changes (coefficient of variation 15%).26
Lipoprotein(a) is a risk factor for MI, stroke, and peripheral arterial disease.14,27 Since Lp(a) has strong prothrombotic and antiangiogenic activity, we hypothesized that Lp(a) could be used as a biomarker to identify patients who might benefit from gene therapy. AdVEGF-D patients in the highest baseline Lp(a) tertile had a significant improvement in MPR as compared with those in the lowest Lp(a) tertile at 12 months. If confirmed, Lp(a) could be used to identify patients who might optimally benefit from AdVEGF-D gene therapy. Lp(a) and oxidized phospholipids they carry are abundantly present in vulnerable plaques, in debris from distal protection devices and in chronic total occlusions, plaque phenotypes that often lead to MI and RA.27,28 Lipoprotein(a) is also highly pro-inflammatory and mediates secretion of cytokines from monocytes that promotes arterial inflammation.
We found a significant improvement in angina pectoris symptoms (CCS class) and a clinically important improvement in QoL (change ≥ +0.015 in the mean 15D score) in the AdVEGF-D patients. A similar trend in symptoms was also found in the controls. As the controls underwent the same intracardiac mapping procedure, we cannot exclude a possible placebo effect, which has been found in previous gene therapy trials.3,4 Establishing clinical significance of the CCS and QoL findings requires further studies.
Randomized controls were catheterized and mapped exactly in the same way as the AdVEGF-D patients but 0.9% NaCl placebo solution was injected without pushing the needle out from the catheter. Only the operator and hospital pharmacy providing AdVEGF-D or placebo solutions were aware of the treatment. Other study personnel responsible for the patient care, data analysis and follow-up were blinded to the treatment. To maintain blinding, all antibody and VEGF-DΔNΔC measurements were performed only at the end of the study. Other options for the placebo administration could have been the use of an empty adenovirus and/or intramyocardial injections of placebo. However, these were considered unethical by the institutional review board.
Limitations of this study include the small number of patients, which precludes any firm conclusions about safety and efficacy. A lack of statistical significance does not necessarily confirm the lack of difference. Particularly this is true for clinical end points. Thus, potential rare complications of the AdVEGF-DΔNΔC gene therapy procedure may not have been detected.
In conclusion, NOGA catheter-mediated intramyocardial delivery of AdVEGF-DΔNΔC was safe and well tolerated and may offer a new option for the treatment of RA. To our knowledge, this is the first study demonstrating a significant improvement in quantitative myocardial blood flow after local gene therapy in the treated areas with impaired perfusion reserve. Elevated plasma Lp(a) may be used as a biomarker to identify patients who could benefit from the AdVEGF-DΔNΔC gene therapy. Phase IIb/III trials are needed to confirm the safety and efficacy of gene therapy in RA patients.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
FinVector Therapies Oy is acknowledged for GMP manufacturing of AdVEGF-DΔNΔC. We thank Dr Hanspeter Fischer (Biologics Delivery Systems, Johnson&Johnson, USA) for the generation of 2D NOGA maps, EU EATRIS infrastructures in Kuopio and Turku for participating in this study, and Ms Marjo-Riitta Salminkoski for preparing the article.
Funding
This study was supported by Kuopio and Turku University Hospitals.
Conflict of interest: S.T. and J.L.W. have patents on the use of oxidation specific antibodies that are held by USCD.
References
- 1. Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, Follath F, Hellemans I, Herlitz J, Lüscher T, Pasic M, Thelle D.. The problem of chronic refractory angina: report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J 2002;23:355–370. [DOI] [PubMed] [Google Scholar]
- 2. Henry TD, Satran D, Jolicoeur EM.. Treatment of refractory angina in patients not suitable for revascularization. Nat Rev Cardiol 2014;11:78–95. [DOI] [PubMed] [Google Scholar]
- 3. Ylä-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J.. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol 2007;49:1015–1026. [DOI] [PubMed] [Google Scholar]
- 4. Yla-Herttuala S, Bridges C, Katz MG, Korpisalo P.. Angiogenic gene therapy in cardiovascular diseases: dream or vision? Eur Heart J 2017;38:1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooke JP, Losordo DW.. Modulating the vascular response to limb ischemia: angiogenic and cell therapies. Circ Res 2015;116:1561–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hedman M, Hartikainen J, Syvänne M, Stjernvall J, Hedman A, Kivelä A, Vanninen E, Mussalo H, Kauppila E, Simula S, Närvänen O, Rantala A, Peuhkurinen K, Nieminen MS, Laakso M, Ylä-Herttuala S.. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation 2003;107:2677–2683. [DOI] [PubMed] [Google Scholar]
- 7. Kastrup J, Jørgensen E, Rück A, Tägil K, Glogar D, Ruzyllo W, Bøtker HE, Dudek D, Drvota V, Hesse B, Thuesen L, Blomberg P, Gyöngyösi M, Sylvén C; Euroinject One Group. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol 2005;45:982–988. [DOI] [PubMed] [Google Scholar]
- 8. Kastrup J, Jørgensen E, Fuchs S, Nikol S, Bøtker HE, Gyöngyösi M, Glogar D, Kornowski R.. A randomised, double-blind, placebo-controlled, multicentre study of the safety and efficacy of BIOBYPASS (AdGVVEGF121.10NH) gene therapy in patients with refractory advanced coronary artery disease: the NOVA trial. EuroIntervention 2011;6:813–818. [DOI] [PubMed] [Google Scholar]
- 9. Stewart DJ, Hilton JD, Arnold JM, Gregoire J, Rivard A, Archer SL, Charbonneau F, Cohen E, Curtis M, Buller CE, Mendelsohn FO, Dib N, Page P, Ducas J, Plante S, Sullivan J, Macko J, Rasmussen C, Kessler PD, Rasmussen HS.. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment. Gene Ther 2006;13:1503–1511. [DOI] [PubMed] [Google Scholar]
- 10. Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, Della Siega A, Bilodeau L, Burton JR, Proulx G, Radhakrishnan S; NORTHERN Trial Investigators. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther 2009;17:1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henry TD, Grines CL, Watkins MW, Dib N, Barbeau G, Moreadith R, Andrasfay T, Engler RL.. Effects of Ad5FGF-4 in patients with angina. an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol 2007;50:1038–1046. [DOI] [PubMed] [Google Scholar]
- 12. Jauhiainen S, Häkkinen SK, Toivanen PI, Heinonen SE, Jyrkkänen HK, Kansanen E, Leinonen H, Levonen AL, Ylä-Herttuala S.. Vascular endothelial growth factor (VEGF)-D stimulates VEGF-A, stanniocalcin-1, and neuropilin-2 and has potent angiogenic effects. Arterioscler Thromb Vasc Biol 2011;31:1617–1624. [DOI] [PubMed] [Google Scholar]
- 13. Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholová I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Ylä-Herttuala S.. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res 2003;92:1098–1106. [DOI] [PubMed] [Google Scholar]
- 14. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol 2017;69:692–711. [DOI] [PubMed] [Google Scholar]
- 15. Harms HJ, Nesterov SV, Han C, Danad I, Leonora R, Raijmakers PG, Lammertsma AA, Knuuti J, Knaapen P.. Clinical value of absolute quantification of myocardial perfusion with 15O-water in coronary artery disease. Circ Cardiovasc Imaging 2011;4:678–684. [DOI] [PubMed] [Google Scholar]
- 16. Westphal M, Ylä-Herttuala S, Martin J, Warnke P, Menei P, Eckland D, Kinley J, Kay R, Ram Z; ASPECT Study Group. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol 2013;14:823–833. [DOI] [PubMed] [Google Scholar]
- 17. Nieminen T, Toivanen PI, Rintanen N, Heikura T, Jauhiainen S, Airenne KJ, Alitalo K, Marjomäki V, Ylä-Herttuala S.. The impact of the receptor binding profiles of the vascular endothelial growth factors on their angiogenic features. Biochim Biophys Acta 2014;1840:454–463. [DOI] [PubMed] [Google Scholar]
- 18. Toivanen PI, Nieminen T, Viitanen L, Alitalo A, Roschier M, Jauhiainen S, Markkanen JE, Laitinen OH, Airenne TT, Salminen TA, Johnson MS, Airenne KJ, Ylä-Herttuala S.. Novel vascular endothelial growth factor D variants with increased biological activity. J Biol Chem 2009;284:16037–16048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nesterov SV, Han C, Mäki M, Kajander S, Naum AG, Helenius H, Lisinen I, Ukkonen H, Pietilä M, Joutsiniemi E, Knuuti J.. Myocardial perfusion quantitation with 15O-labelled water PET: high reproducibility of the new cardiac analysis software (Carimas). Eur J Nucl Med Mol Imaging 2009;36:1594–1602. [DOI] [PubMed] [Google Scholar]
- 20. Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med 2011;33:328–336. [DOI] [PubMed] [Google Scholar]
- 21. Alanne S, Roine RP, Räsänen P, Vainiola T, Sintonen H.. Estimating the minimum important change in the 15D scores. Qual Life Res 2015;24:599–606. [DOI] [PubMed] [Google Scholar]
- 22. Khan TZ, Hsu LY, Arai AE, Rhodes S., Pottle A., Wage R., Banya W., Gatehouse PD., Giri S., Collins P., Pennell DJ., Barbir M.. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross-over trial. Eur Heart J 2017;38:1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rutanen J, Rissanen TT, Markkanen JE, Gruchala M, Silvennoinen P, Kivelä A, Hedman A, Hedman M, Heikura T, Ordén MR, Stacker SA, Achen MG, Hartikainen J, Ylä-Herttuala S.. Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in porcine heart. Circulation 2004;109:1029–1035. [DOI] [PubMed] [Google Scholar]
- 24. Nurro J, Halonen PJ, Kuivanen A, Tarkia M, Saraste A, Honkonen K, Lähteenvuo J, Rissanen TT, Knuuti J, Ylä-Herttuala S.. AdVEGF-B186 and AdVEGF-DΔNΔC induce angiogenesis and increase perfusion in porcine myocardium. Heart 2016;102:1716–1720. [DOI] [PubMed] [Google Scholar]
- 25. Berman DS, Kang X, Slomka PJ, Gerlach J, de Yang L, Hayes SW, Friedman JD, Thomson LE, Germano G.. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol 2007;14:521–528. [DOI] [PubMed] [Google Scholar]
- 26. Saraste A, Ukkonen H, Varis A, Vasankari T, Tunturi S, Taittonen M, Rautakorpi P, Luotolahti M, Airaksinen KE, Knuuti J.. Effect of spinal cord stimulation on myocardial perfusion reserve in patients with refractory angina pectoris. Eur Heart J Cardiovasc Imaging 2015;16:449–455. [DOI] [PubMed] [Google Scholar]
- 27. Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB.. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med 2005;353:46–57. [DOI] [PubMed] [Google Scholar]
- 28. Ravandi A, Leibundgut G, Hung MY, Patel M, Hutchins PM, Murphy RC, Prasad A, Mahmud E, Miller YI, Dennis EA, Witztum JL, Tsimikas S.. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J Am Coll Cardiol 2014;63:1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.