Abstract
Aims
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is characterized by fibrofatty infiltration of the myocardium and ventricular arrhythmias that may lead to sudden cardiac death. It has been observed that male patients develop the disease earlier and present with more severe phenotypes as compared to females. Thus, we hypothesized that serum levels of sex hormones may contribute to major arrhythmic cardiovascular events (MACE) in patients with ARVC/D.
Methods and results
The serum levels of five sex hormones, sex hormone-binding globulin, high sensitivity troponin T, pro-brain natriuretic peptide, cholesterol, triglycerides, insulin, and glucose were measured in 54 ARVC/D patients (72% male). Twenty-six patients (48%) experienced MACE. Total and free testosterone levels were significantly increased in males with MACE as compared to males with a favourable outcome, whereas estradiol was significantly lower in females with MACE as compared to females with a favourable outcome. Increased testosterone levels remained independently associated with MACE in males after adjusting for age, body mass index, Task Force criteria, ventricular function, and desmosomal mutation status. Furthermore, an induced pluripotent stem cell-derived ARVC/D cardiomyocyte model was used to investigate the effects of sex hormones. In this model, testosterone worsened and estradiol improved ARVC/D-related pathologies such as cardiomyocyte apoptosis and lipogenesis, strongly supporting our clinical findings.
Conclusions
Elevated serum testosterone levels in males and decreased estradiol levels in females are independently associated with MACE in ARVC/D, and directly influence disease pathology. Therefore, determining the levels of sex hormones may be useful for risk stratification and may open a new window for preventive interventions.
Keywords: Right ventricular cardiomyopathy , Dysplasia , Arrhythmic outcome , Sex hormones , Risk stratification
Introduction
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is a hereditary myocardial disorder leading to ventricular arrhythmias and an increased risk of sudden cardiac death (SCD), especially in the young athletes.1 It is characterized by progressive fibrofatty replacement of cardiomyocytes (CMs) with increased CM apoptosis, primarily in the right ventricle (RV).2–4 Mutations mostly occur in genes encoding for proteins of the desmosome.1,5
Several prognostic parameters have been associated with adverse outcomes in this entity, but risk stratification in patients with ARVC/D needs further improvement.6–9 It has been shown that increased exercise levels may raise the risk of ventricular arrhythmias in this disease.10,11 Furthermore, gender has been suggested to play an important role. Male patients with ARVC/D have been reported to develop life-threatening ventricular arrhythmias at an earlier age as compared to females, although the disease is usually transmitted as an autosomal dominant trait.4,9,12,13 Thus, sex hormones have been suggested to influence the onset and progression of ARVC/D, but this hypothesis has not been thoroughly investigated.
In an in-vitro ARVC/D model using patient-specific induced pluripotent stem cell-derived CMs (iPSC-CMs), metabolic deregulations due to abnormal activation of peroxisome proliferator-activated receptor-gamma (PPARγ), leading to exaggerated fatty acid oxidation (FAO) and lipogenesis, have been suggested to be main pathogenic mechanisms.3 Inhibition of abnormal PPARγ activation in ARVC/D CMs decreased CM apoptosis and lipogenesis. Other invitro studies suggested that a defective canonical Wnt signalling pathway may enhance adipogenesis driven by PPARγ and also influence protein expression and distribution, especially at the intercalated disc. These mechanisms may exacerbate lipogenesis and apoptosis in ARVC/D CMs.14
Sex hormones are known to regulate metabolic homeostasis of various cell types,15 and therefore may influence ARVC/D disease phenotypes. Testosterone as the principle male sex hormone plays an important role in cell growth. Similar to most sex hormones, it is bound to sex hormone-binding globulin (SHBG). In males, testosterone regulates adipogenesis and lipogenesis of fat cells, and improves insulin sensitivity and muscle mass of skeletal muscles.16 Testosterone has been associated with a higher risk in cardiovascular diseases.17 Furthermore, testosterone has been shown to increase FAO and enhance de novo lipogenesis.18 These testosterone effects in increasing lipid utilization and synthesis, if occurring in CMs, could potentially worsen ARVC/D cardiac phenotypes. Additionally, long-term use of exogenous androgens can cause pathological cardiac hypertrophy, dyslipidemia, myocardial oxidative stress and apoptosis.19 Therefore, the effects of testosterone on ARVC/D phenotypes remain to be investigated. For female hormones, estradiol is the primary female estrogen. It has been shown that estradiol may exert a protective effect on cardiovascular diseases in men and women.20
In terms of cardiac arrhythmias and gender differences, males are at greater risk for atrial fibrillation, ventricular arrhythmias in Brugada syndrome, and SCD after myocardial infarction.12,21 Females have longer QT intervals with a higher risk of drug-induced torsades de pointes as compared to males. Estrogens suppress ischaemia-related arrhythmias and have been associated with fewer idiopathic ventricular arrhythmias originating from the RV outflow tract.21–23 The aim of this study was to investigate the influence of sex hormones on the arrhythmic outcome in patients with ARVC/D and on the pathophysiology of this disease.
Methods
Study population
For this study, a total of 54 patients with ARVC/D according to the 2010 Revised Task Force Criteria with a task force score (TFS) ≥3 were prospectively enrolled from the Zurich ARVC Program (http://www.arvc.ch; 18 January 2017). The TFS was calculated as two points for a major disease criterion and one point for a minor disease criterion.24 Only patients in whom prospective blood withdrawal, physical examination, and transthoracic echocardiography (TTE) were performed were included in this study. The diagnosis of ARVC/D was made at time of blood withdrawal. One patient had heart transplantation during the study period and was excluded. The median study period was 1.1 years (IQR 1.0–1.2 years) in order to minimize hormone variations during the long term. All patients signed an informed consent prior to study inclusion, which was approved by the Ethics Committee of the Canton of Zurich (approval number KEK-ZH-Nr. 2014-0443).
Measurement of serum hormone and biomarker levels
Venous blood samples were collected in standard blood collection tubes containing heparin (Vacutainer™, Beckton Dickinson, Franklin Lakes, NJ, USA) between 8.00 and 11.00 a.m. After blood collection, immediate centrifugation was carried out for 10 min at 2700 g at room temperature. Supernatant was aspirated, centrifuged, aliquoted into cryovials, and stored at –80 °C until assayed. Estradiol, testosterone, progesterone, pro-brain natriuretic peptide (pro-BNP), and high sensitive cardiac troponin T (hs-c TnT) were measured by using electro-chemiluminescence immunoassays and the Cobas 8000 from Roche Diagnostics, (Rotkreuz, Switzerland). Dehydroepiandrosterone-sulfate (DHEA-S), androstenedione, sex hormone-binding globuline (SHBG), and insulin were measured by using chemiluminescent immunoassays on the Immulite 20000XPi from Siemens Healthcare (Zurich, Switzerland). Glucose, triglycerides, total cholesterol, and high-density lipoprotein (HDL) cholesterol were measured using photometric enzymatic tests and the Cobas 8000 from Roche Diagnostics. Free and bioavailable testosterone levels were calculated using the Vermeulen’s method.25 All tests were performed according to the manufacturers’ protocols.
Assessment of clinical data, exercise levels, and genetic mutation status
Transthoracic echocardiography (TTE) was performed at the time of blood withdrawal to determine left (LV) and RV function. Severe RV dysfunction was defined as a RV fractional area change (FAC) below 23%. LV involvement was considered to be present when LV ejection fraction (EF) was <50%, or regional LV wall motion abnormalities were visible, as previously described.6 The individual level and type of physical exercise were assessed during patient interviews based on a prespecified questionnaire. Genetic analysis was performed as previously described (see Supplementary methods for details).26,27
Outcome parameters
A major arrhythmic cardiovascular event (MACE) after study inclusion was defined as SCD, survived SCD, ventricular fibrillation (VF), sustained ventricular tachycardia (VT), or arrhythmic syncope. These data were obtained from clinical reports, during clinical visits, and telephone interviews. Serum hormone and biomarker levels, exercise levels, and other clinical data were compared between patients with MACE and those with a favourable outcome.
In vitro model of ARVC/D using patient-specific iPSC-CMs
A previously established invitro ARVC/D model was used to study the direct effects of testosterone and estradiol on ARVC/D CMs.3 Briefly, using Yamanaka’s 4 pluripotent factors, we have established iPSC lines from fibroblasts of an ARVC/D patient (JK#11 iPSC line) with homozygous c.2484C > T mutations in plakophilin-2 (PKP2), leading to frame-shifted C-terminals that failed to anchor plakoglobin (JUP) to the sarcolemmal membrane. We first induced cardiac differentiation of ARVC/D iPSCs using standard cardiogenic media to form iPSC-CM beating clusters for 30 days.3 We then used a newly developed three factor [3F = 0.5 µM dexamethasone, 1 µg/mL insulin and 0.25 mM IBMX (3-isobutyl-1-methylxanthine)] protocol to induce adult-like, PPARα-dependent, FAO-dominant metabolic maturation of primitive iPSC-CMs in culture for 21 days. Finally, we added 5 µM rosiglitazone and 200 µM indomethacin to the 3F (termed the 5F protocol) for 2 weeks to activate PPARγ activities and subsequent ARVC/D pathologies (apoptosis and lipid accumulation in CMs). To test if sex hormones affect ARVC/D pathologies, 5 ng/mL testosterone or 50 pg/mL estradiol was added to the 5F protocol to determine how each individual hormone affects the survival and lipid accumulation of ARVC/D iPSC-CMs (see Ref. 3 for detailed protocols).
Statistical analysis
Continuous variables are presented as median with interquartile ranges (IQR). Categorical variables are reported as frequency (percentage). Comparisons between patients with MACE and those without were performed by the Mann-Whitney U-test and by Fisher’s exact test, as appropriate. Patients were grouped on the binary dependent variable (MACE yes/no) since the study design was that of a case (MACE yes)–control (MACE no) study. To compare more than two variables, ANOVA and the Kruskal-Wallis test with post-hoc-analysis were performed. A paired Student’s t-test was used to compare means of variables. Furthermore, odds ratios (OR) with 95% confidence intervals (CI) were calculated using binary logistic regression to provide a measure of effect. We performed bivariable and multivariable analyses for age, gender, and other important clinical covariates in order to adjust for confounders. To avoid overfitting, we restricted our multivariable models to a maximum of three variables. ROC analysis was performed using Youdeńs index to determine the cut-off levels with optimal sensitivity and specificity. All statistical analyses were performed using SPSS Statistics version 23 (IBM Switzerland, Zurich, Switzerland) and Prism version 5 software (GraphPad Software Inc., La Jolla, California, USA).
Results
Baseline characteristics and association with clinical outcome
Clinical data and serum levels of sex hormones and biomarkers are presented in Table 1. All patients had a TFS ≥3.24 The median time between blood withdrawal and MACE was 146 days (IQR 88–218 days). Twenty-six patients (48%) experienced a MACE during a median study period of 1.1 years (IQR 1.0–1.2 years) (Table 2). No patient died during follow-up. There were no significant differences in age, blood pressure, body mass index and prevalence of diabetes and physical activity between patients with and without MACE. Patients with MACE had significantly higher TFS as compared to those without. RV-FAC was significantly lower and there was a trend towards lower LVEF in patients with MACE. Glucose and lipid levels were not different between the two groups. Hs-c TnT and pro-BNP were both increased in patients with MACE. As expected, sex hormone levels showed a great range, mostly due to gender differences. Nevertheless, total serum testosterone was significantly higher, and DHEA-S was significantly lower in patients with MACE as compared to those without. We also investigated a potential association of desmosomal mutation status and MACE, but did not observe any statistical differences between desmosomal mutation carriers and the remaining patients (Table 1).
Table 1.
Baseline characteristics of the whole ARVC/D cohort (n = 54) and association with adverse arrhythmic outcomes
| Outcome |
||||
|---|---|---|---|---|
| Variables | All patients n = 54 | Favourable n = 28 | Adverse n = 26 | P-value |
| Men, n (%) | 39 (72) | 20 (71) | 19 (73) | 1 |
| Age (years) | 50 (42–62) | 46 (39–62) | 52 (46–60) | 0 .3 |
| Systolic blood pressure (mmHg) | 122 (110–130) | 119 (110–130) | 124 (110–132) | 0 .77 |
| Diastolic blood pressure (mmHg) | 78 (70–81) | 79 (70–84) | 75 (70–81) | 0 .34 |
| BMI (kg/m2) | 25 (23–28) | 26 (23–30) | 23 (22–27) | 0 .39 |
| ARVC/D Task force score | 5 (3–6) | 4 (3–5) | 6 (5–7) | 0 .0001 |
| LVEF (%) | 57 (50–63) | 61 (52–65) | 56 (46–62) | 0 .07 |
| RV FAC (%) | 28 (21–36) | 32 (25–42) | 24 (20–30) | 0 .01 |
| Physical activity | ||||
| Sedentary, n (%) | 14 (26) | 10 (36) | 4 (15) | 0 .13 |
| Recreational sports, n (%) | 30 (56) | 15 (53) | 15 (58) | 0 .80 |
| Competitive sports, n (%) | 10 (18) | 3 (11) | 7 (27) | 0 .17 |
| Total testosterone (nmol/l) | 10 .4 (0 .6–16.9) | 7.9 (0 .6–12.0) | 15.1 (0 .5–19.5) | 0 .01 |
| Estradiol (pmol/l) | 95.0 (56.5–123.3) | 90 .5 (63.0–155.0) | 101.0 (19.5–112.6) | 0 .42 |
| Progesterone (nmol/l) | 1.1 (0 .9–2.0) | 1.5 (1.0–2.4) | 1.1 (0 .9–1.6) | 0 .23 |
| SHBG (nmol/l) | 40 .0 (31.0–66.6) | 34.4 (27.9–55.6) | 51.3 (35.5–72.2) | 0 .08 |
| DHEA-S (mmol/l) | 3.5 (1.6–6.0) | 4.4 (1.9–7.2) | 3.1 (1.4–4.0) | 0 .04 |
| Androstenedione (nmol/l) | 4.5 (2.8–6.1) | 5.0 (2.9–7.0) | 4.0 (3.0–5.7) | 0 .16 |
| hs-c TnT (ng/l) | 8 (5–17) | 5 (5–12) | 10 (6–22) | 0 .01 |
| pro-BNP (ng/l) | 137 (69–504) | 106 (48–268) | 185 (108–935) | 0 .04 |
| Glucose (mmol/l) | 5.1 (4.8–5.5) | 5.1 (4.7–5.5) | 5.2 (4.9–5.7) | 0 .29 |
| Insulin (mIU/l) | 8.5 (4.8 (18.5) | 8.0 (4.7–16.0) | 9.5 (5.0–21) | 0 .56 |
| Diabetes, n (%) | 9 (17) | 5 (18) | 4 (15) | 1 .00 |
| Total cholesterol (mmol/l) | 5.0 (4.3–5.5) | 5. 1 (4.5–5.8) | 4.7 (4.3–5.4) | 0 .17 |
| HDL cholesterol (mmol/l) | 1.3 (1.1–1.6) | 1.3 (1.1–1.5) | 1.4 (1.0–1.7) | 0 .73 |
| Triglycerides (mmol/l) | 1.1 (1.0 1.9) | 1.2 (1.0–2.0) | 1.1 (0 .8–2.0) | 0 .32 |
| All patients n = 48 | Favourable n = 23 | Adverse n = 25 | ||
| Desmosomal mutations n (%) | 24 | 9 (39) | 15 (60) | 0 .2 |
Data are presented as median (IQR); Mann-Whitney U test was used for comparison of continuous variables, and Fisher’s exact test for categorical variables. P-values < 0 .05 were considered significant. BMI, body mass index; DHEA-S, dehydroepiandrosterone-sulfate; HDL, high-density lipoprotein; hs-c TnT, high sensitive cardiac troponin T; LVEF, left ventricular ejection fraction; pro-BNP, pro brain natriuretic peptide; RV FAC, right ventricular fractional area change; SHBG, sex hormone-binding globulin; Task Force score according to 2010 ARVC/D Task Force Criteria.24
Table 2.
Specification of MACE
| MACE | n = 26 |
|---|---|
| Survived sudden cardiac arrest, n (%) | 1 (4) |
| Ventricular fibrillation, n (%) | 4 (15) |
| Sustained ventricular tachycardia, n (%) | 16 (62) |
| Arrhythmic syncope, n (%) | 5 (19) |
MACE was defined as sudden cardiac death, survived sudden cardiac arrest, ventricular fibrillation, sustained ventricular tachycardia, or arrhythmic syncope.
To assess for confounding effects of a potential variability of hormone levels, in a random sample of 11 patients, serum measurement of the sex hormones testosterone, estradiol, progesterone, SHBG, and DHEAS were repeated after a median of 1.0 day (IQR 0.5–1.8 days). The median intra-individual variation for testosterone in these 11 patients was 10.7% (IQR 6.3–43%), which was lower than the difference between the mean testosterone levels of patients with MACE and those with a favourable outcome. Moreover, serial measurements of serum testosterone levels in these 11 patients did not show any significant differences between baseline and follow-up (see Supplementary material online, Table S1).
Increased serum testosterone levels in male patients with MACE
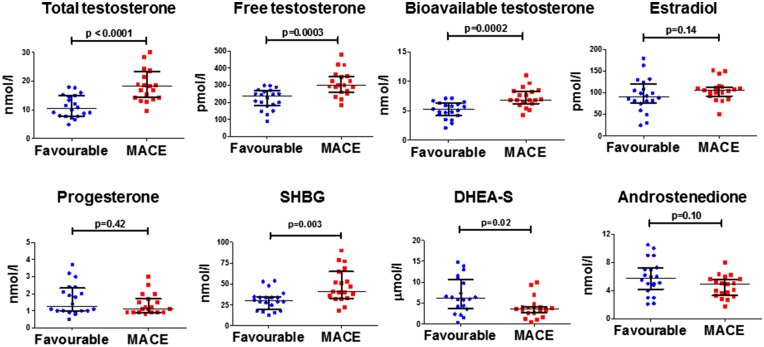
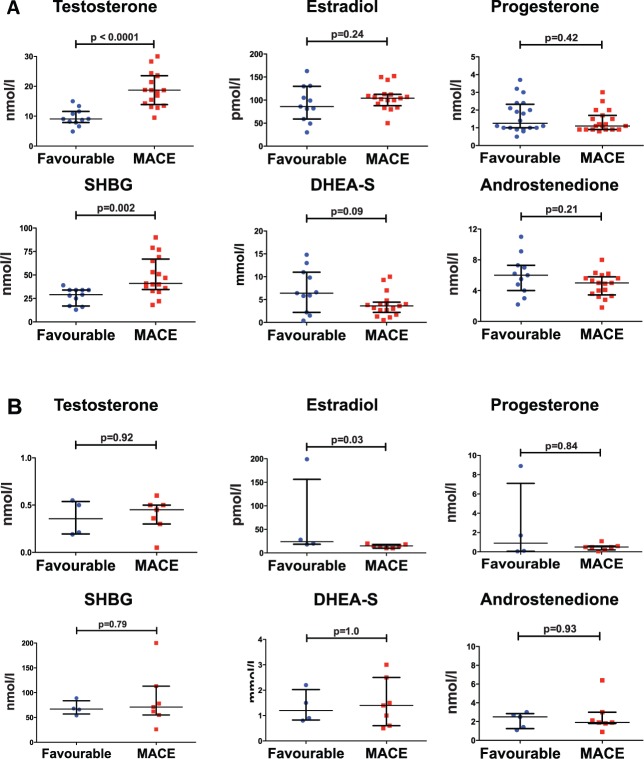
Since sex hormone levels, especially testosterone and estradiol, differ between males and females, and our patient population was predominantly male, we performed a subgroup analysis in which we compared clinical characteristics, sex hormone levels, and other serum biomarkers in male patients with and without MACE (Table 3 and Figure 1). Nineteen male patients (49%) experienced MACE during the study period. Similar to the whole patient population, male patients with a favourable outcome significantly differed from those who experienced MACE. Regarding clinical parameters and serum markers, male patients with MACE had higher TFS, lower RV-FAC levels and a trend towards higher hs-c TNT levels (P = 0.06). Male patients who experienced MACE had higher total free and bioavailable testosterone levels, higher SHBG levels, and lower DHEA-S levels (Figure 1). Moreover, there were no significant differences in testosterone levels between sedentary males, and those who did recreational or competitive sports. However, a trend towards higher testosterone levels in male patients engaging in competitive sports as compared to sedentary patients was observed (P = 0.08). In a subgroup analysis including only male patients with a definite ARVC/D diagnosis and a TFS >4 (n = 28), testosterone and SHBG remained significantly increased, and DHEA-S showed a trend for decreased levels in male patients with MACE (Figure 2).
Table 3.
Baseline clinical characteristics of male patients (n = 39) and association with adverse arrhythmic outcomes
| Outcome |
||||
|---|---|---|---|---|
| Variables | Male patients n = 39 | Favourable n = 20 | Adverse n = 19 | P-value |
| Age (years) | 50 (39–62) | 44 (28–62) | 50 (44–62) | 0 .44 |
| Systolic blood pressure (mmHg) | 125 (114–139) | 121 (110–131) | 126 (117–142) | 0 .31 |
| Diastolic blood pressure (mmHg) | 78 (70–85) | 80 (70–87) | 77 (70–82) | 0 .62 |
| BMI (kg/m2) | 26 (24–28) | 26 (25–31) | 26 (23–28) | 0 .27 |
| ARVC/D Task force Score | 5 (3–6) | 4 (3–6) | 6 (5–7) | 0 .003 |
| LVEF (%) | 57 (51–64) | 60 (52–65) | 56 (49–67) | 0 .28 |
| RV FAC (%) | 29 (21–37) | 32 (26–42) | 22 (20–32) | 0 .02 |
| Physical activity | ||||
| Sedentary, n (%) | 8 (21) | 5 (25) | 3 (16) | 0 .69 |
| Recreational sports, n (%) | 22 (56) | 12 (60) | 10 (53) | 0 .75 |
| Competitive sports, n (%) | 9 (23) | 3 (15) | 6 (31) | 0 .27 |
| hs-c TnT (ng/l) | 9 (5–19) | 5 (5–16) | 14 (5–23) | 0 .06 |
| pro-BNP (ng/l) | 119 (51–311) | 94 (46–178) | 148 (70–391) | 0 .18 |
| Glucose (mmol/l) | 5.2 (4.9–5.5) | 5.1 (4.8–5.6) | 5.2 (4.9–5.5) | 1 .00 |
| Insulin (mIU/l) | 10.0 (5.0–24.0) | 10.0 (5.3–32.0) | 8.0 (5.0–20 .0) | 0 .73 |
| Diabetes, n (%) | 5 (13) | 3 (15) | 2 (11) | 1 .00 |
| Total cholesterol (mmol/l) | 5.0 (4.3–5.4) | 5.1 (4.5–5.8) | 4.7 (4.0–5.2) | 0 .10 |
| HDL cholesterol (mmol/l) | 1.2 (1.1–1.5) | 1.2 (1.1–1.4) | 1.2 (1.0–1.5) | 0 .94 |
| Triglycerides (mmol/l) | 1.2 (1.0–1.9) | 1.2 (1.0–1.9) | 1.1 (1.0–1.9) | 0 .40 |
Data are presented as median (IQR); Mann-Whitney U test was used for comparison of continuous variables, and Fisher’s exact test for categorical variables. P-values < 0 .05 were considered significant.
Figure 1.
Increased serum testosterone and SHBG, and decreased DHEA-S in male patients with MACE. Sex hormone levels (individual data plotted with median and interquartile ranges) of male ARVC/D patients (n = 39) with a favourable outcome as compared to those with MACE. P-values calculated by Mann-Whitney U-test. SHBG, Sex hormone-binding globulin; DHEA-S, dehydroepiandrosterone-sulfate.
Figure 2.
Increased serum testosterone levels and decreased estradiol levels in definite ARVC/D patients with MACE. (A) Increased testosterone and SHBG in definite male ARVC/D patients with MACE (n = 28). (B) Decreased estradiol levels in definite female ARVC/D patients with MACE (n = 11). Individual data plotted with median and interquartile ranges. P-values calculated by Mann-Whitney U-Test. SHBG, Sex hormone-binding globulin; DHEA-S, dehydroepiandrosterone-sulfate.
Increased serum testosterone as an independent predictor of MACE in males
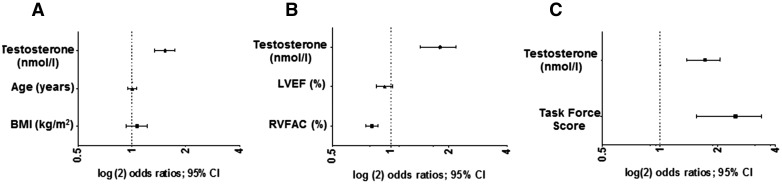
To determine the OR for MACE per 1 nmol/l increase in serum testosterone levels, we performed regression models that were adjusted for other confounding factors such as age, BMI, TFS, LVEF, and RV-FAC (Figure 3A–C and see Supplementary material online, Table S2). These multivariable models revealed that an increase in total serum testosterone levels remained the strongest independent predictor of MACE with a significantly increased OR in the male population after adjusting for confounders. An increased TFS and reduced RV-FAC also remained independent predictors of MACE (Figure 3B and C). To identify the optimum cut-off levels of testosterone to distinguish patients with MACE from those without, receiver-operating characteristics curves were calculated for serum total, free and bioavailable testosterone (Figure 4). Total testosterone levels above 13.5 nmol/l predicted MACE with a sensitivity of 84% and a specificity of 75%. For free testosterone levels, the optimum cut-off of >264 pmol/l had a sensitivity of 74% and a specificity of 75%, and for bioavailable testosterone the optimum cut-off levels were >6.2 nmol/l yielding a sensitivity of 79% and a specificity of 75% (Figure 4).
Figure 3.
Elevated serum testosterone is an independent predictor of MACE in male ARVC/D patients. Multivariable models to determine odds ratios for MACE with increasing serum testosterone (per 1 nmol/l increase) adjusted for other risk factors in male ARVC/D patients (n = 37). (A) Adjusted for increasing age and body mass index. (B) Adjusted for increasing LVEF and RVFAC. (C) Adjusted for an increasing TFS. Odds ratios determined by binary logistic regression; CI, confidence interval.
Figure 4.
Optimal cut-off levels of serum testosterone to predict MACE in male ARVC/D patients. Receiver operating characteristic analysis for testosterone in male ARVC/D patients (n = 39). (A) Cut-off levels for total testosterone; Area under the curve (AUC) = 0.89 (CI 0.79–0.99), P-value <0.001. Total serum testosterone levels of > 13.5 nmol/l provided the best sensitivity of 84% and specificity of 75% for predicting MACE. (B) Cut-off levels of free testosterone; AUC = 0.84 (CI 0.71–0.96), P-value <0.001. Free serum testosterone levels of > 264 pmol/l provided the best sensitivity of 74% and specificity of 75% for predicting MACE. (C) Cut-off levels of bioavailable testosterone; AUC = 0.84 (CI 0.72–0.97), P-value <0.001. Bioavailable serum testosterone levels of > 6.2 nmol/l provided the best sensitivity of 79% and specificity of 75% for predicting MACE. Optimal cut-off levels were determined by Youden’s index. Cut-off levels indicated as black arrows.
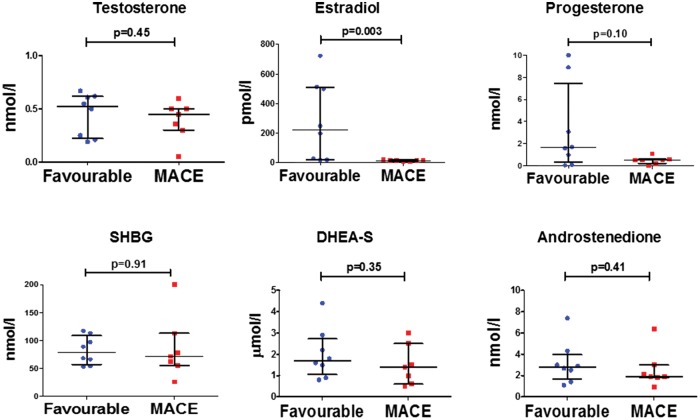
Decreased serum estradiol levels in female patients with MACE
Baseline characteristics are presented separately for our female cohort (Figure 5 and see Supplementary material online, Table S3). Seven out of fifteen (45%) females experienced MACE within the study period. Regarding clinical parameters, female patients with MACE had significantly higher TFS and lower RV-FAC. Regarding exercise, only one out of seven sedentary female patients had MACE (P = 0.03), whereas six out of eight females, who did recreational sports experienced MACE (P = 0.04). None of the female patients engaged in competitive sports. Regarding sex hormones in the female subgroup, estradiol levels were significantly lower in the group with MACE as compared to the group with a favourable outcome. There was also a trend towards higher hs-c TnT levels (P = 0.06) in females. In a subgroup analysis including only female patients with a definite ARVC/D diagnosis and a TFS >4 (n = 11), estradiol remained significantly decreased in patients with MACE (Figure 2).
Figure 5.
Decreased estradiol levels in female ARVC/D patients with MACE. Sex hormone levels of female ARVC/D patients (n = 15) with a favourable outcome as compared to those with MACE. Individual data is plotted with median and interquartile ranges. P-values calculated by Mann-Whitney U-test.
Role of desmosomal mutation status
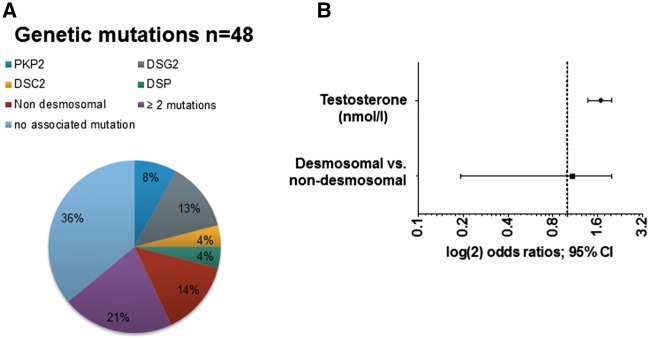
Genetic testing was available in 48 ARVC/D patients and revealed 14 patients harbouring a single heterozygous desmosomal mutation, and seven patients with a heterozygous non-desmosomal mutation (two patients with pathogenic TTN variants, three patients with ryanodine receptor 2 (RYR2) mutations, one patient with a transmembrane protein 43 (TMEM43) mutation, and one patient with a lamin A/C (LMNA) mutation. DSG2 was the most common, and PKP2 was the second most common mutation out of the desmosomal mutations. Ten patients had at least two mutations/pathogenic variants (at least one desmosomal mutation each) (Figure 6A). Furthermore, to determine whether desmosomal mutations had an influence on MACE in male patients, we included genetic mutation status in a bivariable model (Figure 6B). Testosterone remained independently associated with MACE, whereas no significant association between positive desmosomal mutation status and MACE was observed. To assess the impact of gender per se, we compared patients with desmosomal mutations and MACE vs. patients with desmosomal mutations with a favourable outcome (n = 24) stratified by gender, but did not find any statistical differences (10/18 males vs. 5/6 females, P = 0.35). Of note, male desmosomal mutation carriers with MACE had significantly higher serum testosterone levels as compared to male desmosomal mutation carriers with a favourable outcome (19.5 nmol/l [17.3-23.8] vs. 10.5 nmol/l [8.9–12.1], P = 0.0012). Female desmosomal mutation carriers with MACE had lower estradiol levels as compared to female desmosomal mutation carriers with a favourable outcome.
Figure 6.
Genetic mutations in ARVC/D patients and arrhythmic outcomes. (A) Genetic mutation status of our ARVC/D cohort (data available in n = 48). (B) Bivariable model to calculate odds ratios (OR) for total testosterone adjusted for the presence of desmosomal mutations in male ARVC/D patients (n = 33). OR determined by binary logistic regression.
Elevated serum testosterone levels increase, while elevated estradiol levels decrease apoptosis and lipid accumulation of CMs in an invitro ARVC/D model
To explore if sex hormones play a pathogenic role in ARVC/D pathologies, we used a previously published in vitro ARVC/D model and generated iPSC-CMs from an ARVC/D patient with homozygous c.2484C > T mutations in PKP23 to determine if testosterone or estradiol directly affect apoptosis and lipogenesis in ARVC/D iPSC-CMs. In this model, 2 weeks of treatment with 5F pathogenic media induced 16.4 ± 1.2% apoptosis and 1.7 ± 0.1 lipid droplets/CM in ARVC/D iPSC-CMs. Since total testosterone levels above 13.5 nmol/l predicted MACE in our patient cohort, we added 5 ng/mL (17.3nmol/l, a clinically relevant concentration) testosterone to 5F media. Adding 17.3 nmol/l testosterone to 5F led to a small, but significant additional increase in CM apoptosis and lipid accumulation (19.0 ± 2.1% apoptosis and 2.1 ± 0.2 lipid droplets/CM (Figures 7 and 8). To investigate the role of estradiol based on the data in Figure 5, we added 50 pg/mL (183.6 pmol/l) estradiol to 5F media, which significantly decreased CM apoptosis to 6.8 ± 1.5% and lipid accumulation to 1.2 ± 0.1 lipid droplets/CM within 2 weeks of culture (Figures 7 and 8). Of note, normal iPSC-CMs without any desmosomal mutation showed minimal CM apoptosis (<5%) and lipid accumulation after the same treatment with 5F pathogenic media.14 Neither testosterone nor estradiol significantly changed CM apoptosis/lipogenesis in normal iPSC-CMs (not shown). The statistically significant but small detrimental effects of testosterone over a 2-week duration suggest that longer durations of high testosterone might elicit more ARVC/D CM pathologies. Thus, these data support strongly that increased levels of testosterone accelerate ARVC/D pathologies, while premenopausal female estradiol levels slow down exaggerated apoptosis and lipid accumulation in ARVC/D CMs.
Figure 7.

Cardiomyocyte apoptosis is increased by testosterone and decreased by estradiol in ARVC/D iPSC-CMs. (A) Representative images showing that testosterone (T) increased and estradiol (E2) decreased cardiomyocyte (CM) apoptosis (apoptosis shown by TUNEL+ staining in red; DAPI = nucleus in blue, alpha-actinin in green) of 2-week 5F-treated JK#11 ARVC/D iPSC-CMs. (B) Cumulative quantitative data of CM apoptosis. The data from 6–8 slides analysed from 3–4 biological replicates and P-values calculated by ANOVA with the post-hoc Tukey-Kramer test are shown.
Figure 8.
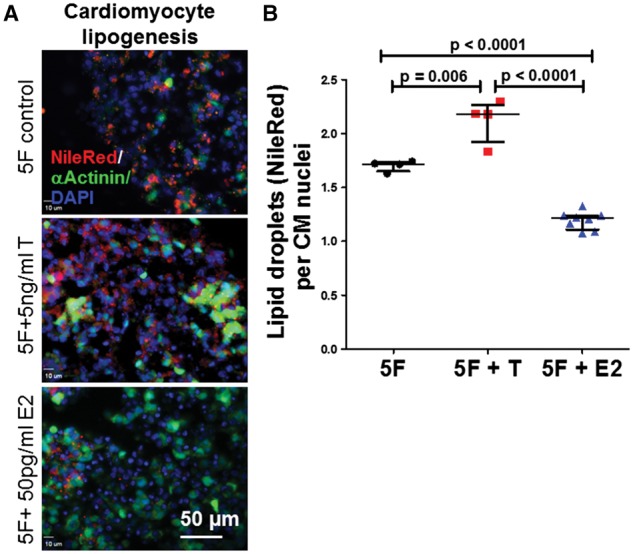
Cardiomyocyte lipid accumulation is increased by testosterone and decreased by estradiol in ARVC/D iPSC-CMs. (A) Representative images showing that testosterone (T) increased and estradiol (E2) decreased lipid accumulation (Nile Red-positive lipid droplets) of 2-week 5F-treated JK#11 ARVC/D iPSC-CMs. (B) Cumulative quantitative data of CM lipogenesis. The data from 4–8 slides analysed from 3–4 biological replicates and P-values calculated by ANOVA with the post-hoc Tukey-Kramer test are shown.
Discussion
This is the first study to investigate the role of sex hormones and their association with MACE in patients with ARVC/D. We were able to show that elevated serum testosterone levels were independently associated with MACE in male ARVC/D patients, whereas in female patients with MACE estradiol levels were significantly decreased. Furthermore, our clinical observations were supported by a human iPSC-CM based experimental model, to provide novel pathogenic insights of an inherited cardiomyopathy and to decipher its disease mechanisms. At clinically relevant concentrations, elevated testosterone levels worsened, whereas normal premenopausal estradiol levels decreased CM apoptosis and lipogenesis. These findings suggest that sex hormones are not just randomly associated with clinical outcomes in ARVC/D, but they also influence disease pathogenesis.
In this study, male patients with MACE had significantly increased serum testosterone levels as compared to those with a favourable outcome, and increased testosterone levels remained a strong independent predictor of MACE after adjusting for established clinical risk factors. Furthermore, we showed that the presence of desmosomal mutations did not influence the association of high testosterone levels with MACE. Our analysis revealed a serum testosterone cut-off value of >13.5 nmol/l that was associated with an optimum sensitivity and specificity for predicting MACE. Previous invitro and invivo studies have suggested that high testosterone levels may have pro-arrhythmic effects through modulation of cardiac contraction and calcium homeostasis.28–31 Other studies suggested that arrhythmias may occur due to higher adrenergic activity increasing apoptosis, which would worsen ARVC/D pathologies.32,33 Since mechanisms of ARVC/D pathology include dysregulation of CM survival and lipogenesis,2,14,15 testosterone could worsen those pathologies or increase the arrhythmic potential in this specific disease constellation, since in our invitro model, the healthy CMs did not show increased lipogenesis and apoptosis after 5F pathogenic induction.3
In female patients with MACE, we observed reduced serum estradiol concentrations, also in the subgroup of patients with definite ARVC/D and desmosomal mutations. This finding supports the role of estradiol as a cardioprotective hormone and is well in line with several studies demonstrating its antiarrhythmic effects.21,23 Another recent study proposed that estradiol prevents apoptosis due to beneficial effects against oxidative stress in non-reproductive tissue.34 Reduced estradiol levels in female patients may therefore lead to an aggravation of ARVC/D after the onset of menopause, and likely explain the late disease onset in females as compared to males.5,9,12
To investigate the pathogenic role of our clinical findings, it was important to use an invitro model to fully recapitulate a pathological phenotype. For this reason, an established human iPSC-CM based ARVC/D model was used to show that sex hormones were directly involved in the disease process. High testosterone levels slowly accelerated the disease process over time and normal pre-menopausal estradiol levels slowed down CM apoptosis and lipogenesis. Together with other invivo and invitro studies showing the direct effects of sex hormones on cardiomyocytes and arrhythmogenesis,5,28–30 our findings emphasize the direct role of sex hormones in influencing disease pathology. With regard to this, our data extend findings from previous publications, and may explain why male ARVC/D patients present with more severe phenotypes as compared to females,5,9 although ARVC/D is usually inherited as an autosomal-dominant trait.
Our finding that low DHEA-S levels were associated with MACE corresponds to previous studies emphasizing the cardio-protective role of DHEA-S in men.35 It has been shown that SHBG acts as a regulator of testosterone, which could explain its association with MACE in this study.36 In line with previous studies, a decrease in LVEF and RV-FAC, a higher TFS, and elevated hs-cTnT and proBNP levels were associated with MACE, well reflecting that patients with more advanced disease stages have a higher risk of arrhythmic events.6–9,37 Elevated cTnT levels have also been associated with arrhythmias, particularly in the so-called hot phases of ARVC/D, likely reflecting on-going cardiac injury.38
Limitations
Although we were able to show that sex hormones directly influence the disease process, we were not able to adjust for all confounders due to our limited patient size. The sample size and genetic heterogeneity did not allow for assessing the effects of different genetic mutations on outcome. Out of 15 patients, who presented with a borderline diagnosis of ARVC/D at study enrolment, 7 patients developed definite ARVC/D at last follow-up. Yet, in the remaining 8 patients, there remains uncertainty about ARVC/D as the underlying pathology. We reduced short-term variations of sex hormones to a minimum and our serial measurements of testosterone levels confirmed that biological variability was lower than the difference between mean testosterone levels of patients with MACE and those with a favourable outcome; nonetheless, it has to be acknowledged that sex hormone levels can vary, particularly during the mid- and long-term for the same individual for various reasons.
In conclusion, we combined a clinical study with an invitro model to provide new pathogenic insights into the role of sex hormones in ARVC/D. Our study shows that elevated testosterone and decreased estradiol serum levels are independently associated with MACE in patients with ARVC/D, and likely have an influence on disease pathology. Thus, determining serum hormone levels besides other established risk factors may serve as a useful tool in the risk stratification process.39,40 This study further suggests that human iPCS-CM based disease models can be used to support the pathogenic roles of clinical observations in an inherited cardiomyopathy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank Prof. Dr. Leonhard Held and Dr. Niels Hagenbuch from the Institute of Epidemiology, Biostatistics and Prevention, University of Zurich, Switzerland for their expert statistical advice.
Funding
The Zurich ARVC/D Program, founded by Drs C Brunckhorst and F Duru from the University Heart Center Zurich, is supported by research grants from the Georg and Bertha Schwyzer-Winiker Foundation Zurich, the Baugarten Foundation Zurich and Swiss National Foundation (SNF). Zurich and the Swiss National Foundation. AM Saguner is supported by a grant from the Siegenthaler Foundation, University of Zurich. D Akdis is supported by the ‘Filling The Gap’ grant of the University of Zurich. K Shah and HSV Chen are supported by California Institute of Regenerative Medicine (CIRM) grants (RB4-06276) and NIH grant (RO1 HL105194) C Wei was supported by a CIRM training grant (TG2-01162).
Conflict of interest: None declared.
References
- 1. Saguner AM, Duru F, Brunckhorst CB.. Arrhythmogenic right ventricular cardiomyopathy: a challenging disease of the intercalated disc. Circulation 2013;128:1381–1386. [DOI] [PubMed] [Google Scholar]
- 2. Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G.. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med 1996;335:1190–1196. [DOI] [PubMed] [Google Scholar]
- 3. Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC, Marine JE, Calkins H, Kelly DP, Judge PJ, Chen H-SV.. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature 2013;494:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akdis D, Medeiros-Domingo A, Gaertner-Rommel A, Kast JI, Enseleit F, Bode P, Klingel K, Kandolf R, Renois F, Andreoletti L, Akdis CA, Milting H, Lüscher TF, Brunckhorst C, Saguner AM, Duru F.. Myocardial expression profiles of candidate molecules in arrhythmogenic right ventricular cardiomyopathy/dysplasia compared with dilated cardiomyopathy and healthy controls. Heart Rhythm 2016;13:731–741. [DOI] [PubMed] [Google Scholar]
- 5. Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JD, Murray B, Te Riele AS, van den Berg MP, Bikker H, Atsma DE, de Groot NM, Houweling AC, van der Heijden JF, Russell SD, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Calkins H, Hauer RN.. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 2015;36:847–855. [DOI] [PubMed] [Google Scholar]
- 6. Saguner AM, Medeiros-Domingo A, Schwyzer MA, On CJ, Haegeli LM, Wolber T, Hürlimann D, Steffel J, Krasniqi N, Rüeger S, Held L, Lüscher TF, Brunckhorst C, Duru F.. Usefulness of inducible ventricular tachycardia to predict long-term adverse outcomes in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2013;111:250–257. [DOI] [PubMed] [Google Scholar]
- 7. Saguner AM, Ganahl S, Kraus A, Baldinger SH, Akdis D, Saguner AR, Wolber T, Haegeli LM, Steffel J, Krasniqi N, Lüscher TF, Tanner FC, Brunckhorst C, Duru F.. Electrocardiographic features of disease progression in arrhythmogenic right ventricular cardiomyopathy/dysplasia. BMC Cardiovasc Disord 2015;15:4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saguner AM, Vecchiati A, Baldinger SH, Rüeger S, Medeiros-Domingo A, Mueller-Burri AS, Haegeli LM, Biaggi P, Manka R, Lüscher TF, Fontaine G, Delacrétaz E, Jenni R, Held L, Brunckhorst C, Duru F, Tanner FC.. Different prognostic value of functional right ventricular parameters in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Cardiovasc Imaging 2014;7:230–239. [DOI] [PubMed] [Google Scholar]
- 9. Rigato I, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E, Migliore F, Marra MP, Lorenzon A, De Bortoli M, Calore M, Nava A, Daliento L, Gregori D, Iliceto S, Thiene G, Basso C, Corrado D.. Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet 2013;6:533–542. [DOI] [PubMed] [Google Scholar]
- 10. Ruwald AC, Marcus F, Estes NA, Link M, McNitt S, Polonsky B, Calkins H, Towbin JA, Moss AJ, Zareba W.. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2015;36:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H.. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyer S, van der Meer P, van Tintelen JP, van den Berg MP.. Sex differences in cardiomyopathies. Eur J Heart Fail 2014;16:238–247. [DOI] [PubMed] [Google Scholar]
- 13. Bauce B, Frigo G, Marcus FI, Basso C, Rampazzo A, Maddalena F, Corrado D, Winnicki M, Daliento L, Rigato I, Steriotis A, Mazzotti E, Thiene G, Nava A.. Comparison of clinical features of arrhythmogenic right ventricular cardiomyopathy in men versus women. Am J Cardiol 2008;102:1252–1257. [DOI] [PubMed] [Google Scholar]
- 14. Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ.. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Res 2014;114:454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, Wu JC, MacRae CA, Kléber AG, Saffitz JE.. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Sci Transl Med 2014;6:240ra274.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab 2011;22:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herring MJ, Oskui PM, Hale SL, Kloner RA.. Testosterone and the cardiovascular system: a comprehensive review of the basic science literature. J Am Heart Assoc 2013;2:e000271.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Magnani JW, Moser CB, Murabito JM, Sullivan LM, Wang N, Ellinor PT, Vasan RS, Benjamin EJ, Coviello AD.. Association of sex hormones, aging, and atrial fibrillation in men: the framingham heart study. Circ Arrhythm Electrophysiol 2014;7:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swinnen JV, Esquenet M, Goossens K, Heyns W, Verhoeven G.. Androgens stimulate fatty acid synthase in the human prostate cancer cell line LNcaP. Cancer Res 1997;57:1086–1090. [PubMed] [Google Scholar]
- 20. Payne JR, Kotwinski PJ, Montgomery HE.. Cardiac effects of anabolic steroids. Heart 2004;90:473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giordano S, Hage FG, Xing D, Chen YF, Allon S, Chen C, Oparil S.. Estrogen and cardiovascular disease: is timing everything? Am J Med Sci 2015;350:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Narayanan K, Havmoeller R, Reinier K, Jerger K, Teodorescu C, Uy-Evanado A, Navarro J, Huertas-Vazquez A, Gunson K, Jui J, Chugh SS.. Sex hormone levels in patients with sudden cardiac arrest. Heart Rhythm 2014;11:2267–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu X, Jiang H, Xu C, Zhou X, Cui B, Lu Z.. Relationship between sex hormones and idiopathic outflow tract ventricular arrhythmias in adult male patients. Transl Res 2009;154:265–268. [DOI] [PubMed] [Google Scholar]
- 24. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W.. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J 2010;31:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vermeulen A, Verdonck L, Kaufman JM.. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- 26. van der Zwaag PA, Jongbloed JD, van den Berg MP, van der Smagt JJ, Jongbloed R, Bikker H, Hofstra RM, van Tintelen JP.. A genetic variants database for arrhythmogenic right ventricular dysplasia/cardiomyopathy. Hum Mutat 2009;30:1278–1283. [DOI] [PubMed] [Google Scholar]
- 27. Medeiros-Domingo A, Saguner AM, Magyar I, Bahr A, Akdis D, Brunckhorst C, Duru F, Berger W.. Arrhythmogenic right ventricular cardiomyopathy: implications of next-generation sequencing in appropriate diagnosis. Europace 2016;5:1532–2092. [DOI] [PubMed] [Google Scholar]
- 28. Ayaz O, Howlett SE.. Testosterone modulates cardiac contraction and calcium homeostasis: cellular and molecular mechanisms. Biol Sex Differ 2015;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parks RJ, Howlett SE.. Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflugers Arch 2013;465:747–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brouillette J, Rivard K, Lizotte E, Fiset C.. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc Res 2005;65:148–157. [DOI] [PubMed] [Google Scholar]
- 31. Fischer TH, Herting J, Eiringhaus J, Pabel S, Harmann NH, Ellenberger D, Friedrich M, Renner A, Gummert J, Maier LS, Zabel M, Hasenfuss G, Sossalla S.. Sex-dependent alterations of Ca2+ cycling in human cardiac hypertrophy and heart failure. Europace 2016;18:1440–1448. [DOI] [PubMed] [Google Scholar]
- 32. Veldhuis JD, Keenan DM, Liu PY, Iranmanesh A, Takahashi PY, Nehra AX.. The aging male hypothalamic-pituitary-gonadal axis: pulsatility and feedback. Mol Cell Endocrinol 2009;299:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsai WC, Lee TI, Chen YC, Kao YH, Lu YY, Lin YK, Chen SA, Chen YJ.. Testosterone replacement increases aged pulmonary vein and left atrium arrhythmogenesis with enhanced adrenergic activity. Int J Cardiol 2014;176:110–118. [DOI] [PubMed] [Google Scholar]
- 34. La Colla A, Vasconsuelo A, Milanesi L, Pronsato L.. 17β-Estradiol Protects Skeletal Myoblasts from Apoptosis through P53, BCL-2 and FoxO Families. J Cell Biochem 2017;118:104–115. [DOI] [PubMed] [Google Scholar]
- 35. Mannic T, Viguie J, Rossier MF.. In vivo and in vitro evidences of dehydroepiandrosterone protective role on the cardiovascular system. Int J Endocrinol Metab 2015;13:e24660.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tint AN, Hoermann R, Wong H, Ekinci EI, MacIsaac RJ, Jerums G, Zajac JD, Grossmann M.. Association of sex hormone-binding globulin and free testosterone with mortality in men with type 2 diabetes mellitus. Eur J Endocrinol 2016;174:59–68. [DOI] [PubMed] [Google Scholar]
- 37. Link MS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear K, Marcus F, Estes NA.. Ventricular arrhythmias in the north american multidisciplinary study of arvc: predictors, characteristics, and treatment. J Am Coll Cardiol 2014;64:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horjen AW, Ulimoen SR, Enger S, Berge T, Ihle-Hansen H, Norseth J, Tveit A.. Impact of atrial fibrillation on levels of high-sensitivity troponin i in a 75-year-old population. Scand J Clin Lab Invest 2015;75:308–313. [DOI] [PubMed] [Google Scholar]
- 39. Sato K, Iemitsu M.. Exercise and sex steroid hormones in skeletal muscle. J Steroid Biochem Mol Biol 2015;145:200–205. [DOI] [PubMed] [Google Scholar]
- 40. Corrado D, Wichter T, Link MS, Hauer R, Marchlinski F, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A, Tandri H, Paul M, Schmied C, Pelliccia A, Duru F, Protonotarios N, Estes NM, McKenna WJ, Thiene G, Marcus FI, Calkins H.. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation 2015;132:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.