Abstract
Botulinum neurotoxin A (BoNT/A) cleaves SNAP25 at the motor nerve terminals and inhibits stimulus evoked acetylcholine release. This causes skeletal muscle paralysis. However, younger neonatal mice (<P7; <7-days old) are resistant to the neuroparalytic effects of BoNT/A. That is, invivo injection of BoNT/A at the innervations of Extensor digitorum longus muscle in the hindlimbs inhibited the toe spread reflex within 24 hours following BoNT/A injection in adult mouse and in older (>P7) mice. However, neonatal mice younger than 7 days-age remained unaffected by BoNT/A injection. Also, BoNT/A inhibited stimulus evoked acetylcholine release and stimulus-evoked twitch tension of diaphragm nerve muscle preparations (NMPs) of adult mouse and >P7 neonates but not that of <P7. Moreover, NMPs of <P7 showed decreased uptake of fluorescent BoNT/A compared to >P7. However, cholesterol depletion using methyl-β-cyclodextrin (MβCD) sensitized <P7 neonates to BoNT/A and facilitated BoNT/A uptake into NMPs obtained from <P7 neonates. Further, MβCD (10 mM; 30 min pretreatment) increased the interaction between synaptic vesicle protein 2 and BoNT/A. Also, cholesterol depletion increased the miniature endplate current in adult NMPs. Interestingly, cholesterol replenishment, invitro, delayed the onset of inhibitory effect of BoNT/A. Collectively, our data suggest that cholesterol rich lipid microdomains are involved in BoNT/A uptake mechanisms during development. Our data demonstrate that cholesterol depletion sensitized neonatal mice (<P7) to BoNT/A while replenishing cholesterol delayed the onset of inhibitory actin of BoNT/A. This suggests that membrane cholesterol modulates neurotoxin sensitivity at the neuromuscular junction (NMJ).
Keywords: neonatal neuromuscular junction, botulinum neurotoxin, cholesterol-rich lipid microdomains, synaptic vesicle protein, methyl-β- cyclodextrin
Cholesterol rich lipid microdomains (LMDs) serve as scaffolds for the interaction of several signaling molecules at the synapses and in the regulation of acetylcholine (ACh) release (Colon-Saez and Yakel, 2011; Kravtsova etal., 2015). Many neurotoxins and pathogens use LMDs for their endocytic processes (Brumell and Grinstein, 2003; Schroeder etal., 2010; Shin etal., 1999). Cholesterol-rich domains (rafts) in the plasma membrane are necessary for SNARE-dependent exocytosis (Rituper etal., 2012; Zefirov and Petrov, 2010). Cholesterol depletion with methyl-β-cyclodextrin (MβCD) inhibited clathrin-dependent endocytosis in multiple preparations (Petrov etal., 2010; Yue and Xu, 2015). Cholesterol rich LMDs are important for the uptake of tetanus toxin and clostridium difficile (Cubi etal., 2013; Herreros etal., 2001; Kerzmann and Feig, 2006; Schwan etal., 2011). Cholesterol depletion blocked the transport of cholera toxin (Shogomori and Futerman, 2001) while enhanced the activity of BoNT/A (Petro etal., 2006) . However, the precise role of cholesterol in the regulation of internalization of these toxins is not well understood.
At the motor nerve terminal (MNT) SNARE complex proteins regulate the docking and fusion of synaptic vesicle. The 7 serotypes of BoNTs (/A through/G) are produced by Clostridium botulinum. Botulinum neurotoxin A (BoNT/A) is the principal serotype and is a potential biothreat agent (Marks, 2004). BoNT/A holotoxin consists of a 100 kDa heavy chain (Hc, receptor binding domain) and a 50 kDa light chain (Lc) linked by a single disulfide bond. BoNT/A Hc binding to its receptors on the neural membrane sets the stage for endocytosis into the MNT. Separation of Lc and Hc takes place after translocation of Lc in the reducing cytosol (Pirazzini etal., 2015). The Hc translocates the Lc into the cytosol where it cleaves SNAP-25 and attenuates ACh release resulting in flaccid neuroparalysis of the innervated muscles in adult mice.
However, in early 1980s younger rats were reported to recover from botulinum-induced paralysis much more quickly than adults. In a study with neonatal mice, it was observed that intragastric administration of Clostridium botulinum spores to mice <7-days old showed resistance to botulinum intoxication (Sugiyama and Mills, 1978). In this research study, we report that neonatal mice, younger than 7 days of age (<P7) showed resistance to BoNT/A and that cholesterol depletion by MβCD sensitized these neonates to the neurotoxin. We analyzed this systematically by investigating the effects of BoNT/A in wild type and MβCD treated diaphragm nerve muscle preparations (NMPs) obtained from <P7, >P7 (older than 7 days of age) and adult mice.
Our data provide evidence for the role of cholesterol in the regulation of BoNT/A uptake mechanism. This knowledge is very useful for ensuring careful clinical use of BoNT/A when administered along with medications that perturb cellular cholesterol levels.
MATERIALS AND METHODS
Surgical Procedure and Injections
Adult and neonatal (age P3-P10) C57BL/6 wild type mice were used for the study. All protocols were conducted as per protocols approved by Institutional Animal Care and Use Committee. Aseptic techniques were used for surgical procedures in mice for invivo experiments. Mice were anesthetized with intraperitoneal injection of a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). A small incision in the skin at the knee region was made in the leg where the peroneal nerve enters into the extensor digitorum longus (EDL) muscle. Three microliters of BoNT/A (1.066 pg/µl) in HEPES Ringer solution (HRS) containing mM 135 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 5 HEPES, and 5.5 glucose was injected into the space surrounding the innervation site of the EDL with a 26-gauge needle attached to a Hamilton syringe. The skin incision was closed with 4-0 nylon suture. HRS (3 µl) injected- contralateral EDL muscles in the left limb served as sham-treated controls. First evaluation of toe spread reflex (TSR) was done at 12 h. after the injections. TSR could not be continued for more than 24 h. since the paralyzed neonates (<p7) did not survive for more than 24 h. following cholesterol depletion with MβCD.
Assessment of TSR
Typically, when the mouse is lifted by its tail, peroneal muscles spread the second, the third, and the fourth toes, and this constitutes peroneal nerve function index (Gutmann etal., 1942). It serves as a sign of functional recovery. The effect of BoNT/A on the TSR in neonate and adult mice was evaluated according to the procedure described in an earlier report (Thyagarajan etal., 2010). The evaluation of TSR is subjective and therefore, the investigators conducting the test were unaware (blinded) of the treatment groups. The TSR measurement was scored from 1 to 5 depending on the number of toes that the mouse could extend from the midline of the foot when the animal was lifted by the tail. The mice were observed every day for a period of 7 days after injection of the toxin.
Recording Twitch Tension
Phrenic nerve diaphragm muscles preparations from adult or neonatal (>P7 and <P7) mice were removed after the mice were fully anesthetized with isoflurane followed by cervical dislocation. Left and right hemidiaphragms with the innervating phrenic nerves were dissected and mounted in a glass chamber (Radnoti Glass Technology, Inc., Monrovia, CA, USA) filled with oxygenated (95% O2-5% CO2) normal Ringer’s solution (pH 7.4, 37 °C) containing 135 mM NaCl, 5 Mm KCl, 1 mM MgCl2, 2 mM CaCl2, 1 mM Na2HPO4, 15 mM NaHCO3, 5.5 mM glucose. The phrenic nerve was drawn into a suction electrode for indirect activation of muscle twitches. The central tendon of the hemidiaphragm muscle was tied to a Grass Force transducer connected to a Digidata 1440A (Molecular Devices, Sunnyvale, California). The data acquisition and analysis of mechanical responses of the isolated diaphragm to nerve stimulation were performed with PCLAMP 10 software (Molecular Devices Inc., USA). The muscle preparations were adjusted to optimal length for force generation and equilibrated for 15 min before stimulation at 1 Hz for data acquisition.
Electrophysiology
Measurement of miniature end plate potentials or currents and stimulus-evoked endplate potentials or currents recorded from diaphragm NMPs
Diaphragm NMPs with phrenic nerve were removed from adult mice or neonatal mice anesthetized with isoflurane followed by cervical dislocation. Isolated NMP were pinned to a Sylgard-lined Plexiglas chamber, and bathed in HRS (22°C) containing (mM) NaCl 135; KCl 5; CaCl2; MgCl2 1; dextrose 5.5 and HEPES 5. In control preparations 0.75 µM µ-conotoxin GIIIB (Alamone Labs, Israel) was added to the bath to inhibit muscle contractions in response to phrenic nerve stimulation. In experiments performed to study the effects of BoNT/A (10 pM) after invitro exposure (bath application), the procedure was as described before (Thyagarajan etal., 2009). The EPPs in control muscles were recorded by blocking the contractions with µ-conotoxin as described earlier. EPPs/EPCs and miniature end plate potentials (mEPPs) (−75 mV holding potential) were recorded from the endplate region by using single or 2 electrode voltage clamp technique as described previously in Thyagarajan etal. (2009). The signals were amplified by Axoclamp-2B amplifier (Axon Instruments, Foster City, California, USA), acquired and digitized (Digidata 1200, Axon Instruments) and analyzed with PCLAMP software (version 9.2, Axon Instruments). All experiments were performed at 22°C.
Uptake of BoNT/a in neonate and adult mouse diaphragm NMPs
Evaluation of neurotoxin uptake was studied in diaphragm preparations removed from neonates (P4 and P8) and adult mice. For analyzing BoNT/A uptake the diaphragms were pinned to a Sylgard-lined Plexiglas chamber and were bathed in 667 pM Alexa Fluor 647-labeled BoNT/A (Alexa Fluor dyes; Invitrogen, Carlsbad, California) in HRS solution. The uptake of neurotoxin (667 pM Alexa Fluor 647) was initiated in response to nerve stimulation (1 Hz). NMPs were fixed in 4% formaldehyde phosphate-buffered saline (PBS) (room temperature [RT]) for 1 h followed by overnight incubation (4 °C) in 100 mM glycine in PBS. NMPs were then permeabilized with 1% Triton X-100 in PBS at 4 °C for 6 h followed by 3 washes with PBS and blocking with 2% bovine serum albumin in PBS overnight. Postsynaptic ACh receptors were labeled by exposing the fixed tissue to 1 ng/ml of Alexa Fluor 488-labeled α-bungarotoxin (α-BnTX) at 4 °C for 6 h. The muscle end-plate region was cut out and mounted with Vectashield on a slide and kept frozen at −20 °C before imaging in Carl Zeiss LSM-710 confocal microscope. The images were captured by exposing slides to appropriate argon laser beams (488 nm for αBnTX) and (647 nm for BoNT/A). Images were saved and represented as TIFF files.
Cell Culture
Mouse cholinergic neuroblastoma (Neuro 2a) cells were cultured in Dulbecco’s modified Eagle’s medium-F12 medium, pH 7.4, supplemented with 10% fetal bovine serum and antibiotics. Cells were exposed 10 mM MβCD preincubation followed by 90 min. of treatment with 1 nM BoNT/A in 40 mM KCl containing HEPES Ringer Solution.
Western Blotting/Immunoprecipitation
Diaphragm NMPs were dissected from isoflurane-anesthetized C57BL/6 mice. NMPs were either treated with buffer or with 10 pM BoNT/A (uptake stimulated either by 1 Hz neural stimulation or by exposing the tissues to 40 mM KCl containing HRS for 90 min. NMPs were then flash frozen in liquid nitrogen and stored at −80 °C until tissue was homogenized in PBS containing 1% Nonidet 40 and complete protease inhibitor cocktail (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Homogenates of Neuro 2a cells were prepared in the same manner. After centrifuging the homogenate at 14 000 rpm for 10 min in the cold, the crude lysate (40 μg) was resolved via SDS-polyacrylamide gel electrophoresis after boiling in 1× aemmli buffer. Immunoblotting was performed with anti-rabbit SV2 polyclonal antibody (detects all 3 SV2- SV2A, SV2B and SV2C; Developmental Study Hybridoma Bank, Iowa City, USA). For immunoprecipitation, the homogenized samples were precleared with Protein A Sepharose 4 beads and incubated with non-immune serum or specific antibody (SV2; AB2315387, DSHB, Iowa City, USA) overnight at 4 °C. Protein A beads was then added to samples for 2 h at 4 °C, pelleted by centrifugation and washed 3 times with lysis buffer (50 mM HEPES, pH 8.0/1 mM EDTA/150 mM NaCl, 1% Triton and protease inhibitors). Proteins were eluted with 1% SDS buffer, resolved by 7.5% SDS/PAGE, and analyzed by immunoblotting with BoNT/A Hc antibody.
Cholesterol Depletion by MβCD and Cholesterol Supplementation
Solutions of 10 mM MβCD or αCD (control) was prepared by dissolving it in sterile filtered HRS. For cholesterol supplementation, we used MβCD saturated with cholesterol (Ch-MβCD). Concentration of 3 μl of 10 mM MβCD, Ch-MβCD, or αCD was injected into right EDL of neonates. For invitro experiments, 10 mM MβCD was used to deplete cholesterol 30 min. prior to 10 pM BoNT/A addition. Typically, for electrophysiological recordings to measure mEPP and EPP were made for the MβCD or αCD treated diaphragm NMPs.
Data Analyses
Data for all figures were expressed as mean ± SD for n = 8 mice, 16 NMPs and 30 endplates. The statistical significance between the population means was determined using Student’s t test and p values ≤ .05 were considered significant * and ** represent statistical significance for p < .05 and .01, respectively.
Chemicals and Drugs
Sources of toxins were: BoNT/A was obtained from Metabiologics Inc., Madison, WI, USA. Alexa 647 labeled BoNT/A was purchased from (BBTech, Dartmouth, Massachusetts). BoNT/A light chain (LC/A) was a kind gift from Dr Bal Ram Singh, INADS, MA. Sources for other reagents were as follows: α-BnTX (Thermoscientific, USA), MβCD, α-cyclodextrin (αCD), water-soluble cholesterol and all other chemicals and drugs were obtained from Sigma-Aldrich (St Louis, MO, USA). SV2 antibody, which recognizes all 3 isoforms of SV2 (AB2315387) was from Developmental Study Hybridoma Bank, Iowa City, USA. SNAP-25 antibody (NBP1-88769) was obtained from Novus Biologicals USA).
RESULTS
BoNT/A Inhibits Stimulus-Evoked EPP and Twitch Tension in Adult Mice and P7 Neonates but Not in P3 Neonates
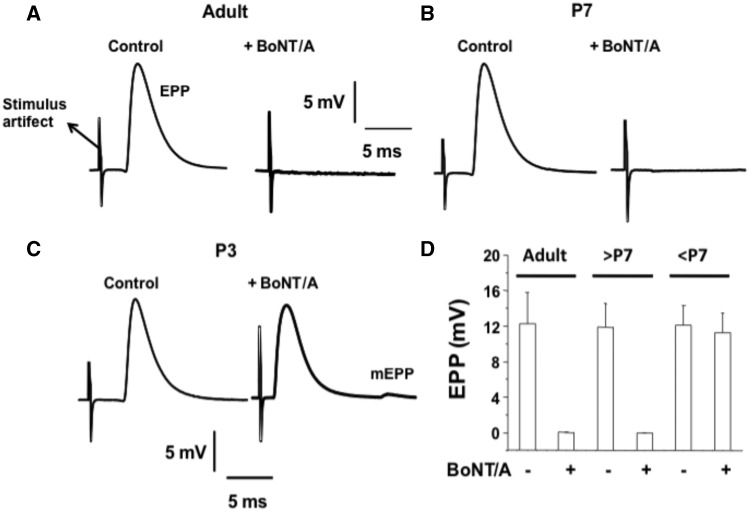
Experiments were performed in diaphragms isolated from adult mice and P7 and P3 neonates. Sample records of EPPs (1 Hz nerve-elicited) and the mean values for their amplitude are shown in Figure 1. Control recordings were obtained in the presence of µ-conotoxin to block muscle twitches (Figs. 1A–C). Control value for the EPPs was around 12 mv for adult, P7 and P3 MNT. Interestingly for adult and P7 diaphragms exposure to BoNT/A (10 pM) for 90 min produced block of neuromuscular transmission (NMT) as seen by lack of EPP response to even increased stimulus strength. On the contrary, NMP of P3 mouse was unaffected by the neurotoxin as shown by a robust EPP of about 11 mV which was followed by a small mEPP (Figure 1C). The mean EPP amplitudes in NMP of muscles from adult, P7 and P3 diaphragm NMPs for before BoNT/A application were 12.22 ± 3.76, 11.94 ± 3.54, and 12.06 ± 3.12 mV, respectively, and 90 min after BoNT/A exposure were 0.10 ± 0.002, 0.08 ± 0.001, and 11.48 ± 3.06 mV, respectively. These results confirmed the finding that the MNT in neonate mice <P7 is resistant to BoNT/A-induced block of NMT.
Figure 1.
P3 neonates are resistant to BoNT/A-mediated inhibition of stimulus-EPPs. A–C, Sample records of stimulus EPPs in diaphragm NMPs of adult (2 months old), P7 and P3 neonates before and after BoNT/A (10 pM; 90 min) exposure. D, Bar graphs summarize the inhibitory effect of BoNT/A on EPPs of adult (2-month old), P7 (and older than P7) and P3 (and younger than P7) neonates. Mean ± SD for n = 8 mice; 16 NMPs and 30 endplates are given.
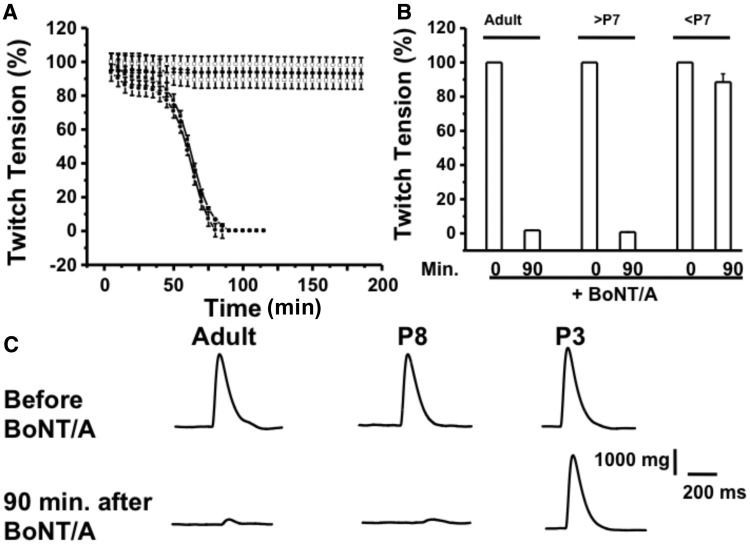
Next, we analyzed the effect of BoNT/A on stimulus-evoked twitch tension in diaphragm NMPs isolated from adult, >P7 and <P7 mice. As illustrated in Figure 2, the stimulus evoked twitch tension values for adult and >P7 neonates were 100% and 1.02 ± 0.001% and 100% and 1.14 ± 0.002%, respectively, while that for <P7 neonates were 100% and 91.521% at 0 min and at 90 min. following BoNT/A treatment.
Figure 2.
BoNT/A inhibits stimulus evoked twitch tension of diaphragm NMPs for the adult and P7 (or older) but not P3 (or younger than P7) neonates. A, Time courses of stimulus evoked twitch tension in adult and neonates (>P7 and <P7 old). B. Bar graphs represent the summary of mean ± SD of stimulus evoked twitch tension, at 0 and 90 min at 37 °C after BoNT/A (10 pM) application in the bath, in these mice (n = 6 for each conditions). C. Sample traces of twitch tension for the above conditions.
Diaphragm NMPs of Neonates Do Not Endocytose BoNT/A
In order to evaluate whether NMPs of neonates younger than 7 days endocytose BoNT/A, we performed stimulus evoked fluorescence BoNT/A uptake experiments in diaphragm of adult, P8 and P4 mice.
Experiments performed with BoNT/A labeled with Alexa Fluor 647 demonstrated that MNTs from diaphragms of mice (P < 7) failed to demonstrate toxin uptake into cholinergic nerves. The right-hand column of Supplementary Figure 1 represents confocal images of motor end-plates in adult, P8 and P4 NMPs labeled with Alexa Fluor 647-BoNT/A and the left-hand column shows the corresponding postsynaptic labeling by Alexa Fluor 488 α-BnTX.
Depletion of Cholesterol by MβCD Sensitizes Neonate Mouse MNT to BoNT/A
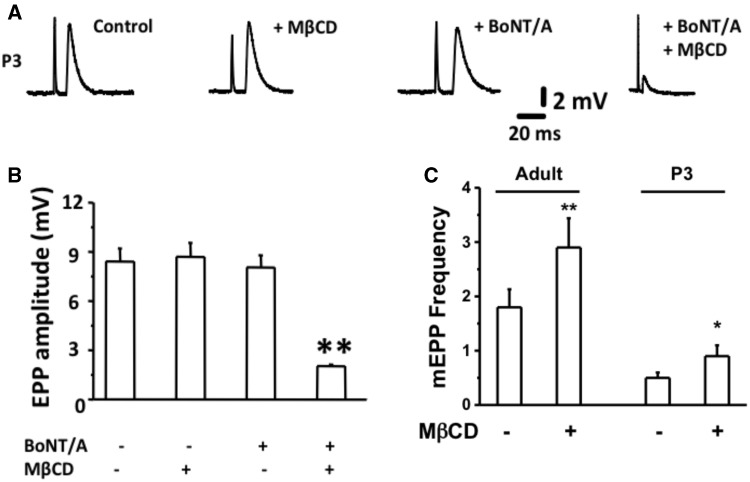
Membrane cholesterol is an important regulator of synaptic vesicle recycling (Puchkov and Haucke, 2013). Membrane cholesterol depletion by MβCD increases spontaneous vesicle fusion rates at the neuromuscular junction while decreased evoked release (Petrov etal., 2014; Rodrigues etal., 2013; Tarakanova etal., 2011). SV2 is a receptor for BoNT/A (Dong etal., 2006; Mahrhold etal., 2006; Weisemann etal., 2016) that is present in vesicles that fuse and release ACh at the MNT. Therefore, we evaluated whether depletion of cholesterol sensitizes neonatal mice to BoNT/A. To achieve this, we pretreated isolated MNPs from < P7 neonates with MβCD (10 mM for 30 min. at RT) and then measured stimulus evoked endplate potentials (EPPs). As described in Figures 3A and B, BoNT/A alone did not inhibit EPPs in P3 MNPs while pretreatment\with MβCD (10 mM for 30 min) conferred sensitivity to BoNT/A in P3 NMPs. The mean EPP amplitude ± STDEV for control (αCD; 10 mM 30 min), MβCD, BoNT/A (10 pM) and BoNT/A + MβCD (30 min pretreatment with 10 mM MβCD followed by 90 min. of BoNT/A treatment) were measured to be 8.76 ± 0.77, 8.94 ± 0.89, 8.44 ± 0.26, and 1.89 ± 0.012 mV, respectively. Further, the mEPP frequency for adult and P3 NMPs before and after MβCD treatment were determined to be 1.82 ± 0.31 and 2.88 ± 0.56 mV (adult) and 0.54 ± 0.14 and 0.88 ± 0.20 mV (P3), respectively.
Figure 3.
Cholesterol depletion by MβCD sensitizes P3 neonates to BoNT/A. A, Samples traces of stimulus evoked EPP for control, MβCD (10 mM for 30 min), BoNT/A (10 pM) and MβCD (pretreatment for 30 min) followed by BoNT/A (10 pM; 90 min) treated conditions. All measurements were made at the end of 90 min of each treatment. B and C, Bar graphs represent the summary of mean EPP and mean mEPP ± SD in these NMPs at the end of 90 min of treatment (n = 8 neonates, 8 NMPs, and 22 endplates).
Neuroparalysis After a Local Injection of BoNT/A in P3 Mice After MβCD
A single local injection of BoNT/A into EDL muscle of right hind limb of adult mouse produced paralysis which was evident as early as 24 h. post injection. The paralysis of right foot was seen by the lack of reflex spreading of toes when the mouse was lifted by its tail. The left foot from HRS injected EDL showed normal TSR (Figure 4). Similar injections of the neurotoxin in P3–P7 mice failed to demonstrate any paralysis of the hind limbs. Normally at this age the mice do not have righting reflex and the ability to move using their fore or hind limbs. This precluded measurement of TSR.
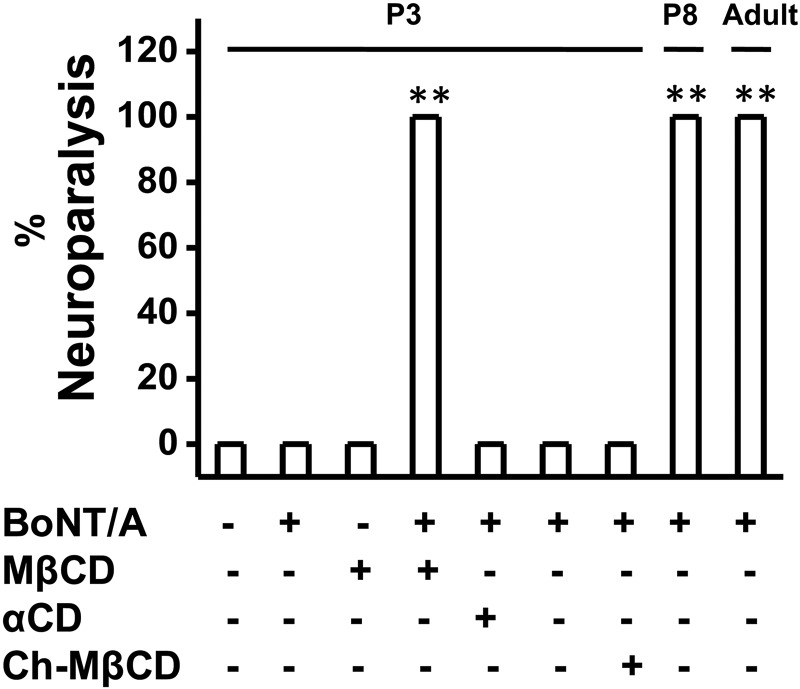
Figure 4.
Injection of MβCD but not αCD or cholesterol saturated MβCD sensitizes BoNT/A and causes neuroparalysis in P3 neonates. Bar graphs represent the percentage of neuroparalysis following the injection of 3 μL of either BoNT/A (3.2 pg), MβCD (10 mM) followed by BoNT/A, αCD (10 mM) followed by BoNT/A or cholesterol saturated MβCD (10 mM) followed by BoNT/A in P3 neonates. P7 and adult mice received only BoNT/A.
None of the neonates (P2–P6) that received BoNT/A showed any paralysis up to 2 months of observation period. The resistance to block of NMT was best demonstrated by injecting MβCD (3 μl of 10 mM) 30 min prior to BoNT/A injection. The percentages of neuroparalysis following the injection of BoNT/A in the adult and P8 mice and that following the injection BoNT/A postαCD, MβCD, or cholesterol saturated MβCD in P3 mice are shown in Figure 4.
Next, we analyzed the ability of BoNT/A to cause inhibition of stimulus-evoked twitch tension in P3 mice diaphragm NMPs following cholesterol depletion with MβCD (Figure 5). The twitch tension percentage at 0, 30, 60 and 90 min. post BoNT/A treatment in αCD or MβCD pretreated (10 mM; 30 min) NMPs are given in Table 1.
Figure 5.
BoNT/A inhibits twitch tension of diaphragm NMPs following cholesterol depletion with MβCD. Time courses of stimulus evoked twitch tension in P3 diaphragm NMPs treated with either αCD (10 mM; 30 min; control), BoNT/A (10 pM; 90 min) or MβCD (10 mM; for 30 min) followed by BoNT/A. (n = 6 for each conditions).
Table 1.
Twitch Tension % Post BoNT/A Treatment
| Time After BoNT/A (10 pM) |
Twitch Tension (%) |
||
|---|---|---|---|
| + Vehicle + BoNT/A | αCD + BoNT/A | MβCD + BoNT/A | |
| 0 min | 100 | 100 | 100 |
| 30 min | 99.02 ± 1.02 | 100 ± 1.11 | 14.44 ± 6.88 |
| 60 min | 95.22 ± 4.67 | 94.18 ± 9.80 | 0.01 ± 0.003 |
| 90 min | 91.00 ± 12.22 | 84.63 ± 14.19 | 0 |
MβCD-Induced Cholesterol Depletion Enhances Uptake of BoNT/A by Nerve Terminal Membrane
Since cholesterol depletion sensitized the P3 neonates to BoNT/A, we evaluated whether MβCD treatment stimulated BoNT/A uptake into P3 NMPs. As shown in Supplementary Figure 2, pretreatment of the NMP with αCD failed to enhance the uptake of florescent labeled BoNT/A while MβCD pretreatment facilitated BoNT/A uptake into P3 (Supplementary Figure 2) and P4 NMPs (Supplementary Figure 5B) at 22°C.
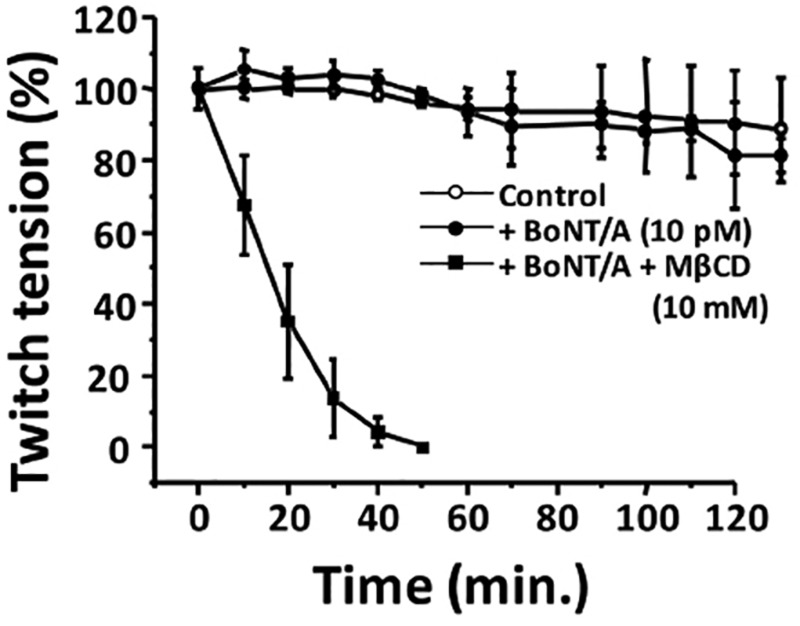
We also measured the effect of MβCD treatment on stimulus-evoked twitch tension at 22°C (RT) and 37°C (Supplementary Figures 3A and B). We calculated the percent of twitch tension in adult diaphragm NMPs at time 0 and at time 120 min following pretreatment with 10 mM MβCD (Supplementary Figure 3C). MβCD pretreatment per se did not significantly decrease stimulus-evoked twitch tension of NMPs.
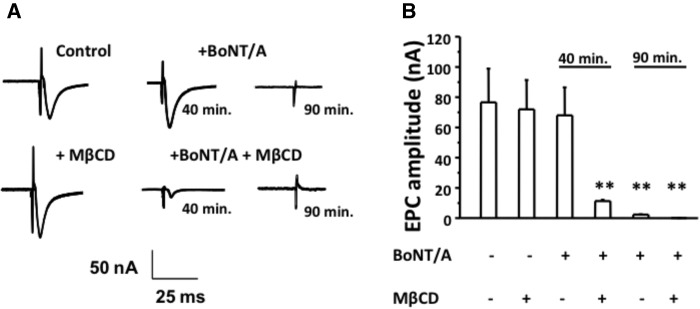
Since cholesterol depletion by MβCD < P7 sensitized neonates to BoNT/A, we evaluated whether MβCD treatment sped up the onset of inhibition of stimulus evoked endplate current (EPCs) in adult NMPs. We performed these experiments in EDS NMPs, which were exposed to either buffer (control; αCD) or MβCD (10 mM for 30 min) before exposure to BoNT/A. The EPC amplitudes (nA) at the beginning of the experiment and at 40 min and 90 min after BoNT/A exposure are shown in Figure 6. Table 2 shows the EPC amplitudes for these conditions.
Figure 6.
Cholesterol depletion with MβCD enhances BoNT/A induced neuroparalysis in adult NMPs and shortens the time to inhibit stimulus-evoked EPCs. A, Samples traces of EPCs for adult diaphragm NMPs treated with BoNT/A (10 pM; 90 min) either following αCD (top panel) or MβCD (bottom panel) at 0, 40, or 90 min of BoNT/A exposure. B, Bar graphs summarize the mean EPC amplitude ± SD for the above conditions for n = 6 mice, 11 NMPs, and 22 endplates.
Table 2.
EPC Amplitudes (nA) Post BoNT/A Treatment
| Time after BoNT/A (10 pM) | EPC amplitude (nA) |
|
|---|---|---|
| αCD + BoNT/A | MβCD + BoNT/A | |
| 0 min | 78.22 ± 21.10 | 77.02 ± 18.66 |
| 40 min | 70.08 ± 18.90 | 12.43 ± 0.88 |
| 90 min | 3.12 ± 0.065 | 0.003 ± 0.001 |
Next, we evaluated the effect of MβCD pretreatment on BoNT/A-induced inhibition of twitch tension in P7 diaphragm NMPs. For this, we pretreated NMPs isolated from P7 (7-day-old mice) with 10 mM MβCD for 30 min and then measured the stimulus evoked twitch tension in the presence of either 10, 3, or 1 pM BoNT/A. We also measured the twitch tension in the presence BoNT/A (10 pM) but without MβCD preincubation, which served as control. As shown in Figure 7, MβCD pretreatment significantly enhanced the inhibitory effect of all 3 concentrations of BoNT/A. Under control (only BoNT/A and no MβCD) conditions, BoNT/A inhibited twitch tensions completely in about 136.45 ± 18.44 min. while MβCD treatment shortened the time to inhibition of twitch tension to about 68.16 ± 14.34 min. Although, no significantly difference was observed among the 3 concentrations of BoNT/A, preincubation with MβCD significantly shortened the time to 50% inhibition of twitch tension by 10 pM of BoNT/A compared with 1 or 3 pM BoNT/A.
Figure 7.
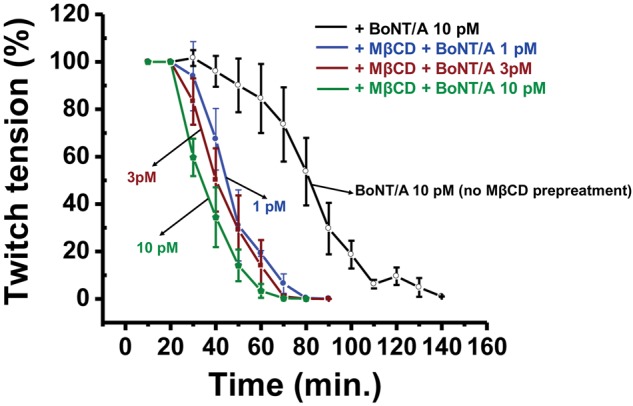
MβCD enhances sensitivity to BoNT/A in the diaphragm NMPs of P7 neonates. Time courses of stimulus evoked twitch tension (mean ± SD) in P7 diaphragm NMPs treated with either αCD (10 mM; 30 min; control) followed by 10 pM BoNT/A (open circle) or MβCD (10 mM; for 30 min) followed by 1, 3 or 10 pM BoNT/A (n = 4–6).
To determine whether MβCD treatment may directly alter the membrane permeability of BoNT/A, we determined the effect of BoNT/A Lc (1 M for 90 min) following MβCD treatment. We expected that if MβCD can directly enhance the internalization of LC/A, then LC/A should suppress the EPP in adult and neonatal (P4) diaphragm NMPs. As shown in Supplementary Figure 5A, LC/A did not cause any paralysis either in the adult or in P4 NMPs.
Previous research suggests that the SNAP-25 isoform determines the differential sensitivity to BoNT/A (Puffer etal., 2001). So, one possibility is that MβCD treatment may enhance the more susceptible form of SNAP-25. We, therefore, analyzed the mRNA levels of SNAP-25 a and b in the developing and adult NMJ but these results were inconclusive due to high cycle time values (data not shown). We therefore, analyzed the expression of SNAP-25 and its cleavage product in the adult and developing NMJ (P4) either treated with BoNT/A (10 pM; 90 min) or pretreatment with αCD (10 mM; 30 min) or MβCD (10 mM; 30 min) prior to BoNT/A treatment (10 pM; 90 min in 40 mM KCl containing HRS). Treatment of BoNT/A (± 10 Mm αCD) did not cause SNAP-25 cleavage in P4 NMPs, while MβCD pretreatment resulted in BoNT/A-induced cleavage of SNAP-25 in P4 NMPs. However, as expected, BoNT/A cleaved SNAP-25 in the adult NMPs. These results are summarized in Supplementary Figure 5C.
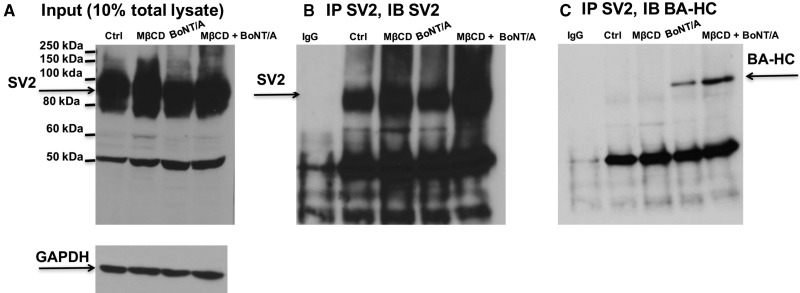
The sensitization of BoNT/A to <P7 neonates and the sped up of onset of inhibitory action of BoNT/A in adult NMPs by MβCD treatment raises an important question. That is, whether depletion of cholesterol by MβCD enhanced the expression of BoNT/A receptor SV2 and its interaction with BoNT/A. We analyzed the expression of SV2 in Neuro 2a cells treated with MβCD (10 mM; 30 min) followed by 90 min. of treatment with BoNT/A in 40 mM KCl containing HRS. As shown in Supplementary Figures 6A and B, MβCD pretreatment increased SV2 expression in the presence of BoNT/A. Next, we evaluated the interaction between SV2 and BoNT/A Hc (designated as BTX-A-HC or BA-HC) by coimmunoprecipitation experiments using antibodies against SV2 and BA-HC (kind gift from Dr Scott Dessain, Lankenau Institute for Medical Research, PA, USA). The expression of SV2 in control (αCD; 10 mM; 30 min), MβCD (10 mM; 30 min) or BoNT/A (1 nM for 90 min after preincubation with either 10 mM αCD or 10 mM MβCD for 30 min) treated Neuro 2a are illustrated in Figure 8A. We performed immunoprecipitation for SV2 and immunoblotted with SV2 antibody (Figure 8B) or BA-HC antibody (Figure 8C) to show that BoNT/A more efficiently interacted with SV2 following MβCD treatment.
Figure 8.
MβCD increases SV2 expression and its interaction with BoNT/A in Neuro 2A cells. A, Western blot showing the expression of SV2 in Input. B, immunoprecipitation with SV2 antibody shows more SV2 under MβCD treated conditions. C, Coimmunoprecipitation showing enhanced interaction of SV2 with BoNT/A under MβCD treated conditions.
Cholesterol Replenishment, via Water Soluble Cholesterol, Delays the Onset of Inhibitory Effect of BoNT/A
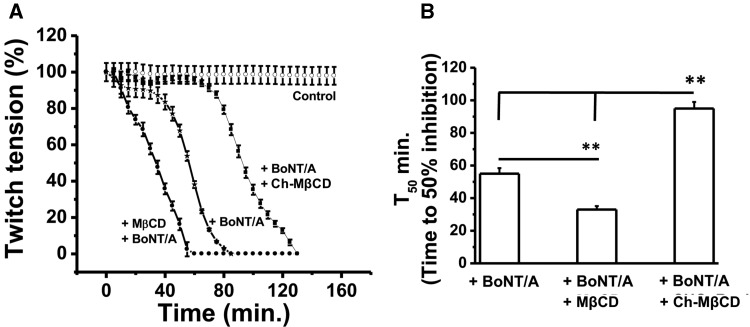
If cholesterol depletion sped up the inhibition of twitch tension by BoNT/A, will cholesterol supplementation with Ch-MβCD prevent this? We performed experiments where NMPs were subjected one of the following treatments: control untreated, BoNT/A treatment in the presence of 10 mM αCD, 30 min in 10 mM MβCD + wash and then BoNT/A or BoNT/A treatment in the presence of 10 mM cholesterol saturated MβCD. The time course of muscle paralysis (Figure 9A) with these treatments clearly points out that BoNT/A produced complete block of NMT by 95 ± 4.43 min while NMPs treated with MβCD and BoNT/A were blocked within about 38.4 ± 2.66 and 51.2 ± 4.85 min, respectively. The beneficial effect of cholesterol supplementation was evident by longer time (133 ± 5.18 min, Figure 9A) required to produce total paralysis of NMP. The data is best shown by calculating time to 50% block with each treatment (Figure 9B).
Figure 9.
Cholesterol supplementation via Ch-MβCD with water soluble cholesterol delays the time to inhibit 50% twitch tension in adult diaphragm NMPs. A. Time courses of stimulus evoked twitch tension in adult diaphragm NMPs treated with either αCD alone (10 mM; 30 min; control), or BoNT/A with and without either MβCD (10 mM; for 30 min preincubation) or MβCD saturated with cholesterol (10 mM; present throughout the experiment). B, Bar graphs represent the mean time to inhibit 50% of stimulus evoked twitch tension ± SEM followed by BoNT/A (10 pM) under the conditions mentioned earlier (n = 5–8 NMPs for each condition).
DISCUSSION
At the adult mammalian NMJ, BoNT/A suppresses ACh release (Baskaran and Thyagarajan, 2014). Although previous research suggests the lack of effect of BoNT/A in neonatal mice following oral administration, the reason for the ability of rat neonates to recover quickly from BoNT/A paralysis or the effect of BoNT/A on immature and developing NMJ have not been studied in detail previously. The enhanced recovery was attributed to return of polyneural innervations in the younger rats (Brown etal., 1981). Also, mice that are <7-days old were resistant to botulism due to negligible systemic absorption of the toxin in these neonates. These data suggest that differential mechanisms regulate toxin’s sensitivity and recovery in neonates compared with adults. However, the role of cholesterol rich LMDs in this process has been analyzed earlier.
This research specifically evaluated the effect of injection of BoNT/A in the hindlimbs of neonates (either <P7 or more than P7) and adult mice. We also evaluated the effect of BoNT/A on the diaphragm NMPs isolated from these groups of mice. We chose to work with diaphragm NMPs due to the ease of access of the tissue as well as due to the nature of diaphragm, being an important tissue for respiratory functions. We present a novel finding that neonatal mice, <7-days old, are resistant to the neuroparalytic effect of BoNT/A, while cholesterol depletion by MβCD sensitized these neonates to BoNT/A.
CDs are routinely used for extracting membrane cholesterol in research. We used 2 kinds of CDs, to perform experiments. αCD, which does not bind cholesterol in the same way as MβCD (Ohtani etal., 1989; Rodal etal., 1999) was used as a control. Since αCD decreased cholera-toxin binding to gangliosides (Ermolinsky etal., 2013), we used it to evaluate its effect on BoNT/A sensitivity at the neonatal and adult NMJ. Both CDs were used at a concentration of 10 mM on neonatal or adult NMJ. Our data suggest that MβCD but not αCD sensitized neonatal mice to BoNT/A. This suggests membrane cholesterol is a critical regulator of BoNT/A sensitivity. Also, it important to note that upon neuroparalysis caused by BoNT/A in MβCD preinjected <P7 neonates, cannibalism was observed with the mother. So, we could not conduct long-term effects of BoNT/A-induced neuroparalysis and recovery aspects in younger neonates.
Cholesterol is an important regulator of synaptic vesicle recycling and neuromuscular functions. Cholesterol depletion suppresses evoked release but enhances spontaneous release at the crayfish NMJ (Zamir and Charlton, 2006). Cholesterol rich LMDs control membrane fluidity and help in the clustering of acetylcholine receptors at the post synaptic nerve terminals (Pato etal., 2008; Stetzkowski-Marden etal., 2006). On the other hand, cholesterol depletion by MβCD enhances BoNT/A activity (Petro etal., 2006) and increases spontaneous neurotransmission and enhances spontaneous vesicle endocytosis (Wasser etal., 2007). Consistently, our data suggest a critical role of cholesterol in regulating endocytic mechanism of BoNT/A in the developing NMJ.
Previous published work suggest that cholesterol depletion by MβCD suppressed stimulus evoked release (Zamir and Charlton, 2006). However, preincubation of NMPs 10 mM MβCD for 30 min followed by washing the NMPs with HEPES Ringer buffer for 30 min did not reduce the EPP or EPC amplitudes however sensitized the NMPs to BoNT/A. Also, performing cholesterol depletion by MβCD at 37°C only slightly decreased (up to 5%) the twitch tension compared with αCD-treated control (Supplementary Figs. 3B and C). These data suggest that perturbation of cholesterol levels at the NMJ facilitated BoNT/A uptake and sensitivity. However, it does not rule out an effect of MβCD that is independent of its ability to chelate cholesterol (Ormerod etal., 2012). Nonetheless, the sensitivity of BoNT/A caused by MβCD was not observed when cholesterol saturated MβCD or αCD was used for invivo BoNT/A injection in the hindlimbs of neonates (Figure 4).
Our data also suggest that the sensitization of neonatal NMPs is associated with a corresponding increase in the frequency of mEPP in both adult and neonatal NMPs (Figure 3C). This observation raised an important question—whether increase in mEPP would facilitate BoNT/A uptake? BoNT/A uptake in NMP is increased upon stimulation (Rummel etal., 2009). It has been shown previously that treatment of NMPs with hyperkalemic buffer (40 or 75 mM KCl extracellularly) significantly facilitated BoNT/A uptake in the adult NMPs as well as Neuro 2a cells (Baskaran etal., 2013; Kitamura etal., 2009; Thyagarajan etal., 2009, 2010). High potassium containing buffers enhanced vesicle fusion density at active zones (Grohovaz etal., 1989) and increased miniature endplate potentials to facilitate the uptake of the neurotoxin. Consistently, MβCD treatment also facilitated BoNT/A uptake (Supplementary Figure 2) and increased mEPPs in neonatal and adult NMPs (Figure 3C). Therefore, it is reasonable to speculate that MβCD would facilitate BoNT/A uptake by increasing spontaneous vesicle fusion, which would expose more synaptic vesicle glycoprotein (SV2) to the membrane to facilitate BoNT/A uptake/. However, the effect of MβCD on gangliosides (another receptor for BoNT/A) still remains to be elucidated at the NMJ. Therefore, an alternate possibility is that MβCD-mediated cholesterol depletion alters the clustering of gangliosides, which would facilitate BoNT/A uptake. Further studies are required to address this mechanism. A third possibility is that the expression of endocytic machinery that governs BoNT/A uptake could not be fully developed in younger neonates. Perhaps, cholesterol depletion induced reorganization of lipid rafts may facilitate BoNT/A endocytosis. Therefore, further studies are required to understand the mechanisms that underlie the resistance to BoNT/A in the developing NMJ.
At the MNTs, synaptic vesicle glycoproteins (SV2), along with gangliosides functions as the receptor for BoNT/A. Experiments performed to evaluate the effect of MβCD on SV2 expression in Neuro 2a cells suggest that MβCD treatment (10 mM for 30 in) significantly increased SV2 expression in these cells. Also, MβCD treatment enhanced the interaction between SV2 and BoNT/A (Figs. 8B and C) in Neuro 2a cells suggesting the facilitation of SV2 availability for toxin binding (Figure 8). Consistent to this observation, a previous report suggests that cholesterol depletion or lipid raft inhibition facilitated the binding of BoNT/A in Neuro 2a cells (Ayyar and Atassi, 2016). Moreover, disruption of lipid rafts enhanced the activity of BoNT/A in cultured Neuro 2a cells possibly by increasing the spontaneous vesicle endocytosis due to cholesterol depletion (Wasser etal., 2007).
If cholesterol depletion sensitizes neonatal mice to BoNT/A and enhanced the effect of BoNT/A in adult NMPs, supplementation of cholesterol via water soluble cholesterol should prevent the effect of MβCD. Consistent to this notion, cholesterol saturated MβCD failed to cause neuroparalysis in younger neonates (P3; Figure 4) and delayed the onset of inhibitory action of BoNT/A on stimulus evoked twitch tension in the adult NMPs (Figure 9).
Collectively, our data suggest that cholesterol is an important determinant of BoNT/A sensitivity at the developing NMJ and perturbation to NMJ cholesterol enhances the uptake and action of the toxin in the neonatal and adult NMJ. This research predominately focused on the effect of toxin following cholesterol depletion. However, how cholesterol depletion modulates the interaction between SNARE complex proteins in developing and adult synapses still remains to be determined. Our data support a model (Supplementary Figure 4) in which cholesterol depletion enhances the effect of BoNT/A by increasing spontaneous vesicle fusion. Nonetheless, how the enhancement of spontaneous vesicle fusion facilitates BoNT/A uptake needs to be answered in the future studies.
The discovery of the lack of sensitivity of BoNT/A in the younger neonates (<7 days of age) and its sensitization by cholesterol depletion suggest that cholesterol is important for the regulation exo-endocytic mechanisms at the NMJ during development.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This work was supported by a pilot project funding from National Institute of General Medical Sciences of the National Institutes of Health under Award Number (8P20GM103432-12) and American Association of Colleges of Pharmacy New Investigator Award to B.T. and F.M. Kirby Foundation Award to J.J.M.
Supplementary Material
REFERENCES
- Ayyar B. V., Atassi M. Z. (2016). Effects of membrane properties on the binding activities of the HN and HC heavy-chain domains of botulinum neurotoxin A. Biochim. Biophys. Acta 1864, 1678–1685. [DOI] [PubMed] [Google Scholar]
- Baskaran P., Lehmann T. E., Topchiy E., Thirunavukkarasu N., Cai S., Singh B. R., Deshpande S., Thyagarajan B. (2013). Effects of enzymatically inactive recombinant botulinum neurotoxin type A at the mouse neuromuscular junctions. Toxicon 72, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran P., Thyagarajan B. (2014). Acute and chronic effects of botulinum neurotoxin a on the mammalian neuromuscular junction. Musc. Nerve 50, 206–215. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Hopkins W. G. (1981). Restoration of focal multiple innervation in rat muscles by transmission block during a critical stage of development. J. Physiol. 318, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumell J. H., Grinstein S. (2003). Role of lipid-mediated signal transduction in bacterial internalization. Cell Microbiol. 5, 287–297. [DOI] [PubMed] [Google Scholar]
- Colon-Saez J. O., Yakel J. L. (2011). The alpha7 nicotinic acetylcholine receptor function in hippocampal neurons is regulated by the lipid composition of the plasma membrane. J. Physiol. 589(Pt 13), 3163–3174. 10.1113/jphysiol.2011.209494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubi R., Candalija A., Ortega A., Gil C., Aguilera J. (2013). Tetanus toxin Hc fragment induces the formation of ceramide platforms and protects neuronal cells against oxidative stress. PLoS One 8, e68055.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M., Yeh F., Tepp W. H., Dean C., Johnson E. A., Janz R., Chapman E. R. (2006). SV2 is the protein receptor for botulinum neurotoxin A. Science 312, 592–596. [DOI] [PubMed] [Google Scholar]
- Ermolinsky B., Peredelchuk M., Provenzano D. (2013). alpha-Cyclodextrin decreases cholera toxin binding to GM1-gangliosides. J. Med. Microbiol. 62(Pt 7), 1011–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohovaz F., Fesce R., Haimann C. (1989). Dual effect of potassium on transmitter exocytosis. Cell Biol. Int. Rep. 13, 1085–1095. [DOI] [PubMed] [Google Scholar]
- Gutmann E., G L., Medawar P. B., Young J. Z. (1942). The rate of regeneration of the nerve. J. Exp. Biol. 19, 14–44. [Google Scholar]
- Herreros J., Ng T., Schiavo G. (2001). Lipid rafts act as specialized domains for tetanus toxin binding and internalization into neurons. Mol. Biol. Cell 12, 2947–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerzmann A., Feig A. L. (2006). Cholesterol, it’s not just for heart disease anymore. ACS Chem. Biol. 1, 141–144. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Matsuka Y., Spigelman I., Ishihara Y., Yamamoto Y., Sonoyama W., Kamioka H., Yamashiro T., Kuboki T., Oguma K. (2009). Botulinum toxin type a (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constriction. Neuroscience 159, 1422–1429. [DOI] [PubMed] [Google Scholar]
- Kravtsova V. V., Petrov A. M., Vasil’ev A. N., Zefirov A. L., Krivoi I. I. (2015). Role of cholesterol in the maintenance of endplate electrogenesis in rat diaphragm. Bull. Exp. Biol. Med. 158, 298–300. [DOI] [PubMed] [Google Scholar]
- Mahrhold S., Rummel A., Bigalke H., Davletov B., Binz T. (2006). The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 580, 2011–2014. [DOI] [PubMed] [Google Scholar]
- Marks J. D. (2004). Medical aspects of biologic toxins. Anesthesiol. Clin. N. Am. 22, 509–532. vii, [DOI] [PubMed] [Google Scholar]
- Ohtani Y., Irie T., Uekama K., Fukunaga K., Pitha J. (1989). Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 186, 17–22. [DOI] [PubMed] [Google Scholar]
- Ormerod K. G., Rogasevskaia T. P., Coorssen J. R., Mercier A. J. (2012). Cholesterol-independent effects of methyl-beta-cyclodextrin on chemical synapses. PLoS One 7, e36395.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pato C., Stetzkowski-Marden F., Gaus K., Recouvreur M., Cartaud A., Cartaud J. (2008). Role of lipid rafts in agrin-elicited acetylcholine receptor clustering. Chem. Biol. Interact. 175, 64–67. [DOI] [PubMed] [Google Scholar]
- Petro K. A., Dyer M. A., Yowler B. C., Schengrund C. L. (2006). Disruption of lipid rafts enhances activity of botulinum neurotoxin serotype A. Toxicon 48, 1035–1045. [DOI] [PubMed] [Google Scholar]
- Petrov A. M., Kasimov M. R., Giniatullin A. R., Tarakanova O. I., Zefirov A. L. (2010). The role of cholesterol in the exo- and endocytosis of synaptic vesicles in frog motor nerve endings. Neurosci. Behav. Physiol. 40, 894–901. [DOI] [PubMed] [Google Scholar]
- Petrov A. M., Yakovleva A. A., Zefirov A. L. (2014). Role of membrane cholesterol in spontaneous exocytosis at frog neuromuscular synapses: Reactive oxygen species-calcium interplay. J. Physiol. 592, 4995–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirazzini M., Azarnia Tehran D., Zanetti G., Lista F., Binz T., Shone C. C., Rossetto O., Montecucco C. (2015). The thioredoxin reductase–Thioredoxin redox system cleaves the interchain disulphide bond of botulinum neurotoxins on the cytosolic surface of synaptic vesicles. Toxicon 107(Pt A), 32–36. [DOI] [PubMed] [Google Scholar]
- Puchkov D., Haucke V. (2013). Greasing the synaptic vesicle cycle by membrane lipids. Trends Cell Biol. 23, 493–503. [DOI] [PubMed] [Google Scholar]
- Puffer E. B., Lomneth R. B., Sarkar H. K., Singh B. R. (2001). Differential roles of developmentally distinct SNAP-25 isoforms in the neurotransmitter release process. Biochemistry 40, 9374–9378. [DOI] [PubMed] [Google Scholar]
- Rituper B., Flasker A., Gucek A., Chowdhury H. H., Zorec R. (2012). Cholesterol and regulated exocytosis: A requirement for unitary exocytotic events. Cell Calcium 52, 250–258. [DOI] [PubMed] [Google Scholar]
- Rodal S. K., Skretting G., Garred O., Vilhardt F., van Deurs B., Sandvig K. (1999). Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10, 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues H. A., Lima R. F., Fonseca Mde C., Amaral E. A., Martinelli P. M., Naves L. A., Gomez M. V., Kushmerick C., Prado M. A., Guatimosim C. (2013). Membrane cholesterol regulates different modes of synaptic vesicle release and retrieval at the frog neuromuscular junction. Eur. J. Neurosci. 38, 2978–2987. [DOI] [PubMed] [Google Scholar]
- Rummel A., Hafner K., Mahrhold S., Darashchonak N., Holt M., Jahn R., Beermann S., Karnath T., Bigalke H., Binz T. (2009). Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J Neurochem 110, 1942–1954. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Huang H., McIntosh A. L., Atshaves B. P., Martin G. G., Kier A. B. (2010). Caveolin, sterol carrier protein-2, membrane cholesterol-rich microdomains and intracellular cholesterol trafficking. Subcell Biochem. 51, 279–318. [DOI] [PubMed] [Google Scholar]
- Schwan C., Nolke T., Kruppke A. S., Schubert D. M., Lang A. E., Aktories K. (2011). Cholesterol- and sphingolipid-rich microdomains are essential for microtubule-based membrane protrusions induced by Clostridium difficile transferase (CDT). J. Biol. Chem. 286, 29356–29365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. S., Gao Z., Abraham S. N. (1999). Bacteria-host cell interaction mediated by cellular cholesterol/glycolipid-enriched microdomains. Biosci. Rep. 19, 421–432. [DOI] [PubMed] [Google Scholar]
- Shogomori H., Futerman A. H. (2001). Cholesterol depletion by methyl-beta-cyclodextrin blocks cholera toxin transport from endosomes to the Golgi apparatus in hippocampal neurons. J. Neurochem. 78, 991–999. [DOI] [PubMed] [Google Scholar]
- Stetzkowski-Marden F., Recouvreur M., Camus G., Cartaud A., Marchand S., Cartaud J. (2006). Rafts are required for acetylcholine receptor clustering. J. Mol. Neurosci. 30, 37–38.. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Mills D. C. (1978). Intraintestinal toxin in infant mice challenged intragastrically with Clostridium botulinum spores. Infect. Immun. 21, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakanova O. I., Petrov A. M., Zefirov A. L. (2011). The role of membrane cholesterol in neurotransmitter release from motor nerve terminals. Dokl. Biol. Sci. 438, 138–140. [DOI] [PubMed] [Google Scholar]
- Thyagarajan B., Krivitskaya N., Potian J. G., Hognason K., Garcia C. C., McArdle J. J. (2009). Capsaicin protects mouse neuromuscular junctions from the neuroparalytic effects of botulinum neurotoxin a. J. Pharmacol. Exp. Ther. 331, 361–371. 10.1124/jpet.109.156901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan B., Potian J. G., Garcia C. C., Hognason K., Capkova K., Moe S. T., Jacobson A. R., Janda K. D., McArdle J. J. (2010). Effects of hydroxamate metalloendoprotease inhibitors on botulinum neurotoxin A poisoned mouse neuromuscular junctions. Neuropharmacology 58, 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser C. R., Ertunc M., Liu X., Kavalali E. T. (2007). Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J. Physiol. 579(Pt 2), 413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisemann J., Stern D., Mahrhold S., Dorner B. G., Rummel A. (2016). Botulinum neurotoxin serotype a recognizes its protein receptor SV2 by a different mechanism than botulinum neurotoxin B synaptotagmin. Toxins (Basel) 85, pii: E154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue H. Y., Xu J. (2015). Cholesterol regulates multiple forms of vesicle endocytosis at a mammalian central synapse. J. Neurochem. 134, 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir O., Charlton M. P. (2006). Cholesterol and synaptic transmitter release at crayfish neuromuscular junctions. J Physiol 571(Pt 1), 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zefirov A. L., Petrov A. M. (2010). [Lipids in the process of synaptic vesicle exo- and endocytosis]. Ross Fiziol. Zh. Im. I. M. Sechenova. 96, 753–765. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.