Abstract
Background: There is limited knowledge about the optimal timing of antiretroviral treatment initiation in older children and adolescents.
Methods: A total of 20 576 antiretroviral treatment (ART)-naïve patients, aged 1-16 years at enrolment, from 19 cohorts in Europe, Southern Africa and West Africa, were included. We compared mortality and growth outcomes for different ART initiation criteria, aligned with previous and recent World Health Organization criteria, for 5 years of follow-up, adjusting for all measured baseline and time-dependent confounders using the g-formula.
Results: Median (1st;3rd percentile) CD4 count at baseline was 676 cells/mm3 (394; 1037) (children aged ≥ 1 and < 5 years), 373 (172; 630) (≥ 5 and < 10 years) and 238 (88; 425) (≥ 10 and < 16 years). There was a general trend towards lower mortality and better growth with earlier treatment initiation. In children < 10 years old at enrolment, by 5 years of follow-up there was lower mortality and a higher mean height-for-age z-score with immediate ART initiation versus delaying until CD4 count < 350 cells/mm3 (or CD4% < 15% or weight-for-age z-score < -2) with absolute differences in mortality and height-for-age z-score of 0.3% (95% confidence interval: 0.1%; 0.6%) and -0.08 (-0.09; -0.06) (≥ 1 and < 5 years), and 0.3% (0.04%; 0.5%) and -0.07 (-0.08; -0.05) (≥ 5 and < 10 years). In those aged > 10 years at enrolment we did not find any difference in mortality or growth with immediate ART initiation, with estimated differences of -0.1% (-0.2%; 0.6%) and -0.03 (-0.05; 0.00), respectively. Growth differences in children aged < 10 years persisted for treatment thresholds using higher CD4 values. Regular follow-up led to better height and mortality outcomes.
Conclusions: Immediate ART is associated with lower mortality and better growth for up to 5 years in children < 10 years old. Our results on adolescents were inconclusive.
Keywords: Antiretroviral treatment, paediatrics, g-formula, causal inference
Introduction
The World Health Organization (WHO) recommendations on when to start treatment in children and adolescents have changed substantially in recent years. In 2006, antiretroviral treatment (ART) was only recommended for children and adolescents with advanced or severe HIV-associated clinical disease or if CD4 count (or CD4%) fell below an age-dependent critical value.1,2 Reasons for delaying therapy initiation were due to concerns about toxicities, non-adherence, drug resistance, logistical challenges, cost considerations and limited future options for patients failing therapy.3–8
An increasing body of evidence from recent years suggests that delaying ART initiation for too long may be harmful: the results of the CHER trial, which showed a striking mortality benefit for immediate ART initiation in children less than 3 months of age, prompted WHO to recommend ART in all HIV-positive children presenting under the age of 1 year.9 CD4 treatment-initiation thresholds for older children and adolescents persist, but were gradually increased in 2010 and 2013.10–12 From 2013, WHO recommended ART for children older than 5 years and for non-pregnant adolescents with asymptomatic or mild clinical disease when CD4 count is below 500 cells/mm3. Children younger than 5 years were to be started immediately irrespective of CD4 count. These changes were motivated by programmatic and operational considerations favouring simplified treatment guidelines, the rapid decline in CD4 among children presenting with CD4 above the threshold for treatment initiation and no demonstrated harm of immediate treatment in the PREDICT trial or causal modelling studies.13–17 The newly released WHO 2015 guidelines recommend universal ART for all.18
However, large evidence gaps remain: the PREDICT trial enrolled children aged 1-12 years but was underpowered (due to the lower than expected event rate) and did not include older adolescents.15 Causal modelling studies only included children younger than 5 years.16,17 The optimal timing of treatment initiation in adolescents, a key population in the HIV epidemic, is unknown. This is concerning, as findings from adult studies, including the morbidity benefit associated with immediate ART found in the START and TEMPRANO trials,19,20 may not apply to adolescents – due to different lifestyle factors, adherence, modes of infection and the effects of puberty, including rapid physical and neurological change. Moreover, all the above studies implicitly assume that children come for regular visits, typically 3-monthly. It is possible that in real-world settings (with less frequent or missed visits and lag in ART initiation after meeting the treatment initiation criteria) the effects of different treatment initiation criteria differs from idealized study conditions. Also, there are no data after 3 years of follow-up although possible disadvantages of immediate treatment initiation may only be visible in the long term, for example for children failing multiple treatments or with drug complications. In addition, all mentioned studies evaluate criteria which are not exactly identical and comparable to WHO criteria.
In order to inform the WHO 2015 guidelines on the timing of treatment initiation, we attempted to address the above evidence gaps by analysing observational data from West Africa, Southern Africa and Europe, adjusting for time-dependent confounders affected by previous treatment by using the g-formula.21–24 We chose the g-formula since traditional multivariate regression techniques may yield biased treatment effect estimates in our context. We compared mortality and growth outcomes for different treatment initiation rules, aligned with previous and recent WHO criteria, for patients 1-16 years of age and with up to 5 years of follow-up. We consider both a best-case scenario, which uses similar assumptions to other studies regarding visit frequency and prompt ART initiation once treatment thresholds are met, and an alternative scenario which uses assumptions that aim to resemble the real-world situation in some of our cohorts.
Methods
Study population
We used data from 19 cohorts of the IeDEA Southern Africa, IeDEA West Africa and COHERE in EuroCoord collaborations, representing 11 countries (Supplementary Table 1, available as Supplementary data at IJE online).25–29 All contributing cohorts obtained ethical approval from the relevant institutions before submitting anonymized patient data to the networks. Patients aged ≥ 1 year of age and presenting before their 16th birthday were included if their first clinic visit was no earlier than 1 January 1996 to ensure that every patient received combination antiretroviral therapy, and those that received antiretrovirals had at least one pre-ART visit recorded. Database closure was 31 December 2014.
Variables and definitions
For our analysis we used children’s age at enrolment, health care facility, sex, date of ART initiation and year of enrolment as well as CD4 count, CD4%, weight-for-age z-scores (WAZ, if ≤ 10 years), and body mass index (BMI)-for-age z-scores (BMIAZ, if ≥ 5 years)–both at time of enrolment and during follow-up. The outcomes variables were height-for-age z-score (HAZ) and death. Most sites measured supine length until a child was comfortable to stand, though some sites worked with supine length until the age of 2 years. All z-scores were based on WHO definitions, i.e. standards developed by the WHO Multicenter Growth Reference Study conducted between 1997 and 2003 in multifaceted settings.30 Motivated by historical initiation criteria, we defined the following three age groups: 1-5 years (AG1, ≥ 1 and < 5 years), 5-10 years (AG2, ≥ 5 and < 10 years), and 10-16 years (AG3, ≥ 10 and < 16 years).
Follow-up data were evaluated at 3-monthly intervals for a period of up to 5 years. Data were defined to be missing if no data were available for a particular interval. Children were defined as lost to follow-up (LTFU), and censored, if LTFU was confirmed or if at the time of database closure they had had no contact with their health care facility for at least 12 months since their last recorded visit.
We carried forward previous values for missing CD4 count, CD4%, WAZ and HAZ follow-up data until a patient was censored or died. To deal with missing baseline data we used the expectation-maximization-bootstrap algorithm for multiple imputation.31 The imputation model included all baseline and follow-up variables (including lagged and lead versions of them), death, LTFU, both a carry-forward and an attended visit indicator variable and region. The algorithm accounted for the non-linear and longitudinal structure of the data.
Statistical analyses
Both baseline and follow-up data were summarized with medians (first;third quartile) and by proportions (categorical data). HAZ trajectories, stratified by age and region, were displayed smoothly using additive models.32,33 We used the g-formula21–23 to estimate cumulative mortality and growth (mean HAZ) for up to 5 years of follow-up for different treatment initiation criteria. The criteria differ by age group and are based on recent and old guidelines, see Table 1 for a comprehensive overview.
Table 1.
Intervention rules used in all g-formula analyses
| Strategy | Motivationa | ||
|---|---|---|---|
| ≥ 1 & < 5 years | i) | Start ART immediately, irrespective of CD4 count | =WHO 2013 criterion |
| ii) | Start ART if CD4 count < 750 cells/mm3 or CD4% < 25% or WAZ < -2 (as a proxy for a severe event) | ≈WHO 2010 criterion | |
| iii) | Start ART if CD4 count < 350 cells/mm3 or CD4% < 15% or WAZ < -2 (as a proxy for a severe event) | ≈WHO 2006 criterionb | |
| iv) | Do not start ART | reference criterion | |
| ≥ 5 & < 10 years | i) | Start ART immediately, irrespective of CD4 count | =WHO 2015 criterion |
| ii) | Start ART if CD4 count < 500 cells/mm3 or WAZ < -2 (as a proxy for a severe event) | ≈WHO 2013 criterion | |
| iii) | Start ART if CD4 count < 350 cells/mm3 or WAZ <-2 (as a proxy for a severe event) | ≈WHO 2010 criterion | |
| iv) | Start ART if CD4 count < 200 cells/mm3 or WAZ < -2 (as a proxy for a severe event) | ≈WHO 2006 criterionc | |
| v) | Do not start ART | reference criterion | |
| ≥ 10 & < 16 yearsd | i) | Start ART immediately, irrespective of CD4 count | =WHO 2015 criterion |
| ii) | Start ART if CD4 count < 500 cells/mm3 | ≈WHO 2013 criterion | |
| iii) | Start ART if CD4 count < 350 cells/mm3 | ≈WHO 2010 criterion | |
| iv) | Start ART if CD4 count < 200 cells/mm3 | ≈WHO 2006 criterion | |
| v) | Do not start ART | reference criterion |
See [http://www.who.int/hiv/pub/guidelines/en] for WHO guideline documents
For children aged 36-59 months.
WHO 2006 criteria for children aged ≥ 5 years also supported ART initiation if CD4% < 15%.
We do not use WAZ for treatment assignment because there are no WAZ standards for children aged ≥ 10 years. We also do not use BMIAZ for treatment assignment since there is no clear threshold to approximate severe clinical events.
With the g-formula we took into account time-dependent confounding affected by previous treatment. Time-dependent variables which affected both treatment assignment and the outcome were clinical stage, CD4 count and CD4% (for children ≤ 10). We followed the approach of previous work16,17 and approximated stage with WAZ (BMIAZ for AG3) since many stage-defining events in our context, such as persistent diarrhoea or tuberculosis, are likely to affect a child’s weight. Our algorithm implementing the g-formula required comprehensive model fitting for the time-varying variables and the outcome, which we utilized using additive models, non-linear interactions and model selection (Supplementary Textbox 1, available as Supplementary data at IJE online).
In our main analysis, we used similar assumptions as Schomaker et al.:16 we estimated all counterfactual outcomes under no administrative censoring, no loss to follow-up, full adherence to the regimen, immediate ART initiation after reaching eligibility and regular (3-monthly) follow-up. We further assumed no unmeasured confounding and no model mis-specification. We present results separately for each age group and for all patients presenting with a CD4 count > 500 cells/mm3. In an alternative secondary analysis, we changed our assumptions: we do not assume regular follow-up, but rather infrequent follow-up which we modelled based on the visit frequency in our data. In addition, we assumed that treatment is started at one visit after reaching eligibility; see Supplementary Textbox 1 and other work for implementation details.16,34,35 To explore whether our implicit assumptions of correct model specification and non-informative censoring were likely met or not, we compared the estimates of the g-formula under no treatment strategy (‘natural course scenario’) with the observed data. All results are presented with 95% nonparametric bootstrap confidence intervals (CI). Our analyses were implemented in R.36
Results
Descriptive r esults
Of the 20 576 patients included in our study, most came from the youngest age group (42.1%) and from Southern Africa (78.9%). The median follow-up time was 900 (366;1827) days and 37.2% of our patients did not start ART during follow-up (Table 2). About 29%, 35% and 7% of patients met our LTFU definition in Southern Africa, West Africa and Europe, respectively. Most deaths (53.7%) occurred during the first 6 months after the first visit (Supplementary Table 2, available as Supplementary data at IJE online).
Table 2.
Patient characteristics at the first visit, stratified by region and age group
| Europe (N = 991) | Southern Africa (N = 16230) | West Africa (N = 3355) | 1-5 years (N = 8665) | 5-10 years (N = 7358) | 10-16 years (N = 4553) | Total (N = 20576) | |
|---|---|---|---|---|---|---|---|
| Sex | 991 (100%) | 16206 (99.9%) | 3355 (100%) | 8656 (99.9%) | 7352 (99.9%) | 4544 (99.8%) | 20552 (99.0%) |
| male | 468 (47.2%) | 7888 (48.7%) | 1708 (50.9%) | 4419 (51.1%) | 3644 (49.6%) | 2001 (44.4%) | 10064 (49.0%) |
| Age | 991 (100%) | 16230 (100%) | 3355 (100%) | 8665 (100%) | 7358 (100%) | 4553 (100%) | 20576 (100%) |
| Median (1st; 3rd quartile) | 6.08 (3.18; 9.73) | 5.99 (2.98; 9.64) | 5.51 (2.9; 8.78) | 2.56 (1.7; 3.74) | 7.19 (6.06; 8.46) | 12.42 (11.15; 14.04) | 5.94 (2.98; 9.48) |
| CD4 count | 835 (84.3%) | 11077 (68.3%) | 2651 (79.0%) | 6019 (69.5%) | 5272 (71.7%) | 3272 (71.9%) | 14563 (70.8%) |
| Median (1st; 3rd quartile) | 555 (299; 879) | 421 (200; 725) | 489 (198; 841) | 676 (394; 1037) | 373 (172; 630) | 237.5 (88; 425) | 440 (205; 757) |
| CD4% | 799 (80.6%) | 9668 (59.6%) | 2012 (60.0%) | 5471 (63.1%) | 4515 (61.4%) | 2493 (54.8%) | 12479 (60.7%) |
| Median (1st; 3rd quartile) | 20 (13; 27) | 15 (9; 23) | 15 (8; 22) | 17 (11; 24) | 15 (8; 23) | 13 (6; 20) | 16 (9; 23) |
| WAZ | 615 (62.1%)a | 8189 (50.5%)a | 2002 (59.7%)a | 5956 (68.7%) | 4850 (65.9%) | 10806 (52.5%)a | |
| Median (1st; 3rd quartile) | −0.07 (-0.87; 0.69) | −1.46 (-2.49; -0.6) | −1.94 (-3.34; -0.98) | −1.46 (-2.66; -0.5) | −1.47 (-2.48; -0.62) | −1.46 (-2.57; -0.56) | |
| HAZ | 767 (77.4%) | 8545 (52.7%) | 2125 (63.3%) | 4834 (55.8%) | 4178 (56.8%) | 2425 (53.3%) | 11437 (55.6%) |
| Median (1st; 3rd quartile) | −0.65 (-1.44; 0.21) | −2.24 (-3.17; -1.29) | −1.98 (-2.94; -0.97) | −2.37 (-3.43; -1.25) | −1.81 (-2.71; -0.94) | −2.12 (-3.01; -1.21) | −2.08 (-3.07; -1.1) |
| BMIAZ | 474 (47.8%)1 | 4905 (30.2%)a | 1125 (33.5%)a | 4116 (55.9%) | 2388 (52.5%) | 6504 (31.6%)a | |
| Median (1st; 3rd quartile) | 0.24 (-0.46; 0.94) | −0.56 (-1.45; 0.21) | −1.4 (-2.72; -0.43) | −0.42 (-1.3; 0.32) | −1.02 (-2.08; -0.15) | −0.62 (-1.61; 0.19) | |
| ART started | 991 (100%) | 16230 (100%) | 3355 (100%) | 8665 (100%) | 7358 (100%) | 4553 (100%) | 20576 (100%) |
| ever | 803 (81.0%) | 10289 (63.4%) | 2031 (60.5%) | 5602 (64.7%) | 4723 (64.2%) | 2798 (61.5%) | 13123 (63.8%) |
| Treatment start | 991 (100%) | 16230 (100%) | 3355 (100%) | 8665 (100%) | 7358 (100%) | 4553 (100%) | 20576 (100%) |
| (1995, 1999] | 142 (14.3%) | 69 (0.4%) | 1 (0.0%) | 133 (1.5%) | 64 (0.9%) | 15 (0.3%) | 212 (1.0%) |
| (1999, 2004] | 433 (43.7%) | 1663 (10.3%) | 679 (20.2%) | 1432 (16.5%) | 983 (13.4%) | 360 (7.9%) | 2775 (13.5%) |
| (2004, 2007] | 235 (23.7%) | 5571 (34.3%) | 1289 (38.4%) | 3165 (36.5%) | 2629 (35.7%) | 1301 (28.6%) | 7095 (34.5%) |
| (2007, 2010] | 132 (13.3%) | 6033 (37.2%) | 1047 (31.2%) | 2763 (31.9%) | 2605 (35.4%) | 1844 (40.5%) | 7212 (35.1%) |
| (2010, 2014] | 49 (4.9%) | 2894 (17.8%) | 339 (10.1%) | 1172 (13.5%) | 1077 (14.6%) | 1033 (22.7%) | 3282 (16.0%) |
| Follow-up time (days) | |||||||
| Median (1st; 3rd quartile) | 1827 (1612; 1827) | 824 (285; 1785) | 829 (366; 1813) | 969 (366; 1827) | 982 (366; 1827) | 656 (217; 1538) | 900 (366; 1827) |
Note that WAZ is not calculated for children > 10 years, and BMIAZ is not calculated for children < 5 years; thus, reported percentages seem small.
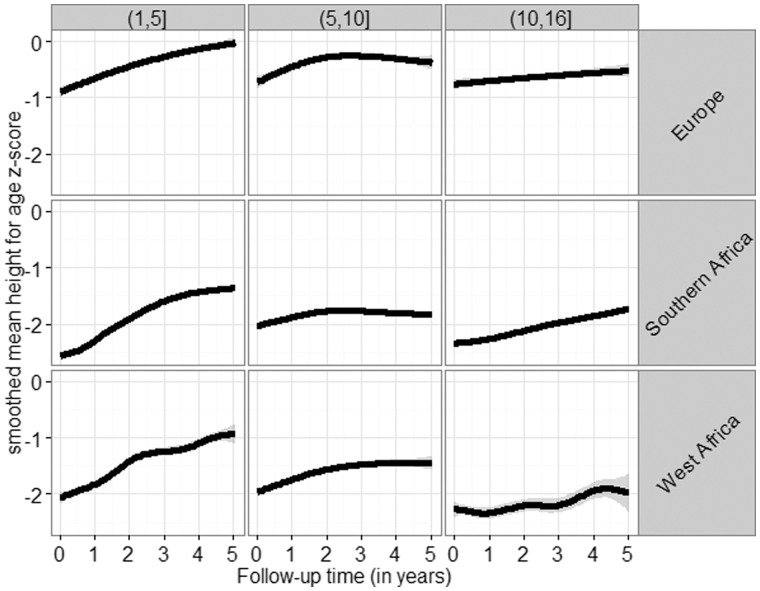
Both baseline and follow-up characteristics differed markedly between regions and age groups (Table 2; Supplementary Table 3, available as Supplementary data at IJE online): European children presented with substantially higher CD4 count, CD4%, WAZ, HAZ and BMIAZ than African children. Children from both African regions had similar baseline characteristics, though West African children had higher HAZ but lower WAZ at baseline. Older children had lower CD4 counts and lower CD4% than younger children. All characteristics improved gradually during follow-up (Supplementary Table 3). Of note, the shapes of growth trajectories were very similar for all three regions but differed by age group: children aged 1-5 showed clear improvement during the whole follow-up period, whereas growth in children aged 5-10 plateaued after 2-3 years. On average, adolescents showed a slow but steady increase in mean HAZ (Figure 1).
Figure 1.
Estimated growth trajectories in the raw data, stratified by region and age group.
About 79.1% of the European adolescents were confirmed perinatally infected, and for 13.2% the mode of infection was unknown. We had no data on mode of infection for African adolescents.
A nalyses using g-formula
Our implementations of the g-formula were in general able to reproduce the relevant data characteristics in the natural course scenario (Supplementary Figures 8-9, available as Supplementary data at IJE online). Some deviations for the mean HAZ measurements in the 5th year of follow-up for AG2 indicate caution with respect to these results. We therefore report only results up to 4 years for the growth analysis of this age group.
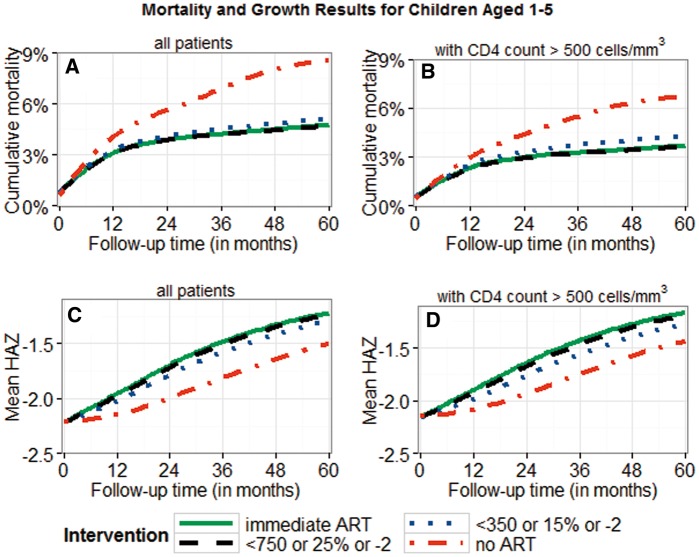
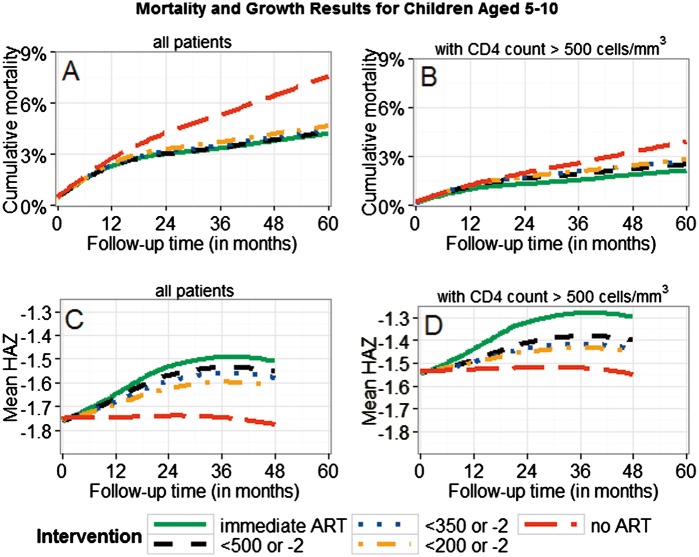
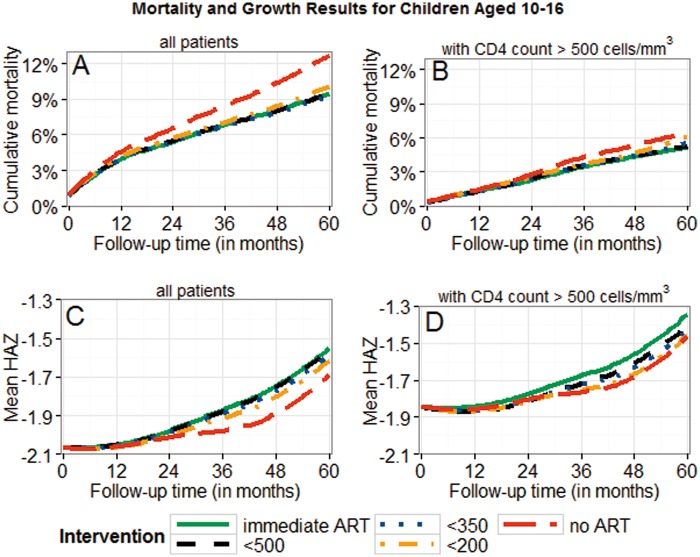
There was a trend towards lower mortality and better growth for earlier treatment initiation (Figures 2–4; Supplementary Figure 7, available as Supplementary data at IJE online). The mortality differences between immediate ART initiation and thresholds using CD4 count ≤ 350 cells/mm3 were clear in children ≤ 10 years, but not very clear for higher thresholds or for adolescents (95% CIs in Table 3a). Growth differences with respect to different treatment interventions were more pronounced than the mortality differences, suggesting clear benefits of immediate ART initiation in all age groups.
Figure 2.
Cumulative mortality and mean HAZ for children aged ≥ 1 and < 5 years. Results are displayed for different intervention strategies and patient groups; 95% bootstrap confidence intervals for absolute estimates and estimated differences between strategies are listed in Table 3a. All results were obtained using the g-formula. Treatment thresholds refer to CD4 count (< 350/750), CD4% (< 15%/25%), and WAZ < -2). Panels B and D are restricted to patients presenting with CD4 count > 500 cells/mm3.
Table 3.
Estimates obtained from the g-formula: cumulative mortality at 5 years, cumulative mortality difference (MD) between interventions at 5 years, mean measured HAZ at 5 years, and mean measured HAZ difference (MHD) at 5 years. Results are shown (a) for the main scenario (3-month follow-up as in many trials) and for the alternative scenario (irregular follow-up with visit frequency modelled based on our data). All results are reported with 95% bootstrap confidence intervals. Treatment thresholds refer to CD4 count (< 200/350/500/750), CD4% (< 15%/25%), and WAZ (< -2)
| (a) Regular follow-up (3-monthly, as in a trial) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | MD | Mean HAZ | MHD | |||||||||
| Age 1-5 | 95% CI | 95% CI | 95% CI | 95% CI | ||||||||
| no ART | 8.4% | 7.5% | 10.4% | 3.7% | 3.5% | 5.5% | −1.53 | −1.65 | −1.41 | −0.29 | −0.36 | −0.21 |
| < 350/15%/-2 | 5.0% | 4.6% | 5.9% | 0.3% | 0.1% | 0.6% | −1.32 | −1.41 | −1.21 | −0.08 | −0.09 | −0.06 |
| < 750/25%/-2 | 4.7% | 4.3% | 5.5% | 0.0% | −0.1% | 0.2% | −1.26 | −1.36 | −1.16 | −0.02 | −0.03 | −0.004 |
| Immediate | 4.7% | 4.3% | 5.5% | – | – | – | −1.24 | −1.34 | −1.15 | – | – | – |
| Age 5-10 | ||||||||||||
| no ART | 7.4% | 6.5% | 9.3% | 3.3% | 2.3% | 4.9% | −1.88 | −1.94 | −1.77 | −0.34 | −0.37 | −0.27 |
| < 200/-2 | 4.6% | 4.1% | 5.5% | 0.5% | 0.2% | 0.8% | −1.66 | −1.73 | −1.59 | −0.12 | −0.14 | −0.09 |
| < 350/-2 | 4.4% | 3.9% | 5.3% | 0.3% | 0.04% | 0.5% | −1.61 | −1.69 | −1.55 | −0.07 | −0.08 | −0.05 |
| < 500/-2 | 4.2% | 3.8% | 5.2% | 0.1% | −0.1% | 0.3% | −1.59 | −1.65 | −1.52 | −0.05 | −0.06 | −0.02 |
| Immediate | 4.1% | 3.7% | 5.1% | – | – | – | −1.54 | −1.62 | −1.48 | – | – | – |
| Age 10-16 | ||||||||||||
| no ART | 12.5% | 11.1% | 15.3% | 3.2% | 2.4% | 4.8% | −1.72 | −1.85 | −1.49 | −0.15 | −0.21 | −0.07 |
| < 200 | 10.0% | 9.1% | 12.0% | 0.7% | 0.2% | 1.1% | −1.63 | −1.75 | −1.41 | −0.06 | −0.09 | −0.03 |
| < 350 | 9.2% | 8.7% | 11.3% | −0.1% | −0.2% | 0.6% | −1.60 | −1.60 | −1.39 | −0.03 | −0.05 | 0.0 |
| < 500 | 9.4% | 8.6% | 11.1% | 0.1% | −0.3% | 0.4% | −1.58 | −1.70 | −1.38 | −0.01 | −0.03 | 0.01 |
| Immediate | 9.3% | 8.5% | 11.1% | – | – | – | −1.57 | −1.69 | −1.36 | – | – | – |
|
| ||||||||||||
|
(b) Irregular follow-up (visit frequency modelled based on our data) |
||||||||||||
| Mortality | MD | Mean HAZ | MHD | |||||||||
|
| ||||||||||||
| Age 1-5 | 95%CI | 95%CI | 95%CI | 95%CI | ||||||||
| no ART | 7.7% | 6.8% | 9.0% | 2.0% | 1.2% | 2.9% | −1.66 | −1.78 | −1.55 | −0.28 | −0.33 | −0.26 |
| < 350/15%/-2 | 5.9% | 5.4% | 6.7% | 0.2% | 0.0% | 0.6% | −1.47 | −1.59 | −1.36 | −0.09 | −0.11 | −0.07 |
| < 750/25%/-2 | 5.8% | 5.3% | 6.5% | 0.1% | −0.2% | 0.3% | −1.40 | −1.51 | −1.29 | −0.02 | −0.04 | −0.01 |
| Immediate | 5.7% | 5.2% | 6.4% | – | – | – | −1.38 | −1.48 | −1.27 | – | – | – |
| Age 5-10 | ||||||||||||
| no ART | 7.1% | 5.7% | 8.4% | 1.9% | 0.8% | 2.6% | −1.77 | −1.85 | −1.72 | −0.12 | −0.15 | −0.10 |
| < 200/-2 | 5.5% | 4.7% | 6.3% | 0.3% | 0.0% | 0.5% | −1.71 | −1.80 | −1.66 | −0.06 | −0.08 | −0.05 |
| < 350/-2 | 5.5% | 4.7% | 6.2% | 0.3% | −0.1% | 0.4% | −1.70 | −1.78 | −1.65 | −0.05 | −0.06 | −0.03 |
| < 500/-2 | 5.4% | 4.5% | 6.2% | 0.2% | −0.1% | 0.3% | −1.68 | −1.76 | −1.63 | −0.03 | −0.04 | −0.02 |
| Iimmediate | 5.2% | 4.6% | 6.0% | – | – | – | −1.65 | −1.74 | −1.60 | – | – | – |
| Age 10-16 | ||||||||||||
| no ART | 12.2% | 10.8% | 14.2% | 1.9% | 1.3% | 2.6% | −1.77 | −1.91 | −1.67 | −0.04 | −0.05 | −0.01 |
| < 200 | 11.0% | 9.6% | 12.4% | 0.7% | 0.0% | 0.9% | −1.76 | −1.89 | −1.67 | −0.03 | −0.04 | 0.00 |
| < 350 | 10.6% | 9.4% | 12.1% | 0.3% | −0.2% | 0.6% | −1.65 | −1.89 | −1.66 | −0.01 | −0.03 | 0.01 |
| < 500 | 10.4% | 9.2% | 11.9% | 0.1% | −0.3% | 0.4% | −1.74 | −1.88 | −1.66 | −0.01 | −0.01 | 0.01 |
| Immediate | 10.3% | 9.2% | 12.0% | – | – | – | −1.73 | −1.88 | −1.66 | – | – | – |
Patients presenting with CD4 count > 500 cells/mm3 had lower mortality and higher mean HAZ values than other patients (Figures 2-4). The mortality and growth differences at 5 years for immediate ART initiation versus delaying ART until CD4 count < 750 cells/mm3 (or CD4% < 25% or WAZ < -2), as shown in Figure 2, were 0% (-0.1%;0.3%) and 0.03 (0.01;0.04) for AG1. Comparing immediate ART initiation with deferring ART until CD4 count < 500 cells/mm3 (or WAZ < -2), as shown in Figures 3 and 4, yielded differences of 0.4% (0.1%;0.6%) and 0.10 (0.07;0.12) for AG2 and 0.1% (-0.1%;0.9%) and 0.06 (-0.01;0.09) for AG3.
Figure 3.
Cumulative mortality and mean HAZ for children aged ≥ 5 and < 10 years. Results are displayed for different intervention strategies and patient groups; 95% bootstrap confidence intervals for absolute estimates and estimated differences between strategies are listed in Table 3a. All results were obtained using the g-formula. Treatment thresholds refer to CD4 count (< 200/350/500) and WAZ (< -2). Panels B and D are restricted to patients presenting with CD4 count > 500 cells/mm3.
Figure 4.
Cumulative mortality and mean HAZ for adolescents aged ≥ 10 and < 16 years. Results are displayed for different intervention strategies and patient groups; 95% bootstrap confidence intervals for absolute estimates and estimated differences between strategies are listed in Table 3a. All results were obtained using the g-formula. Treatment thresholds refer to CD4 count (< 200/350/500). Panels B and D are restricted to patients presenting with CD4 count > 500 cells/mm3.
In our alternative scenario, with infrequent visits and slightly delayed treatment assignment, mortality was typically higher and the mean HAZ lower than in our main scenario. However, the comparative effectiveness of the different interventions was in general similar, and was slightly attenuated only in a few comparisons (Table 3).
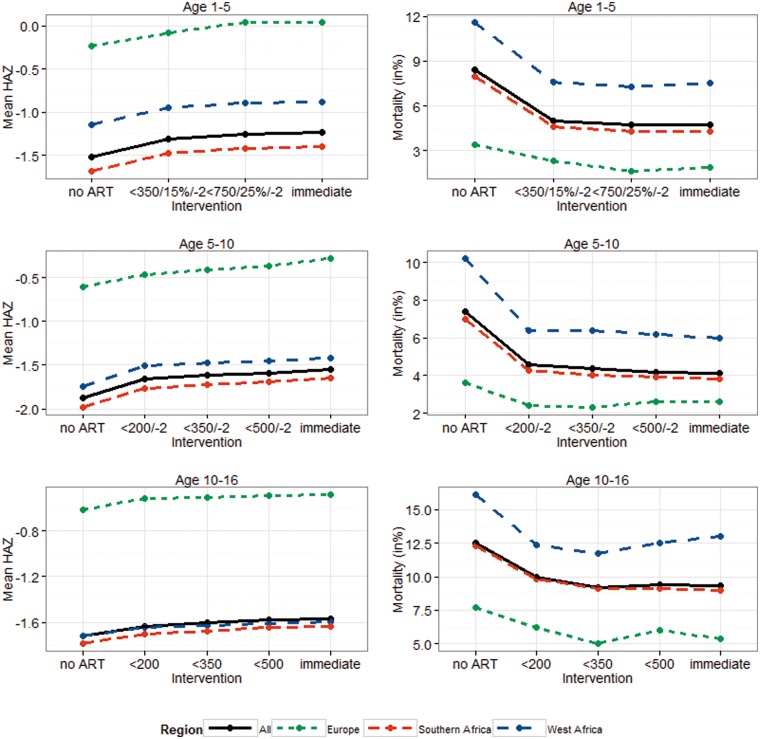
Our results for the effectiveness of the different treatment interventions were consistent in all three regions (Figure 5; Supplementary Figures 1-6, available as Supplementary data at IJE online). As in the raw data, results were best for European patients, followed by West African patients when looking at growth and South African patients when evaluating mortality.
Figure 5.
Mean HAZ and cumulative mortality overall and for different regions, stratified by age group. Treatment thresholds refer to CD4 count (< 200/350/500/750), CD4% (15%/25%), and WAZ (< -2).
Discussion
Statement of principal findings
Our study suggests better growth and lower or equal mortality for early ART initiation in children < 10 years, but results were inconclusive for adolescents. Outcomes were better, with more pronounced benefits of immediate ART initiation, for children presenting with CD4 ≥ 500 cells/mm3 and under regular follow-up assumptions. The comparative effectiveness of the different initiation criteria was similar in all regions and for irregular follow-up and slightly delayed initiation.
Strengths of the study
We included a large study population from three different contexts of HIV care, which enabled us to investigate the generalizability of our results. Our choice of treatment initiation criteria and assumptions provide a good comparison to WHO criteria and former trials and modelling studies. Moreover, the present study is the first to include adolescents, to evaluate outcomes after 3 years and to contrast estimates from idealized STUDY SETTINGS with estimates from more realistic settings. We believe that our results therefore give a comprehensive and concise overview of the key implications of the timing of ART in children and adolescents.
Limitations
We were constrained with respect to the availability of some data: our African cohorts did not collect regular information on clinical stage, the outcomes of children LTFU were not known and we had missing baseline data. Moreover, we did not look at secondary outcomes such as immune recovery and other morbidity measures.
To deal with the unavailability of stage data, we used WAZ as a proxy measure. This may be appropriate, as argued in other studies,16,17 but for adolescents we had to use BMIAZ since there are no WHO-based z-scores for weight. BMIAZ may not respond as quickly to clinically severe events as WAZ, but the results from the natural course scenario (Supplementary Figure 9, available as Supplementary data at IJE online) gives some reassurance of its use. In general, our sensitivity checks suggest that we may be able to estimate our outcomes reasonably well and that unmeasured confounding and model mis-specification may not be severe in our analysis, though these assumptions can never be tested completely from the data. The g-formula can deal with LTFU as long as censoring is uninformative. However, we cannot exclude the possibility that particularly sick children get lost and die soon thereafter–possibly because some of them may stay with caregivers struggling to maintain adherence and clinic visits. Absolute mortality estimates and comparisons between regions should therefore be interpreted with caution. We have, however, successfully imputed missing baseline data. Patients with missing data may have different characteristics and therefore outcomes, but comparisons between interventions may not differ much as suggested in another study.16
Interpretation of the study
Our study highlights the heterogeneity and characteristics of the different age groups in our cohorts. The youngest age group consists of infants who were infected perinatally. This group is affected by high early mortality, but those who survive can expect steady improvement in height with immediate ART initiation, and in Europe even up to the level of HIV-uninfected children.
Older children, aged 5-10 years, are long-term survivors who tend to present at health care facilities much sicker than younger children. Since they are long-term survivors, their early mortality after presentation is not as high. However, likely because of the longer time period they have been exposed to HIV, it may be more difficult to restore their immune system and other physiological functions, which in turn results in slower growth. When the children have reached an age of 8-13 years, 2-3 years after presentation, their growth slows down as seen in both the raw data and the counterfactual outcomes. This could be explained by a delay in the start of puberty.37,38 It is not surprising that immediate ART initiation proves to have the most beneficial effect compared with the other criteria in this age group.
Adolescents comprise a mixed population of long-term perinatally infected survivors and some newly sexually infected patients. Their growth trajectories look different from those of younger patients, but potential changes in adolescence, for example lifestyle and adherence, may complicate comparisons. Moreover, the poor baseline characteristics and overall high mortality highlight the vulnerability of this group. It remains unclear whether behavioural factors or baseline characteristics drive the results of this age group, in particular the identical results for immediate ART initiation and delayed initiation until CD4 count < 350 cells/mm3.
Another important finding is that, irrespective of the treatment strategy, growth is better and mortality lower when patients have frequent visits and start ART as soon as they are eligible. It is not surprising that this is the case, but the differences we found quantify this benefit and highlight how results from trials need to be interpreted carefully when translated into policy in real-world settings. Moreover, it shows the importance of retaining children in regular care to achieve optimal treatment outcomes.
Results in context
The comparative effectiveness of the different treatment strategies in the youngest age group is almost identical to a recent causal modelling study which included a subset of our data (compare Figure 2a and c with Figures 3 and 4 in Schomaker et al.16). Smaller differences, particularly with respect to our higher and smoother growth curves, can be explained by different sample sizes, the inclusion of European data and small differences with respect to eligibility criteria, LTFU definitions and imputation models.
The PREDICT trial, enrolling Asian children aged 1-12 years, showed no mortality benefit between immediate ART initiation and deferring ART until either the CD4% was below 15% or any CDC category C event occurred. The trial did however show better height gain for children who start ART immediately. Our results support the claim that immediate ART initiation enhances growth in children. However, our results also suggest mortality benefits of immediate ART initiation in older children. The differences of the two studies may be because of: (i) the small number of children aged 5-10; and (ii) the lower than expected event rate in the trial. The mortality benefit we have found is small in absolute terms and it would be surprising to see this effect in a trial with only few events which, in addition, did not enrol many children aged 5-10. Moreover, the children in the trial presented as healthier than ours and eligibility criteria differed, i.e. only children with CD4% between 15% and 24% were considered in the trial.
We did not find major negative consequences of delaying initiation of ART in adolescents until immunological criteria are met, even when considering a threshold of 350 cells/mm3 or restricting to individuals with a high CD4 count at baseline. This yields similar interpretations to a recent causal modelling study conducted in over 50 000 adults in North America and Europe.39 Preliminary findings from the START and TEMPRANO trials point towards a morbidity benefit of immediate ART initiation but, as Lodi et al.39 have also highlighted, different populations with different co-infections, different follow-up times and different assumptions complicate comparisons between different settings. Furthermore, findings from adults can likely not be transferred to adolescents due to different patient care systems, durations of infection, issues with non-adherence, drug combinations and lifestyle factors. It remains important to couple ART initiation strategies in children and adolescents with appropriate patient adherence and support strategies including better drug formulations, to reduce the risk of treatment failure and to monitor neurodevelopmental progress.
Conclusions
Immediate ART initiation is likely of benefit for all children aged ≤ 10 years. However, more research on adolescents and long-term outcomes is required.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by: the US National Institute of Allergy and Infectious Diseases (NIAID) through the International epidemiological Databases to Evaluate AIDS, Southern Africa (IeDEA-SA) and West Africa, grant numbers 5U01AI069924-05 and U01AI069919; the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD); the National Cancer Institute (NCI); the Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS); the HIV Monitoring Foundation; the Augustinus Foundation; the European Union Seventh Framework Programme (FP7/2007-2013) under EuroCoord grant agreement no. 260694; and the World Health Organization (WHO). The opinions expressed herein are those of the authors and do not necessarily reflect the views of any of the funders. No funder had a role in data collection and analysis, decision to publish or preparation of the manuscript. FT was supported by South African MRC Flagship (MRC-RFA-UFSP-012013/UKZN HIVEPI) and NIH (R01 HD084233) grants as well as a UK Academy of Medical Sciences Newton Advanced Fellowship (NA150161).
Key Messages
We found lower mortality and better growth with immediate versus delayed antiretroviral treatment initiation in children < 10 years of age after 5 years of follow-up.
We showed neither benefits nor harms with immediate treatment initiation in adolescents aged 10-16.
The best outcomes were observed in European children who attained growth outcomes comparable to HIV-negative children. The effects for the different ART initiation criteria were similar in Southern Africa, West Africa and Europe.
Irregular clinic visits led to worse outcomes than with regular follow-up, but the comparative effectiveness of different ART initiation criteria were not affected.
Supplementary Material
Acknowledgments
The authors are grateful to all patients’ families and staff at the HIV care programmes included in this analysis and to the staff at the data centres. Computations were performed using facilities provided by the University of Cape Town’s ICTS High Performance Computing team. We would like to acknowledge the IeDEA-WA, IeDEA-SA and COHERE in EuroCoord steering groups listed in Supplementary Textbox 2 (available as Supplementary data at IJE online).
Conflict of interest: M.S. and M-A.D. received funding from WHO for consultancy work related to timing of ART initiation in 2013 and 2015. WHO had no role in the preparation of the manuscript or the decision to publish.
References
- 1. WHO. Antiretroviral Therapy of HIV Infection in Infants and Children. 2006. http://www.who.int/hiv/pub/guidelines/en/
- 2. WHO. Antiretroviral Therapy for HIV Infection in Adults and Adolescents. 2006. http://www.who.int/hiv/pub/guidelines/en/
- 3. Prendergast AJ, Penazzato M, Cotton M. et al. Treatment of young children with HIV infection: using evidence to inform policymakers. Plos Med 2012;9:e1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puthanakit T, Bunupuradah T.. Early versus deferred antiretroviral therapy in children in low-income and middle-income countries. Curr Opin HIV/AIDS 2010;5:12-17. [DOI] [PubMed] [Google Scholar]
- 5. Schomaker M. Implications of causal modelling studies on the question of when to start antiretroviral treatment in young children. SACEMA Q 2014. http://sacemaquarterly.com/wp-content/uploads/2014/11/Michael_Implications-of-article-1.pdf
- 6. Siegfried N, Davies MA, Penazzato M, Muhe LM, Egger M.. Optimal time for initiating antiretroviral therapy (ART) in HIV-infected, treatment-naive children aged 2 to 5 years old. Cochrane Database Syst Rev 2013;10:CD010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turkova A, Webb RH, Lyall H.. When to start, what to start and other treatment controversies in pediatric HIV infection. Paediatr Drugs 2012;14:361-76. [DOI] [PubMed] [Google Scholar]
- 8. Welch SB, Gibb D.. When should children with HIV infection be started on antiretroviral therapy? Plos Med 2008;5:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Violari A, Cotton MF, Gibb DM. et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008;359:2233-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WHO. Antiretroviral Therapy for HIV Infection in Adults and Adolescents. 2010. http://www.who.int/hiv/pub/guidelines/en/
- 11. WHO. Antiretroviral Therapy for HIV Infection in Infants and Children. 2010. http://www.who.int/hiv/pub/guidelines/en/
- 12. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. 2013. http://www.who.int/hiv/pub/guidelines/en/
- 13. Edmonds A, Yotebieng M, Lusiama J. et al. The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: a cohort study. Plos Med 2011;8:e1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puthanakit T, Ananworanich J, Vonthanak S. et al. Cognitive Function and Neurodevelopmental Outcomes in HIV-infected Children Older Than 1 Year of Age Randomized to Early Versus Deferred Antiretroviral Therapy: The PREDICT Neurodevelopmental Study. Pediatr Infect Dis J 2013;32: 501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puthanakit T, Saphonn V, Ananworanich J. et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. Lancet Infect Dis 2012;12:933-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schomaker M, Davies MA, Malateste K. et al. Growth and mortality outcomes for different antiretroviral therapy initiation criteria in children aged 1-5 years: a causal modelling analysis from West and Southern Africa. Epidemiology 2016;27:237-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schomaker M, Egger M, Ndirangu J. et al. When to start antiretroviral therapy in children aged 2-5 years: a collaborative causal modelling analysis of cohort studies from southern Africa. Plos Med 2013;10:e1001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. WHO. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. 2015. http://www.who.int/hiv/pub/guidelines/en/ [PubMed]
- 19. Danel C, Moh R, Gabillard D. et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015;373:808-22. [DOI] [PubMed] [Google Scholar]
- 20. Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015;373:795-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daniel RM, De Stavola BL, Cousens SN. G-formula: Estimating causal effects in the presence of time-varying confounding or mediation using the g-computation formula. Stata J 2011;11:479-517. [Google Scholar]
- 22. Robins J. A new approach to causal inference in mortality studies with a sustained exposure period - application to control of the healthy worker survivor effect. Math Modelling 1986;7:1393-512. [Google Scholar]
- 23. Robins J, Hernan MA. Estimation of the causal effects of time-varying exposures In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G (eds). Longitudinal Data Analysis. London: Chapman Hall/CRC Press, 2009. [Google Scholar]
- 24. Robins J, Hernan MA, Siebert U. Comparative quantication of health risks: global and regional burden of disease attributable to selected major risk factors In: Ezzati M, Murray C, Lopez A (eds). Effects of Multiple Interventions. Geneva: World Health Organization, 2004. [Google Scholar]
- 25. Egger M, Ekouevi DK, Williams C. et al. Cohort Profile: The international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012;41:1256-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fenner L, Brinkhof MWG, Keiser O. et al. Early Mortality and Loss to Follow-up in HIV-Infected Children Starting Antiretroviral Therapy in Southern Africa. J Acquir Immune Defic Syndr 2010;54:524-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ekouevi DK, Azondekon A, Dicko F. et al. 12-month mortality and loss-to-program in antiretroviral-treated children: The IeDEA pediatric West African Database to evaluate AIDS (pWADA), 2000-2008. BMC Public Health 2011;11:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. IeDEA Pediatric Working Group. A survey of paediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa - the International epidemiologic Databases to Evaluate AIDS (IeDEA). J Int AIDS Soc 2013;16:17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sabin CA, Smith CJ, Monforte AD. et al. Response to combination antiretroviral therapy: variation by age - The Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study group. AIDS 2008;22:1463-73. [DOI] [PubMed] [Google Scholar]
- 30. WHO. The WHO Child Growth Standards. Geneva: WHO, 2008. [Google Scholar]
- 31. Honaker J, King G, Blackwell M.. Amelia II: A Program for Missing Data. J Stat Software 2011;45:1-47. [Google Scholar]
- 32. Wood SN. Thin plate regression splines. J R Stat Soc B 2003;65:95-114. [Google Scholar]
- 33. Wood SN. Generalized Additive Models: An Introduction with R. London: Chapman and Hall/CRC, 2006.
- 34. Westreich D, Cole SR, Young JG. et al. The parametric g-formula to estimate the effect of highly active antiretroviral therapy on incident AIDS or death. Stat Med 2012;31:2000-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Young JG, Cain LE, Robins JM, O'Reilly EJ, Hernan MA.. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Stat Biosci 2011;3:119-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation For Statistical Computing, 2014. [Google Scholar]
- 37. Szubert AJ, Musiime V, Bwakura-Dangarembizi M. et al. Pubertal development in HIV-infected African children on first-line antiretroviral therapy. AIDS 2015;29:609-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams PL, Abzug MJ, Jacobson DL. et al. Pubertal onset in children with perinatal HIV infection in the era of combination antiretroviral treatment. AIDS 2013;27:1959-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lodi S, Phillips A, Logan R. et al. Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. Lancet HIV 2015;2:e335-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.