ABSTRACT
Background: Chronic kidney disease (CKD) affects ∼10% of the adult population. The majority of patients with CKD are managed by primary care physicians, and despite the availability of effective treatment options, the use of evidence-based interventions for CKD in this setting remains suboptimal. Clinical pathways have been identified as effective tools to guide primary care physicians in providing evidence-based care. We aimed to describe the availability, characteristics and credibility of clinical pathways for adult CKD using a scoping review methodology.
Methods: We searched Medline, Embase, CINAHL and targeted Internet sites from inception to 31 October 2014 to identify studies and resources that identified adult CKD clinical pathways for primary care settings. Study selection and data extraction were independently performed by two reviewers.
Results: From 487 citations, 41 items were eligible for review: 7 published articles and 34 grey literature resources published between 2001 and 2014. Of the 41 clinical pathways, 32, 24 and 22% were from the UK, USA and Canada, respectively. The majority (66%, n = 31) of clinical pathways were static in nature (did not have an online interactive feature). The majority (76%) of articles/resources reported using one or more clinical practice guidelines as a resource to guide the clinical pathway content. Few articles described a dissemination and evaluation plan for the clinical pathway, but most reported the targeted end-users.
Conclusions: Our scoping review synthesized available literature on CKD clinical pathways in the primary care setting. We found that existing clinical pathways are diverse in their design, content and implementation. These results can be used by researchers developing or testing new or existing clinical pathways and by practitioners and health system stakeholders who aim to implement CKD clinical pathways in clinical practice.
Keywords: chronic kidney disease (CKD), clinical pathways, guideline-concordant care, primary care, scoping review
INTRODUCTION
Chronic kidney disease (CKD) is defined by an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, and it affects ∼10% of adults [1–3]. CKD is associated with increased morbidity, mortality and substantial health care costs [4, 5]. Timely recognition of CKD is important, as lifestyle and pharmacological interventions can prevent or slow progression of CKD to kidney failure [6–8]. The majority of patients with CKD are managed by primary care physicians [9]; however, despite effective treatment options, the use of evidence-based interventions for CKD in this setting remains suboptimal [10, 11]. This is related in part to the volume and complexity of clinical practice guidelines that primary care physicians are expected to implement and the limited time available per patient encounter [12, 13]. Since translating evidence into appropriate patient care can be challenging, tools to assist the incorporation of evidence into practice are needed to enhance guideline-concordant CKD care in primary care [14].
Although clinical practice guidelines are intended to address evidence-practice gaps, their passive dissemination has not led to improvements in patient care [15–17]. Clinical pathways have been identified as an effective tool to guide clinicians in providing evidence-based health care [18–20]. In contrast to clinical practice guidelines, clinical pathways are a tool to enhance uptake of clinical practice guidelines in routine care and are explicit about the sequence, timing and provision of interventions [21]. Clinical pathways aim to incorporate evidence-based clinical practice guideline recommendations and therefore have the potential to improve the quality of care [22, 23] by guiding clinical assessments and interventions at the point of care [18–20]. Further, clinical pathways may lead to improved clinical outcomes and reduced health care costs [21, 24].
Clinical pathways may optimize care of patients with CKD in primary care by facilitating diagnosis, management and nephrology referral. To our knowledge, no study has systematically synthesized information on available clinical pathways for adults with CKD. Our scoping review aimed to examine the availability, characteristics and credibility of clinical pathways for adults with CKD managed in the primary care setting. Findings from this review may be used by researchers, clinicians and health system stakeholders to inform the development and implementation of CKD clinical pathways in clinical care.
METHODS
A scoping review is a form of knowledge synthesis that allows researchers to examine the extent and range of existing research findings, summarize them and identify potential gaps in the literature [25]. Our scoping review was guided by the framework outlined by Arksey and O'Malley [25] and refined by Levac et al. [26]. This framework includes (i) identifying the research question, (ii) identifying relevant studies, (iii) study selection, (iv) charting the data and (v) collating, summarizing and reporting the results. For this review, we aimed to determine the scope of clinical pathways for the primary care management of adults with CKD and specifically to report on their availability, characteristics and credibility.
Definitions, information sources and search strategies
Given the lack of a unified definition of a ‘clinical pathway’ [27], we considered a clinical pathway to be a ‘methodology for the mutual decision making and organization of care for a well-defined group of patients during a well-defined period’ [28]. Search terms for clinical pathways in this scoping review included ‘clinical pathway, algorithms, care pathway and critical pathway or a combination of the terms critical care pathway’ [27]. We included the following terms for CKD: ‘chronic kidney disease, renal insufficiency, chronic renal insufficiency, kidney disease and chronic kidney failure’. Searches were expanded using truncation symbols and Boolean operators to combine search terms where appropriate. Articles were limited to English language only. The electronic databases Medline, Embase, CINAHL and targeted Internet sites were searched from inception to 31 October 2014. The following websites and/or libraries were also searched to identify additional relevant resources: organizations associated with CKD care and research [including the Kidney Foundation of Canada, the National Kidney Foundation, the National Institute for Health and Care Excellence (NICE), Caring for Australians with Renal Impairment and the National Kidney Disease Education Program], government health agencies (including the Public Health Agency of Canada and the National Institutes of Health), Internet search engines (www.google.ca, www.google.co.uk, www.google.com.nrc, www.google.ca and www.google.com.au), graduate (Master's and PhD) theses and the Cochrane Database of Systematic Reviews. A librarian with expertise in systematic and scoping reviews assisted in developing the grey literature search strategies based on the Canadian Agency for Drugs and Technologies in Health Grey Matters checklist and relevant library resources [29–31]. To ensure that all relevant literature was captured, we hand searched references of eligible studies where references were available.
Article selection
A broad range of information sources from both scholarly (i.e. academic and peer-reviewed) and non-scholarly (i.e. professional organizations, individuals and government agencies) resources were considered for inclusion [32]. We defined ‘published’ articles as publications by an organization or journal whose primary purpose is to publish, whereas we classified ‘grey literature’ as information that is unpublished or published for non-commercial purposes (i.e. websites, reports and manuals) [33]. Two reviewers (S.G. and B.R.H.) independently screened titles, abstracts of articles and titles of resources to determine whether a ‘clinical pathway for CKD care’ was described. Articles indicating clinical pathway use in end-stage renal disease, transplant, dialysis, acute kidney injury, paediatric or conservative care settings were excluded. Two reviewers (S.G. and M.J.E.) then reviewed full-text articles and resources to determine their final eligibility based on evidence of (i) clinical pathway in descriptive (text format) and/or visual (step-by-step guide, visual algorithm or flow chart) format, (ii) integrated patient management plan that could be contained within an accessible research or clinical document or as a stand-alone tool [28, 34] and (iii) adult CKD in an outpatient setting (i.e. community-based or outpatient clinic). Disagreements between reviewers were discussed and a third reviewer (M.D.) was consulted if necessary to achieve consensus.
Data extraction and synthesis
A data extraction form was developed a priori to collect information on availability, characteristics and credibility of the included articles and resources. To describe the availability of the research, we recorded publication information (periodical type and country). We reported the following key clinical pathway characteristics: (i) format—static (one-way transfer of information) or online interactive tool (defined as ‘the continuous two-way transfer of information between a user and a central point of a communication system to obtain data or commands and to give immediate results or update information’) [33]; (ii) content—CKD screening/diagnosis, drug and lifestyle management, nephrology referral; (iii) implementation—dissemination plan and pathway end-users and (iv) evaluation—pathway evaluation and cost (related to the impact on downstream health care costs from use of the clinical pathway).
We evaluated clinical pathway credibility by assessing the quality of the clinical practice guidelines referenced within the clinical pathway design, recommendations and/or development and by the editorial ownership (authorship) and control (year of publication or revision) [35]. Where clinical pathways referenced clinical practice guidelines, these guidelines were evaluated using the AGREE II instrument to assess their quality, reporting and applicability of the cited recommendations for adults with non-dialysis CKD [36]. Using this tool, each of the referenced clinical practice guidelines was appraised in six domains and assigned an overall score that encompasses whether the guideline would be recommended for use in practice.
Two reviewers (S.G. and M.J.E.) independently extracted data from all included articles and resources. Descriptive statistics were used to summarize data on clinical pathway availability, characteristics and credibility, and as reporting of these criteria was highly variable between included clinical pathways, qualitative descriptions were provided where appropriate. As the objective of this scoping review was to broadly examine existing published and grey literature on adult CKD clinical pathways, formal assessment of study quality was not undertaken.
RESULTS
Search strategy, study selection and data extraction
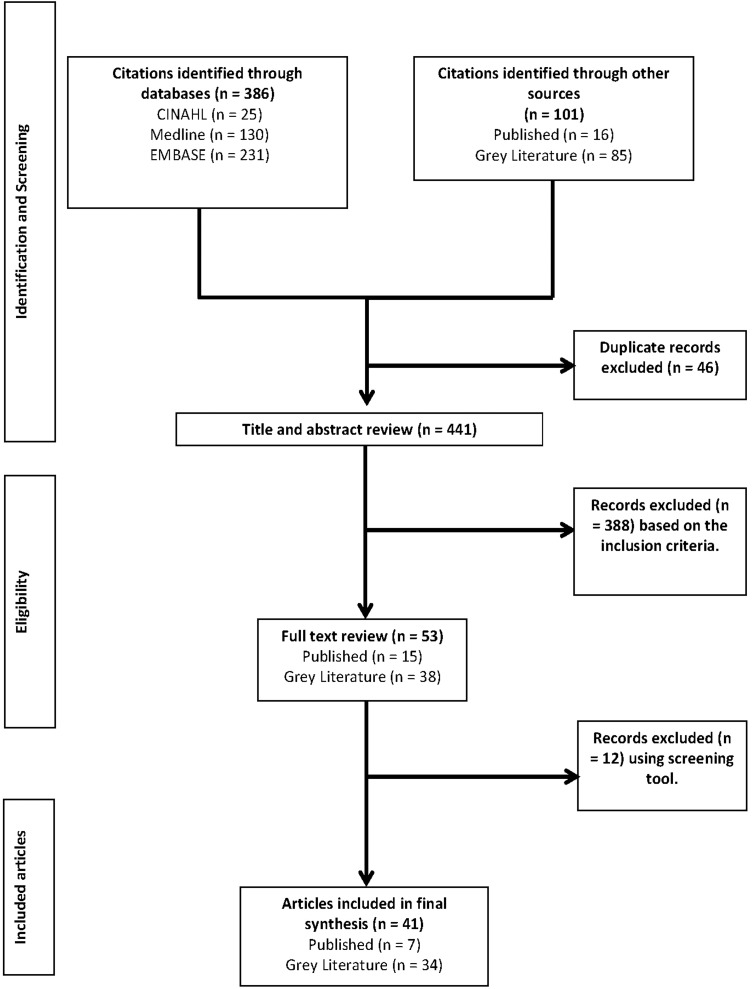
The results of the search strategy and study selection are outlined in Figure 1. After duplicates were removed, 441 articles were identified for title and abstract review (346 published and 95 grey literature). Of these, 53 articles were retrieved for full-text review, 12 of which were excluded for not meeting the eligibility criteria. The final synthesis included 41 different clinical pathways, 7 published articles and 34 grey literature resources. Interrater reliability of the study selection was moderate {κ = 0.59 [95% confidence interval (CI) 0.42–0.79]}, with published articles having excellent agreement [κ = 0.85 (95% CI 0.21–1.00)] and grey literature fair agreement [κ = 0.38 (95% CI 0.16–0.65)].
FIGURE 1.
Flow diagram indicating selection of articles and resources.
Summary of CKD clinical pathway availability
The majority of the articles and resources were from grey literature sources (85%, n = 34), and no systematic or scoping reviews were found. Of the 41 clinical pathways, the majority (32%) were from the UK, with 24% from the USA and 22% from Canada (Table 1). The earliest available clinical pathway was from 2001 [37], although the year of publication was not reported for several (n = 13).
Table 1.
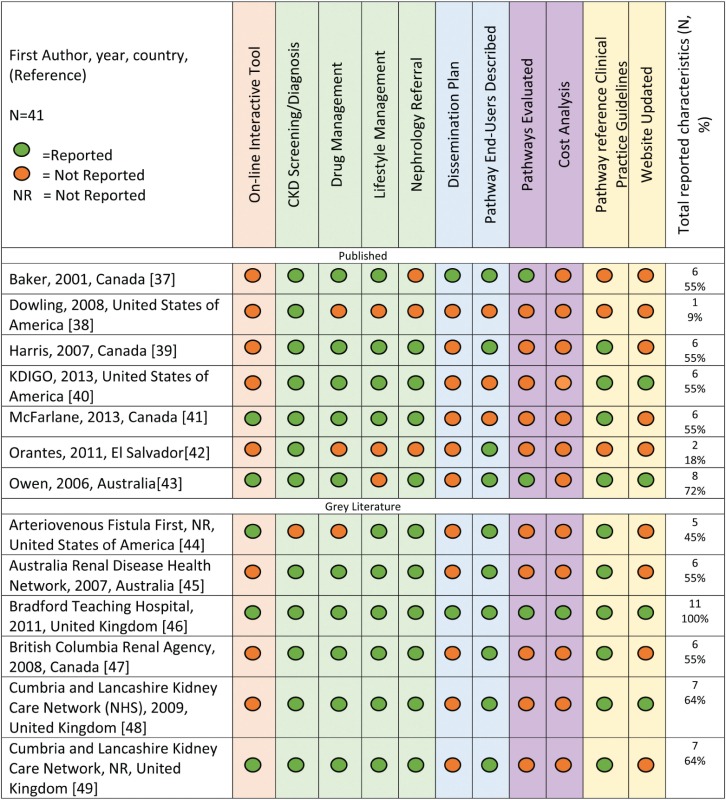
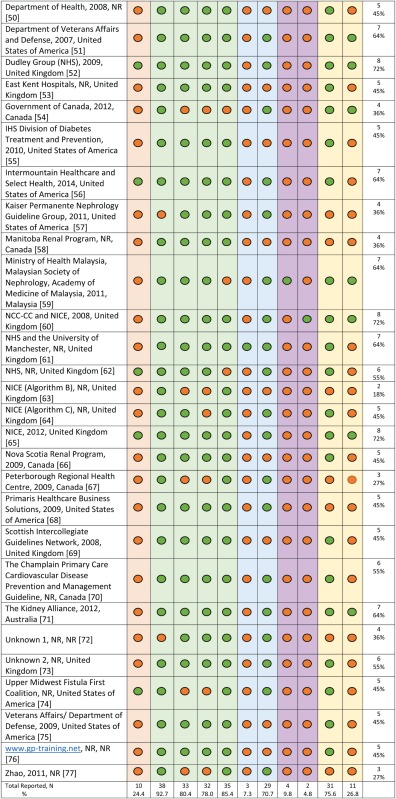
Characteristics and content of adult CKD clinical pathways
 |
 |
Summary of clinical pathways' characteristics
The majority (66%, n = 31) of clinical pathways were static and did not indicate that they had an online interactive feature (Table 1). In regards to content, 93% (n = 38) of the clinical pathways included content related to CKD screening/diagnosis and 76% (n = 31) described both drug and lifestyle management. However, the specific recommendations were highly variable across clinical pathways. For example, the recommended patient populations for whom CKD screening should be considered and the screening tests themselves differed. Similarly, while most clinical pathways described mainstays of CKD treatment such as angiotensin-converting enzyme inhibitors to slow progression, specific treatment recommendations were not consistently reported and varied in their level of detail. The majority (85%, n = 35) of clinical pathways had information on when to initiate nephrology referral, and most provided criteria for referral, including eGFR and proteinuria cut-offs. Seventy-one per cent (n = 29) described intended clinical pathway end-users, and of these, the majority (n = 20) were designed for use in primary care settings [39, 45–49, 51, 52, 56, 57, 59–61, 64, 65, 68, 70, 71, 73, 77] and two [37, 62] were intended for use by patients with CKD.
Information regarding clinical pathway implementation, specifically a dissemination plan, was reported for only 7% (n = 3) of articles. Clinical pathway evaluation and cost were reported in 10% (n = 4) [37, 43, 46, 59] and 5% (n = 2) [46, 60] of articles, respectively (Table 1). This included reporting of qualitative and quantitative outcomes pre/post-pathway implementation, pathway usefulness and comprehension and health care costs associated with use of the clinical pathway. Only one article [46] reported 100% of the data extraction variables based on criteria of availability, characteristics and credibility.
Summary of clinical pathways' credibility
The majority (76%, n = 31) of articles reported using one or more clinical practice guidelines as a resource to develop content. Overall, 20 clinical practice guideline documents were used along with 6 consensus statement documents, frameworks or standards (Supplementary data, Tables S1 and S2). Based on guideline assessments using the AGREE II instrument, 75% (n = 15) of the clinical practice guidelines were recommended for use in practice, and those not recommended generally contained recommendations more specific to dialysis than to earlier stages of CKD (Supplementary data, Table S1). Authorship was not reported for two articles, and publication year was reported for only 32% (n = 13) of the clinical pathways. Twenty-seven per cent (n = 11) of the articles reported when the clinical pathway was revised or planned for revision.
DISCUSSION
Using an established methodology for scoping reviews [25], we identified 41 scholarly and non-scholarly articles pertaining to adult CKD clinical pathways. The Internet has increased access to health-related information, thus it was important to search both published and grey literature to ensure we identified all relevant clinical pathways from organizations, groups and individuals. In fact, the majority (34 of 41) of the clinical pathways were from grey literature sources. We were able to locate available CKD clinical pathways dating back to 2001, and from six countries. The majority of clinical pathways were static (i.e. not interactive) in nature and reported using one or more clinical practice guidelines as a resource for developing pathway content. While few clinical pathways described their dissemination and evaluation plan, many reported intended pathway end-users.
As primary care physicians manage the vast majority of patients with CKD [9], it is not unsurprising that the majority of clinical pathways we identified were designated for use in the primary care setting. In patients with earlier stages of CKD, management focuses on identifying those at increased risk of progressing, offering targeted interventions to slow disease progression and appropriately referring for specialist nephrology care in advanced or complicated cases [40]. Given that patients with CKD often have several comorbidities, care for these individuals can be complex and encompass chronic disease treatment recommendations from multiple sources. Indeed, it has been reported that for primary care physicians to apply all preventive recommendations by the US Preventive Service Task Force (USPSTF), 7.4 h/working day would be required [12]. Given the time constraints in primary care practice, the development of clinical tools that engage primary care physicians in efficient and evidence-based CKD care is paramount. Clinical pathways are such tools that provide the opportunity for improved collaboration and communication between primary care physicians and nephrologists in providing optimal CKD care. In a systematic review, integrated care pathways were found to be effective tools in ensuring patients receive timely and appropriate interventions and/or assessments and in promoting practitioner adherence to clinical practice guideline recommendations [18]. Thus, the development and evaluation of quality clinical pathways for CKD management may be a strategy to enhance quality care for patients with CKD in the primary care setting.
Clinical pathways are more explicit than clinical practice guidelines about the timing and provision of interventions and assessments and aim to be directly incorporated into routine patient care [27]. It has been shown that online applications can increase pathway compliance [78]. Our findings show that only 25% of the available clinical pathways incorporated an interactive feature—the majority were static with a single flow diagram concept, and no tool allowed for integration of patient information into the clinical pathway. With the proliferation of electronic medical records, demand for available and accessible online interactive clinical pathways will potentially increase.
While the majority (>75%) of clinical pathways included content related to screening, diagnosis, management and referral, the level of detail was highly variable and the content typically relevant to a local setting, limiting their generalizability. Many clinical pathways omitted information such as the date the clinical pathways were developed, plans for updating and sources used. These features are important to determine currency and relevance to best available evidence.
The clinical pathways reviewed had sparse information on implementation, evaluation and cost. Few articles described their dissemination plan, although most did indicate their intended end-users. Strategies for effective dissemination and implementation are critical to ensure uptake of the clinical pathway. Evaluating the effect of a clinical pathway is a relatively new area in health care research, and essential to guarantee guideline-concordant care for patients with CKD.
The clinical pathway from the Bradford Teaching Hospital [46] included information on all variables we considered important for this review. It describes the local implementation, quality evaluation and cost outcomes of an e-consultation initiative and provides an interactive algorithm to guide the management of adults with CKD. Four other articles and resources reported on 72% of the features and were deemed credible based on the clinical practice guidelines used to develop the content [43, 52, 60, 65]. Two of these resources [60, 65] were developed by the NICE guidance. The NICE 2012 document [65] outlines an interactive algorithm for CKD management and links directly to guideline recommendations for screening, management and specialist referral. These documents could serve as a useful resource for one seeking to develop a CKD clinical pathway.
Clinicians, organizations and policy makers may want to consider the following when choosing to use or develop a CKD clinical pathway. First, ‘buyer beware’, as there is no gold standard to guide development, implementation and evaluation of clinical pathways in the primary care setting. It is important to examine clinical pathway characteristics (i.e. format, content) and credibility (i.e. guideline referencing, authorship) to ensure they meet end-user needs. Second, availability and accessibility are not interchangeable—we reviewed only the articles and resources available to us, but other clinical pathways may exist that are only accessible to end-users internally within organizations. Third, in the future, standardized reporting of clinical pathway development, implementation and evaluation in the literature must be emphasized in the research and clinical communities.
Due to challenges inherent to scoping reviews, limitations of this review must be acknowledged in the overall interpretation of our findings. First, the articles were diverse in their content and focus, making it challenging to select articles, extract data and synthesize results. Interrater reliability for published article selection was excellent, but fair for grey literature, reflecting the difficulty reviewing the various article formats and content. While criteria have been developed to objectively evaluate clinical pathways [27], these criteria were established for clinical pathways based in a hospital setting and therefore were less relevant for the clinical pathways included in our scoping review. Second, we limited articles to those published in English, which may limit overall generalizability. Third, by definition, scoping reviews aim to broadly describe the state of the literature on a given topic without formal assessment of the quality of the identified articles. Therefore, it is difficult to identify the strengths and weaknesses of included articles based on the validity of the research findings and assessment of methodology. However, it was reassuring to see that 76% of the literature did identify clinical practice guidelines as a source for their content, and we used the AGREE II instrument to score the quality of the clinical practice guidelines considered. Fourth, for practical reasons, we were unable to contact authors or have access to internal organizational documents or websites to obtain further detail on pathway development, implementation and evaluation, although we do believe that the majority of articles and resources have been captured in this review.
Clinical pathways are an effective tool to increase uptake of guidelines and may help optimize the recognition and management of patients with CKD. Our scoping review has synthesized the available literature for CKD clinical pathways and may be of use to researchers, practitioners and health system stakeholders who are interested in implementing, adapting or developing new CKD clinical pathways.
S UPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Diane Lorenzetti for assisting us with the electronic searches and Adriane Lewin and Robert Weaver for their assistance with the analysis. We would also like to acknowledge Dr Sharon Straus and Selina Allu for their expertise and guidance throughout the project. This research was supported by the Canadian Institutes of Health Research and an interdisciplinary team grant from Alberta Innovates – Health Solutions. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
AUTHORS’ CONTRIBUTIONS
Research idea and study design: B.R.H., M.D. and S.G.; data acquisition: M.D., S.G. and M.J.E.; data analysis/interpretation: M.D., S.G. and M.J.E. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. B.R.H. takes responsibility that this study has been reported honestly, accurately and transparently; that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Wu XC, Liu BC, Wang YL et al. Prevalence of chronic kidney disease in Chinese hospitalized adult patients: investigation of 13,383 cases. Zhonghua Yi Xue Za Zhi 2007; 87: 2672–2676 [PubMed] [Google Scholar]
- 2. Coresh J, Astor BC, Greene T et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003; 41: 1–12 [DOI] [PubMed] [Google Scholar]
- 3. Arora P, Vasa P, Brenner D et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ 2013; 185: E417–E423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet 2005; 365: 331–340 [DOI] [PubMed] [Google Scholar]
- 5. Go AS, Chertow GM, Fan D et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 6. Jafar TH, Schmid CH, Landa M et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135: 73–87 [DOI] [PubMed] [Google Scholar]
- 7. Lewis EJ, Hunsicker LG, Clarke WR et al. Renoprotective effect of the angiotensin-receptor antagonist Irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [DOI] [PubMed] [Google Scholar]
- 8. Wright JT, Bakris G, Greene T et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease—results from the AASK trial. JAMA 2002; 288: 2421–2431 [DOI] [PubMed] [Google Scholar]
- 9. Manns B, Tonelli M, Culleton B et al. A cluster randomized trial of an enhanced eGFR prompt in chronic kidney disease. Clin J Am Soc Nephrol 2012; 7: 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tonelli M, Bohm C, Pandeya S et al. Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency. Am J Kidney Dis 2001; 37: 484–489 [PubMed] [Google Scholar]
- 11. Shlipak MG, Fried LF, Crump C et al. Cardiovascular disease risk status in elderly persons with renal insufficiency. Kidney Int 2002; 62: 997–1004 [DOI] [PubMed] [Google Scholar]
- 12. Yarnall KS, Pollak KI, Ostbye T et al. Primary care: is there enough time for prevention? Am J Public Health 2003; 93: 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ostbye T, Yarnall KSH, Krause KM et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med 2005; 3: 209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hemmelgarn BR, Manns BJ, Straus S et al. Knowledge translation for nephrologists: strategies for improving the identification of patients with proteinuria. J Nephrol 2012; 25: 933–943 [DOI] [PubMed] [Google Scholar]
- 15. Grimshaw JM, Eccles MP. Is evidence-based implementation of evidence-based care possible? Med J Aust 2004; 180: S50–S51 [DOI] [PubMed] [Google Scholar]
- 16. Agency for Healthcare Research and Quality (AHRQ). Translating Research into Practice (TIP0-11). http://archive.ahrq.gov/research/findings/fact she ets/ translating/tripfac/trip2fac.html (21 November 2015, date last accessed)
- 17. Grimshaw JM, Thomas RE, MacLennan G et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004; 8: iii-iv, 1–72 [DOI] [PubMed] [Google Scholar]
- 18. Allen D, Gillen E, Rixson L. Systematic review of the effectiveness of integrated care pathways: what works, for whom, in which circumstances? Int J Evid Base Healthc 2009; 7: 61–74 [DOI] [PubMed] [Google Scholar]
- 19. Sulch D, Perez I, Melbourn A et al. Evaluation of an integrated care pathway for stroke unit rehabilitation. Age Ageing 2000; 29: 87. [DOI] [PubMed] [Google Scholar]
- 20. Cunningham S, Logan C, Lockerbie L et al. Effect of an integrated care pathway on acute asthma/wheeze in children attending hospital: cluster randomized trial. J Pediatr 2008; 152: 315–320 [DOI] [PubMed] [Google Scholar]
- 21. Rotter T, Kinsman L, James E et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 2010; 3: CD006632. [DOI] [PubMed] [Google Scholar]
- 22. Whittle C, Hewison A. Integrated care pathways: pathways to change in health care? J Health Organ Manag 2007; 21: 297–306 [DOI] [PubMed] [Google Scholar]
- 23. Scott SD, Grimshaw J, Klassen TP et al. Understanding implementation processes of clinical pathways and clinical practice guidelines in pediatric contexts: a study protocol. Implement Sci 2011; 6: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rotter T, Kugler J, Koch R et al. A systematic review and meta-analysis of the effects of clinical pathways on length of stay, hospital costs and patient outcomes. BMC Health Serv Res 2008; 8: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8: 19–32 [Google Scholar]
- 26. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010; 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kinsman L, Rotter T, James E et al. What is a clinical pathway? Development of a definition to inform the debate. BMC Med 2010; 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Bleser L, Depreitere R, De Waele K et al. Defining pathways. J Nurs Manag 2006; 14: 553–563 [DOI] [PubMed] [Google Scholar]
- 29. Canadian Agency for Drugs and Technologies in Health. CADTH Peer Review Checklist for Search Strategies. https://www.cadth.ca/resources/finding-evidence (December 2012, date last accessed)
- 30. Lorenzetti D. Health Information Network Calgary (HIN) Grey Literature. Alberta, Canada: University of Calgary, 2012 [Google Scholar]
- 31. Heather Ganshorn. Advanced Google Searching. Alberta, Canada: University of Calgary, 2011 [Google Scholar]
- 32. Cornell University Library. Distinguishing Scholarly from Non-Scholarly Periodicals: A Checklist of Criteria: Introduction & Definitions. http://guides.library.cornell.edu/c.php?g=31867&p=201758 (5 November 2015, date last accessed)
- 33. Schöpfel J, Farace DJ. Grey literature. In: Bates MJ, Maack MN (eds). Encyclopedia of Library and Information Sciences, 3rd edn Boca Raton, FL: CRC Press, 2010, 2029–39 [Google Scholar]
- 34. Panella M, Marchisio S, Di Stanislao F. Reducing clinical variations with clinical pathways: do pathways work? Int J Qual Health Care 2003; 15: 509–521 [DOI] [PubMed] [Google Scholar]
- 35. Eysenbach G. Credibility of health information and digital media: new perspectives and implications for youth. In: Flanagin AJ, Metzger MJ (eds). Digital Media, Youth and Credibility. Cambridge, MA: MIT Press, 2008, 123–54 [Google Scholar]
- 36. Brouwers MC, Kho ME, Browman GP et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010; 182: E839–E842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baker J, Rogers K, Thomas A. A patient pathway to renal replacement therapy: implementation in the progressive renal insufficiency population. CANNT J 2001; 11: 18–22 [PubMed] [Google Scholar]
- 38. Dowling T. Nephrology 1—Controversies in Assessing Kidney Function. Pharmacotherapy Self-Assessment Program, 6th edn, 2008, 1–14 [Google Scholar]
- 39. Harris K, Stribling B, Farooqi A. Diabetic nephropathy: implications of the renal NSF for primary care. Diabetes Primary Care 2007; 9: 50–57 [Google Scholar]
- 40. Kidney Disease: Improving Global Outcomes. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 136–150 [DOI] [PubMed] [Google Scholar]
- 41. McFarlane P, Gilbert RE, MacCallum L et al. Chronic kidney disease in diabetes. Can J Diabetes 2013; 37(Suppl 1): S129–S136 [DOI] [PubMed] [Google Scholar]
- 42. Orantes C, Herrera R, Almaguer M et al. Chronic kidney disease and associated risk factors in the Bajo Lempa Region of El Salvador: Nefrolempa Study, 2009. MEDICC Rev 2011; 13: 14–22 [DOI] [PubMed] [Google Scholar]
- 43. Owen JE, Walker RJ, Edgell L et al. Implementation of a pre-dialysis clinical pathway for patients with chronic kidney disease. Int J Qual Health Care 2006; 18: 145–151 [DOI] [PubMed] [Google Scholar]
- 44. Arteriovenous Fistula First. Care of Patients at Risk for or with Chronic Kidney Disease. http://www.therenalnetwork.org/qi/3Pdocs/W04_Hosp i tal_ Care_Plan.pdf (21 March 2014, date last accessed)
- 45. Renal Diseases Health Network. Chronic Kidney Disease Model of Care. http://www.healthnetworks.health.wa.gov.au/modelsofcare/docs/CKD_Mo del_of_Care.pdf (6 September 2012, date last accessed)
- 46. Bradford Teaching Hospital. Electronic Consultation: Chronic Kidney Disease. arms.evidence.nhs.uk/resources/qipp/29440/attachment (6 September 2012, date last accessed)
- 47. British Columbia Renal Agency. Chronic Kidney Disease Management. http:www.bcrenalagency.ca/patients/ChronDisMgmt.html (6 September 2012, date last accessed)
- 48. Cumbria and Lancashire NHS. Chronic Kidney Disease (CKD) Algorithm– One Page Version. http://www.gplocumcover.co.uk/docstore/CKD Algo rithm.pdf (21 March 2014, date last accessed)
- 49. Cumbrian and Lancashire kidney care network NHS. Chronic Kidney Disease (CKD) Algorithm. http://www.cumbria.nhs.uk/ProfessionalZone/MedicinesManagement/Guidelines/chronic-kidney-disease.pdf (20 March 2014, date last accessed)
- 50. Department of Health. 18 Week Commissioning Pathway–Chronic Kidney Disease 2008. http://www.billpeckham.com/files/download-pdf-file-1.pdf (31 August 2012, date last accessed)
- 51. Department of Veterans Affairs. VA/DoD Clinical Practice Guidelines for Management of Chronic Kidney Disease in Primary Care. http://www.healthquality.va.gov/ckd/ckd_v478.pdf (19 March 2014, date last accessed)
- 52. Dudley Group (NHS). Chronic Kidney Disease in the Primary Care Setting. http://www.dudley.nhs.uk/sections/publications/documents%5CFOI28246053000.pdf (29 August 2012, date last accessed)
- 53. East Kent Hospitals University. Kidney Care Guidelines 2009. Kent Adult Chronic Kidney Disease Management and Referral Guidelines. http://www.ekhuft.nhs.uk/EasySiteWeb/GatewayLink.aspx?alId=214970 (13 September 2012, date last accessed)
- 54. Government of Canada. Operations Directorate, Health Branch Immigration Medical Examination Instructions. http://www.cic.gc.ca/english/department/partner/pp/pdf/IMEI_Renal_disease.pdf (21 March 2014, date last accessed)
- 55. Indian Health Service (IHS) Division of Diabetes Treatment and Prevention. Type 2 Diabetes–Chronic Kidney Disease. http://www.ihs.gov/MedicalPrograms/Diabetes/HomeDocs/Tools/Algorithms/DM_algorithm_CKD_508c.pdf (21 March 2014, date last accessed)
- 56. Intermountain Healthcare and Selecthealth. Management of Chronic Kidney Disease. https://intermountainhealthcare.org/ext/Dcmnt?ncid= 521 39 5847 (21 March 2014, date last accessed)
- 57. Kaiser Permanente Nephrology Guideline Group. KP Hawaii Algorithm for Management of Patients with Chronic Kidney Disease in Primary Care. http://www.delfini.org/KP%20CKD/CKD%20algorithm%20final_2011_May%2018.pdf (21 March 2014, date last accessed)
- 58. Manitoba Renal Program. Kidney Disease Renal Pathway. http://www.kidneyhealth.ca/wp/healthcare-professionals/egfr-referral-pathways/mrp- kidney-disease-referral-pathway/ (10 September 2012, date last accessed)
- 59. Ministry of Health Malaysia, Malaysian Society of Nephrology, Academy of Medicine of Malaysia. Management of Chronic Kidney Disease in Adults. http://www.msn.org.my/doc/publicdoc_pb/cpgmanagement ofchronic kid ne y d isease.pdf (21 March 2014, date last accessed)
- 60. National Collaborating Centre for Chronic Conditions. Chronic Kidney Disease National Clinical Guideline for Early Identification and Management in Adults in Primary Care and Secondary Care. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0009554/pdf/TOC.pdf (6 September 2012, date last accessed)
- 61. National Health Service (NHS) and the University of Manchester. Improving Diagnosis and Care for People with Chronic Kidney Disease. http://clahrc-gm.nihr.ac.uk/cms/wp-content/uploads/CLAHRC-CKD-Improvement-Guide.pdf (21 March 2014, date last accessed)
- 62. National Health Service (NHS). Kidney Disease, Chronic–Treatment. http://www.nhs.uk/Conditions/Kidney-disease-chronic/Pages/Treatment.aspx (19 March 2014, date last accessed)
- 63. National Institute for Health and Care Excellence (NICE). Algorithm B—Diagnosis and Referral of Patients with Chronic Kidney Disease and Without Diabetes. http://www.nice.org.uk/nicemedia/pdf/CKDAlgorithmB.pdf (21 March 2014, date last accessed)
- 64. National Institute for Health and Care Excellence (NICE). Algorithm C—Diagnosis and Referral of Patients with Diabetes and Chronic Kidney Disease. http://www.nice.org.uk/nicemedia/pdf/CKDAlgorithmC.pdf (21 March 2014, date last accessed)
- 65. National Institute for Health and Clinical Excellence (NICE). Chronic Kidney Disease Overview. http://pathways.nice.org.uk/pathways/chronic-kidney-disease (13 September 2012, date last accessed) [PubMed]
- 66. Nova Scotia Renal Program. Detection, Monitoring & Referral of Chronic Kidney Disease Using eGFR. http://www.nsrp.gov.ns.ca/sites/default/files/publications/ckd-prevention-early-detection/Renal%20Algorithm%20for%20Website.pdf (13 September 2012, date last accessed)
- 67. Peterborough Regional Health Centre. Chronic Kidney Disease Screening Project Workflow Algorithm. http://www.centraleastlhin.on.ca/uploaded Files/ Home_Page/Connected_with_Care/CKDS-WORKFLOW_Algorithm-HFHG-0907311.pdf (21 March 2014, date last accessed)
- 68. Primaris Healthcare Business Solutions. CKD Assessment Algorithm, Identification, Treatment, Referral. http://primaris.org/sites/default/files/resources/Chronic%20Kidney%20Disease/CKDAssessmentAlgorithm09.pdf (21 March 2014, date last accessed)
- 69. Scottish Intercollegiate Guidelines Network. Diagnosis and Management of Chronic Kidney Disease. http://www.sign.ac.uk/pdf/qrg103.pdf (21 March 2014, date last accessed)
- 70. Champlain Primary Care Cardiovascular Disease Prevention and Management Guideline. Detection and Referral of Chronic Kidney Disease (CKD). http://ccpnetwork.ca/wp-content/uploads/2013/05/CKD.pdf (21 March 2014, date last accessed)
- 71. Kidney Health Australia. Chronic Kidney Disease (CKD) Management in General Practice. http://www.kidney.org.au (4 September 2012, date last accessed)
- 72. Clinical Pathways for Chronic Kidney Disease. xa.yimg.com/kq/groups/21359473/1257462310/name/revised (22 August 2012, date last accessed)
- 73. Care Pathway for Management of Chronic Kidney Disease. http://www.northsomersetpathways.co.uk/documents/pathways/renal_medicine/revisedckdpathway2009final.pdf (4 September 2012, date last accessed)
- 74. Upper Midwest Fisula First Coalition. Chronic Kidney Disease (CKD) Assessment Algorithm. http://www.esrdnet11.org/assets/coalition/er_algorithm.pdf (21 March 2014, date last accessed)
- 75. Department of Veterans Affairs. VA/DoD Clinical Practice Guidelines. Management of Chronic Kidney Disease. http://www.onlinecpg.com/ahlta/CKD-pocket/ALT-CKD-110518.pdf (21 March 2014, date last accessed)
- 76. GP Training. Chronic Kidney Disease. http://www.gp-training.net/protocol/genitourinary/ckd.html (14 September 2012, date last accessed)
- 77. Zhao HJ, Culpepper M, Rutecki G. Kidney disease: a straightforward diagnostic approach. Consultant 2011; 51 [Google Scholar]
- 78. Wakamiya S, Yamauchi K. What are the standard functions of electronic clinical pathways? Int J Med Inform 2009; 78: 543–550 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.