Abstract
Background
Pancreatic cancer is one of the most lethal. Most countries have exhibited a stable or decreasing incidence over time. The aim of this study was to provide updated French temporal trends in pancreatic cancer incidence and mortality over the past three decades.
Methods
Incidence was estimated using the French National Network of Cancer Registries (FRANCIM) and mortality using the French Mortality Statistics Office. World age-standardized incidence and mortality were modelled by age-period-cohort models. The net cumulative risk of developing pancreatic cancer by birth cohort was calculated, as were annual percentage changes (APCs) in incidence and mortality.
Results
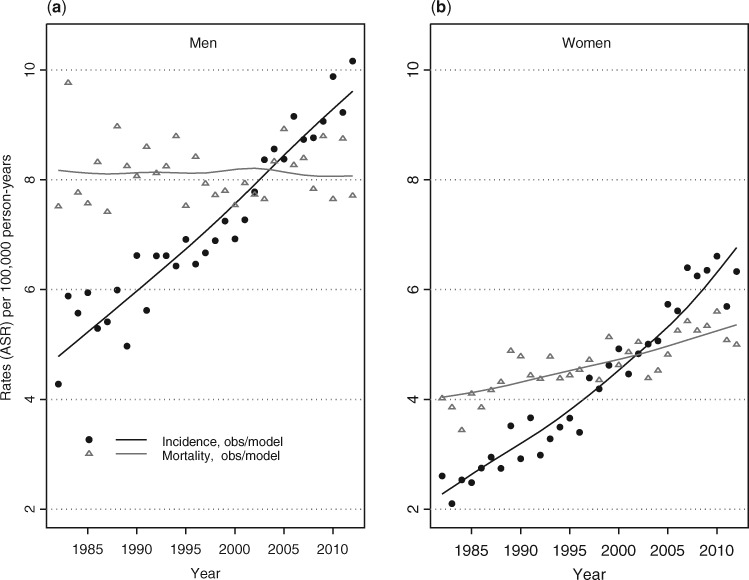
Between 1982 and 2012, age-standardized incidence increased from 4.8 in 1980 to 9.6 per 100 000 in men and from 2.3 to 6.8 in women. The mean APC was 2.3% (2.1–2.6) and 3.6% (3.3–3.9), respectively. The cumulative risk of developing pancreatic cancer before age 75 rose from 0.62% for males born around 1920 to 1.17% for those born around 1950. It was respectively 0.31% and 0.86% for women. Mortality did not vary in men (8.1 per 100 000). It slightly increased in women from 4.0 in 1982 to 5.4 in 2012.
Conclusion
Pancreatic cancer incidence and mortality exhibited diverging trends. Incidence increased over the last 30 years in France whereas mortality did not vary in men and moderately increased in women. Incidence remained lower than mortality up to 2002. One cannot exclude the possibility that a similar trend may appear in other countries. Etiological studies are required to further explain this increase.
Keywords: pancreatic cancer, incidence, mortality, cancer registry, epidemiology
Key Messages
Lifestyle factors may influence incidence of pancreatic cancer.
Most countries have exhibited a stable or decreasing incidence over time.
Incidence increased in both sexes over the last 30 years in France.
Mortality did not vary in men and moderately increased in women.
Similar patterns may appear in other countries in the future.
Introduction
Cancer of the pancreas is a major challenge in oncology due to its lethality. Despite advances in diagnostic procedures, the prognosis of pancreatic cancer remains one of the worst in oncology.1–3 This situation has stimulated the interest of clinicians and researchers. The incidence of pancreatic cancer is higher in developed countries than in other countries, suggesting the potential influence of lifestyle factors such as diet or smoking. There are many reasons for studying its time trends. Secular trends in the incidence by time period and birth cohort would provide hypotheses crucial to better understanding of the disease. From a public health viewpoint, by observing changes in risk, it should be possible to anticipate the burden of this cancer in the near future.
Population-based studies, which accurately record all cases diagnosed in a well-defined population and thus provide unbiased measurements, are the best way to assess changes in pancreatic cancer incidence. Temporal trends in incidence are related to three main components: age, period of diagnosis and birth cohort. Age-period-cohort models are commonly used to study cancers, as they may provide better understanding of the observed trends and the associated aetiological factors. These models also make it possible to estimate the net probability of developing cancer for a given birth cohort.4 Up to now, most available observed data on pancreatic cancer incidence from different countries have not exhibited any major increase in incidence, in contrast to what was observed in Burgundy, a region of France.5–7 The aim of the present study was to provide updated temporal trends in pancreatic cancer incidence and mortality over the past three decades in France using a larger dataset.
Methods
Data sources
The network of French population-based cancer registries FRANCIM, in collaboration with the biostatistics department of the Hospices Civils de Lyon, the French Institute for Public Health Surveillance (InVS, Institut de veille sanitaire) and the French National Cancer Institute (INCa, Institut national du cancer) manage a common database of all cancers diagnosed in well-defined administrative areas called ‘départements’. Quality checks are carried out at both the registry level, using local controls, and at the common database level, using in particular the DepEdit software provided by the International Agency for Research on Cancer. The quality and exhaustiveness of these registries are certified every 4 years by an audit of the National Institute of Health and Medical Research (INSERM), the InVS and the INCa. Cases are notified by many sources: public and private pathology laboratories, regional databases of the National Health System, and public and private hospital discharge databases. French registries do not record incident cases that are notified by death certificates only. Death certificates mentioning pancreatic cancer and which escaped the registration process during life were individually traced back. The few cases which were not traceable were not registered.
Malignant pancreatic tumours (coded as C25 according to the International Classification of Diseases in Oncology, third revision) diagnosed over the period 1982–2012 in eight ‘départements’ (Table 1) were extracted from the common French registries database (N = 8908 and 7967, number of cases in men and women, respectively). Among the whole study population, the proportion of cases with histologic confirmation ranged from 62% in 1982 to 72% in 2012. The mortality data were provided by the official French Mortality Statistics Office (CepiDc-INSERM). All pancreatic cancer deaths (anonymous records) that occurred in the eight concerned Départments between 1982 and 2012 were extracted (ICD-8 and ICD-9 codes157 and ICD-10 code C25).
Table 1.
Population-based cancer registries included in the study
| ‘Département’ covered by cancer registry | Type of registry | First year of registration | Population of the ‘département’ in 2012 |
|---|---|---|---|
| Bas Rhin (67) | General | 1975 | 1 117 803 |
| Calvados (14) | Digestive | 1978 | 692 049 |
| Côte d’Or (21) | Digestive | 1976 | 529 967 |
| Doubs (25) | General | 1978 | 533 494 |
| Isère (38) | General | 1979 | 1 237 946 |
| Saône et Loire (71) | Digestive | 1982 | 556 993 |
| Somme (80) | General | 1982 | 575 153 |
| Tarn (81) | General | 1982 | 385 356 |
| Total | 5 628 761 |
Person-years were estimated from the population data provided by the French national institute of statistics (INSEE), by département, sex, calendar year and annual age from 1982 to 2013.
Methodology
Incidence and mortality in the registry area were analysed in men and women separately (i.e. four separate analyses) using age-period-cohort models (Poisson regression).8,9 Data were aggregated over the registry area by annual age a, annual birth cohort c and thus by period (calendar year) p = c + a. Each model included continuous functions of a and c (cubic splines with one knot at the median age or cohort, respectively) and, if statistically significant (likelihood ratio test, α = 1%), a linear age-cohort interaction was added [which is equivalent to adding a period (year) term p2]. Based on this procedure, in the end, an age-cohort model was selected for the four analyses.
Annual percentage changes (APCs) between 1982 and 2012 in age-standardized incidence λ were derived from the formula: λ2012 = λ1982 × (1 + APC)30 and similarly for mortality. Confidence intervals (CIs) at 95% were derived using the delta-method,10 the parameters and their variance-covariance matrix estimated from the model.
The net probability of developing pancreatic cancer before age 74 was calculated by birth cohort by extrapolating up to 74 years for the most recent cohorts. This net probability represents the probability of developing pancreatic cancer before age 74 that would be observed if people could not die before this age (i.e. in the absence of mortality). This indicator is thus not sensitive to an increase in life expectancy. Statistical analyses were performed using Splus- software, version 6.2.
Results
Time trends in incidence and mortality by calendar year of diagnosis
Pancreatic cancer age-standardized incidence rates (ASRs) doubled over time for men, from 4.8 per 100 000 person-years (PY) in 1982 to 9.6 per 100 000 PY in 2012, and nearly tripled in women from 2.3 to 6.8 per 100 000 PY (Table 2 and Figure 1). The corresponding overall mean APC was +2.3% (95% CI: 2.1–2.3) in men and +3.6% (3.3–3.9) in women. The increase was regular over time in both men and women and relatively similar across registries (see Supplementary Figure 1, available as Supplementary Data at IJE online). Across the study period, the male–female incidence rate ratio decreased steadily from 2.6 in 1982 to 1.4 in 2012. Over the same time period, the pancreatic cancer age-standardized mortality rate remained stable for men, at 8.1 per 100 000, whereas it slightly increased for women, from 4.0 per 100 000 in 1982 to 5.4 per 100 000 in 2012 (APC: +1.0%, 95% CI: 0.7–1.3). The incidence/mortality ratio regularly increased and has exceeded unity since 2003 for both sexes. According to age, incidence reached the same level as mortality from the early 1990s for age 50 to the year 2008 for age 80 (figure not shown).
Table 2.
Age-standardized incidence and mortality ratesa of pancreatic cancers in France in men and women between 1982 and 2012
| 1982 | 1992 | 1997 | 2002 | 2007 | 2012 | Mean annual percent change (APC) | |
|---|---|---|---|---|---|---|---|
| Incidence | |||||||
| Men | 4.8 | 6.3 | 7.1 | 7.9 | 8.8 | 9.6 | 2.3% (2.1–2.6) |
| Women | 2.3 | 3.4 | 4.1 | 4.8 | 5.7 | 6.8 | 3.6% (3.3–3.9) |
| Mortality | |||||||
| Men | 8.2 | 8.1 | 8.1 | 8.2 | 8.1 | 8.1 | 0% (–0.3–0.2) |
| Women | 4.0 | 4.4 | 4.6 | 4.8 | 5.1 | 5.4 | 1% (0.7–1.3) |
aPer 100 000.
Figure 1.
Time trends in age-standardized (world) incidence and mortality from pancreatic cancer in France (registry areas) from 1982 to 2012: modelled vs observed rates in men (a) and women (b).
Time trends in incidence and mortality by birth cohort
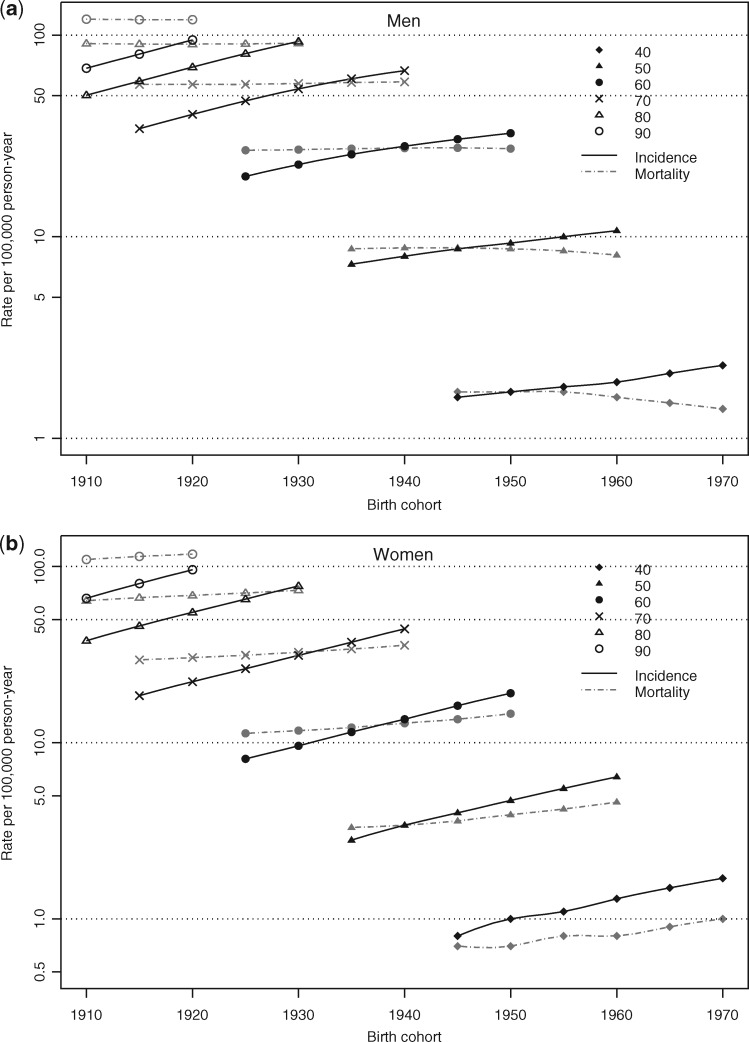
Figure 2 shows the incidence and mortality rates by birth cohorts, for different ages (40–90) in men (a) and women (b). Whatever the age, incidence increased with the birth cohort in both sexes, whereas mortality remained stable in men and increased with the birth cohort only in women. Note that the slight decrease in men in recent cohorts and in the youngest ages is subject to very wide variability.
Figure 2.
Incidence and mortality rates (per 100 000 PY) of pancreatic cancer according to birth cohorts for selected age, in men (a) and women (b).
Incidence caught up with mortality in a similar way in both sexes. Incidence exceeded mortality after age 40 in the youngest cohorts born after 1950, whereas, in the 1930 cohort, incidence only reached the same level as mortality at age 70.
The net cumulative risk of developing or dying from pancreatic cancer before age 75 according to birth cohort is given by sex in Table 3. The cumulative risk of developing pancreatic cancer increased in both sexes, and more markedly in women; it rose from 0.62% for men born around 1920 to 1.17% for those born around 1950 and from 0.31% to 0.73% in women. The cumulative risk of mortality exhibited an unchanged risk in men and a slight increase in risk in women, from 0.44% for women born around 1920 to 0.59% for those born around 1950. In both sexes, the cumulative risk of incidence has exceeded the cumulative risk of mortality since the 1935 birth cohort.
Table 3.
Trends in the net probability (%) of developing or of dying from a pancreatic cancer before age 75 according to birth cohort, in men and women
| Birth cohort | |||||||
|---|---|---|---|---|---|---|---|
| 1920 | 1925 | 1930 | 1935 | 1940 | 1945 | 1950 | |
| Incidence | |||||||
| Men | 0.62 | 0.72 | 0.82 | 0.92 | 1.01 | 1.10 | 1.17 |
| Women | 0.31 | 0.36 | 0.43 | 0.51 | 0.61 | 0.73 | 0.86 |
| Mortality | |||||||
| Men | 0.91 | 0.92 | 0.92 | 0.93 | 0.94 | 0.94 | 0.93 |
| Women | 0.44 | 0.45 | 0.47 | 0.49 | 0.52 | 0.55 | 0.59 |
Discussion
The major findings from our study are that pancreatic cancer incidence has increased over the last 30 years in France whereas mortality did not vary in men and moderately increased in women. The increase in incidence was greater in women than in men (APC 3.6 vs 2.3%), and affected all ages. The observed differences in incidence and mortality trends are unexpected for this very lethal cancer site and raise questions about the causes. Several possible explanations are discussed below.
In France, information concerning cancer incidence is provided by cancer registries working at the level of geographic administrative area ‘départements’. Cancer registries have created a huge amount of population-based data, which are collected and managed in a standardized procedure. These data encompass all cancers, including those diagnosed in very old patients and those without histological confirmation, which is of particular interest for a cancer often diagnosed without a histological biopsy.11 Thus, this study provides an informative picture of pancreatic cancer incidence in a general population over a 30-year period.
This study has shown that incidence and mortality rates for pancreatic cancer exhibited different trends in France. When these findings are compared with similar data reported from other countries, the steady rise in incidence for pancreatic cancers seems to be specific to France. High-quality data on cancer incidence, provided by population-based cancer registries for all countries around the world, are published by the International Agency for Research on Cancer (IARC) and the International Association of Cancer Registries (IACR).6,7,12–15 The last five volumes of Cancer Incidence in Five Continents cover the period 1983–2007 and use the common world standard population. Therefore, and unlike many other publications, it allows geographical comparisons of rates. Pancreatic cancer is relatively rare in Oceania, Central and South America and most Asian countries, and rare in Africa. The highest incidence in these countries was reported in Japan, where there has been no significant increase in incidence over the past 25 years. Pancreatic cancer incidence is among the highest in Northern America. In the USA, recent data from the SEER-9 programme, which included all races and sexes, showed an average rise in incidence of 0.8% each year from 2002 to 2012.2 The incidence of pancreatic cancer in Canada remained relatively stable from 1992 to 2005, although the incidence decreased somewhat in men16 (Table 4). In contrast, different trends are reported in Europe. In 2012, the mean world-standardized incidence rate in Europe was 8.3 per 100 000 PY in men and 5.4 per 100 000 in women.1 The incidence rates in France were thus very close to the mean European rates in 2012, but the increasing trends observed in France are atypical. Indeed, a slight decrease in incidence was seen in the Netherlands,17 Sweden15 and Switzerland.15 In Norway,18 Ireland19 and in the UK20 and Denmark,21,22 incidence rates of pancreatic cancer have been relatively stable since the mid-1990s. Incidence did not vary in Eastern European countries such as Slovenia or Poland, except for a slight increase in Slovakia from 8 per 100 000 PY in 1982 to 11.9 per 100 000 PY in 2007. South European registries data showed no variation in incidence in males and a slight increase until 2007 for females in Italy and Spain.15 Data on time trends in incidence by age are scarce. Those available did not exhibit any variation according to age classes.16,17,23
Table 4.
International comparisons of trends in incidence for pancreatic cancer
| Country | Authors | Year | APCa | ||
|---|---|---|---|---|---|
| Global | Males | Females | |||
| Canada | Flook et al. | 1992–2005 | – | –10% | 0% |
| Denmark | Edvardsson et al. | 2000–12 | ‘Unchanged’ | ||
| Denmark | Cancer stat factsheets NORDCAN | 2003–13 | 0% | 0% | 0% |
| England | Coupland et al. | 1998–2007 | ‘Unchanged’ | ||
| Ireland | Cancer stat factsheets www.ncri.ie | 1994–2013 | – | 0.50% | 0.40% |
| Netherlands | Nienhuijs et al. | 1989–2008 | –0.14% | –0.44% | 0.28% |
| Norway | Soreide et al. | 1985–2007 | ‘Unchanged’ | ||
| USA, SEER Program | Howlader et al. | 1992–2012 | 0.80% | ||
| France | The present study | 1982–2012 | 2.30% | 3.60% | |
aAnnual Percentage Change.
The hypothesis of an artefactual increase related to an improvement in pancreatic cancer registration in the concerned French cancer registries does not seem plausible as the only explanation, although, compared with other solid-organ cancers, pancreatic cancer has a low rate of histology-confirmed diagnosis.11 In order to avoid possible underestimation of incidence at the beginning of the registration for recent cancer registries under construction, which may have endured under-documentation in their early years, we only included data from longstanding French cancer registries. The use of several sources of notification and a network of expertise allows these longstanding registries to gather all incident cases. Although data are collected and managed using standardized procedures, the enlargement of registry identification sources over time, by including administrative databases for example, could affect the incidence reported by registries. However, with decentralized sources of cancer notification, all of the registries should not have been affected simultaneously, yet all of them exhibited a similar regular increase in pancreatic cancer incidence. When a new identification procedure is set up in a registry, it mostly improves registration in elderly patients, who are less likely to be detected in multiple clinical sources. Besides, trends revealed by national health insurance data, which have been available since 1997 in France, are consistent with the incidence trends observed in cancer registries: requests for full reimbursement of costs related to newly diagnosed pancreatic cancer have increased regularly since 1997, and more markedly in women than in men.24,25
The possible effect of an improvement in medical imaging (identification of previously unspecific lesions) on pancreatic cancer incidence cannot be ruled out, but it should not have concerned women more than men and should have had a similar impact in other industrialized countries. Moreover, in making diagnosis easier and less interventional, these improvements would probably have concerned elderly patients more than others.
Mortality was higher than incidence at the beginning of the study period. The ratio of mortality to incidence progressively decreased from 1.7 in 1980, reaching unity in 2005 and 0.8 in 2012. This pattern differs from data published in Cancer Incidence in the Five Continents: in almost all other European countries, the mortality to incidence ratio ranged between 0.9 and 1.2 in the 1988–92 period. In the last period (2003–07), the ratio varied from 0.9 to 1.3 in Northern European countries.6,7,12–15 There is no clear explanation for these trends. One could hypothesize that French clinicians may have previously over-reported pancreatic cancer in mortality statistics in the absence of easily available diagnostic tools for patients dying with jaundice. Until the early 1980s, exploration of the pancreas was difficult, and diagnosis was made on operative findings in nearly 40% of cancers, then diagnosis became progressively easier with the development of imaging.11 International publications concerning pancreatic cancer death certificates are scarce. To our knowledge, only one Spanish publication partly concerned the accuracy of death certificates and did not underline any issues with pancreas cancer.26 Five-year net survival only slightly increased in France in both sexes from 6% during the period 1989–93 to 8% during the period 2005–10.27 Similar trends in incidence and mortality are therefore expected. Incidence increased more than mortality, suggesting that the improvement in diagnosis has reduced the over-notification of pancreatic cancer as the cause of death, balancing the increase in mortality following the increase in incidence.
It should be noted that the international practice to consider Death Certificate Only (DCO) cases in incidence estimations should not weaken these comparisons. DCO cases are theoretically the remaining cases for which no information source other than a death certificate mentioning pancreatic cancer can be found. They should represent the residuum of the trace-back process of the death certificates mentioning pancreatic cancer. Data from the last Cancer Incidence in Five Continents publications showed that DCO were not uniformly taken into account over the world and over time in incidence estimations.6,7,12–14 The highest percentages of DCO, higher than 25%, concerned Asia, South America and Germany. It could probably be related to the incomplete trace-back of death certificates. Of lesser importance, the percentage of DCO ranged from 8 to 16% during the first two periods and decreased thereafter to less than 6% in Poland and Spain. Elsewhere in European countries, as well as in Canada, Australia or North America, the percentage of DCO was low and represented less than 5% of incident cases. Residual DCO cases have never been taken into account in French, Dutch and Swedish cancer registries. Thus, one might think that international comparisons in trends in incidence for pancreatic cancer are not markedly affected by the inclusion of DCO.
Apart from rare cases of familial pancreatic cancer, the aetiology of pancreatic cancer is still not well understood. In many studies, the reported data are limited to period analyses. Tobacco smoking is considered a well-established risk factor, with an attributable risk of around 25%.28 Changing patterns of smoking in France could partly explain the larger increase in incidence in women than in men. Whereas tobacco consumption has decreased in men and women in most European countries, smoking rates are still increasing in women in France.29,30 Diverging trends in pancreatic cancer incidence over the world could, at least partly, reflect the different patterns in tobacco consumption in successive generations of men and women. The fact that women have now taken up the smoking habits of men may have had a latent effect on the risk of pancreatic cancer. In France, considering the cumulative life exposure to tobacco smoking in people aged 60–64 (indicated by the mean number of daily cigarettes smoked since the age 15), life exposure increased in men from 1970 to 2000 and then stabilized, whereas it regularly increased in women.31 However, the increase in pancreatic cancer incidence contrasts sharply with the stable lung cancer rates in men, suggesting the contribution of other factors besides smoking. The risk of pancreatic cancer appears to be greater in people with a high-energy diet and lower in those with a diet rich in fruits and vegetables, though more etiological studies are needed to support this hypothesis.32,33 One might think that the increasing prevalence of overweight may contribute to the observed cohort effect but, if this were the case, this effect should also have been observed in countries with a similar way of life.
Even though cancer registry data are considered the gold standard for cancer surveillance, several limitations in the data and methods may have influenced interpretations of the findings in this study. This study included data from all French registries (n = 8) with at least 30 years of records. These registry areas covered 9% of the French population, which raises the question of the representativeness of the trends observed. However, as mentioned above, incidence trends in these areas were consistent with the trends revealed in national health insurance data, which suggests that the trends observed are not specific to our registry areas. Another limitation is that detailed data, such as stage at diagnosis or size of tumour, were not available. Future studies incorporating additional epidemiological risk factors, such as smoking or obesity, are needed. Changes in detection practices due to the introduction or increased use of diagnostic techniques, which may contribute to a temporal increase in observed incidence rates, were not measurable. Statistics on cancer occurrence cannot be routinely reported according to racial and ethnic populations in France. Wide variations in the cancer burden by ethnicity may occur and trends in incidence may be partly related to temporal migration.
It is thus difficult to explain this trend in the frequency of a severe cancer in France. One cannot exclude the possibility that a similar trend may appear in other countries in the future. Indeed, very recent predictions suggest that the burden of pancreatic cancer is expected to rise over the next 15 years regardless of age and sex in European regions as well as in other regions defined according to the WHO.34 The WHO also relies on worldwide population-based data and thus cannot disentangle causality from extension at presentation. Nonetheless, the WHO underlines the need to improve knowledge of this disease. The recent developments in molecular biology have so far failed to identify high-risk populations for pancreatic cancer. Future epidemiological research should investigate whether there is any difference in trends in incidence between exposed groups for plausible risk factors and between different countries.
Supplementary Data
Supplementary data are available at IJE online.
Supplementary Material
Acknowledgements
The authors thank all the private and public physicians, specialists and pathologists for their collaboration, Santé Publique France (SPF) and the National Cancer Institute (INCa) for supporting the Registry. Members of the FRANCIM network are as follow: Bas Rhin: M. Velten; Calvados: G. Launoy, V. Bouvier, A.V. Guizard; Doubs: A.S. Woronoff; Finistère: M. Robaszkiewicz; Gironde: A. Monnereau, G. Coureau; Haut-Rhin: E. Marrer; Hérault: B. Trétarre; Isère: M. Colonna; Limousin: N. Leone; Loire-Atlantique-Vendée: F. Molinié; Manche: S. Bara; Nord: K. Ligier; Somme: O. Ganry; Tarn: P. Grosclaude.
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1. Ferlay J, Soerjomataram I, Ervik M. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon: International Agency for Research on Cancer, 2013. [Google Scholar]
- 2. Howlader N, Noone AM, Krapcho M. et al. SEER Cancer Statistics Review, 1975–2012, National Cancer Institute. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda, MD, 2016. http://seer.cancer.gov/csr/1975_2013/ (28 April 2017, date last accessed). [Google Scholar]
- 3. Lepage C, Capocaccia R, Hackl M. et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: results of EUROCARE-5. Eur J Cancer 2015;51:2169–78. [DOI] [PubMed] [Google Scholar]
- 4. Coleman M, Esteve J, Damiecki P. et al. Trends in Cancer Incidence and Mortality, IARC ed. Lyon: Scientific Publication, 1993. [DOI] [PubMed] [Google Scholar]
- 5. Bouvier AM, David M, Jooste V. et al. Rising incidence of pancreatic cancer in France. Pancreas 2010;39:1243–6. [DOI] [PubMed] [Google Scholar]
- 6. Curado MP, Edwards B, Shin HR. et al. Cancer Incidence in Five Continents, Vol. IX 160 vol Lyon: IARC, 2007. [Google Scholar]
- 7. Forman D, Bray F, Brewster DH. et al. Cancer Incidence in Five Continents, Vol. X.IARC Lyon: International Agency for Research on Cancer, Scientific Publication, 2013. http://ci5.iarc.fr (28 April 2017, date last accessed). [Google Scholar]
- 8. Belot A, Velten M, Grosclaude P. et al. Estimation nationale de l’incidence et de la mortalité par cancer en France entre 1980 et 2005, 2008. www.invs.sante.fr (31 March 2017, date last accessed).
- 9. Binder-Foucard F, Bossard N, Delafosse P. et al. Cancer incidence and mortality in France over the 1980–2012 period: solid tumors. Rev Epidemiol Sante Publique 2014;62:95–108. [DOI] [PubMed] [Google Scholar]
- 10. Kotz S, Johnson NL, Read CB. Encyclopaedia of Statistical Sciences. New York: Editions John Wiley, 1988, 646–7. [Google Scholar]
- 11. David M, Lepage C, Jouve JL. et al. Management and prognosis of pancreatic cancer over a 30-year period. Br J Cancer 2009;101:215–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parkin DM, Whelan S, Ferlay J. et al. Cancer Incidence in Five Continents, Vol. VIII Lyon: International Agency for Research on Cancer, Scientific Publication, 2002. [Google Scholar]
- 13. Parkin DM, Muir CS, Whelan SL. et al. Cancer Incidence in Five Continents, Vol. VI Lyon: International Agency for Research on Cancer, Scientific Publication, 1992. [Google Scholar]
- 14. Parkin D, Whelan S, Ferlay J. et al. (eds). Cancer Incidence in Five Continents, Vol. VII Lyon: International Agency for Research on Cancer, Scientific Publication, 1997. [Google Scholar]
- 15. Forman D, Bray F, Brewster DH. et al. Cancer Incidence in Five Continents, Vol. X Lyon: International Agency for Research on Cancer, Scientific Publication, 2013. http://ci5.iarc.fr (3 April 2017, date last accessed). [Google Scholar]
- 16. Flook R, van Zanten SV. Pancreatic cancer in Canada: incidence and mortality trends from 1992 to 2005. Can J Gastroenterol 2009;23:546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nienhuijs SW, van den Akker SA, de Vries E. et al. Nationwide improvement of only short-term survival after resection for pancreatic cancer in the Netherlands. Pancreas 2012;41:1063–6. [DOI] [PubMed] [Google Scholar]
- 18. Soreide K, Aagnes B, Moller B. et al. Epidemiology of pancreatic cancer in Norway: trends in incidence, basis of diagnosis and survival 1965–2007. Scand J Gastroenterol 2010;45:82–92. [DOI] [PubMed] [Google Scholar]
- 19. National Cancer Registry Ireland. Survival Statistics 2014. http://www.ncri.ie/data/survival-statistics (3 April 2017, date last accessed).
- 20. Coupland VH, Kocher HM, Berry DP. et al. Incidence and survival for hepatic, pancreatic and biliary cancers in England between 1998 and 2007. Cancer Epidemiol 2012;36:e207–14. [DOI] [PubMed] [Google Scholar]
- 21. Engholm G, Ferlay J, Kejs A. et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.2 (16.12.2015). Danish Cancer Society; http://www.ancr.nu (3 April 2017, date last accessed). [Google Scholar]
- 22. Bjerregaard JK, Mortensen MB, Pfeiffer P. Trends in cancer of the liver, gall bladder, bile duct, and pancreas in elderly in Denmark, 1980–2012. Acta Oncol 2016;55(Suppl 1):40–5. [DOI] [PubMed] [Google Scholar]
- 23. SEER. Surveillance, Epidemiology, and End Results Program. Stat Fact Sheets: Pancreas Cancer; 2015. www.cancer.gov (3 April 2017, date last accessed). [Google Scholar]
- 24. Bonaldi C, De Maria F. Surveillance nationale de l’incidence des cancers: tendances récentes des ALD de la Caisse d’Assurance Maladie des Travailleurs Salariés, période 1997–2014. Document technique 2016, Santé publique France. [Google Scholar]
- 25. Uhry Z, Remontet L, Grosclaude P. et al. Tendances récentes des données d’affections de longue durée (ALD): intérêt pour la surveillance nationale de l’incidencedes cancers. Période 1997–2009. Bull Epidémiol Hebd 2012;5:58–63. [Google Scholar]
- 26. Perez-Gomez B, Aragones N, Pollan M. et al. Accuracy of cancer death certificates in Spain: a summary of available information. Gac Sanit 2006;20(Suppl 3):42–51. [DOI] [PubMed] [Google Scholar]
- 27. Cowppli-Bony A, Uhry Z, Remontet L. et al. Survie des personnes atteintes de cancer en France métropolitaine 1989–2013. Partie 1 Tumeurs solides. Saint Maurice; ( France: ), 2016. [Google Scholar]
- 28. Silverman DT, Dunn JA, Hoover RN. et al. Cigarette smoking and pancreas cancer: a case-control study based on direct interviews. J Natl Cancer Inst 1994;86:1510–16. [DOI] [PubMed] [Google Scholar]
- 29. Levi F, Bosetti C, Fernandez E. et al. Trends in lung cancer among young European women: the rising epidemic in France and Spain. Int J Cancer 2007;121:462–5. [DOI] [PubMed] [Google Scholar]
- 30. Lortet-Tieulent J, Renteria E, Sharp L. et al. Convergence of decreasing male and increasing female incidence rates in major tobacco-related cancers in Europe in 1988–2010. Eur J Cancer 2015;51:1144–63. [DOI] [PubMed] [Google Scholar]
- 31. Hill C, Jougla E, Beck F. Le point sur l’épidémie de cancer du poumon dû au tabagisme. Bull Epidémiol Hebd 2010;19:210–13. [Google Scholar]
- 32. Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol 2015;44:186–98. [DOI] [PubMed] [Google Scholar]
- 33. Song M, Giovannucci E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among white adults in the United States. JAMA Oncol 2016;2:1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Are C, Chowdhury S, Ahmad H. et al. Predictive global trends in the incidence and mortality of pancreatic cancer based on geographic location, socio-economic status, and demographic shift. J Surg Oncol 2016;114:736–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.