Abstract
Aims
Post-stroke hypertension is associated with poor short-term outcome, although the results have been conflicting. Our objective was to evaluate the association of blood pressure (BP) and in-hospital outcomes in patients with acute ischaemic stroke.
Methods and results
Patients in the Get With The Guidelines-Stroke registry with acute ischaemic stroke were included. Admission systolic and diastolic BP was used to compute mean arterial pressure (MAP) and pulse pressure (PP). The outcomes of interest were: in-hospital mortality, not discharged home, inability to ambulate independently at discharge and haemorrhagic complications due to thrombolytic therapy. A total of 309 611 patients with an ischaemic stroke were included. There was a J-shaped/U-shaped relationship between systolic BP and outcomes. Both lower and higher systolic BP values, compared with a central reference value, had higher risk of in-hospital death [e.g. adjusted odds ratio (95% confidence interval) (OR[CI]) = 1.16[1.13–1.20] for 120 vs. 150 mmHg and 1.24[1.19–1.30] for 200 vs. 150 mmHg], not discharged home (OR[CI] = 1.11[1.09–1.13] for 120 vs. 150 mmHg and 1.15[1.12–1.18] for 200 vs. 150 mmHg), inability to ambulate independently at discharge (OR[CI] = 1.16[1.13–1.18] for 120 vs. 150 mmHg and 1.09[1.06–1.11] for 200 vs. 150 mmHg). However, risk of haemorrhagic complications of thrombolytic therapy was lower with lower systolic BP (OR[CI] = 0.89[0.83–0.97] for 120 vs. 150 mmHg), while higher with higher systolic BP (OR[CI] = 1.21[1.11–1.32] for 200 vs. 150 mmHg). The results were largely similar for admission diastolic BP, MAP, and PP.
Conclusion
In patients hospitalized with ischaemic stroke, J-shaped, or U-shaped relationships were observed between BP variables and short-term outcomes. However, haemorrhagic complications with thrombolytic therapy were lower with lower BP.
Keywords: Blood pressure, Diastolic, Ischaemic stroke, Mean arterial pressure, Pulse pressure, Systolic, Stroke, Transient ischaemic attack
Introduction
Hypertension is a major risk factor for stroke.1 In patients with ischaemic stroke, post-stroke hypertension has been shown to have a beneficial effect due to a pressure-dependent cerebral blood flow to the ischaemic regions.2 However, other studies have shown that post-stroke hypertension is associated with an increased incidence of recurrent stroke,3 haemorrhagic transformation and cerebral oedema in the short term.4,5 As such observational studies on the relationship between blood pressure (BP) and short-term outcomes have been conflicting.3,6–12 Moreover, limited data exist on the association of either the pulsatile component of BP [pulse pressure (PP)] or the steady component [mean arterial pressure (MAP)] on outcomes.13
Our objectives were: (i) to evaluate the association between admission BP on in-hospital outcomes in patients with an acute ischaemic stroke; (ii) to evaluate if the above relationships change with the receipt of thrombolytic therapy; and (iii) to evaluate if MAP or PP are stronger predictors of in-hospital mortality than systolic or diastolic BP alone.
Methods
Patient selection
Patients in the Get With The Guidelines-Stroke (GWTG-Stroke) registry hospitalized with ischaemic stroke were considered for this study. The GWTG-Stroke program is a voluntary quality improvement program across the USA, which collects information on stroke admission.14 The institutional review boards at the participating centre determined that the study was exempt and hence no individual patient consent was required.14 The reliability of the data collected in GWTG-Stroke has been shown to be excellent.15
Inclusion and exclusion criteria
Patients with a diagnosis of ischaemic stroke, where patients presented <4.5 h after symptom onset, between 2003 and 2014 were included. Patients with symptoms lasting <24 h but with radiographic evidence of ischaemic injury, or those with symptoms lasting ≥24 h, were classified as ischaemic stroke.16 Case ascertainment of admissions for ischaemic stroke was conducted by prospective clinical identification and retrospective identification with International Classification of Diseases (9th revision) discharge codes (433.xx, 434.xx, and 436 for ischaemic stroke), followed by chart review to confirm case eligibility, or a combination of both approaches. Sites were instructed to follow AHA/ASA guidelines for definition of ischaemic stroke17; these include that ischaemic stroke can be diagnosed when symptoms are present beyond 24 h even in the absence of neuroimaging evidence of brain infarction. To exclude haemorrhagic stroke, neuroimaging is required. A limited number of admissions had a final diagnosis of stroke of uncertain type, which were predominantly cases where neuroimaging could not be performed. In this analysis, we have limited the study cohort to those with a final diagnosis of ischaemic stroke.
Patients were excluded if: (i) they were transferred in from another facility; (ii) transferred out to another acute care facility; (iii) left against medical advice or if the discharge status was unknown; and (iv) admission systolic or diastolic BP was missing or if the BP values were out of valid range (diastolic BP ≥systolic BP pulse pressure <20 mmHg or >230 mmHg, MAP <30 mmHg or >220 mmHg).
Blood pressure variables
Admission systolic and diastolic BP was recorded for each patient. Mean arterial pressure was calculated using the formula MAP = [systolic BP + (2*diastolic BP)]/3. Pulse pressure was calculated as the difference between systolic and diastolic pressures.
Outcomes of interest
Outcomes of interest were: (i) in-hospital mortality; (ii) inability to ambulate independently at discharge (defined as inability to ambulate without the assistance of another person with or without device use, and derived from chart notes including assessments by physical therapists); and (iii) not discharged home (includes those patients that died in hospital or were discharged to another (non-acute) care facility or to hospice care). In addition, haemorrhagic complications of thrombolytic therapy (defined as symptomatic intracranial haemorrhage (sICH) or life threatening haemorrhage within 36 h) were evaluated. Symptomatic intracranial haemorrhage was defined as intracerebral haemorrhage within 36 h, documented by computed tomography or magnetic resonance imaging and by the treating physician’s notes indicating clinical deterioration attributable to haemorrhage. This definition is based on the criteria for sICH established in the National Institute of Neurological Disorders and Stroke (NINDS) tPA trials.18 The AHA/ASA guidelines provide recommendations for treatment criteria for thrombolysis, and GWTG recognition programs reward hospitals that adhere to AHA/ASA guidelines. Nonetheless, ultimately the decision to administer thrombolytic therapy is made at the discretion of the treating physician.
Statistical analysis
Both unadjusted and adjusted (multivariable) logistic regression analyses were performed to evaluate the relationship between admission BP variables and outcomes. Multivariable models included the following covariates: age, sex, race, body mass index (BMI), arrival time (on vs. off hours), arrival mode, medical history (atrial fibrillation/flutter, coronary artery disease (CAD), prior stroke/transient ischaemic attack (TIA), carotid stenosis, diabetes, peripheral vascular disease (PVD), hypertension, dyslipidaemia, smoking), and site characteristics (region, teaching hospital, number of beds, rural vs. urban hospital). Missing values of covariates (BMI was missing in 16%; all other covariates were missing in <3%) were imputed using multiple imputations (25 imputations). All models used generalized estimating equations to account for within-hospital clustering.
Each admission BP variable was assessed for the linearity of its relationship with each outcome. In each case, a 5th order polynomial best captured the non-linearity. To demonstrate these relationships, for each BP variable, a reference BP value was chosen corresponding to the point of lowest predicted risk (rounded to nearest 10 mmHg) in the in-hospital mortality model. This corresponded to a systolic BP of 150 mmHg, diastolic BP of 70 mmHg, MAP of 100 mmHg, and a PP of 80 mmHg. We then tabulated odds ratio (OR) and their 95% confidence interval (CI) for outcomes at BP values in 10 mmHg increments, over the full range of values, compared with the reference value. To display the relationship between BP and outcomes, we graphed the adjusted OR compared with the reference values, along with observed event rates for groups defined by 10 mmHg increments. The range for each BP variable shown in the graphs is approximately the 1st–99th percentile of its distribution
To evaluate the interaction between BP variables and thrombolytic treatment and outcomes, terms for thrombolytic treatment and the interaction between thrombolytic treatment and each BP measurement were added to the above models. To evaluate the relative contribution of BP variables to in-hospital mortality models we used the F-statistics from the above models. The F-test assesses whether the measure is associated with the outcome, conditional on all other covariates, with the largest F-statistic demonstrating the closest association with in-hospital mortality risk. In addition, the C-index was calculated for each BP variable.
Sensitivity analysis was performed in the subset of patients with NIH stroke scale data. These models included the NIH stroke scale as a covariate in the model. NIH stroke scale score is a measure of neurologic deficits ranging from 0 to 42, with higher score for greater stroke severity.19
All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA). All P-values were 2-sided, with P < 0.05 considered statistically significant.
Results
A total of 309 611 patients with an ischaemic stroke fulfilled our criteria (see Supplementary material online, Figure S1). Among them, 81 358 (26%) patients received thrombolytic therapy of whom 4564 (5.6%) had a haemorrhagic complication (3726 had sICH; 738 had life-threatening systemic haemorrhage; and 100 had both).
Baseline characteristics
Of the 309 611 patients, systolic BP ≥140 mmHg was observed in 73% and diastolic BP ≥90 mmHg in 34%. Moreover, systolic BP ≥185 mmHg was observed in 19% and diastolic BP ≥110 mmHg in 8% of patients. Patients who were in the higher quintile of systolic BP were more often older, women, black, presented during off-hours, had higher LDL cholesterol levels, lower NIH stroke scale but less likely to arrive using the emergency medical service compared with those in the lower quintile (Table 1). They were less likely to have atrial fibrillation/flutter, prior stroke/TIA, CAD/prior myocardial infarction, smoker, chronic heart failure but more likely to have hypertension, diabetes or carotid stenosis when compared with those in the lower quintile of systolic BP (Table 1). Moreover, patients who were in the higher quintile of systolic BP were less likely to be on antithrombotics, antiplatelets, anticoagulants or receive thrombolytic therapy but more likely to be on antihypertensive agents when compared with those in the lower quintile (Table 1).
Table 1.
Baseline characteristics by quintile of admission systolic blood pressure in patients presenting with ischaemic stroke*
| Variable | Overall (N = 309 611) | SBP 50–133 (N = 62 737) | SBP 134–149 (N = 64 865) | SBP 150–163 (N = 60 872) | SBP 164–183 (N = 60 871) | SBP 184–250 (N = 60 266) |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age | 74 (62–84) | 73 (59–83) | 74 (61–83) | 75 (63–84) | 76 (64–84) | 75 (63–84) |
| Female | 52.2 | 50.9 | 49.9 | 50.5 | 52.3 | 57.9 |
| Race | ||||||
| White | 73.2 | 73.5 | 74.4 | 74.5 | 73.7 | 69.9 |
| Black | 14.5 | 14.2 | 13.3 | 13.4 | 14.3 | 17.7 |
| Hispanic | 6.6 | 6.7 | 6.5 | 6.5 | 6.4 | 6.7 |
| Asian | 2.4 | 2.2 | 2.4 | 2.3 | 2.4 | 2.4 |
| Presentation | ||||||
| On-hour arrival (M-F 7a-6p) | 51.7 | 53.3 | 52.7 | 51.3 | 50.9 | 50.0 |
| Arrival mode EMS | 72.8 | 75.4 | 72.9 | 72.5 | 72.3 | 70.7 |
| NIH stroke scale | 5 (2–13) | 6 (2–14) | 6 (2–13) | 5 (2–13) | 5 (2–12) | 5 (2–12) |
| Labs | ||||||
| Serum creatinine (mg/dL) | 1.0 (0.8–1.3) | 1.0 (0.8–1.4) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) |
| LDL (mg/dL) | 94 (71–121) | 86 (66–112) | 91 (69–116) | 93 (71–120) | 96 (73–123) | 103 (78–131) |
| Medical history | ||||||
| Atrial fib/flutter | 22.7 | 24.6 | 23.9 | 23.4 | 22.4 | 19.0 |
| Prior stroke or TIA | 32.5 | 34.4 | 32.4 | 32.2 | 32.1 | 31.6 |
| CAD/prior MI | 27.0 | 29.5 | 27.0 | 26.9 | 26.6 | 24.9 |
| Hypertension | 76.0 | 68.3 | 71.8 | 76.1 | 79.9 | 84.6 |
| Diabetes mellitus | 29.2 | 28.1 | 27.3 | 28.5 | 29.8 | 32.8 |
| Smoker | 15.4 | 17.2 | 16.0 | 14.6 | 14.5 | 14.8 |
| Dyslipidaemia | 44.3 | 43.3 | 44.2 | 45.3 | 45.2 | 43.3 |
| CHF | 10.0 | 13.2 | 10.4 | 9.4 | 8.7 | 7.9 |
| PVD | 4.7 | 5.2 | 4.5 | 4.5 | 4.5 | 4.6 |
| Carotid stenosis | 3.8 | 3.9 | 3.5 | 3.6 | 3.9 | 4.1 |
| Medications prior to admission | ||||||
| Antithrombotic | 56.1 | 57.8 | 56.9 | 57.2 | 56.4 | 52.1 |
| Antiplatelet | 47.9 | 48.6 | 47.9 | 48.8 | 48.7 | 45.5 |
| Anticoagulant | 12.3 | 14.5 | 13.4 | 12.5 | 11.5 | 9.6 |
| Antihypertensive | 71.8 | 69.6 | 70.0 | 72.1 | 74.1 | 73.5 |
| Thrombolytic therapy | 26.3 | 26.4 | 27.5 | 27.2 | 26.5 | 23.7 |
Continuous variables are medians with 25th and 75th percentiles.
CAD, coronary artery disease; CHF, congestive heart failure; EMS, emergency medical service; LDL, low-density lipoprotein cholesterol; MI, myocardial infarction; PVD, peripheral vascular disease; SBP, systolic blood pressure; TIA, transient ischaemic attack.
P < 0.0001 for all comparisons.
Systolic blood pressure and outcomes
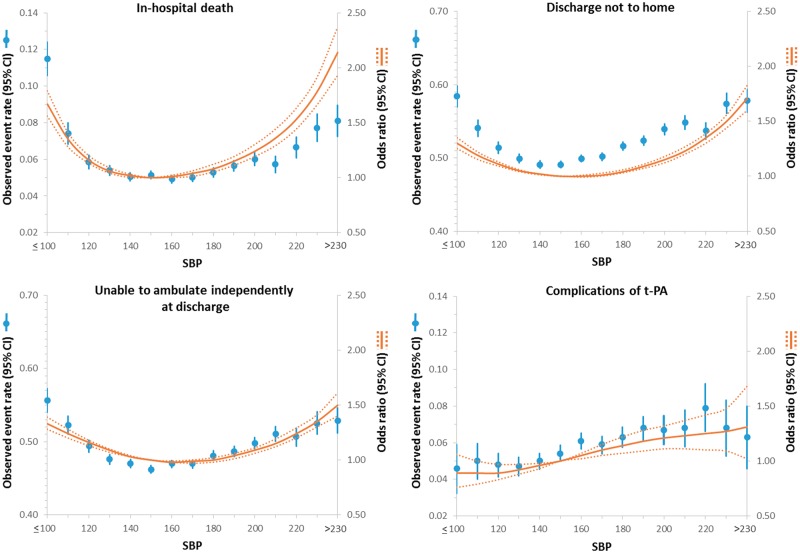
There was a J-shaped/U-shaped relationship between systolic BP and in-hospital death, not discharged home, and inability to ambulate independently at discharge such that both at higher and lower systolic BP below and above 150 mmHg, the odds (unadjusted and adjusted) of these outcomes increased (Figure 1 and see Supplementary material online, Table S1). For example, compared with systolic BP 150 mmHg (reference), patients with systolic BP 120 mmHg had higher odds of in-hospital death (ORadj = 1.16 [1.13–1.20]), not discharged home (ORadj = 1.11 [1.09–1.13]), and inability to ambulate independently at discharge (ORadj = 1.16 [1.13–1.18]). Likewise, patients with systolic BP higher than 150 mmHg had worse outcomes. For example, patients with systolic BP 200 mmHg had higher odds of in-hospital death (ORadj = 1.24 [1.19–1.30]), not discharged home (ORadj = 1.15 [1.12–1.18]) and inability to ambulate independently (ORadj = 1.09 [1.06–1.11]). However, the odds of complications of thrombolytic therapy were lower with lower systolic BP throughout the entire range of systolic BP (Figure 1). For example, when compared with patients who presented with systolic BP 150 mmHg, there was a lower risk of haemorrhagic complications of thrombolytic therapy for a systolic BP of 120 mmHg (ORadj = 0.89 [0.83–0.97]) and a higher risk at systolic BP of 200 mmHg (ORadj = 1.21 [1.11–1.32]).
Figure 1.
Relationship between systolic blood pressure and outcomes in patients presenting with an ischaemic stroke. Adjusted odds ratio (OR) and 95% confidence interval (CI) are shown for each 10 mmHg change away from the reference value (150 mmHg).
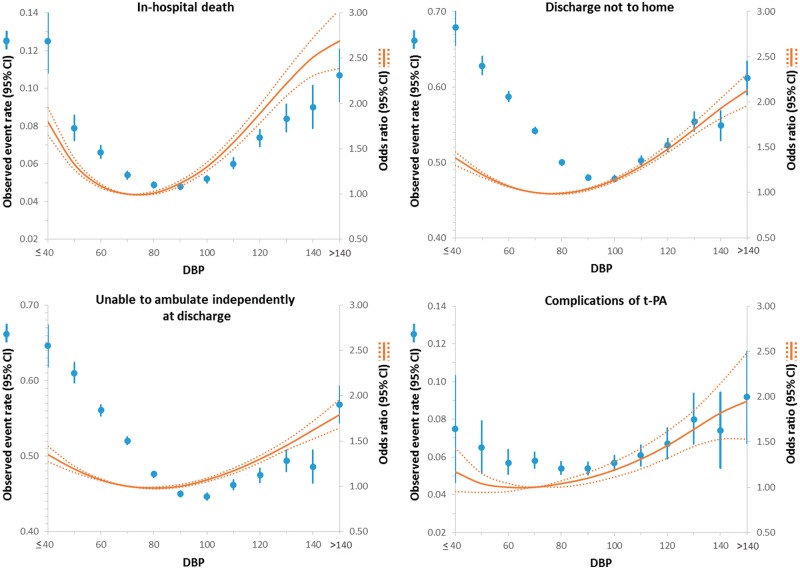
Diastolic blood pressure and outcomes
Similar to the above, there was a J-shaped/U-shaped relationship between diastolic BP and in-hospital death, not discharged home, and inability to ambulate independently at discharge such that below and above 70 mmHg, the odds (unadjusted and adjusted) of these outcomes increased (Figure 2 and see Supplementary material online, Table S2). However, the J-shaped/U-shaped relationship was not seen for complications of thrombolytic therapy (Figure 2).
Figure 2.
Relationship between diastolic blood pressure and outcomes in patients presenting with an ischaemic stroke. Adjusted odds ratio (OR) and 95% confidence interval (CI) are shown for each 10 mmHg change away from the reference value (70 mmHg).
Mean arterial pressure and outcomes
Similarly, there was a J-shaped/U-shaped relationship between MAP and in-hospital death, not discharged home, and inability to ambulate independently at discharge such that below and above 100 mmHg, the odds (unadjusted and adjusted) of these outcomes increased (see Supplementary material online, Figure S2 and Table S3). However, the J-shaped/U-shaped relationship was not seen for complications of thrombolytic therapy (see Supplementary material online, Figure S2 and Table S3).
Pulse pressure and outcomes
Similarly, there was a J-shaped/U-shaped relationship between PP and in-hospital death, not discharged home and inability to ambulate independently at discharge such that below and above 80 mmHg, the odds (unadjusted and adjusted) of these outcomes increased (see Supplementary material online, Figure S3 and Table S4). However, the J-shaped/U-shaped relationship was not seen for complications of thrombolytic therapy (see Supplementary material online, Figure S3 and Table S4).
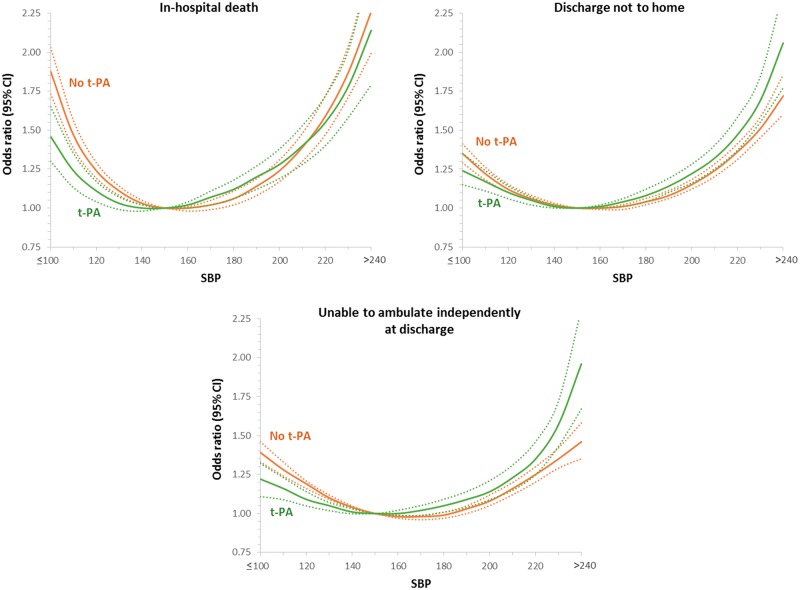
Influence of thrombolytic therapy
For systolic BP and outcomes, the test for interaction was significant for the outcomes of in-hospital mortality (P < 0.0001), inability to ambulate independently (P < 0.0001), or not discharged home (P = 0.001) such that at low values of systolic BP, patients not treated with thrombolytic therapy had a higher odds of these adverse outcomes than did patients treated with thrombolytic therapy (Figure 3). For high values of systolic BP, the odds of adverse outcomes were higher in those treated with thrombolytic therapy than those who were not treated with thrombolytic therapy (Figure 3). For PP and outcomes, the results were largely similar to the above (see Supplementary material online, Figure S6). For diastolic BP (see Supplementary material online, Figure S4) and MAP (see Supplementary material online, Figure S5), the test for interaction was significant for in-hospital mortality such that for extremes of BP values, the odds of in-hospital death were higher in those not treated with thrombolytic therapy than those who were treated with thrombolytic therapy.
Figure 3.
Interaction of thrombolytic use on the relationship between systolic blood pressure and outcomes in patients presenting with an ischaemic stroke.
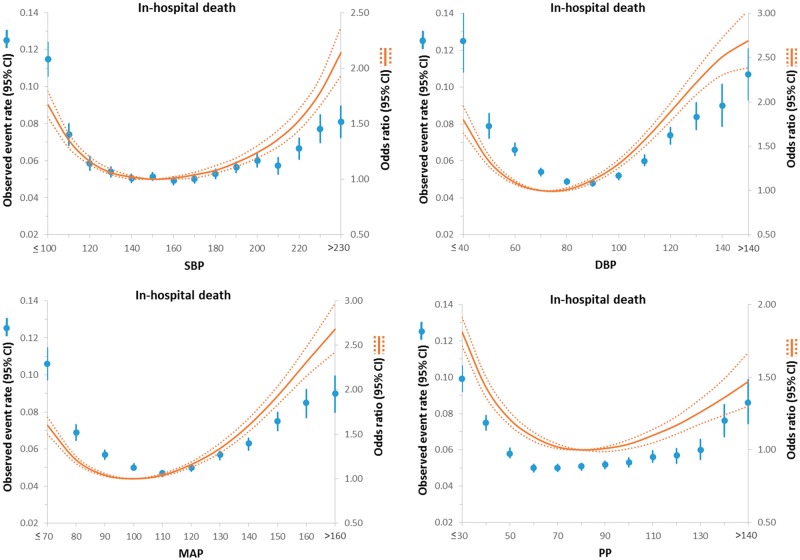
Central Figure.
Relationship between various blood pressure indices and in-hospital death in patients presenting with an ischaemic stroke. Adjusted odds ratio (OR) and 95% confidence interval (CI) are shown for each 10 mmHg change away from the reference value (150 mmHg).
Sensitivity analysis
Sensitivity analyses restricted to the cohort with data on NIH stroke scale were largely similar (see Supplementary material online, Table S5).
Relative contributions of blood pressure variables to in-hospital mortality
All four BP variables were significant predictors of in-hospital mortality with the F-statistic being highest for MAP (180.83) followed by diastolic BP (152.51), systolic BP (139.80) and pulse pressure (92.96). However the C-statistics of the models were largely similar (0.7167, 0.7162, 0.7154, and 0.7161, respectively).
Discussion
The present study of 309 611 patients with ischaemic stroke, the largest to date, showed a J-shaped/U-shaped relationship between admission BP variables (systolic, diastolic, MAP, or pulse pressure) and short-term outcomes including in-hospital death, not discharged home, and inability to ambulate independently at discharge such that the odds of these outcomes were higher in those with presenting BP less than or greater than the reference range of BP (systolic BP of 150 mmHg, diastolic BP of 70 mmHg, MAP of 100 mmHg, and a PP of 80 mmHg). However, no J-shaped/U-shaped association was seen for haemorrhagic complications associated with thrombolytic therapy. Moreover, all four BP variables were predictors of in-hospital death with the strongest predictor being MAP.
Patients with acute ischaemic stroke commonly present with post-stroke hypertension as a result of pre-existing hypertension,20 mental stress,20 centrally mediated mechanism,21 or neuroendocrine factors (activation of sympathetic nervous system, renin–angiotensin system, and glucocorticoid systems).20,22 In our study, 73% and 34% of patients presented with post-stroke hypertension (systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg, respectively). Moreover, 19% and 8% of patients presented with a systolic BP ≥185 mmHg or diastolic BP ≥110 mmHg, respectively, a BP range that is considered to be a relative contraindication for thrombolytic therapy. Although the majority of patients with acute stroke present with elevated BP, data suggest that there is spontaneous reduction of BP within days following the acute event in the majority of patients.7,23
Clinical studies of the autoregulation of the cerebral blood flow suggest a pressure-dependent cerebral blood flow to the ischaemic regions of the brain and hence a potential beneficial effect of post-stroke hypertension.2 As such few studies have shown better functional recovery in patients who presented with higher BP.6–8 However, other studies suggest a deleterious effect of post-stroke hypertension including increased incidence of recurrent stroke,3 haemorrhagic transformation, and cerebral oedema.4,5 As such, data from the International Stroke Trial (IST) of 17 398 patients showed that the risk of early death was independently associated with higher systolic BP.2 However, the results from observational studies have been conflicting with a meta-analysis of 32 studies with 10 892 patients failed to show an association between systolic BP, diastolic BP or MAP on death, although each of these were associated with the composite outcome of death or dependency in ischaemic stroke.11 Other studies have shown no statistically significant relationship between casual BP measures including systolic, diastolic and MAP and mortality in patients with acute stroke.12 On the other hand, a few other studies have shown a U-shaped/J-shaped relationship between BP and outcomes such that not only high BP but also low BP was associated with poor outcome.3,9,10
There are a number of limitations of the prior studies including inclusion of patients with both ischaemic and haemorrhagic stroke, use of very few categorical cut-points for BP (likely driven by sample size), the use of linear models to evaluate association of BP and outcomes and not adjusting for baseline confounders. Moreover, there are limited studies evaluating MAP and pulse pressure on outcomes. In addition, the relative prognostic value of various BP variables has not been explored previously. Our study in over 300 000 patients with ischaemic stroke is the largest series thus far and provides important insights into the association of BP variables and outcomes: (i) there was a U-shaped/J-shaped relationship between BP variables and outcomes suggesting that both low- and high-BP values are associated with poor outcomes; (ii) the J-shaped/U-shaped relationship was not seen for the outcome of haemorrhagic complications of thrombolytic therapy, suggesting that the risk of haemorrhagic complications with thrombolytic therapy increases with higher BP values (consistent with prior literature); (iii) an interaction was observed between systolic pressure and thrombolytic therapy and in-hospital death such that at low values of systolic BP, patients not treated with thrombolytic therapy had higher in-hospital death, lower likelihood of independent ambulation and were less likely to be discharged home than patients treated with thrombolytic therapy; and (iv) all four BP variables were predictors of in-hospital death with the strongest predictor being MAP.
Although the association between BP variable, thrombolytic therapy and outcomes may not be causal, this may be explained by the fact that patients who are not reperfused (with thrombolytic therapy) are less likely to tolerate lower cerebral perfusion resulting from a low systolic pressure. This could also represent prescription bias in that patients with low values of systolic BP may have increased comorbidities and frailty where thrombolytic therapy may have been held due to overall poor prognosis. On the other hand, for high values of systolic BP, the odds of poor outcomes were higher in those treated with thrombolytic therapy than those who were not treated with thrombolytic therapy, suggesting perhaps that at higher systolic BP the risk of thrombolytic therapy may outweigh the benefits.
The J-curve association and especially the higher event rate at lower BP values seen in this study can be hypothesized by a number of factors: (i) The worse outcomes in patients with lower BP may be attributed to decreased cerebral perfusion in patients with ischaemic stroke who are critically dependent on pressure-dependent perfusion of ischaemic area; (ii) The worse outcomes with lower BP could also be related to increased cardiovascular events in patients with low BP24–26; and (iii) Low BP may represent inability to mount an hypertensive response resulting from increased comorbidities and frailty and thus may be an epiphenomenon of comorbidity burden.
Study limitations
The study did not assess follow-up BP or use of antihypertensive therapy on outcomes. Admission BP can be influenced by anxiety, frustration, etc. However, the finding that bleeding from thrombolytic therapy increased with elevated BP (consistent with prior literature) perhaps points to the fact that these measurements are likely not spurious. In addition, the study evaluated in-hospital outcomes only. Social factors could also have impacted the discharge destination. However, since the intention of the analysis was to compare this outcome between BP subgroups, this should not differentially affect any one BP subgroup. In addition, prior analyses show that stroke severity is the major determinant in home discharge and higher NIHSS is strongly associated with not discharged home. Although the multivariable models adjusted for many baseline confounders it does not account for unmeasured confounders such as frailty. The decision to use thrombolytic therapy for patients with ischaemic stroke was left to the treating physician. In addition, the study tests associations and cannot rule out whether a high/low BP is a marker of worse outcomes.
Conclusions
In summary, data from over 300 000 patients with ischaemic stroke showed a J-shaped/U-shaped relationship between BP variables and in-hospital outcomes, including death, such that there were higher adverse events in the groups with higher and lower BP above the reference range (Central Figure). However, no J-shaped/U-shaped relationship was seen for the outcome of haemorrhagic complications of thrombolytic therapy. The test for interaction was significant for systolic BP and thrombolytic therapy such that patients who underwent thrombolytic therapy had lower odds of in-hospital outcomes at lower BP values indicating perhaps that they tolerated lower systolic BP better than those who did not undergo thrombolytic therapy. Finally, all four BP variables were predictors of in-hospital death.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The GWTG-Stroke program is provided by the AHA/American Stroke Association. The GWTG-Stroke program is currently supported in part by a charitable contribution from Janssen Pharmaceutical Companies of Johnson & Johnson. GWTG Stroke has been funded in the past through support from Boehringer-Ingelheim, Merck, Bristol-Myers Squib/Sanofi Pharmaceutical Partnership, and the AHA Pharmaceutical Roundtable. The industry sponsors of GWTG Stroke had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the article.
Conflict of interest: G.C.F. reports the following: Employment—UCLA Employee, which holds a patent on stroke retriever devices. PCORI and NIH—grants and grants pending. D.L.B. discloses the following relationships—Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. All other authors have no disclosures. Dr. Schwamm reports being the principal investigator of an investigator-initiated study of extended-window intravenous thrombolysis funded by the National Institutes of Neurological Disorders and Stroke (clinicaltrials.gov/show/NCT01282242) for which Genentech provides alteplase free of charge to Massachusetts General Hospital as well as supplemental per-patient payments to participating sites; serving as chair of the AHA/ASA GWTG stroke clinical work group and hospital accreditation Science Committee; serving as a stroke systems consultant to the Massachusetts Department of Public Health; and serving as a scientific consultant to Medtronic (Victory AF and Stroke AF trials). All authors had access to the data and a role in writing the manuscript.
Supplementary Material
References
- 1. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA.. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 2. Yatsu FM, Zivin J.. Hypertension in acute ischemic strokes. Not to treat. Arch Neurol 1985;42:999–1000. [DOI] [PubMed] [Google Scholar]
- 3. Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA.. Group ISTC. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002;33:1315–1320. [DOI] [PubMed] [Google Scholar]
- 4. Bowes MP, Zivin JA, Thomas GR, Thibodeaux H, Fagan SC.. Acute hypertension, but not thrombolysis, increases the incidence and severity of hemorrhagic transformation following experimental stroke in rabbits. Exp Neurol 1996;141:40–46. [DOI] [PubMed] [Google Scholar]
- 5. O'Brien MD, Jordan MM, Waltz AG.. Ischemic cerebral edema and the blood-brain barrier. Distributions of pertechnetate, albumin, sodium, and antipyrine in brains of cats after occlusion of the middle cerebral artery. Arch Neurol 1974;30:461–465. [DOI] [PubMed] [Google Scholar]
- 6. Allen CM. Predicting the outcome of acute stroke: a prognostic score. J Neurol Neurosurg Psychiatry 1984;47:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chamorro A, Vila N, Ascaso C, Elices E, Schonewille W, Blanc R.. Blood pressure and functional recovery in acute ischemic stroke. Stroke 1998;29:1850–1853. [DOI] [PubMed] [Google Scholar]
- 8. Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS.. Effect of blood pressure and diabetes on stroke in progression. Lancet 1994;344:156–159. [DOI] [PubMed] [Google Scholar]
- 9. Okumura K, Ohya Y, Maehara A, Wakugami K, Iseki K, Takishita S.. Effects of blood pressure levels on case fatality after acute stroke. J Hypertens 2005;23:1217–1223. [DOI] [PubMed] [Google Scholar]
- 10. M'Buyamba-Kabangu JR, Longo-Mbenza B, Tambwe MJ, Dikassa LN, Mbala-Mukendi M.. J-shaped relationship between mortality and admission blood pressure in black patients with acute stroke. J Hypertens 1995;13(12 Pt 2):1863–1868. [PubMed] [Google Scholar]
- 11. Willmot M, Leonardi-Bee J, Bath PM.. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004;43:18–24. [DOI] [PubMed] [Google Scholar]
- 12. Dawson SL, Manktelow BN, Robinson TG, Panerai RB, Potter JF.. Which parameters of beat-to-beat blood pressure and variability best predict early outcome after acute ischemic stroke? Stroke 2000;31:463–468. [DOI] [PubMed] [Google Scholar]
- 13. Selvaraj S, Steg PG, Elbez Y, Sorbets E, Feldman LJ, Eagle KA, Ohman EM, Blacher J, Bhatt DL; Investigators REACH Registry. Pulse pressure and risk for cardiovascular events in patients with atherothrombosis: from the REACH Registry. J Am Coll Cardiol 2016;67:392–403. [DOI] [PubMed] [Google Scholar]
- 14. Schwamm LH, Fonarow GC, Reeves MJ, Pan W, Frankel MR, Smith EE, Ellrodt G, Cannon CP, Liang L, Peterson E, Labresh KA.. Get with the guidelines-stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation 2009;119:107–115. [DOI] [PubMed] [Google Scholar]
- 15. Xian Y, Fonarow GC, Reeves MJ, Webb LE, Blevins J, Demyanenko VS, Zhao X, Olson DM, Hernandez AF, Peterson ED, Schwamm LH, Smith EE.. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J 2012;163:392–398, 398.e1. [DOI] [PubMed] [Google Scholar]
- 16. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40:2276–2293. [DOI] [PubMed] [Google Scholar]
- 17. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 19. Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V.. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 20. Carlberg B, Asplund K, Hagg E.. Factors influencing admission blood pressure levels in patients with acute stroke. Stroke 1991;22:527–530. [DOI] [PubMed] [Google Scholar]
- 21. Olsson T, Marklund N, Gustafson Y, Nasman B.. Abnormalities at different levels of the hypothalamic-pituitary-adrenocortical axis early after stroke. Stroke 1992;23:1573–1576. [DOI] [PubMed] [Google Scholar]
- 22. Murros K, Fogelholm R, Kettunen S, Vuorela AL.. Serum cortisol and outcome of ischemic brain infarction. J Neurol Sci 1993;116:12–17. [DOI] [PubMed] [Google Scholar]
- 23. Wallace JD, Levy LL.. Blood pressure after stroke. JAMA 1981;246:2177–2180. [PubMed] [Google Scholar]
- 24. Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, LaRosa JC.. J-curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J 2010;31:2897–2908. [DOI] [PubMed] [Google Scholar]
- 25. Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG; CLARIFY Investigators. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016;388:2142–2152. [DOI] [PubMed] [Google Scholar]
- 26. McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E.. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016;68:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.