ABSTRACT
Background: Data on the effectiveness of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) in reducing cardiovascular (CV) risk in patients undergoing peritoneal dialysis (PD) are limited. We investigated the association between ACEI/ARB use and CV outcomes in patients initiating PD.
Methods: In this observational cohort study, we identified from the United States Renal Data System all adult patients who initiated PD from 2007 to 2011 and participated in Medicare Part D, a federal prescription drug benefits program, for the first 90 days of dialysis. Patients who filled a prescription for an ACEI or ARB in those 90 days were considered users. We applied Cox regression to an inverse probability of treatment weighted cohort to estimate the hazard ratios (HRs) for the combined outcome of death, ischemic stroke or myocardial infarction (MI) and each outcome individually.
Results: Among 4879 patients, 2063 (42%) used an ACEI/ARB. Patients were followed up for a median of 1.2 years. We recorded 1771 events, for a composite rate of 25 events per 100 person-years. ACEI/ARB use (versus nonuse) was associated with a reduced risk of the composite outcome {HR 0.84 [95% confidence interval (CI) 0.76–0.93]}, all-cause mortality [HR 0.83 (95% CI 0.75–0.92)] and CV death [HR 0.74 (95% CI 0.63–0.87)], but not MI [HR 0.88 (95% CI 0.69–1.12)] or ischemic stroke [HR 1.06 (95% CI 0.79–1.43)]. Results were similar in as-treated analyses. In a subgroup analysis, we did not find any effect modification by residual renal function.
Conclusions: ACEI/ARB use is common in patients initiating PD and is associated with a lower risk of fatal CV outcomes.
Keywords: angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, cardiovascular, peritoneal dialysis, renin angiotensin system blockers
INTRODUCTION
Patients with end-stage renal disease (ESRD) experience a high burden of cardiovascular (CV) disease. Mortality exceeds 20% in the first year after initiation of dialysis, and 42% of these deaths are attributed to CV causes [1]. In patients with chronic kidney disease (CKD) not on dialysis, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) slow the progression of diabetic nephropathy and reduce CV risk [2–8]. However, data on their effectiveness in patients with ESRD have been mixed. Notably, three randomized clinical trials involving patients with ESRD on hemodialysis and one in kidney transplant recipients found no reduction in CV outcomes with the use of an ACEI or ARB [9–12].
Yet, ACEIs and ARBs may be of some benefit to patients with ESRD on peritoneal dialysis (PD), in contrast to patients on hemodialysis. These medications may preserve residual renal function in patients on PD by decreasing inflammation and glomerulosclerosis [13–16], and residual renal function is consistently associated with better CV outcomes and overall survival rates in PD patients [17–22]. However, previous studies testing the effectiveness of ACEIs/ARBs on CV outcomes in patients on PD are sparse and limited by small patient populations [13, 23, 24].
In this study, we investigated the associations of the use of ACEIs or ARBs with both fatal and nonfatal CV outcomes in a large cohort of patients across the USA initiating PD between 2007 and 2011.
MATERIALS AND METHODS
Study population
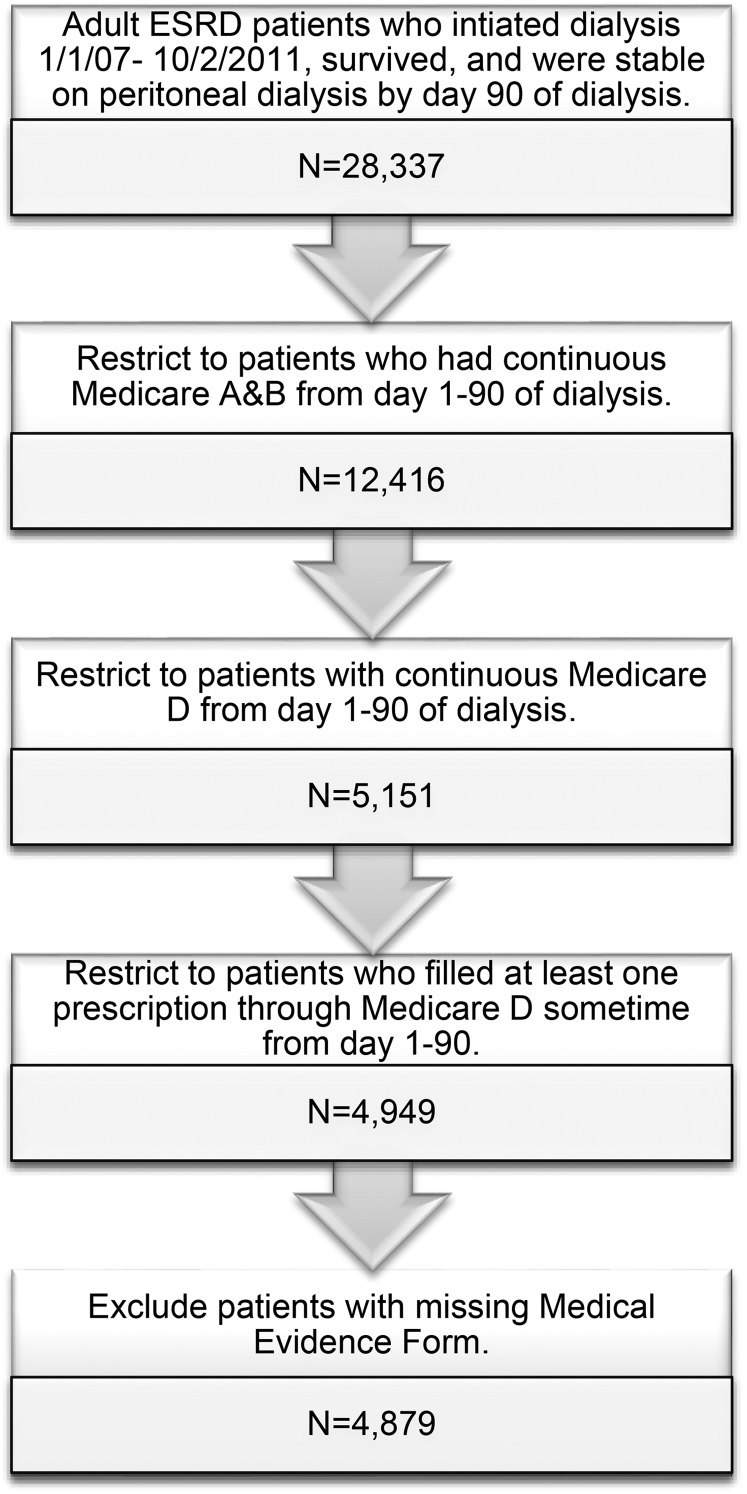
From the United States Renal Data System (USRDS), we identified all adult (≥18 years old) patients with ESRD who initiated dialysis between 1 January 2007 and 2 October 2011 (Figure 1). We restricted the cohort to patients who survived and were stable on PD (i.e. on the modality for at least 60 days) by Day 90 of dialysis, the index date. Thus, index dates ranged from 1 April 2007 to 31 December 2011. Inclusion criteria also included continuous Medicare Parts A, B, and D coverage (a federal health insurance program for people who are ≥65 years of age, certain younger people with disabilities and people with ESRD) from Day 1–90 of dialysis and having had at least one prescription filled during that time as an indication of active participation in Part D, the prescription drug benefit program.
FIGURE 1.
Study population selection from the USRDS. We selected a cohort of adult patients initiating PD between 2007 and 2011 who survived to Day 90 of dialysis and who had continuous Medicare Parts A, B and D coverage from Day 1 to 90.
ACEI/ARB use
Use of an ACEI/ARB (versus no use) was the exposure of interest and defined using Medicare Part D claims. Prescription claims contain not only the generic substance and dose but also the number of days of drug supply dispensed. Patients were categorized as ACEI/ARB users if they filled a prescription for either an ACEI or ARB within 90 days of initiating dialysis; everyone else was considered a nonuser. For analyses using an approach that corresponds to an ‘intention-to-treat’ (ITT) analysis in trials, baseline exposure was carried forward indefinitely. ‘As-treated’ (AT) analyses considered patients exposed for 60 days after the recorded supply from their previously filled prescription was exhausted (refill grace period). If patients failed to fill a subsequent prescription during this 60-day grace period, the follow-up time was censored. Conversely, follow-up for nonusers was censored if an ACEI/ARB prescription was filled.
Outcomes
For the survival analyses, our primary outcome was a composite of death from any cause, ischemic stroke and myocardial infarction (MI). We also analyzed each outcome individually in addition to death from CV causes. Nonfatal outcomes were ascertained from validated claims-based algorithms [25, 26]. Death and cause-specific mortality were ascertained from the USRDS death file (Supplementary data, Table S1).
Patient characteristics
We ascertained demographics [age, sex, race (white, black and other), Hispanic ethnicity, Medicaid at time of dialysis initiation], comorbidities, body mass index (BMI) and laboratory measurements [hemoglobin, albumin, estimated glomerular filtration rate (eGFR)], baseline medication use, dialysis characteristics (year initiated dialysis, predialysis referral to nephrologist) and facility characteristics (size of the PD program, rural/urban location, US census division) from the Medical Evidence Report (form CMS-2728), the ESRD Facility Survey (form CMS-2744) conducted in the year a patient initiated dialysis and all available Medicare claims data from the first 90 days of dialysis. Details about these algorithms have been previously described and can be found in Supplementary data, Table S2 [27].
For a subset of patients who initiated dialysis with DaVita, a large national dialysis provider, we had additional laboratory measurements. Patients were classified as having residual renal function if none of the 24-h urine volumes in the first 90 days of dialysis were <200 mL. We also ascertained the first hemoglobin and albumin measurements made within 90 days of dialysis initiation since these data were more complete than those derived from the CMS-2728.
Statistical analysis
We tabulated the characteristics of ACEI/ARB users and nonusers using percentages and means (± SD) or medians (interquartile range). We compared the two groups using standardized differences, with differences >10 indicating significant imbalance between the two groups [28].
We conducted an inverse probability of treatment-weighted (IPTW) survival analysis, a novel method to control for selection bias by observed characteristics between ACEI/ARB users and nonusers [29]. Patients are weighted by their probability of being exposed for those exposed, and the probability of being unexposed for those unexposed, to create a pseudo-population with a similar percentage of patients exposed in each level of the covariates as the overall percentage in the study population, simulating the balance ideally achieved in a randomized study. The weights are based on propensity scores for ACEI/ARB that were estimated using a multivariable logistic regression that included the variables listed in Table 1 with the exception of vital signs and laboratory measurements as these data were not available for all patients. Note that we achieved balance in the IPTW cohort for the vital signs and laboratory measurements even though they were excluded from the propensity score modeling. See Supplementary data for detailed information on this method.
Table 1.
Characteristics of patients initiating PD from 2007 to 2011 who participated in Medicare Part D for the first 90 days of dialysis
| Variable | Full cohort |
IPTW cohort |
||||
|---|---|---|---|---|---|---|
| Nonusers, (N = 2816) | ACEI/ARB users, (N = 2063) | Std. diff. (%) | Nonusers | ACEI/ARB users | EEStd. diff. (%) | |
| Demographics | ||||||
| Age (years), mean ± SD | 67 ± 13 | 65 ± 13 | 14.8 | 67 ± 14 | 66 ± 13 | 0.3 |
| Male sex | 53 | 49 | 6.6 | 51 | 51 | 0.3 |
| Race | ||||||
| Black | 16 | 19 | 8.2 | 17 | 18 | 0.3 |
| White | 79 | 75 | 8.6 | 77 | 77 | 0.2 |
| Other | 5 | 5 | 2.3 | 5 | 5 | 0.0 |
| Hispanic ethnicity | 8 | 12 | 11.9 | 10 | 10 | 0.4 |
| Medicaid at time of dialysis initiation | 26 | 33 | 13.4 | 29 | 29 | 0.1 |
| Reported comorbidities | ||||||
| Cancer | 11 | 8 | 10.6 | 9 | 9 | 1.0 |
| Cardiac disease, othera | 29 | 24 | 12.1 | 27 | 26 | 0.5 |
| Cerebrovascular disease | 12 | 12 | 0.7 | 12 | 12 | 0.1 |
| Coronary artery disease | 27 | 26 | 2.0 | 27 | 26 | 0.1 |
| Diabetes mellitus | 57 | 65 | 16.9 | 61 | 61 | 0.3 |
| Heart failure | 35 | 31 | 8.0 | 33 | 33 | 0.1 |
| Hyperkalemia | 5 | 5 | 1.3 | 5 | 5 | 0.1 |
| Hyperlipidemia | 19 | 20 | 4.2 | 19 | 19 | 0.3 |
| Hypertension | 92 | 96 | 14.5 | 94 | 94 | 1.7 |
| Liver disease | 4 | 3 | 1.7 | 4 | 4 | 0.2 |
| Peripheral vascular disease | 17 | 18 | 0.7 | 17 | 17 | 0.3 |
| Pulmonary disease | 17 | 15 | 7.0 | 16 | 16 | 0.3 |
| Tobacco use | 7 | 8 | 2.8 | 8 | 8 | 0.2 |
| Days hospitalized in the first 90 days of dialysis, median (IQR) | 0 (0–3) | 0 (0–3) | 1.1 | 0 (0–3) | 0 (0–3) | 0.4 |
| Baseline medication use | ||||||
| ACEI or ARB | 0 | 100 | NA | 0 | 100 | NA |
| ACEI | 0 | 64 | NA | 0 | 64 | NA |
| ARB | 0 | 44 | NA | 0 | 44 | NA |
| Both | 0 | 8 | NA | 0 | 8 | NA |
| β-blocker | 60 | 66 | 11.1 | 63 | 64 | 1.1 |
| Calcium channel blocker | 51 | 62 | 23.4 | 56 | 57 | 1.4 |
| Diuretic | 54 | 65 | 21.8 | 59 | 59 | 0.4 |
| Other antihypertensiveb | 40 | 46 | 10.9 | 43 | 44 | 1.2 |
| Statin | 47 | 55 | 15.6 | 51 | 51 | 0.4 |
| Clopidogrel | 14 | 14 | 2.4 | 14 | 14 | 0.3 |
| Warfarin | 9 | 8 | 5.3 | 9 | 9 | 0.5 |
| Other CV medc | 22 | 25 | 7.2 | 23 | 23 | 0.1 |
| Levothyroxine | 19 | 18 | 2.1 | 19 | 18 | 3.1 |
| Dialysis characteristics | ||||||
| Saw nephrologist prior to dialysis initiation | 86 | 87 | 2.1 | 87 | 87 | 0.6 |
| Year initiated dialysis | ||||||
| 2007 | 17 | 19 | 5.4 | 18 | 18 | 0.4 |
| 2008 | 18 | 20 | 4.2 | 19 | 19 | 0.5 |
| 2009 | 21 | 19 | 3.2 | 20 | 20 | 0.0 |
| 2010 | 24 | 22 | 2.8 | 23 | 23 | 0.3 |
| 2011 | 20 | 19 | 3.2 | 20 | 20 | 0.2 |
| CAPD (versus CCPD) | 42 | 44 | 4.8 | 43 | 43 | 0.4 |
| Vital signs and laboratory measurements, mean ± SD | ||||||
| BMId | 28.4 ± 6.7 | 29.2 ± 7.0 | 10.5 | 28.7 ± 6.8 | 28.9 ± 6.9 | 4.0 |
| Hemoglobin (g/dL)e | 10.6 ± 1.6 | 10.6 ± 1.5 | 1.6 | 10.6 ± 1.5 | 10.6 ± 1.5 | 0.6 |
| Albumin (g/dL)f | 3.6 ± 0.6 | 3.6 ± 0.6 | 1.1 | 3.6 ± 0.6 | 3.6 ± 0.6 | 0.2 |
| eGFR (mL/min)g | 12 ± 4 | 12 ± 4 | 0.5 | 12 ± 4 | 12 ± 4 | 1.9 |
| Facility characteristics | ||||||
| Number of PD patients, median (IQR)h | 24 (14–40) | 24 (13–42) | 3.7 | 24 (14–40) | 24 (13–42) | 2.5 |
| ≥20 | 61 | 61 | 0.4 | 61 | 61 | 0.4 |
| Rurali | 15 | 14 | 1.9 | 15 | 15 | 0.4 |
| Geographic location (US census division)j | ||||||
| East North Central | 16 | 17 | 1.6 | 17 | 17 | 0.1 |
| East South Central | 10 | 10 | 1.1 | 10 | 10 | 0.3 |
| Mid-Atlantic | 10 | 7 | 9.4 | 9 | 9 | 0.6 |
| Mountain | 4 | 5 | 1.8 | 4 | 4 | 0.0 |
| New England | 4 | 4 | 1.4 | 4 | 4 | 0.3 |
| Pacific | 11 | 14 | 10.7 | 12 | 12 | 0.4 |
| South Atlantic | 23 | 22 | 3.0 | 22 | 22 | 0.4 |
| West North Central | 8 | 7 | 3.4 | 8 | 8 | 0.4 |
| West South Central | 13 | 14 | 2.8 | 14 | 14 | 0.1 |
All values are percentages unless indicated otherwise. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CAPD, continuous ambulatory peritoneal dialysis; CCPD, continuous cycling peritoneal dialysis; eGFR, estimated glomerular filtration rate; IQR, interquartile range; IPTW, inverse probability of treatment weighted; NA, not applicable; PD, peritoneal dialysis; SD, standard deviation; Std. diff., standardized difference.
aAtrial fibrillation, arrhythmias, implanted cardiac defibrillators, pacemakers and valvular disease.
bAlfuzosin, aliskiren, clonidine, doxazosin, guanfacine, hydralazine, isosorbide, methyldopa, minoxidil, prazosin, ranolazine and terazosin.
cEzetimibe, simvastatin, niacin, amiodarone, aspirin/dipyridamole, colesevelam, colestipol, digoxin, dipyridamole, dronedarone, fenofibrate, flecainide, gemfibrozil, mexiletine, nitroglycerin, omega-3 acid ethyl esters, procainamide, propafenone and quinidine.
dMissing for 1% of nonusers and 1% of users.
eMissing for 10% of nonusers and 10% of users.
fMissing for 23% of nonusers and 20% of users.
gMissing for 1% of nonusers and 1% of users.
hBased on the year the patient initiated dialysis.
iFacilities were considered urban if they were classified as a metropolitan area in the Rural–Urban Commuting Area (RUCA) Codes version 2.0, which are based on 2000 census commuting data and 2004 zip codes; all other areas were considered to be rural [30].
jFacilities were categorized into one of nine U.S. Census Bureau Divisions based on their state [31].
Given the potential for confounding in this observational study, we examined the robustness of our primary results in three sensitivity analyses:
We restricted the cohort to patients with a low-income subsidy (LIS) for Medicare Part D. This subsidy reduces or eliminates premiums and copayments for prescription medications based on patients' household income level, reducing the likelihood that they will obtain their medications outside of the program, making ascertainment of drug use via claims data more accurate [32].
We used a new user cohort. Studying prevalent users can lead to bias since these patients have tolerated and adhered to the medication and thus tend to be healthier than those who may have discontinued the drug shortly after initiation [33]. We created a new user cohort by first restricting the population to those ≥66 years of age so that we would have at least 6 months of Medicare Part D claims data prior to the initiation of dialysis. We then excluded anyone who filled a prescription for an ACEI/ARB during that 6-month period. Consequently, patients who filled a prescription for an ACEI/ARB during the first 90 days of dialysis were considered new users.
We restricted the cohort to users who dialyzed with DaVita, the only group for whom we had information on residual renal function. Residual renal function is a potential confounder since (a) ACEIs/ARBs preserve residual renal function, and could be used more often in patients with residual renal function [13, 14], and (b) residual renal function is associated with better CV outcomes and survival rates in PD patients [17–22]. For these analyses, we included residual renal function in the propensity score model.
All survival analyses were conducted using Cox proportional hazards regression with robust standard errors. As patients may have had multiple events, we only analyzed the first event they experienced. For the outcome of death, patients were censored on end of study (1 January 2012). For all other outcomes, patients were censored for end of study, loss of Medicare Parts A and B coverage and kidney transplantation. For AT analyses, patients were additionally censored for discontinuation of Part D, specifically for ACEI/ARB users when their most recent recorded prescription expired plus the 60-day grace period, and for nonusers if an ACEI/ARB prescription was filled. Violation of the proportional hazards assumption was checked using interaction terms with time. All hazard ratios (HRs) were accompanied by their corresponding 95% confidence interval (CI). We assessed effect modification by age (<66 or ≥66 years, the mean age), sex, race, history of diabetes mellitus, history of coronary artery disease, history of heart failure and residual renal function.
All analyses were performed using SAS Enterprise Guide 6.1 (SAS Institute, Cary, NC). The institutional review boards of Stanford University and Baylor College of Medicine approved the study.
RESULTS
Patient characteristics
Of the 4879 patients we identified as having initiated PD from 2007 to 2011, and who fulfilled the other stated criteria, 42% (2063) were ACEI/ARB users. ACEI/ARB users were younger and more likely to be Hispanic and receiving Medicaid (Table 1). Although there was no difference in the baseline prevalence of coronary artery disease between the two groups, diabetes mellitus and hypertension were more common among users, while heart failure was more prevalent among nonusers. Users had a higher rate of antihypertensive and statin use. However, their use of other medications was comparable with those of non-ACEI/ARB users. On average, ACEI/ARB users had higher BMIs than nonusers, but comparable eGFRs at initiation. The two groups also had similar dialysis and facility characteristics. After weighting the cohort by their inverse probability of treatment with an ACEI/ARB [34], all observed characteristics were balanced between users and nonusers (Table 1).
Association of ACEI/ARB use with outcomes
In the ITT analysis, we recorded 1771 events (death, stroke or MI) over 7131 person-years of follow-up, for a composite event rate of 25 events per 100 person-years. For each individual outcome, we recorded 20.9 deaths, 9.0 CV deaths, 3.9 MIs and 2.5 ischemic strokes per 100 person-years. The rates of the composite outcome, all-cause mortality and CV death were all significantly lower for ACEI/ARB users than nonusers [HR (95% CI): 0.84 (0.76–0.93), 0.83 (0.75–0.92) and 0.74 (0.63–0.87), respectively]. The rates of nonfatal events were no different between the two groups (Table 2, Figure 2). Age (≥66 versus <66 years), sex, race, history of diabetes mellitus, history of coronary artery disease, history of heart failure and diuretic use did not modify any of the associations (data not shown). The AT analyses yielded generally similar results with lower point estimates for the HRs and wider confidence limits (Table 2, Figure 2).
Table 2.
Number of events, follow-up time, incidence rates and HRs for all study outcomes based on an IPTW population of 2063 (42%) ACEI/ARB users and 2816 (58%) nonusers
| Outcome | Analysis | Exposure group | Number of events | Follow-up time (years) |
Incidence rate (per 100 person-years) | HR (95% CI) | |
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | ||||||
| Death, ischemic stroke or MI | ITT | ACEI/ARB | 695 | 1.50 ± 1.17 | 1.21 | 22.5 | 0.84 (0.76–0.93) |
| Nonuser | 1076 | 1.44 ± 1.14 | 1.16 | 26.6 | |||
| AT | ACEI/ARB | 256 | 0.72 ± 0.80 | 0.44 | 17.1 | 0.66 (0.57–0.76) | |
| Nonuser | 770 | 1.03 ± 1.00 | 0.71 | 26.5 | |||
| All-cause mortality | ITT | ACEI/ARB | 622 | 1.61 ± 1.20 | 1.33 | 18.8 | 0.83 (0.75–0.92) |
| Nonuser | 976 | 1.54 ± 1.18 | 1.27 | 22.6 | |||
| AT | ACEI/ARB | 206 | 0.74 ± 0.82 | 0.46 | 13.4 | 0.61 (0.52–0.72) | |
| Nonuser | 682 | 1.07 ± 1.02 | 0.75 | 22.6 | |||
| CV death | ITT | ACEI/ARB | 249 | 1.61 ± 1.20 | 1.33 | 7.5 | 0.74 (0.63–0.87) |
| Nonuser | 440 | 1.54 ± 1.18 | 1.27 | 10.2 | |||
| AT | ACEI/ARB | 85 | 0.74 ± 0.82 | 0.46 | 5.5 | 0.69 (0.54–0.89) | |
| Nonuser | 244 | 1.07 ± 1.02 | 0.75 | 8.1 | |||
| Ischemic stroke | ITT | ACEI/ARB | 82 | 1.54 ± 1.19 | 1.26 | 2.6 | 1.06 (0.79–1.43) |
| Nonuser | 102 | 1.48 ± 1.17 | 1.21 | 2.4 | |||
| AT | ACEI/ARB | 40 | 0.74 ± 0.81 | 0.45 | 2.6 | 1.06 (0.71–1.59) | |
| Nonuser | 69 | 1.06 ± 1.01 | 0.73 | 2.3 | |||
| MI | ITT | ACEI/ARB | 115 | 1.54 ± 1.18 | 1.25 | 3.6 | 0.88 (0.69–1.12) |
| Nonuser | 170 | 1.47 ± 1.15 | 1.21 | 4.1 | |||
| AT | ACEI/ARB | 47 | 0.73 ± 0.80 | 0.45 | 3.1 | 0.80 (0.57–1.13) | |
| Nonuser | 116 | 1.05 ± 1.00 | 0.72 | 3.9 | |||
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; AT, as treated; CI, confidence interval; CV, cardiovascular; HR, hazard ratio; IPTW, inverse probability of treatment weighted; ITT, intention to treat; MI, myocardial infarction; SD, standard deviation.
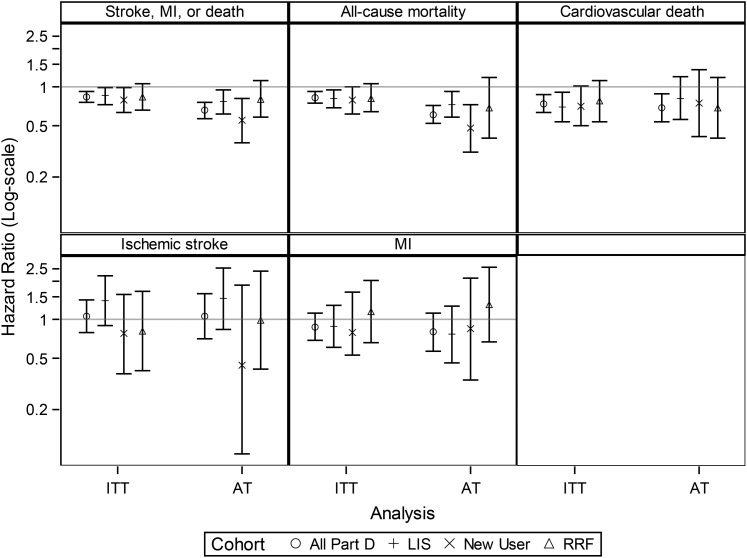
FIGURE 2.
HRs for all study outcomes for both the primary analyses [based on the full (all Part D) IPTW cohort] and the sensitivity analyses [based on the LIS cohort, new user cohort and cohort in which we adjusted for residual renal function]. AT, as-treated analysis where patients were censored 60 days after their drug supply ran out; HR, hazard ratio; IPTW, inverse probability of treatment weighted; ITT, intention to treat; LIS, low-income subsidy; MI, myocardial infarction; RRF, residual renal function.
The sensitivity analyses yielded results that were mostly similar except that HRs were nonsignificant for the outcome of CV death, and for the cohort that adjusted for residual renal function, none of the outcomes reached statistical significance even though the point estimates for the HRs were similar to the main analyses (Figure 2, Supplementary data, Tables S3–S8).
DISCUSSION
In a large cohort of patients with incident ESRD undergoing PD, we found that use of an ACEI/ARB was associated, with lower risks of CV outcomes, predominantly driven by a reduction in fatal outcomes. These results were robust across several sensitivity analyses that probed the potential impact of prevalent versus incident use of these drugs and in a subset of patients in whom we had information on residual renal function. Note that in the latter cohort, the HRs were similar but did not achieve statistical significance due to a smaller sample size. Thus, our findings support the use of ACEI/ARB medications in patients undergoing PD in whom previous evidence had been rather limited.
To our knowledge, only a single randomized trial on this topic in the PD population exists, a randomized control trial of the effect of ramipril versus placebo in 60 prevalent PD patients on the preservation of residual renal function [13]. This study showed no differences in the secondary outcomes of all-cause mortality [HR 1.56 (95% CI 0.24–10.05)] and CV events [HR 1.00 (95% CI 0.19–5.40)]. However, it was underpowered to detect a meaningful difference in these outcomes. In contrast, in the only observational study addressing the issue in PD patients only, ACEI/ARB use was associated with a 62% (95% CI 47–77) reduction in mortality in 306 patients who initiated PD [24]. The magnitude of the association seen in that study versus our own was much larger (HR 0.38 versus 0.83). This difference may have stemmed from their classification of users as anyone with 6 months of ACEI/ARB use during the follow-up period, which could have led to survivor treatment selection bias and an overestimate of the benefits of ACEIs/ARBs [35].
Observational studies on ACEI/ARB use that have included mixed populations of patients on hemodialysis and PD have not consistently shown a beneficial association of ACEI/ARB use [36–42]. Randomized trials involving only patients on hemodialysis have similarly failed to show a consistent benefit of ACEI/ARB treatment. The Fosinopril in Dialysis (FOSIDIAL) study randomized 397 patients on hemodialysis to fosinopril or placebo and found no significant reduction in CV events [HR 0.79 (95% CI 0.59–1.10)] [9]. In contrast, an open-label trial that randomized 360 patients on hemodialysis to either an ARB (valsaratan, candesartan or losartan) or no ARB treatment found that treatment was associated with a reduction in CV events [HR 0.51 (95% CI 0.33–0.79)], even though the authors note that the large effect may have been a chance finding owing to the small sample size [43]. A smaller trial of 80 patients on hemodialysis randomized to candesartan versus no treatment also found a lower rate of CV events in the treated group (45.9 versus 17.3%) [44]. However, a meta-analysis that pooled the result of these three trials found no significant association of ACEI/ARB use and the risk of CV events [HR 0.66 (95% CI 0.35–1.25)]. Later studies confirmed the negative finding. One such trial randomized 469 patients on hemodialysis to either olmesartan or a non-ACEI/ARB antihypertensive regimen and found no difference in a composite outcome of death, ischemic stroke, MI or coronary revascularization [HR 1.00 (95% CI 0.71–1.40)] [10]. Finally, a trial of 200 patients undergoing hemodialysis who were randomized to either lisinopril or atenolol was terminated early due to an ‘increased’ risk of CV events in the ACEI group [incident rate ratio 1.36 (95% CI 1.36–4.23)] [11].
It is plausible that ACEI/ARB use might confer benefit in patients with ESRD on PD but not in those on hemodialysis. A possible mechanism of action might be in the putative ability of ACEIs/ARBs to preserve residual renal function in patients on PD, since residual renal function has been consistently linked to better outcomes [13, 14, 17–22]. While there are limited data showing that ACEIs/ARBs might similarly preserve residual renal function in patients on hemodialysis, the effect on CV outcomes might be limited in the hemodialysis population since they lose their residual renal function sooner than those on PD [46–48]. An alternative explanation is that ACEIs/ARBs may help preserve the peritoneal membrane, providing better ultrafiltration and improved CV function [49]. Finally, ACEIs/ARBs aid in the remodeling of myocardial and endothelial tissue, as evidenced by their ability to reduce left ventricular hypertrophy in patients on chronic dialysis [50, 51]. Perhaps the protective effects of such remodeling are mitigated in patients on hemodialysis, who are subject to large fluctuations in blood pressure and frequent cardiac stunning to which patients on PD are not habitually exposed [52].
Our study has potential implications for clinical practice. While we found that ACEI/ARB use is common among incident patients on PD (42%), it is not as high as it could be, judging by the prevalence of hypertension and the use of other antihypertensives in nonusers. Our data suggest that ACEIs/ARBs are not being used as first-line antihypertensives despite several national guidelines that recommend use for those with diabetic nephropathy with proteinuria and those on PD with residual renal function [53, 54]. One possible explanation is that ACEIs/ARBs may have been discontinued in the late stages of non-dialysis-dependent CKD due to hyperkalemia and concerns about decreased eGFR, and never restarted once the patient transitioned to PD. Indeed, there is an ongoing multicenter randomized controlled trial of ACEI/ARB withdrawal in patients with Stage 4 or 5 CKD that stemmed from such concerns [55]. Clinicians should not shy away from restarting ACEIs/ARBs in incident PD patients since they are much more likely to exhibit hypokalemia than hyperkalemia due to the continuous nature of the dialysis. If ACEIs/ARBs decrease mortality, consideration should be given to encouraging their use as a first-line antihypertensive for most patients on PD.
Our study has limitations. We could not control for unmeasured confounders, most significantly blood pressure and the specific indication for the drug. It is certainly possible that the ACEI/ARB group had a lower rate of events because they had better control of their blood pressure. We also could not control for physician effects; it is possible that ACEIs/ARBs were prescribed more often by physicians who were more experienced with PD, and that this is driving the association with better outcomes rather than the actual drug use. We tried to mitigate this effect by controlling for the size of the PD program (larger programs have been shown to have better outcomes) [56]. Because our cohort was restricted to those receiving Medicare Part D when they initiated PD, the results may not be generalizable to those who do not qualify for this drug benefit, a group that tends to be younger. As always, the limitations must be balanced against the strengths of the study, which include a large, national cohort, the use of IPTW to minimize indication bias and results that were consistent across ITT and AT analyses as well as sensitivity analyses restricted to patients with a LIS, new users and those with residual renal function information available.
CONCLUSIONS
In a large, nationally representative cohort of patients on PD, we found ACEI/ARB use to be associated with a decreased risk of fatal CV outcomes. Further clinical trials are warranted to show whether this is a causal association, as it could change clinical practice.
S UPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
The manuscript was reviewed and approved for publication by an officer of the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK). Data reported herein were supplied by the USRDS. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. This work was supported by grants F32DK096765 and K23DK103972 (to J.I.S.) and K23DK095914 (to T.I.C.) from the NIDDK. The Stanford Nephrology fellowship program was supported by grant T32DK007357. J.I.S. was also supported by the Satellite Dialysis Clinical Investigator Award from the National Kidney Foundation, grant KL2TR000122 from the National Institutes of Health/National Center for Advancing Translational Science (NCATS) and a generous gift honoring the life and work of nephrologist Henry Shavelle, M.D.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. United States Renal Data System. USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 2. Andersen S, Tarnow L, Rossing P et al. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int 2000; 57: 601–606 [DOI] [PubMed] [Google Scholar]
- 3. Maschio G, Alberti D, Janin G et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med 1996; 334: 939–945 [DOI] [PubMed] [Google Scholar]
- 4. Ruggenenti P, Perna A, Gherardi G et al. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet 1998; 352: 1252–1256 [DOI] [PubMed] [Google Scholar]
- 5. Hou FF, Zhang X, Zhang GH et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 2006; 354: 131–140 [DOI] [PubMed] [Google Scholar]
- 6. Jafar TH, Schmid CH, Landa M et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135: 73–87 [DOI] [PubMed] [Google Scholar]
- 7. Mann JF, Gerstein HC, Pogue J et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med 2001; 134: 629–636 [DOI] [PubMed] [Google Scholar]
- 8. Tokmakova MP, Skali H, Kenchaiah S et al. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the Survival and Ventricular Enlargement (SAVE) study. Circulation 2004; 110: 3667–3673 [DOI] [PubMed] [Google Scholar]
- 9. Zannad F, Kessler M, Lehert P et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int 2006; 70: 1318–1324 [DOI] [PubMed] [Google Scholar]
- 10. Iseki K, Arima H, Kohagura K et al. Effects of angiotensin receptor blockade (ARB) on mortality and cardiovascular outcomes in patients with long-term haemodialysis: a randomized controlled trial. Nephrol Dial Transplant 2013; 28: 1579–1589 [DOI] [PubMed] [Google Scholar]
- 11. Agarwal R, Sinha AD, Pappas MK et al. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant 2014; 29: 672–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knoll GA, Fergusson D, Chasse M et al. Ramipril versus placebo in kidney transplant patients with proteinuria: a multicentre, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4: 318–326 [DOI] [PubMed] [Google Scholar]
- 13. Li PK, Chow KM, Wong TY et al. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med 2003; 139: 105–112 [DOI] [PubMed] [Google Scholar]
- 14. Suzuki H, Kanno Y, Sugahara S et al. Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis 2004; 43: 1056–1064 [DOI] [PubMed] [Google Scholar]
- 15. Patel N, Hu SL. Preserving residual renal function in dialysis: what we know. Semin Dial 2015; 28: 250–258 [DOI] [PubMed] [Google Scholar]
- 16. Zhang L, Zeng X, Fu P et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for preserving residual kidney function in peritoneal dialysis patients. Cochrane Database Syst Rev 2014; doi:10.1002/14651858.CD009120.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12: 2158–2162 [DOI] [PubMed] [Google Scholar]
- 18. Paniagua R, Amato D, Vonesh E et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002; 13: 1307–1320 [DOI] [PubMed] [Google Scholar]
- 19. Termorshuizen F, Korevaar JC, Dekker FW et al. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis 2003; 41: 1293–1302 [DOI] [PubMed] [Google Scholar]
- 20. Marron B, Remon C, Perez-Fontan M et al. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl 2008; 108: S42–S51 [DOI] [PubMed] [Google Scholar]
- 21. Wang AY, Wang M, Woo J et al. A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int 2002; 62: 639–647 [DOI] [PubMed] [Google Scholar]
- 22. Wang AY, Wang M, Woo J et al. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol 2004; 15: 2186–2194 [DOI] [PubMed] [Google Scholar]
- 23. Akbari A, Knoll G, Ferguson D et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in peritoneal dialysis: systematic review and meta-analysis of randomized controlled trials. Perit Dial Int 2009; 29: 554–561 [PubMed] [Google Scholar]
- 24. Fang W, Oreopoulos DG, Bargman JM. Use of ACE inhibitors or angiotensin receptor blockers and survival in patients on peritoneal dialysis. Nephrol Dial Transplant 2008; 23: 3704–3710 [DOI] [PubMed] [Google Scholar]
- 25. Kiyota Y, Schneeweiss S, Glynn RJ et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004; 148: 99–104 [DOI] [PubMed] [Google Scholar]
- 26. Kumamaru H, Judd SE, Curtis JR et al. Validity of claims-based stroke algorithms in contemporary Medicare data: reasons for geographic and racial differences in stroke (REGARDS) study linked with medicare claims. Circ Cardiovasc Qual Outcomes 2014; 7: 611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang TI, Shilane D, Kazi DS et al. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol 2012; 23: 2042–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28: 3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11: 550–560 [DOI] [PubMed] [Google Scholar]
- 30. WWAMI Ruca Rural Health Research Center. http://depts.washington.edu/uwruca/ruca-data.php(5 January 2012, date last accessed)
- 31. Census Bureau Regions and Divisions with State FIPS Codes. http://www.census.gov/geo/www/reg_div.txt (5 January 2012, date last accessed)
- 32. Park H, Rascati KL, Lawson KA et al. Adherence and persistence to prescribed medication therapy among Medicare part D beneficiaries on dialysis: comparisons of benefit type and benefit phase. J Manag Care Spec Pharm 2014; 20: 862–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003; 158: 915–920 [DOI] [PubMed] [Google Scholar]
- 34. Normand ST, Landrum MB, Guadagnoli E et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001; 54: 387–398 [DOI] [PubMed] [Google Scholar]
- 35. Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008; 167: 492–499 [DOI] [PubMed] [Google Scholar]
- 36. Abbott KC, Trespalacios FC, Agodoa LY et al. Beta-blocker use in long-term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med 2004; 164: 2465–2471 [DOI] [PubMed] [Google Scholar]
- 37. Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol 2003; 42: 201–208 [DOI] [PubMed] [Google Scholar]
- 38. Ishani A, Herzog CA, Collins AJ et al. Cardiac medications and their association with cardiovascular events in incident dialysis patients: cause or effect? Kidney Int 2004; 65: 1017–1025 [DOI] [PubMed] [Google Scholar]
- 39. Kestenbaum B, Gillen DL, Sherrard DJ et al. Calcium channel blocker use and mortality among patients with end-stage renal disease. Kidney Int 2002; 61: 2157–2164 [DOI] [PubMed] [Google Scholar]
- 40. Trespalacios FC, Taylor AJ, Agodoa LY et al. Incident acute coronary syndromes in chronic dialysis patients in the United States. Kidney Int 2002; 62: 1799–1805 [DOI] [PubMed] [Google Scholar]
- 41. Trespalacios FC, Taylor AJ, Agodoa LY et al. Heart failure as a cause for hospitalization in chronic dialysis patients. Am J Kidney Dis 2003; 41: 1267–1277 [DOI] [PubMed] [Google Scholar]
- 42. Wetmore JB, Shireman TI. The ABCs of cardioprotection in dialysis patients: a systematic review. Am J Kidney Dis 2009; 53: 457–466 [DOI] [PubMed] [Google Scholar]
- 43. Suzuki H, Kanno Y, Sugahara S et al. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis 2008; 52: 501–506 [DOI] [PubMed] [Google Scholar]
- 44. Takahashi A, Takase H, Toriyama T et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis—a randomized study. Nephrol Dial Transplant 2006; 21: 2507–2512 [DOI] [PubMed] [Google Scholar]
- 45. Tai DJ, Lim TW, James MT et al. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: a meta-analysis. Clin J Am Soc Nephrol. 2010; 5: 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jansen MA, Hart AA, Korevaar JC et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002; 62: 1046–1053 [DOI] [PubMed] [Google Scholar]
- 47. Xydakis D, Papadogiannakis A, Sfakianaki M et al. Residual renal function in hemodialysis patients: the role of angiotensin-converting enzyme inhibitor in its preservation. ISRN Nephrol 2013; 2013: 184527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kjaergaard KD, Peters CD, Jespersen B et al. Angiotensin blockade and progressive loss of kidney function in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis 2014; 64: 892–901 [DOI] [PubMed] [Google Scholar]
- 49. Nessim SJ, Perl J, Bargman JM. The renin-angiotensin-aldosterone system in peritoneal dialysis: is what is good for the kidney also good for the peritoneum? Kidney Int 2010; 78: 23–28 [DOI] [PubMed] [Google Scholar]
- 50. Paoletti E, Cassottana P, Bellino D et al. Left ventricular geometry and adverse cardiovascular events in chronic hemodialysis patients on prolonged therapy with ACE inhibitors. Am J Kidney Dis 2002; 40: 728–736 [DOI] [PubMed] [Google Scholar]
- 51. Yang LY, Ge X, Wang YL et al. Angiotensin receptor blockers reduce left ventricular hypertrophy in dialysis patients: a meta-analysis. Am J Med Sci 2013; 345: 1–9 [DOI] [PubMed] [Google Scholar]
- 52. Selby NM, McIntyre CW. Peritoneal dialysis is not associated with myocardial stunning. Perit Dial Int 2011; 31: 27–33 [DOI] [PubMed] [Google Scholar]
- 53. Kidney Disease: Outcomes Quality Initiative. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43: S1–S290 [PubMed] [Google Scholar]
- 54. Peritoneal Dialysis Adequacy Work Group. Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis 2006; 48 (Suppl 1): S98–S [DOI] [PubMed] [Google Scholar]
- 55. Bhandari S, Ives N, Brettell EA et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31: 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schaubel DE, Blake PG, Fenton SS. Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 2001; 60: 1517–1524 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.