This editorial refers to ‘Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT’†, by I.C. Van Gelder et al., on page 1339.
As the number of our patients with implantable cardiac devices grows, we are increasingly faced with reports of previously concealed ‘subclinical’ atrial fibrillation (AF) that are now routinely captured by such devices. However, how to respond to this kind of information is often unclear. To some patients, an incidental diagnosis of subclinical AF, even if it lasts for several days, may be irrelevant. This is commonly the case when patients are known to have clinical AF or when they are already on appropriate risk-modifying treatments. To other patients, however, such incidental findings may well have management implications.
A growing body of evidence suggests that even short episodes of subclinical AF are important markers of risk, in particular of stroke and systemic embolism. In light of these findings, some have argued that the next question is not whether such patients should be treated differently but how and when intensive treatment, typically in the form of oral anticoagulation, is to be commenced.1 In the absence of robust evidence on the natural history of subclinical AF and the impact of treatments, however, current ESC guidelines give no specific advice on a differential management of patients with device-detected subclinical AF, nor do they make any recommendations for more active screening of subclinical AF.2
In this issue of the journal, Van Gelder et al. make an important contribution to the evidence base on this topic.3 They use the ASSERT database to investigate elegantly the relationship between maximum duration of subclinical AF and future risk of stroke and systemic embolism in a cohort of patients without a prior history of clinical AF. They postulate that if we were able to classify patients into two groups based on the duration of subclinical AF and their risk of stroke and systemic embolism, then we might be able to formulate clearer recommendations about when to start anticoagulation and when not. In other words, they assume that across the whole spectrum of subclinical AF durations, there is a natural threshold above which patients would behave like those with clinical AF (and hence should be treated as such) and below which the risk is not materially different from those without subclinical AF (hence, where additional treatment is not indicated).
To identify this risk threshold, the investigators ranked patients into four groups based on the maximum duration of subclinical AF. At baseline, all patients were in the reference group with no recording of subclinical AF. During follow-up, some patients developed subclinical AF and from then onwards were ranked into one of the three categories with boundaries conveniently defined as maximum duration of subclinical AF: (i) 6 min to <6 h; (ii) 6 h to <24 h; and (iii) >24 h. Patients remained in one of these categories until the end of the study unless a longer subclinical AF episode was recorded, in which case the patient was up-classified. Of note, 125 patients with a maximum duration of subclinical AF <6 min were excluded from the primary analysis, although in a sensitivity analysis they were combined with the reference group, with no major impact on the main risk estimates.
In their main analysis, the authors compared the risk of developing stroke or systemic embolism in each of the subclinical AF groups with the reference group of no subclinical AF and found the risk to be about three times higher among those with the longest duration of subclinical AF (>24 h category). In contrast, the risk in the other two groups with shorter durations was not statistically different from that of the reference group. This led to the conclusion that ‘subclinical AF > 24 h is associated with an increased risk of ischaemic stroke or systemic embolism’, and the authors go on to discuss that ‘subclinical AF > 24 h may be a threshold for a higher stroke risk’ which could help with the decision as to ‘whether or not and when to start anticoagulation, [while we are] awaiting the results of randomized trials.’
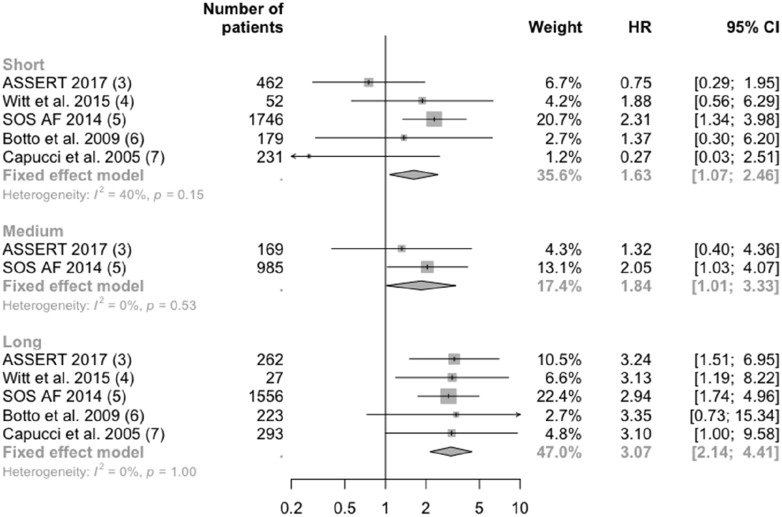
Have we now identified the natural threshold for making binary decisions about when to start anticoagulation in patients with subclinical AF? I believe not, partly because ASSERT was significantly limited by sample size, rendering its findings about the existence or level of a threshold unreliable. In such a situation, it might help if we consider the new ASSERT findings in the context of the wider literature. To date, several other studies have reported risks associated with subclinical AF. These studies differ in many respects, including the population included, definition of outcomes, and statistical methods used. Thus, a meta-analysis of their findings has to be interpreted with great caution. A few of these studies, however, chose very similar exposure categories and outcomes to those in ASSERT.4–7 Notwithstanding some remaining clinical and methodological differences among them, a meta-analysis of their group-specific associations shows their findings to be fairly consistent (Figure 1). More importantly, they collectively lend no support to the existence of a threshold at which relative risks change qualitatively. In fact, the risk of stroke and embolic events appears to rise continuously with increasing duration of subclinical AF, a finding consistent with the literature showing a 60% stronger association in risk of stroke and death in patients with non-paroxysmal AF compared with those with shorter spells of paroxysmal AF.8
Figure 1.
Association of risk of stroke and embolism by categories of duration of subclinical atrial fibrillation (AF). Inclusion criteria for patients were: Witt et al., implantable cardioverter defibrillator (ICD) and no clinical AF; SOS AF, implantable devices and no clinical AF; Botto et al., pacemaker and history of AF; Capucci et al., patients with bradycardic pacing; ASSERT, pacemaker, history of hypertension, older than 65 years, and no history of AF. Witt et al., Botto et al., and Capucci et al. chose stroke, transient ischaemic attack (TIA), and peripheral arterial embolism as their outcome. SOS AF chose ischaemic stroke and TIA. ASSERT chose stroke and systemic embolism. Short duration of subclinical AF is defined as 6 min to 24 h in Witt et al., 5 min to 24 h in Botto et al. and Cappuci et al., 5 min to 6 h in SOS AF, and 6 min to 6 h in ASSERT. Medium duration of subclinical AF is defined as 6–23 h in SOS AF and 6–24 h in ASSERT. Long duration of subclinical AF is defined as > 23 h in SOS AF and >24 h in all other studies. CI, confidence interval; HR, hazard ratio.
If subclinical AF really increases the risk of stroke in a graded and continuous way, then how might we be able to use this information to refine our decisions about patient management? Certainly, at a glance, the slope of the association seems to be steep enough to have a meaningful impact on decision-making. In particular, the three times stronger association observed among those with the longest duration of subclinical AF would be expected to have a non-negligible impact on risk stratification for use of anticoagulation. However, this would only be true if the observed associations were independent of established risk factors for stroke, and in the present study it is not clear whether this condition is met. While Van Gelder et al. adjust for important variables at ‘baseline’ of the ASSERT cohort, in their time-dependent models ‘baseline’ is not the beginning of the cohort for all patients. As some patients go on to develop subclinical AF and their baseline shifts to a later time point, their risk profile for developing stroke will change too. They will become older and might then have a higher blood pressure, additional co-morbidities (including clinical AF), and different treatments known to affect their risk of stroke. Therefore, a simple adjustment for co-variates at the beginning of study without updated information about risk profiles would be an incomplete adjustment of differences between patients at the time of risk assessment. As previous studies have shown, lack of consideration of time-dependent changes in variables could have unpredictable effects on the observed associations.9 However, apart from use of anticoagulation during follow-up, Van Gelder et al. did not take account of such time-dependent changes, presumably because of the limited number of events available or incomplete updated information about risk factors.
The study by Van Gelder et al. reminds us that our arbitrary classification criteria for AF which are based on opportunistic screening methods are too crude for optimal risk stratification. The study further highlights the need for much larger observational studies to better understand the consequences of shorter spells of AF. Such studies would complement ongoing randomized trials that are assessing the effect of anticoagulation in patients at high risk of stroke and in the presence of long durations of subclinical AF. Large-scale observational studies can go beyond these trials by evaluating risks across a much wider range of AF durations and on less well investigated outcomes. Emerging evidence from recent large-scale studies show that clinical AF is associated not only with a higher risk for stroke and embolism but also several other vascular events, such as heart failure, myocardial infarction, peripheral vascular disease, renal disease, and vascular dementia,10 and even non-vascular events such as cancer.11 Limited evidence suggests that device-detected AF is also associated with increased risk of heart failure hospitalization and cardiovascular death.12,13 However, the strength of associations and the extent to which they might be able to add to existing multivariate risk prediction models needs further research.
Acknowledgements
The author’s research is supported by grants from the NIHR Oxford BRC, NIHR CDF, and Oxford Martin School.
Funding
Dr. Rahimi reports grants from NIHR Oxford BRC, grants from NIHR CDF, grants from Oxford Martin School, during the conduct of the study.
Conflict of interest: none declared.
References
- 1. Chen-Scarabelli C, Scarabelli TM, Ellenbogen KA, Halperin JL.. Device-detected atrial fibrillation. J Am Coll Cardiol 2015;65:281–294. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothoracic Surg 2016;50:e1–e88. [DOI] [PubMed] [Google Scholar]
- 3. Van Gelder IC, Healey JS, Crijns HJGM, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau C-P, Morillo CA, Hobbelt AH, Rienstra M, Connolly SJ. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 4. Witt CT, Kronborg MB, Nohr EA, Mortensen PT, Gerdes C, Nielsen JC.. Early detection of atrial high rate episodes predicts atrial fibrillation and thromboembolic events in patients with cardiac resynchronization therapy. Hear Rhythm 2015;12:2368–2375. [DOI] [PubMed] [Google Scholar]
- 5. Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, Gasparini M, Lewalter T, Camm JA, Singer DE.. Device-detected atrial fibrillation and risk for stroke: an analysis of > 10 000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J 2014;35:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi F, Favale S, Molon G, Ricci R, Biffi M, Russo G, Vimercati M, Corbucci G, Boriani G.. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol 2009;20:241–248. [DOI] [PubMed] [Google Scholar]
- 7. Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani G, Ricci R, Favale S, Zolezzi F, Di Belardino N, Molon G, Drago F, Villani GQ, Mazzini E, Vimercati M, Grammatico A; Italian AT500 Registry Investigators. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol 2005;46:1913–1920. [DOI] [PubMed] [Google Scholar]
- 8. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P, McGavigan AD.. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J 2016;37:1591–1602. [DOI] [PubMed] [Google Scholar]
- 9. van Walraven C, Davis D, Forster AJ, Wells GA.. Time-dependent bias was common in survival analyses published in leading clinical journals. J Clin Epidemiol 2004;57:672–682. [DOI] [PubMed] [Google Scholar]
- 10. Emdin C,, Anderson A,, Salimi-Khorshidi G,, Woodward M,, MacMahon M,, Dwyer T,, Rahimi K.. Usual blood pressure, atrial fibrillation and vascular risk: evidence from 4.3 million adults. Int J Epidemiol 2016;36:dyw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, Albert CM.. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol 2016;1:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shanmugam N, Boerdlein A, Proff J, Ong P, Valencia O, Maier SK, Bauer WR, Paul V, Sack S.. Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure–cardiac resynchronization therapy population. Europace 2012;14:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonzalez M, Keating RJ, Markowitz SM, Liu CF, Thomas G, Ip JE, Lerman BB, Cheung JW.. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm 2014;11: 2214–2221. [DOI] [PubMed] [Google Scholar]