Abstract
FICZ and TCDD, two high-affinity AhR ligands, are reported to have opposite effects on T cell differentiation with TCDD inducing regulatory T cells and FICZ inducing Th17 cells. This dichotomy has been attributed to ligand-intrinsic differences in AhR activation, although differences in sensitivity to metabolism complicate the issue. TCDD is resistant to AhR-induced metabolism and produces sustained AhR activation following a single dose in the μg/kg range, whereas FICZ is rapidly metabolized and AhR activation is transient. Nonetheless, prior studies comparing FICZ with TCDD have generally used the same 10–50 μg/kg dose range, and thus the two ligands would not equivalently activate AhR. We hypothesized that high-affinity AhR ligands can promote CD4+ T cell differentiation into both Th17 cells and Tregs, with fate depending on the extent and duration of AhR activation. We compared the immunosuppressive effects of TCDD and FICZ, along with two other rapidly metabolized ligands (ITE and 11-Cl-BBQ) in an acute alloresponse mouse model. The dose and timing of administration of each ligand was optimized for TCDD-equivalent Cyp1a1 induction. When optimized, all of the ligands suppressed the alloresponse in conjunction with the induction of Foxp3– Tr1 cells on day 2 and the expansion of natural Foxp3+ Tregs on day 10. In contrast, a low dose of FICZ induced transient expression of Cyp1a1 and did not induce Tregs or suppress the alloresponse but enhanced IL-17 production. Interestingly, low doses of the other ligands, including TCDD, also increased IL-17 production on day 10. These findings support the conclusion that the dose and the duration of AhR activation by high-affinity AhR ligands are the primary factors driving the fate of T cell differentiation.
Keywords: aryl hydrocarbon receptor, TCDD, FICZ, Tr1, Foxp3, Th17
The aryl hydrocarbon receptor (AhR) is a ligand-activated heterodimeric transcription factor that is a member of the basic helix-loop-helix-Per/ARNT/Sim family. Following ligand binding in the cytoplasm, AhR dissociates from its complex with Hsp90 and the AhR-interacting protein, XAP2, allowing for ligated AhR to translocate into the nucleus. There, AhR dimerizes with the AhR nuclear translocator (ARNT), that then binds to xenobiotic response elements (XREs), promoting the up- or downregulation of a multitude of target genes in many different tissues. Through this canonical pathway, AhR also regulates the metabolism of AhR ligands by inducing a battery of genes including Cyp1a1 and Cyp1b1, which are often used as biomarkers of AhR activation. Furthermore, AhR can interact with several other signaling pathways (eg, NFκB, estrogen receptor, KLF6), expanding its influence on gene regulation (Beischlag et al., 2008; Denison et al., 2011; Nebert, 2017; Wright et al., 2017).
AhR activation influences many aspects of immunological function, but its role in the suppression of adaptive immune responses is most thoroughly documented. Based primarily on studies with TCDD, a high-affinity AhR ligand, immune suppression results from activation of AhR in CD4+ T cells and dendritic cells (DCs) during early stages of the response to antigen (Bankoti et al., 2010; Duarte et al., 2013; Funatake et al., 2005; Hauben et al., 2008; Kerkvliet et al., 2002; Quintana et al., 2010; Vorderstrasse and Kerkvliet, 2001). AhR activation in CD4+ T cells directly alters gene expression and, subsequently, T cell differentiation into IL-10 producing, Foxp3– type 1 regulatory T cells (Tr1 cells) (Apetoh et al., 2010; Ehrlich et al., 2017; Marshall et al., 2008). In addition, AhR activation in DCs has been shown to promote a tolerogenic phenotype that increases the percentage of Foxp3+ Tregs (Benson and Shepherd, 2011b; Hauben et al., 2008; Quintana et al., 2010; Vogel et al., 2008). The increase in the frequency of regulatory T cells following AhR activation has been associated with the suppression of several immune-mediated diseases (Benson and Shepherd, 2011a; Ehrlich et al., 2016; Funatake et al., 2005; Hauben et al., 2008; Quintana et al., 2008; Schulz et al., 2011; Singh et al., 2011; Zhang et al., 2010). These studies provide support for the development of AhR ligands for therapeutic use (Ehrlich and Kerkvliet, 2017).
In contrast to an increase in regulatory T cells by TCDD, another high-affinity AhR ligand, FICZ, has been reported to enhance Th17 responses and exacerbate immune-mediated diseases in several mouse models (Quintana et al., 2008; Schulz et al., 2012; Singh et al., 2016; Veldhoen et al., 2008). These divergent outcomes following AhR activation have been interpreted as reflecting ligand-intrinsic effects on CD4+ T cell differentiation, although no definitive explanation for such differences have been reported. However, one issue with this interpretation is that FICZ has been administered in the same dose range as TCDD, despite major differences in their susceptibility to metabolic breakdown (Gasiewicz et al., 1983; Mukai and Tischkau, 2007; Wheeler et al., 2014). TCDD, which is resistant to AhR-induced metabolism, has a long half-life and induces sustained AhR activation following a single dose in the μg/kg range. FICZ, in contrast, is rapidly metabolized. Thus, when a single μg/kg dose of FICZ is administered, AhR will be transiently activated for only a few hours whereas the same dose of TCDD will maintain AhR activation for at least 1 to 2 weeks.
In the current study, we hypothesized that high-affinity AhR ligands have similar effects on CD4+ T cell differentiation, with cell fate dependent on the extent and duration of AhR activation. To test this hypothesis, low and high doses of several high-affinity AhR ligands (TCDD, FICZ, ITE, 11-Cl-BBQ) were used to determine the effects on CD4+ T cell differentiation using a parent-into-F1 alloresponse mouse model (Kerkvliet et al., 2002; Puliaev et al., 2005). The results show that all of the ligands induced AhR-Tr1 cells on day 2 and increased Foxp3+ Tregs on day 10 in conjunction with suppression of the alloresponse when administered at doses/dose rates that activate AhR to the same extent as the Treg-inducing dose of TCDD. Furthermore, we found that the increase in Foxp3+ Tregs represented an expansion of donor-derived natural Tregs rather than differentiation of naïve CD4+ T cells. In contrast, low doses of the ligands, even when given daily, did not induce Tregs nor alter the alloresponse, but instead increased the percentage of CD4+ cells that produce IL-17. Collectively, the data suggest that AhR activation produces a hormetic-like response; when AhR activation is minimal, it will enhance profinflammatory immune responses while stronger and sustained AhR activation will result in immune suppression.
MATERIALS AND METHODS
Animals
C57BL/6J (B6; H-2b/b) and B6D2F1 (F1; H-2b/d) mice were obtained from The Jackson Laboratory (Sacramento, CA) and maintained in the specific pathogen-free animal facility at Oregon State University. All experiments used female age-matched mice between 9 and 12 weeks of age. All animal procedures were carried out following protocols approved by the Institutional Animal Care and Use Committee.
Donor cell transfer
Donor cells were collected from the spleen and peripheral lymph nodes of B6 mice and transferred by tail vein injection into F1 host mice at a concentration equivalent to 3–4 × 107 donor T cells. For the day 2 studies, cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Life Technologies, Carlsbad, CA) at a final concentration of 5 μM prior to donor cell injection to delineate the donor cell population by flow cytometric analysis. For the natural Treg depletion studies, anti-CD25 microbeads and magnetic cell separation were used to deplete CD25+ cells in the donor cell population prior to cell transfer (Miltenyi Biotec, Bergisch Gladbach, Germany).
AhR ligand treatments
TCDD was dissolved in anisole, diluted in peanut oil, and administered at 15 μg/kg in 0.15% anisole (high dose) or 0.6 μg/kg in 0.006% anisole (low dose). For high-dose studies, Cl-BBQ (a 35:65 mixture of 10- and 11-Cl-BBQ, ChemBridge, San Diego, CA), 10-Cl-BBQ (ChemBridge), 11-Cl-BBQ (ChemBridge), ITE (Tocris, Bristol, United Kingdom), FICZ (Tocris, Bristol, United Kingdom), and DIM (a kind gift from Dr David Williams) were dissolved in DMSO and diluted in peanut oil for a final concentration of 1% DMSO. A 1% DMSO solution in peanut oil was used for the vehicle. For low-dose studies, the high-dose concentration of 11-Cl-BBQ, ITE, and FICZ was further diluted in the vehicle, so that the final concentration of DMSO remained at 1%. All treatments were administered by i.p. injection. Doses and timing of administration are indicated in the Results section and figure legends.
Real-time PCR
Livers were removed from mice and placed in RNAlater (Qiagen, Hilden, Germany) for immediate stabilization prior to RNA isolation using RNeasy columns (Qiagen, Hilden, Germany). cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). qPCR reactions were performed on an ABI PRISM 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA) using SYBR Green/ROX Master Mix (SA Biosciences, Frederick, MD). Cyp1a1 and Cyp1b1 levels were normalized to Actb using primers from SA Biosciences (Frederick, MD).
Flow cytometry
Following removal of red blood cells via hypotonic lysis, splenocytes were stained for flow cytometric analysis. Cells were incubated with rat IgG to block Fc receptors and stained with the following antibodies, San Diego, CA: CD45RB (C363-16A), CD44 (1M7), CD8 (53.6.7), CD19 (1D3), CD4 (RM4-5), CD25 (PC61.5), and CCR9 (CW-1.2) from eBioscience, San Diego, CA; CD62L (R1-2) from BD Bioscience (San Jose, CA); and CCR4 (2G12) and H2D (34-2-12) from Biolegend (San Diego, CA). For intracellular Foxp3 staining, cells were fixed and permeabilized using Foxp3 Fixation/Permeabilization buffer (eBioscience, San Diego, CA) and stained with Foxp3 (FJK-16s, eBioscience, San Diego, CA). For IL-17 staining, cells were stimulated with PMA, ionomycin, brefeldin A, and monensin (eBioscience, San Diego, CA) for 4 h in culture prior to surface staining. Cells were then fixed with Cytofix/Cytoperm (BD Biosciences, San Jose, CA) and stained with anti-IL-17 antibody (eBio17B7, eBioscience, San Diego, CA). Data were acquired on a FC-500 or Cytoflex flow cytometer (Beckman Coulter, Brea, CA). Data were compensated and analyzed using FlowJo (Treestar, Ashland, OR) software. Fluorescence minus one controls were used for setting gates.
ELISA
Splenocytes were stimulated ex vivo with PMA/ionomycin (eBioscience, San Diego, CA) for 6 h. Culture supernatants were removed and IL-17 was measured using the eBioscience Ready Set Go IL-17 ELISA Kit, according to the manufacturer’s protocol.
In silico studies
Mouse and human AhR homology models of the Per-ARNT-Sim B (PASB)-ligand binding domains were developed based on the 3D-coordinates of the resolved structure of the HIF-2α-PASB (PDB 1P97) using both TCDD- and FICZ-guided optimization (Hubbard et al., 2015; Perkins et al., 2014). Molecular docking was performed as previously reported (Perkins et al., 2014), using the ICM-Virtual Ligand Screening (Molsoft ICM, San Diego, CA) procedure. The scoring function provides a good approximation of the binding free energy between a ligand and a receptor and is usually a function of different energy terms based on a force-field. The ICM scoring function is weighted according to the following parameters (1) internal force-field energy of the ligand, (2) entropy loss of the ligand between bound and unbound states, (3) ligand–receptor hydrogen bond interactions, (4) polar and nonpolar solvation energy differences between bound and unbound states, (5) electrostatic energy, (6) hydrophobic energy, and (7) hydrogen bond donor or acceptor desolvation. The lower the ICM score, the higher the likelihood that the ligand is a good fit within the receptor (Perkins et al., 2014).
Statistics
All statistical analyses were performed using Graphpad Prism (La Jolla, CA). One-way ANOVA with Tukey’s test for multiple comparisons was used to determine differences between treatment groups, with p ≤ .05 considered statistically significant.
RESULTS
In Silico Molecular Modeling and Docking of AhR Ligands
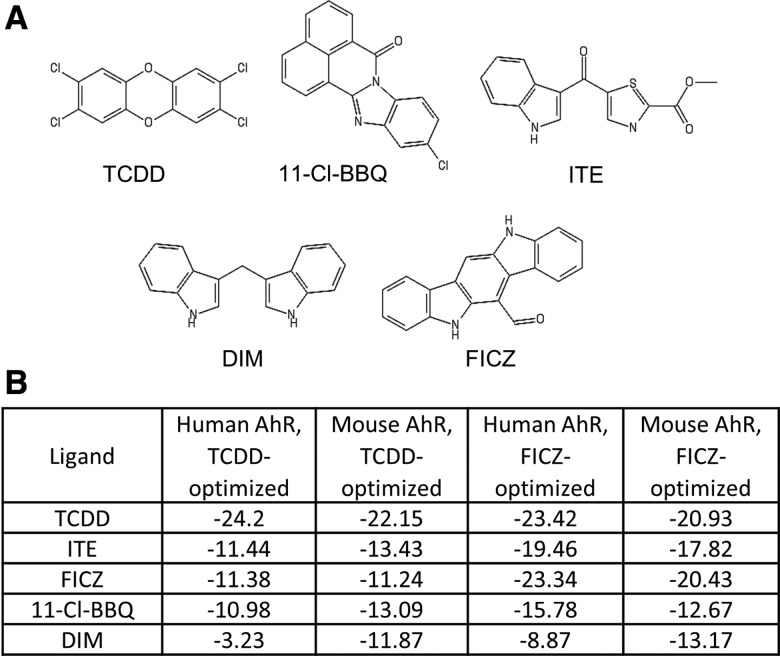
Initial studies were carried out to compare the AhR-binding properties of several known high-affinity AhR ligands that have been previously reported to influence CD4+ T cell differentiation, including TCDD, FICZ, ITE, 11-Cl-BBQ, and DIM (Figure 1A). Using TCDD- and FICZ-optimized models of mouse and human AhR-PASB ligand binding domains (Hubbard et al., 2015; Perkins et al., 2014), docking simulations were performed for each ligand. As shown in Figure 1B, TCDD docking generated the lowest score in both species and models. FICZ, ITE, and 11-Cl-BBQ had comparable docking scores in the mouse and human TCDD-optimized models. As expected, FICZ had a lower score than the other rapidly metabolized ligands in the FICZ-optimized model. 11-Cl-BBQ had a higher docking score than expected in the FICZ-optimized model given its known high affinity for AhR (Punj et al., 2014). The docking score of DIM was highest in the series for both species in the TCDD-optimized model and in the human FICZ-optimized model. Overall, the prevailing view that FICZ is a unique AhR ligand could not be explained based on differences in molecular docking.
Figure 1.
AhR ligand structures and docking scores for AhR. (A) Structures of TCDD, 11-Cl-BBQ, ITE, DIM, and FICZ. (B) Molecular docking scores for human and mouse AhR ligand binding domains using TCDD- and FICZ-guided models.
Determination of Dosing Regimens for Rapidly Metabolized AhR Ligands to Achieve AhR Activation Comparable to TCDD
In a parent-into-F1 alloresponse model, a single dose of 15 μg/kg TCDD maintains AhR activation for more than 2 weeks as measured by Cyp1a1 induction in the liver. This dose of TCDD skews CD4+ T cell differentiation toward a Tr1-like phenotype on day 2 of the alloresponse and suppresses the development of the CTL response (Funatake et al., 2005). Similar Tr1-like skewing of CD4+ T cell differentiation occurred when mice were treated daily with Cl-BBQ, delivered at a dose that maintained AhR activation similarly to TCDD (Punj et al., 2014). Subsequent studies with Cl-BBQ have shown that AhR activation in donor CD4+ T cells during the first 3 days of the alloresponse is sufficient to suppress the alloresponse. During this window of time, the differentiation of the CD4+ T cells is skewed toward AhR-Tr1 cells that inhibit T effector cell development (Ehrlich et al., 2017).
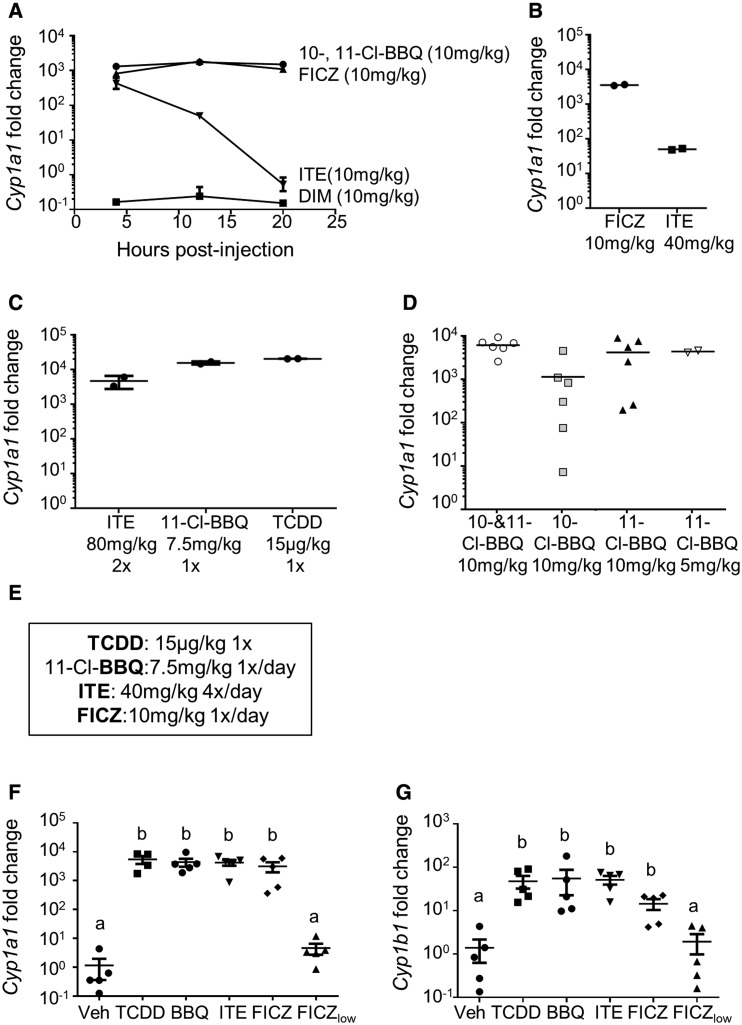
To determine if other high affinity, rapidly metabolized AhR ligands induce AhR-Tr1 cells and suppress the alloresponse, we first determined the dose and dose rate for each ligand of interest that induces hepatic Cyp1a1 to the same extent as 15 μg/kg TCDD as measured at 20 h. The individual 10- and 11-Cl-BBQ congeners in Cl-BBQ as well as DIM, FICZ, and ITE were chosen for this study, and optimized treatment regimens were determined empirically (Figs. 2A–D). Initially, each ligand was administered i.p. at 10 mg/kg and Cyp1a1 was measured at 4, 12, and 20 h (Figure 2A). Although Cl-BBQ and FICZ maintained a high level of Cyp1a1 induction throughout this period, Cyp1a1 induction by ITE peaked at 4 h and then declined to a low level by 20 h. DIM failed to induce Cyp1a1 at any time point, consistent with its high docking score (Figure 1B). Cyp1a1 induction remained low in ITE-treated mice, at 20 h even after increasing the dose to 40 mg/kg (Figure 2B). When the dose of ITE was increased to 80 mg/kg and administered at 0 and 12 h, Cyp1a1 induction was increased; however, mice showed signs of overt toxicity (Figure 2C). Ultimately, ITE was administered at 40 mg/kg every 6 h, which maintained high Cyp1a1 induction without toxicity. 10-Cl-BBQ, used in prior studies (Ehrlich et al., 2016; Punj et al., 2014), was later identified as a 35:65 mixture of 10- and 11-Cl-BBQ congeners. To determine if there were differences in potency, Cyp1a1 induction at 20 h was compared in mice treated with 10 mg/kg of either the Cl-BBQ mixture or the individual congeners (Figure 2D). Based on in vitro data suggesting that 11-Cl-BBQ was more potent than 10-Cl-BBQ at inducing Cyp1a1 in hepatocytes (data not shown), a 5 mg/kg dose of 11-Cl-BBQ was also tested. Results showed that 11-Cl-BBQ was more potent than 10-Cl-BBQ (Figure 2D), and therefore 11-Cl-BBQ was used for subsequent studies. The final optimized dosing regimen for each ligand is shown in Figure 2E.
Figure 2.
AhR ligand dose optimization. (A–D) Cyp1a1 expression was measured in the liver of B6 mice following i.p. injection of AhR ligands at annotated doses. (A) FICZ, ITE, DIM, or Cl-BBQ (a 35:65 mixture of 10- and 11-Cl-BBQ) was injected one time and Cyp1a1 was measured at 4, 12, and 20 h postadministration. (B) FICZ or ITE was injected 1×/day for 2 days and Cyp1a1 was measured 20 h following the last dose. (C) 11-Cl-BBQ and TCDD were administered one time. ITE was administered at 0 and 12 h. Cyp1a1 was measured at 20 h postadministration of the first dose. (D) 10-Cl-BBQ, 11-Cl-BBQ, or the mixture of both isomers was injected one time, and Cyp1a1 was measured at 20 h postadministration. (E) Optimized doses that led to TCDD-equivalent Cyp1a1 induction. (F, G) Spleen and peripheral lymph node cells from B6 mice were transferred into F1 host mice (day 0). Host mice were treated with optimized doses of AhR ligands: TCDD (15 μg/kg on day 0), 11-Cl-BBQ (7.5 mg/kg on days 0 and 1, BBQ), ITE (40 mg/kg every 6 hours on days 0 and 1), and FICZ (10 mg/kg on days 0 and 1). Treatment with a low dose of FICZ (50 μg/kg on days 0 and 1, FICZlow) was also included. Mice were sacrificed on day 2, and Cyp1a1 (F) and Cyp1b1 (G) were measured in the liver. Cyp1a1 and Cyp1b1 expression were normalized to Actb and fold-change was calculated by the ΔΔCt method using vehicle-treated sample values as the control. Each point represents an individual mouse. Treatment groups with different letters are significantly different. p ≤ .05.
The ability of 11-Cl-BBQ, ITE, and FICZ administered according to the optimized dosing regimen to maintain Cyp1a1 expression to the same extent as 15 μg/kg TCDD was assessed. Treatment began at the time of donor cell transfer, and Cyp1a1 and Cyp1b1 were measured on day 2 of the alloresponse. A low dose of FICZ (50 μg/kg; FICZlow) that has been previously reported to promote IL-17 production (Quintana et al., 2008; Schulz et al., 2012; Singh et al., 2016) was used for comparison to the optimized dose of FICZ (10 mg/kg). On day 2 after donor cell transfer, host mice treated with the optimized doses of the different ligands showed an approximately 5000-fold increase in Cyp1a1 induction over vehicle-treated mice. In contrast, daily FICZlow treatment led to an 8-fold increase in Cyp1a1 expression (Figure 2F). A similar pattern was observed with Cyp1b1 induction (Figure 2G). These data demonstrate that repeated administration of rapidly metabolized high-affinity AhR ligands can maintain AhR activation to the same extent as TCDD.
Optimized Doses of TCDD, 11-Cl-BBQ, ITE, and FICZ Suppress the Alloresponse
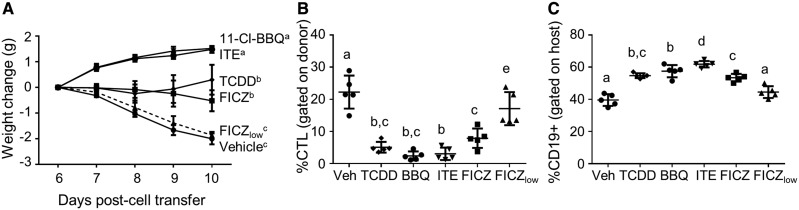
To determine if rapidly metabolized AhR ligands suppress CD4+ T cell responses similarly to TCDD, mice were treated with the optimized high-dose regimen of 11-Cl-BBQ, ITE, and FICZ during the CD4-dependent phase of the alloresponse (days 0–3), and the development of allo-CTL was assessed on day 10.
Consistent with previous studies (Kerkvliet et al., 2002; Rohlman et al., 2013), 15 μg/kg of TCDD prevented the alloresponse-associated weight loss that progresses in vehicle-treated mice after 6 days in association with the development of the allo-CTL response (Figure 3A). In addition, TCDD treatment suppressed the development of allo-CTL, which was reflected by the prevention of host cell destruction as measured by host B cell depletion (Figs. 3B and 3C). Like TCDD, treatment with the optimized doses of the rapidly metabolized ligands, 11-Cl-BBQ, ITE, and notably FICZ, also suppressed the allo-CTL response (Figs. 3A–C). FICZlow treatment did not influence the alloresponse (Figs. 3A–C).
Figure 3.
TCDD, 11-Cl-BBQ, ITE, and FICZ suppress the alloresponse. Spleen and peripheral lymph node cells from B6 mice were transferred into F1 host mice (day 0). Host mice were treated with TCDD (15 μg/kg on day 0), 11-Cl-BBQ (7.5 mg/kg on days 0-3, BBQ), ITE (40 mg/kg every 6 h on days 0–3), FICZ (10 mg/kg on days 0–3), or FICZ (50 μg/kg on days 0–3, FICZlow). (A) Mice were weighed from days 6 to 10 to calculate the weight change associated with the alloresponse. On day 10, splenocytes were analyzed by flow cytometry for the percentage of (B) donor cells that expressed the CTL phenotype (CD8+CD44highCD45RBlow) and (C) host cells that expressed CD19. Each point represents an individual mouse. Treatment groups with different letters are significantly different. p ≤ .05.
TCDD, 11-Cl-BBQ, ITE, and FICZ Induce AhR-Tr1 Cells on Day 2 of the Alloresponse
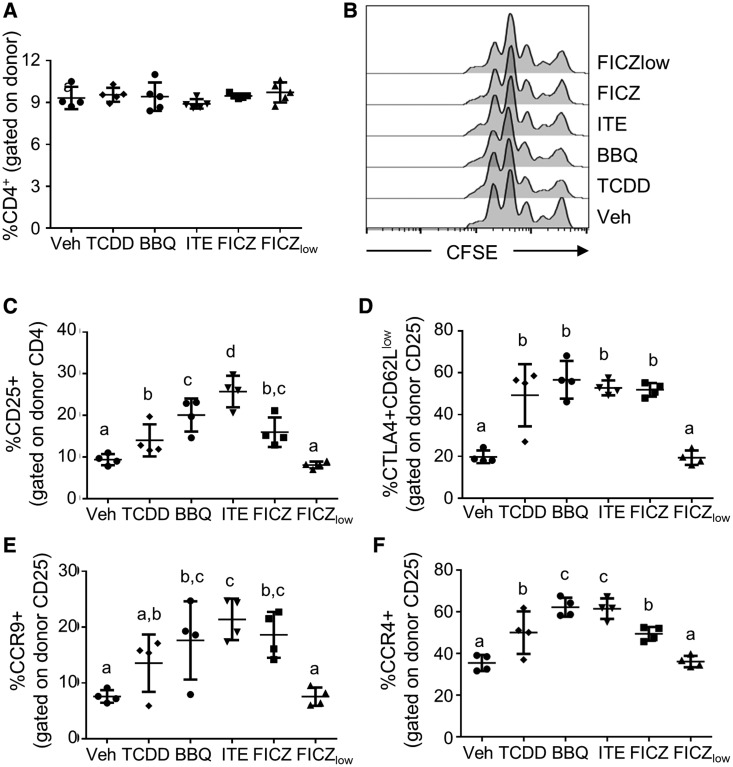
AhR activation with TCDD or Cl-BBQ promotes the differentiation of AhR-Tr1 cells on day 2 of the alloresponse (Funatake et al., 2005; Punj et al., 2014). These activated AhR-Tr1 cells are characterized by high expression of CD25 and CTLA4 as well as low expression of CD62L (Funatake et al., 2005). Furthermore, AhR-Tr1 cells express the chemokine receptors CCR9 and CCR4, which promote the migration of AhR-Tr1 cells toward mucosal tissues (Ehrlich et al., 2017). To determine if other rapidly metabolized high-affinity AhR ligands also promote CD4+ T cell differentiation into the AhR-Tr1 cell phenotype, protein expression on donor CD4+ T cells was analyzed by flow cytometry. On day 2, AhR activation did not alter the percentage of donor cells that expressed CD4 (Figure 4A), nor the proliferation status of donor CD4+ T cells (Figure 4B). Treatment with optimized doses of TCDD, 11-Cl-BBQ, ITE, and FICZ led to an increased percentage of CD4+ cells which expressed CD25 (Figure 4C), and a greater percentage of these CD25+ cells were CTLA4+CD62Llow (Figure 4D) as well as CCR9+ and CCR4+ (Figs. 4E and 4F) in comparison with vehicle-treated mice. Treatment with FICZlow did not alter the percentage of cells expressing the AhR-Tr1 cell phenotype (Figs. 4C–F).
Figure 4.
TCDD, 11-Cl-BBQ, ITE, and FICZ treatment leads to Tr1 cell differentiation on day 2 of the alloresponse. Spleen and peripheral lymph node cells from B6 mice were labeled with CFSE and transferred into F1 host mice (day 0). Host mice were treated with TCDD (15 μg/kg on day 0), 11-Cl-BBQ (7.5 mg/kg on days 0 and 1, BBQ), ITE (40 mg/kg every 6 h on days 0 and 1), FICZ (10 mg/kg on days 0 and 1), or FICZ (50 μg/kg on days 0 and 1, FICZlow). On day 2, splenocytes were stained and analyzed for (A) CD4 expression on donor cells, (B) donor CD4+ T cell proliferation, and (C) CD25 expression on donor CD4+ cells. The donor CD4+CD25+ population was then analyzed for the percentage of (D) CTLA4+CD62Llow, (E) CCR9+, and (F) CCR4+ cells. Each point represents an individual mouse. Treatment groups with different letters are significantly different. p ≤ .05.
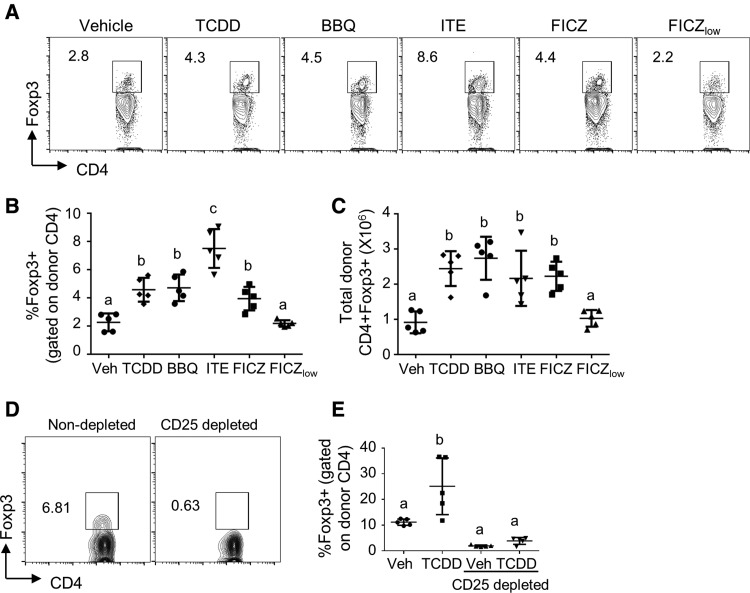
TCDD, 11-Cl-BBQ, ITE, and FICZ Increase Foxp3+ Tregs on Day 10 of the Alloresponse
Foxp3 expression in CD4+ cells is not directly regulated by TCDD on day 2 of the alloresponse (Marshall et al., 2008); however, AhR activation in DCs has been shown to indirectly increase the frequency of Foxp3+ Tregs (Benson and Shepherd, 2011b; Hauben et al., 2008; Quintana et al., 2010; Vogel et al., 2008). To determine if Foxp3+ cells are increased by high-affinity AhR ligands later in the alloresponse, we measured the percentage of Foxp3+ Tregs on day 10. Indeed, optimized treatments with TCDD, 11-Cl-BBQ, ITE, and FICZ led to an increased percentage and total number of CD4+ T cells expressing Foxp3 (Figs. 5A–C). To determine if the increase in Foxp3+ Tregs later in the alloresponse was due to the de novo induction of Foxp3+ Tregs or due to the expansion of existing Foxp3+ Tregs present in the donor inoculum, mice were injected with Foxp3-depleted or intact donor cells and treated with TCDD. Surprisingly, depletion of the Foxp3+ cells from the donor cell pool by treatment with anti-CD25 prior to their transfer negated the AhR-mediated increase in Foxp3+ Tregs (Figs. 5D and 5E), suggesting that AhR activation in host DCs promotes the expansion of donor-derived natural Tregs during the alloresponse. Treatment with FICZlow did not alter the frequency or total number of Foxp3+ cells on day 10 (Figs. 5A–C). These results suggest that a threshold of AhR activation is needed to expand Foxp3+ natural Tregs.
Figure 5.
TCDD, 11-Cl-BBQ, ITE, and FICZ treatment results in an expansion of donor-derived Foxp3+ T cells. Spleen and peripheral lymph node cells from B6 mice were transferred into F1 host mice (day 0). Host mice were treated with TCDD (15 μg/kg on day 0), 11-Cl-BBQ (7.5 mg/kg on days 0–3, BBQ), ITE (40 mg/kg every 6 h on days 0–3), FICZ (10 mg/kg on days 0–3), or FICZ (50 μg/kg on days 0–3, FICZlow). (A–C) On day 10, splenocytes were stained and analyzed for Foxp3+ Tregs. (A) Representative FACS plots for Foxp3 expression gated on donor CD4+ cells. The percentage (B) and total number (C) of donor CD4+Foxp3+ cells were calculated. (D, E) Spleen and peripheral lymph node cells from B6 mice were transferred into F1 host mice. For the CD25 depleted group, CD25+ cells were removed from donor cells by magnetic bead isolation prior to injection. Mice were treated with either TCDD (15 μg/kg) or vehicle at the time of donor cell injection. (D) Efficacy of CD25 depletion in removing Foxp3+ cells from the donor cell population. (E) Donor cells expressing Foxp3 were assessed on day 15 after donor cell transfer. Each point represents an individual mouse. Treatment groups with different letters are significantly different. p ≤ .05.
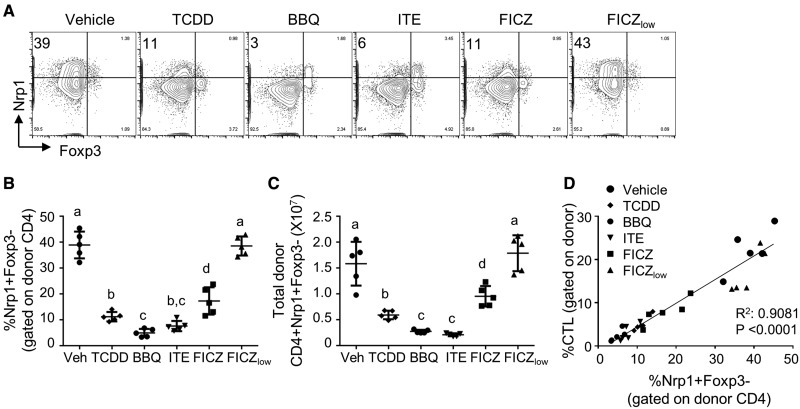
TCDD, 11-Cl-BBQ, ITE, and FICZ Reduce the Percentage and Number of CD4+ T Effector Cells on Day 10 of the Alloresponse
In the parent-into-F1 acute alloresponse model, CD4+ T effector (Teff) cells are required for and participate in the immunopathogenic response. To determine if AhR activation by different ligands affected CD4+ Teff cell development, we quantified the percentage of neuropilin-1 (Nrp1)+Foxp3– donor CD4+ cells on day 10 of the alloresponse. Nrp1 expression on CD4+Foxp3– cells has been previously associated with an effector/memory phenotype (Tordjman et al., 2002; Weiss et al., 2012). Furthermore, we have shown that the percentage of CD4+Foxp3– cells that express Nrp1 is strongly correlated with islet infiltration in nonobese diabetic mice. Reduction in the percentage of CD4+Nrp1+Foxp3– cells proved to be a useful biomarker for suppression of insulitis in mice treated with Cl-BBQ and TCDD (Ehrlich et al., 2016). In this study, mice treated with the optimized doses of TCDD, 11-Cl-BBQ, ITE, or FICZ had a significantly reduced percentage and total number of donor CD4+ cells that were Nrp1+Foxp3– in comparison with vehicle-treated mice (Figs. 6A–C). The reduction in the percentage of Nrp1+Foxp3– donor CD4+ T cells strongly correlated with the reduction in the percentage of allo-CTL cells on day 10 (Figure 6D). The reduction in Teff cells on day 10 in AhR ligand-treated mice is likely a consequence of the suppression of Teff differentiation by AhR-Tr1 cells on day 2 (Ehrlich et al., 2017).
Figure 6.
TCDD, 11-Cl-BBQ, ITE, and FICZ treatment decreases the percentage and number of CD4+Nrp1+Foxp3− T effector cells on day 10 of the alloresponse. Spleen and peripheral lymph node cells from B6 mice were transferred into F1 host mice (day 0). Host mice were treated with TCDD (15 μg/kg on day 0), 11-Cl-BBQ (7.5 mg/kg on days 0–3, BBQ), ITE (40 mg/kg every 6 h on days 0–3), FICZ (10 mg/kg on days 0–3), or FICZ (50 μg/kg on days 0–3, FICZlow). On day 10, splenocytes were analyzed by flow cytometry. (A) Gating strategy and representative FACS plots of Nrp1 and Foxp3 staining gated on donor CD4+ cells. The percentage (B) and total number (C) of Nrp1+Foxp3– cells gated on donor CD4+ cells. (D) Correlation between the percentage of Nrp1+Foxp3– cells and CTL. Each point represents an individual mouse. Treatment groups with different letters are significantly different. p ≤ .05.
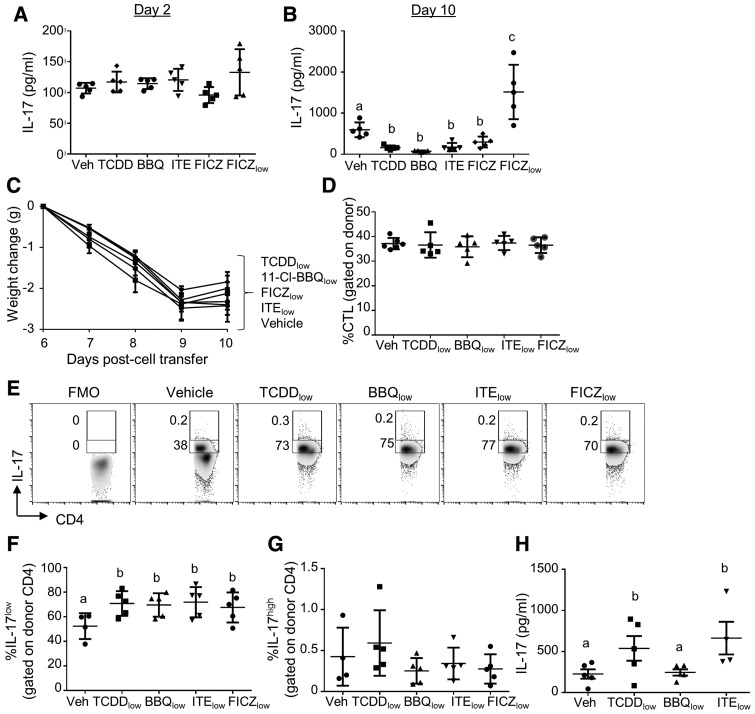
Low Doses of AhR Ligands Increase IL-17 Production
Treatment of mice with 50 μg/kg of FICZ has been shown to induce a Th17 response and to exacerbate central nervous system autoimmunity in a mouse model of experimental autoimmune encephalomyelitis (EAE) (Quintana et al., 2008). To determine if this dose of FICZ would also enhance IL-17 production in the alloresponse model, we measured ex vivo production of IL-17 by ELISA on day 2 and day 10 of the alloresponse. Splenocytes from mice treated with FICZlow showed a trend toward increased IL-17 production on day 2 (Figure 7A) and significantly increased IL-17 production on day 10 of the alloresponse (Figure 7B). As expected, optimized doses of TCDD, 11-Cl-BBQ, ITE, and FICZ did not increase IL-17 production, but instead significantly reduced IL-17 production when compared with vehicle-treated mice.
Figure 7.
Treatment with low, but not high, doses of AhR ligands increases IL-17 production on day 10 of the alloresponse. (A and B) Spleen and peripheral lymph node cells from B6 mice were transferred into F1 host mice (day 0). Host mice were treated with TCDD (15 μg/kg on day 0), 11-Cl-BBQ (7.5 mg/kg on days 0 and 1, BBQ), ITE (40 mg/kg every 6 h on days 0 and 1), FICZ (10 mg/kg on days 0 and 1), or FICZ (50 μg/kg on days 0 and 1, FICZlow). On day 2 (A) and day 10 (B), splenocytes were cultured ex vivo in the presence of PMA/ionomycin, and IL-17 was measured in the culture supernatant. (C–G) Host mice were treated with low doses of TCDD (0.6 μg/kg on day 0), 11-Cl-BBQ (50 μg/kg on days 0–3, BBQ), ITE (50 μg/kg on days 0–3), or FICZ (50 μg/kg on days 0–3). (C) Mice were weighed from days 6 to 10 to calculate the weight change because of the alloresponse. (D) On day 10, splenocytes were analyzed by flow cytometry for the percentage of donor cells that expressed the CTL phenotype (CD8+CD44highCD45RBlow). (E–G) On day 10, splenoyctes were cultured with PMA/ionomycin/brefeldin A/monensin then stained for IL-17. (E) Representative FACS plots gated on donor CD4+ cells. Percentage of donor cells that were IL-17low (F) and IL-17high (G). (H) IL-17 was measured in culture supernatants of splenocytes stimulated with PMA/ionomycin. Each point represents an individual mouse. In panel B, groups receiving optimized doses of AhR ligands were analyzed separately from the FICZlow group due to lack of homoegeneity of variance. Treatment groups with different letters are significantly different. p ≤ .05.
To determine if low doses of the other rapidly metabolized AhR ligands could induce IL-17 production, host mice were treated with 50 μg/kg of 11-Cl-BBQ or ITE on days 0–3 of the alloresponse. Likewise, TCDD was tested for its ability to induce IL-17 production using a single dose of 0.6 μg/kg, which was previously shown to increase IL-17 production during peanut sensitization (Schulz et al., 2011). Treatment of mice with low doses of any of the ligands did not affect the generation of the alloresponse (Figs. 7C and 7D). However, on day 10, all of the ligands induced an increase in the percentage of CD4+ T cells that were IL-17+ (Figs. 7E and 7F). The IL-17+ cells appeared to represent two subpopulations based on the fluorescence intensity of IL-17, a large population of low-expressing cells and a small population of high-expressing cells. AhR ligands increased the percentage of CD4+ cells that expressed low but not high levels of IL-17 (Figs. 7F and 7G). In a separate experiment, the low dose of TCDD and ITE but not 11-Cl-BBQ, increased the production of IL-17 by splenocytes cultured ex vivo (Figure 7H). The lack of effect of Cl-BBQ on IL-17 production may be due to the fact that the 50 μg/kg dose used was not optimized for enhancing IL-17 production. A full dose characterization would be necessary to determine an optimal dose for promotion of IL-17 production. Nonetheless, our data clearly show that low-level AhR activation by diverse AhR ligands can promote IL-17 production by CD4+ T cells.
DISCUSSION
Over the past 10 years, a paradigm in AhR immunobiology has become established that high-affinity AhR ligands promote the induction of either Tregs or Th17 cells. This paradigm is based primarily on studies of EAE in which treatment of mice with TCDD induced Tregs and suppressed disease whereas treatment with FICZ induced Th17 cells and exacerbated disease (Quintana et al., 2008; Veldhoen et al., 2008). The Treg-inducing ability of additional AhR ligands, such as ITE, Cl-BBQ, I3C, and VAG539, has since been documented in several different disease models (Hauben et al., 2008; Nugent et al., 2013; Punj et al., 2014; Quintana et al., 2010; Singh et al., 2016); however, FICZ remains the only AhR ligand that has been shown to induce Th17 cell differentiation in vivo. Proposed explanations for AhR ligand-specific effects on CD4+ T cell differentiation include differences in (1) receptor conformations following the binding of structurally different AhR ligands, (2) genes regulated by XRE- and non-XRE-dependent pathways, (3) interactions with microRNAs, (4) cytokine microenvironments, (5) the route of administration of the ligands, and (6) persistence of AhR activation (Denison and Faber, 2017; Julliard et al., 2014; Mohinta et al., 2015; Singh et al., 2016; Stockinger et al., 2014). In this study, we challenge the existing paradigm by showing that several AhR ligands can promote IL-17 production and that FICZ can also induce regulatory T cells. Importantly, we demonstrate that the outcome of CD4+ T cell differentiation is determined by the ligand dose and dose-rate used to activate the AhR, and not by the specific ligand. These data suggest that the duration and strength of AhR activation determines when or if Th17 cells or regulatory T cells (AhR-Tr1 cells and Foxp3+ Tregs) are induced.
Several reports have provided clues that the extent of AhR activation could play a role in the outcome of CD4+ T cell differentiation. In 2012, Schulz et al. compared the effects of treatment with TCDD (15 μg/kg) and FICZ (50 μg/kg, 2 times/week) on peanut sensitization and found that TCDD, but not FICZ, induced Tregs and suppressed peanut antigen-specific immune responses. Subsequent studies showed that 500 μg/kg FICZ increased Cyp1a1 by approximately 8-fold when measured 24 h after the final of three doses over an 8-day period in comparison to a 5000-fold increase by a single dose of TCDD. The authors speculated that the inability of FICZ to suppress peanut sensitization or induce Tregs was due to its rapid metabolism and inability to maintain activation of AhR to the same extent as TCDD.
Wheeler et al. (2014) were the first to try to account for differences in AhR ligand metabolism while directly comparing the effects of TCDD and FICZ on the CD8+ T cell response to influenza. After noting that AhR was only transiently activated following a single dose of 10 μg/kg FICZ in vivo, they utilized two approaches to enhance FICZ’s AhR-activating potential. The first approach employed micro-osmotic pumps for continuous FICZ exposure at a rate of 10 μg/kg/h (240 μg/kg/day). Although this approach led to a more sustained activation of AhR, Cyp1a1 induction was still approximately 10-fold less than the level induced by a 10 μg/kg dose of TCDD. In a second set of experiments, Cyp1a1-knockout mice were used to reduce the metabolism of FICZ delivered by the micro-osmotic pump, presumably increasing the FICZ serum concentration. Using this combination approach, they found that FICZ treatment led to a reduction in IFNγ-producing CD8+ T cells and a trend toward fewer influenza-specific CD8+ T cells. Although the extent of the reduction of these CD8+ T cell responses did not reach that of a single dose of TCDD, by increasing the exposure rate and reducing metabolism, FICZ began to “behave” more like TCDD. The comparative effects of TCDD and FICZ on Th17 differentiation were not examined in these studies.
Building upon the Wheeler study, our experimental approach was designed to further optimize the dose rate of rapidly metabolized ligands to match the extent of AhR activation that is seen with 15 μg/kg TCDD. We used hepatic Cyp1a1 induction as a biomarker for systemic AhR activation to determine the TCDD-equivalent daily dose. For the most part, this approach was successful, and a similar in vivo efficacy to induce regulatory T cells and to suppress the allo-CTL response was observed with all of the AhR ligands tested, including FICZ. However, despite similar Cyp1a1 induction at 20 h, there were quantitative differences in the magnitude of CD4+ T cell responses and in degree of suppression of body weight loss. Most notably, ITE treatment consistently resulted in a higher percentage of CD4+ T cells expressing AhR-Tr1 markers on day 2 and Foxp3+ Treg markers on day 10 in comparison with the other ligands. This difference could be due to the fact that ITE was administered every 6 h which may have led to higher spikes of Cyp1a1 expression. Conversely, the CD4+ T cell response to 10 mg/kg FICZ tended to be lower than the other ligands, even though hepatic Cyp1a1 levels were comparable at 20 h. Although it is possible that hepatic Cyp1a1 expression levels did not accurately reflect the level of AhR activation in CD4+ T cells themselves, we have shown that Cyp1a1 induction in alloresponding CD4+ T cells from mice treated i.p. with TCDD or Cl-BBQ is equivalent when using hepatic Cyp1a1-normalized dosing (Ehrlich et al., 2017).
Measuring AhR activation directly in target cells may be more critical when trying to compare the effects of AhR ligands delivered by different treatment routes. This is evident from past studies with FICZ where both the dose and route of administration differed and Cyp1a1 was not measured. For example, in the EAE mouse model, s.c. administration of 600 ng FICZ (Veldhoen et al., 2008) increased the number of Th17 cells and exacerbated disease, as did a single i.p. dose of 1 μg FICZ (Quintana et al., 2008). In contrast, i.p. administration of a single dose of 10 mg/kg FICZ ameliorated EAE, although not as robustly as TCDD (Duarte et al., 2013). Based on our studies, it is likely that daily, rather than a single dose of 10 mg/kg FICZ would have led to TCDD-equivalent Cyp1a1 induction and equal suppression of EAE. The authors proposed that the contradictory disease outcomes between mice treated with FICZ at 10 mg/kg by i.p. injection and mice treated with 600 ng by s.c. injection resulted from the different routes of administration (Duarte et al., 2013). Certainly, different routes of administration in combination with different doses will lead to different pharmacokinetics, and thus the amount of ligand that reaches the target cells. Knowing the degree of AhR activation systemically and within the target cells would be required to make meaningful conclusions about the influence of route of exposure on the immune response.
Although the dose–response paradigm explains much of the conflicting data on AhR ligand-mediated immunomodulation, there are several studies which show that low doses of FICZ are anti-inflammatory rather than proinflammatory. In DSS-, TNBS-, and T cell transfer-induced models of colitis, 1 μg FICZ suppressed disease, downregulated inflammatory cytokines and upregulated IL-22 in colonic lamina propria mononuclear cells (Monteleone et al., 2011). Likewise in a DSS-induced colitis model, daily i.p. injection of 1 μg FICZ ameliorated disease, prevented the disease-related reduction in CD8αα+TCRαβ+ intraepithelial lymphocytes (IELs), increased IL-10-producing IELs, and reduced IFNγ-producing IELs (Chen et al., 2017). Furthermore, i.p. treatment of B6 mice with 100 μg/kg FICZ also ameliorated imiquimod-induced psoriasis (Di Meglio et al., 2014). This therapeutic effect was attributed to AhR activation in keratinocytes, which exhibited an approximately 2-fold induction in Cyp1a1. The impact of FICZ on CD4+ T cell differentiation was not reported, precluding direct comparison to our studies despite the same route of delivery and mouse strain. However, these findings appear to highlight differences in the sensitivity of distinct cell types (eg, colonic lamina propria cells, IELs, keratinocytes, CD4+ T cells) to immune modulation by AhR ligands.
One unresolved issue regarding the current study is the absence of exacerbation of the alloresponse by low-dose AhR ligand treatment despite the increase in IL-17 production. Although a Th1 response is known to promote the development of allo-CTL in the nonirradiated acute parent-into-F1 alloresponse model used in our studies, little is known about the role of IL-17 in this model. Specifically, it is not clear if the alloresponse generates a cytokine microenvironment conducive to the AhR-mediated enhancement of bona fide Th17 cells. It is also possible that the CD4+IL-17+ T cells generated in the alloresponse model are derived from non-Th17 Teff populations (eg, IL-17-producing Tregs, Th1 cells). If so, increased IL-17 production by low-dose AhR activation would not be evident in the endpoints that we measured on day 10 of the alloresponse (ie, body weight loss, %CTL). In the future, it would be interesting to test the dose response paradigm in a model that is known to be sensitive to both Treg and Th17-mediated perturbations, such as EAE (Komiyama et al., 2006).
In conclusion, with the exception of FICZ, there have been numerous reports demonstrating the immunosuppressive effects of high affinity, rapidly metabolized AhR ligands (Hauben et al., 2008; Nugent et al., 2013; Punj et al., 2014; Rouse et al., 2013). In these studies, the AhR ligands were administered in the mg/kg range, rather than the μg/kg range, to suppress immune-mediated diseases. It is therefore surprising that FICZ dosing in the μg/kg range continues to be studied in comparison to TCDD. By considering metabolic sensitivity and the extent of AhR activation in the experimental design, future studies could more effectively identify real differences in the immunomodulatory potential of AhR ligands, whether because they are selective AhR agonists, lead to alternative receptor conformations, have additional signaling partners, regulate unique gene targets, or have indirect activity through their metabolites. In our studies, ligand-intrinsic effects were not apparent when comparing TCDD, ITE, 11-Cl-BBQ, and FICZ in both in silico and in vivo studies. All of the ligands activated the canonical pathway when given daily at an optimized dose. Additional studies to define the shape of the dose–response curve for inducing IL-17-producing cells and identification of the genes that are induced in CD4+ T cells following weak versus strong activation of AhR may shed light on how CD4+ T cell responses are regulated by AhR activation. A better understanding of the mechanistic basis for the dose-dependent changes in CD4+ T cell differentiation induced by AhR activation will be important for optimizing the beneficial effects of AhR-targeted drugs.
FUNDING
This work was supported by the National Institute of Environmental Health Sciences [grant numbers 5R01ES016651, 5T32ES007060-35] and by the American Cancer Society [RSG-132-01-CDD to SK].
ACKNOWLEDGMENTS
The authors would like to thank Matthew Newman and Matthew Kaiser for assay support with ELISAs and qPCR.
REFERENCES
- Apetoh L., Quintana F. J., Pot C., Joller N., Xiao S., Kumar D., Burns E. J., Sherr D. H., Weiner H. L., Kuchroo V. K. (2010). The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankoti J., Rase B., Simones T., Shepherd D. M. (2010). Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol. Appl. Pharmacol. 246, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag T. V., Luis Morales J., Hollingshead B. D., Perdew G. H. (2008). The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 18, 207–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. M., Shepherd D. M. (2011a). Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn's disease. Toxicol. Sci.: Off. J. Soc. Toxicol. 120, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. M., Shepherd D. M. (2011b). Dietary ligands of the aryl hydrocarbon receptor induce anti-inflammatory and immunoregulatory effects on murine dendritic cells. Toxicol. Sci.: Off. J. Soc. Toxicol. 124, 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Pu A., Sheng B., Zhang Z., Li L., Liu Z., Wang Q., Li X., Ma Y., Yu M., et al. (2017). Aryl hydrocarbon receptor activation modulates CD8alphaalpha+TCRalphabeta+ IELs and suppression of colitis manifestations in mice. Biomed. Pharmacother. 87, 127–134. [DOI] [PubMed] [Google Scholar]
- Denison M. S., Faber S. C. (2017). And now for something completely different: Diversity in ligand dependent activation of Ah receptor responses. Curr. Opin. Toxicol. 2, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M. S., Soshilov A. A., He G., DeGroot D. E., Zhao B. (2011). Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci.: Off. J. Soc. Toxicol. 124, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio P., Duarte J. H., Ahlfors H., Owens N. D., Li Y., Villanova F., Tosi I., Hirota K., Nestle F. O., Mrowietz U., et al. (2014). Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity 40, 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J. H., Di Meglio P., Hirota K., Ahlfors H., Stockinger B., Basso A. S. (2013). Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS One 8, e79819.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich A. K., Kerkvliet N. I. (2017). Is chronic AhR activation by rapidly metabolized ligands safe for the treatment of immune-mediated disesaes? Curr. Opin. Toxicol. 2, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich A. K., Pennington J. M., Tilton S., Wang X., Marshall N. B., Rohlman D., Funatake C., Punj S., O’Donnell E., Yu Z., et al. (2017). AhR activation increases IL-2 production by alloresponding CD4+ T cells initiating the differentiation of mucosal-homing Tim3+Lag3+ Tr1 cells. Eur. J. Immunol., doi: 10.1002/eji.201747121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich A. K., Pennington J. M., Wang X., Rohlman D., Punj S., Lohr C. V., Newman M. T., Kolluri S. K., Kerkvliet N. I. (2016). Activation of the aryl hydrocarbon receptor by 10-Cl-BBQ prevents insulitis and effector T cell development independently of Foxp3+ regulatory T cells in nonobese diabetic mice. J. Immunol. 196, 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatake C. J., Marshall N. B., Steppan L. B., Mourich D. V., Kerkvliet N. I. (2005). Cutting edge: Activation of the aryl hydrocarbon receptor by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol. 175, 4184–4188. [DOI] [PubMed] [Google Scholar]
- Gasiewicz T. A., Geiger L. E., Rucci G., Neal R. A. (1983). Distribution, excretion, and metabolism of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab. Dispos.: Biol. Fate Chem. 11, 397–403. [PubMed] [Google Scholar]
- Hauben E., Gregori S., Draghici E., Migliavacca B., Olivieri S., Woisetschlager M., Roncarolo M. G. (2008). Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood 112, 1214–1222. [DOI] [PubMed] [Google Scholar]
- Hubbard T. D., Murray I. A., Bisson W. H., Lahoti T. S., Gowda K., Amin S. G., Patterson A. D., Perdew G. H. (2015). Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 5, 12689.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julliard W., Fechner J. H., Mezrich J. D. (2014). The aryl hydrocarbon receptor meets immunology: Friend or foe? A little of both. Front. Immunol. 5, 458.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet N. I., Shepherd D. M., Baecher-Steppan L. (2002). T lymphocytes are direct, aryl hydrocarbon receptor (AhR)-dependent targets of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD): AhR expression in both CD4+ and CD8+ T cells is necessary for full suppression of a cytotoxic T lymphocyte response by TCDD. Toxicol. Appl. Pharmacol. 185, 146–152. [DOI] [PubMed] [Google Scholar]
- Komiyama Y., Nakae S., Matsuki T., Nambu A., Ishigame H., Kakuta S., Sudo K., Iwakura Y. (2006). IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177, 566–573. [DOI] [PubMed] [Google Scholar]
- Marshall N. B., Vorachek W. R., Steppan L. B., Mourich D. V., Kerkvliet N. I. (2008). Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. J. Immunol. 181, 2382–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohinta S., Kannan A. K., Gowda K., Amin S. G., Perdew G. H., August A. (2015). Differential regulation of Th17 and T regulatory cell differentiation by aryl hydrocarbon receptor dependent xenobiotic response element dependent and independent pathways. Toxicol. Sci.: Off. J. Soc. Toxicol. 145, 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone I., Rizzo A., Sarra M., Sica G., Sileri P., Biancone L., MacDonald T. T., Pallone F., Monteleone G. (2011). Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflmamation in the gastrointestinal tract. Gastroenterology 141, 237–248. [DOI] [PubMed] [Google Scholar]
- Mukai M., Tischkau S. A. (2007). Effects of tryptophan photoproducts in the circadian timing system: Searching for a physiological role for aryl hydrocarbon receptor. Toxicol. Sci.: Off. J. Soc. Toxicol. 95, 172–181. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. (2017). Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog. Lipid Res., doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent L. F., Shi G., Vistica B. P., Ogbeifun O., Hinshaw S. J., Gery I. (2013). ITE, a novel endogenous nontoxic aryl hydrocarbon receptor ligand, efficiently suppresses EAU and T-cell-mediated immunity. Invest. Ophthalmol. Vis. Sci. 54, 7463–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A., Phillips J. L., Kerkvliet N. I., Tanguay R. L., Perdew G. H., Kolluri S. K., Bisson W. H. (2014). A structural switch between agonist and antagonist bound conformations for a ligand-optimized model of the human aryl hydrocarbon receptor ligand binding domain. Biology 3, 645–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puliaev R. A., Puliaeva I. A., Ryan A. E., Via C. S. (2005). The parent-into-F1 model of graft-vs-host disease as a model of in vivo T cell function and immunomodulation. Curr. Med. Chem. Immunol. Endocr. Metab. Agents 5, 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punj S., Kopparapu P., Jang H. S., Phillips J. L., Pennington J., Rohlman D., O’Donnell E., Iversen P. L., Kolluri S. K., Kerkvliet N. I., Forsthuber T. (2014). Benzimidazoisoquinolines: A new class of rapidly metabolized aryl hydrocarbon receptor (AhR) ligands that induce AhR-dependent Tregs and prevent murine graft-versus-host disease. PLoS One 9, e88726.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana F. J., Basso A. S., Iglesias A. H., Korn T., Farez M. F., Bettelli E., Caccamo M., Oukka M., Weiner H. L. (2008). Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71. [DOI] [PubMed] [Google Scholar]
- Quintana F. J., Murugaiyan G., Farez M. F., Mitsdoerffer M., Tukpah A. M., Burns E. J., Weiner H. L. (2010). An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA. 107, 20768–20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman D., Punj S., Pennington J., Bradford S., Kerkvliet N. I. (2013). Suppression of acute graft-versus-host response by TCDD is independent of the CTLA-4-IFN-gamma-IDO pathway. Toxicol. Sci.: Off. J. Soc. Toxicol. 135, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse M., Singh N. P., Nagarkatti P. S., Nagarkatti M. (2013). Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Brit. J. Pharmacol. 169, 1305–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz V. J., Smit J. J., Huijgen V., Bol-Schoenmakers M., van Roest M., Kruijssen L. J. W., Fiechter D., Hassing I., Bleumink R., Safe S., et al. (2012). Non-dioxin-like AhR ligands in a mouse peanut allergy model. Toxicol. Sci.: Off. J. Soc. Toxicol. 128, 92–102. [DOI] [PubMed] [Google Scholar]
- Schulz V. J., Smit J. J., Willemsen K. J., Fiechter D., Hassing I., Bleumink R., Boon L., van den Berg M., van Duursen M. B., Pieters R. H. (2011). Activation of the aryl hydrocarbon receptor suppresses sensitization in a mouse peanut allergy model. Toxicol. Sci.: Off. J. Soc. Toxicol. 123, 491–500. [DOI] [PubMed] [Google Scholar]
- Singh N. P., Singh U. P., Rouse M., Zhang J., Chatterjee S., Nagarkatti P. S., Nagarkatti M. (2016). Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from proinflammatory Th17 cells to anti-inflammatory regulatory T cells through regulation of microRNA. J. Immunol. 196, 1108–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. P., Singh U. P., Singh B., Price R. L., Nagarkatti M., Nagarkatti P. S., Stoddart C. A. (2011). Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One 6, e23522.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B., Di Meglio P., Gialitakis M., Duarte J. H. (2014). The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 32, 403–432. [DOI] [PubMed] [Google Scholar]
- Tordjman R., Lepelletier Y., Lemarchandel V., Cambot M., Gaulard P., Hermine O., Romeo P. H. (2002). A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 3, 477–482. [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Westendorf A. M., Buer J., Dumoutier L., Renauld J. C., Stockinger B. (2008). The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109. [DOI] [PubMed] [Google Scholar]
- Vogel C. F., Goth S. R., Dong B., Pessah I. N., Matsumura F. (2008). Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2, 3-dioxygenase. Biochem. Biophys. Res. Commun. 375, 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorderstrasse B. A., Kerkvliet N. I. (2001). 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin affects the number and function of murine splenic dendritic cells and their expression of accessory molecules. Toxicol. Appl. Pharmacol. 171, 117–125. [DOI] [PubMed] [Google Scholar]
- Weiss J. M., Bilate A. M., Gobert M., Ding Y., Curotto de Lafaille M. A., Parkhurst C. N., Xiong H., Dolpady J., Frey A. B., Ruocco M. G., et al. (2012). Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 209, 1723–1742. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J. L., Martin K. C., Resseguie E., Lawrence B. P. (2014). Differential consequences of two distinct AhR ligands on innate and adaptive immune responses to influenza A virus. Toxicol. Sci.: Off. J. Soc. Toxicol. 137, 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. J., Pereira De Castro K., Joshi A. D., Elferink C. J. (2017). Canonical and non-canonical aryl hycrocarbon receptor signaling pathways. Curr. Opin. Toxicol. 2, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ma J., Takeuchi M., Usui Y., Hattori T., Okunuki Y., Yamakawa N., Kezuka T., Kuroda M., Goto H. (2010). Suppression of experimental autoimmune uveoretinitis by inducing differentiation of regulatory T cells via activation of aryl hydrocarbon receptor. Investig. Ophthalmol. Vis. Sci. 51, 2109–2117. [DOI] [PubMed] [Google Scholar]