Abstract
Background. Posttransplant hyperglycemia is an important predictor of new-onset diabetes after transplantation, and both are associated with significant morbidity and mortality. Precise estimates of posttransplant hyperglycemia and diabetes in children are unknown. Low magnesium and potassium levels may also lead to diabetes after transplantation, with limited evidence in children.
Methods. We conducted a cohort study of 451 pediatric solid organ transplant recipients to determine the incidence of hyperglycemia and diabetes, and the association of cations with both endpoints. Hyperglycemia was defined as random blood glucose levels ≥11.1 mmol/L on two occasions after 14 days of transplant not requiring further treatment. Diabetes was defined using the American Diabetes Association Criteria. For magnesium and potassium, time-fixed, time-varying and rolling average Cox proportional hazards models were fitted to evaluate the association with hyperglycemia and diabetes.
Results. Among 451 children, 67 (14.8%) developed hyperglycemia and 27 (6%) progressed to diabetes at a median of 52 days (interquartile range 22–422) from transplant. Multi-organ recipients had a 9-fold [hazard ratio (HR) 8.9; 95% confidence interval (CI) 3.2–25.2] and lung recipients had a 4.5-fold (HR 4.5; 95% CI 1.8–11.1) higher risk for hyperglycemia and diabetes, respectively, compared with kidney transplant recipients. Both magnesium and potassium had modest or no association with the development of hyperglycemia and diabetes.
Conclusions. Hyperglycemia and diabetes occur in 15 and 6% children, respectively, and develop early posttransplant with lung or multi-organ transplant recipients at the highest risk. Hypomagnesemia and hypokalemia do not confer significantly greater risk for hyperglycemia or diabetes in children.
Keywords: diabetes, hyperglycemia, hypokalemia, hypomagnesemia, solid organ transplant
INTRODUCTION
New-onset diabetes mellitus is one of the main metabolic complications after solid organ transplantation and is an independent risk factor for long-term cardiovascular morbidity and mortality [1]. The development of diabetes posttransplant results from an interplay between the non-modifiable (age, ethnicity and genetic susceptibility) and modifiable (immunosuppressive medications, obesity and electrolyte disturbances) risk factors. Early posttransplant hyperglycemia, i.e. within 1 week after transplant, is also a strong predictor of new-onset diabetes after kidney transplantation with 2.4 times higher risk compared with those without hyperglycemia among adults [2]. In the general population, the 3-year risk of developing diabetes in 86 512 adults with stress hyperglycemia (random glucose >11.1 mmol/L) during a hospital admission is 9.9% (9.2–10.6%) [3], thereby emphasizing the association of hyperglycemia and diabetes. Despite a strong evidence in adults, hyperglycemia is not often highlighted as a risk factor for diabetes in children.
There is a considerable variation in the reported incidence of diabetes in children after transplantation, and precise estimates are lacking due to small sample sizes, limited follow-up and inconsistent definitions of diabetes [4, 5]. It is important to understand the burden of diabetes in children posttransplant as well as potential modifiable risk factors to plan effective preventive strategies.
Potentially modifiable risk factors such as low levels of magnesium or potassium are also associated with diabetes in the general [6–8] and adult transplant [9–13] populations. Electrolyte disorders are quite common in the immediate posttransplant period due to immunosuppression medications such as calcineurin inhibitors (CNI), diuretics and possible renal tubular dysfunction [9]. Although low magnesium is a risk factor for diabetes in adult transplant recipients, the evidence of hypomagnesemia and the risk of hyperglycemia and diabetes in pediatric solid organ transplantation is scarce, whereas the role of hypokalemia has never been evaluated. Given that children are more vulnerable to having electrolyte disturbances than adults, it is important to determine the association of these cations with diabetes.
This study aims to determine the incidence of hyperglycemia and diabetes among children with solid organ transplantation including heart, lung, kidney, liver and multiple organ recipients, and the associated risk with low magnesium and potassium levels.
MATERIALS AND METHODS
Study design and population
We conducted a single-center, nonconcurrent, cohort study of children from birth to 18 years of age who underwent their first solid organ transplant (liver, heart, lung, kidney and multiple organs) at the Hospital for Sick Children in Toronto, Canada between 1 January 2002 and 31 December 2011. Children with pretransplant diabetes were excluded. Children were followed until the development of hyperglycemia and/or diabetes, transfer to adult care at age 18 years, or 30 June 2012, whichever occurred first. Clinical and laboratory data were retrieved from electronic patient records. The study was approved by the Hospital for Sick Children’s Research Ethics Board.
Outcomes assessment
The primary outcome was the development of hyperglycemia or diabetes. Hyperglycemia was defined as random inpatient or outpatient blood glucose levels ≥11.1 mmol/L (200 mg/dL) occurring on at least two consecutive days after the first 14 days of transplantation and the second day was considered as the date of the event. We selected glucose levels after 14 days posttransplant since many children receive glucose-containing intravenous fluids that may elevate levels. Average glucose levels were calculated each day when multiple values were available. Diabetes was defined as per the American Diabetes Association criteria as typical symptoms of diabetes with a random blood glucose level ≥11.1 mmol/L (200 mg/dL), or need for antidiabetic medications persisting beyond the first month after transplantation [14]. In order to reduce misclassification bias, medical records of children with hyperglycemia were reviewed to confirm the development of diabetes and treatment with insulin. No child received oral medications for the treatment of diabetes.
Exposure assessment
Hypomagnesemia and hypokalemia were the main exposure variables of interest and were analyzed as both categorical [serum magnesium level <0.7 mmol/L (1.7 mg/dL) and serum potassium <3.5 mmol/L (3.5 mEq/L), respectively] and continuous (per 0.1 mmol/L decrease) variables. Since magnesium and potassium levels fluctuate in the posttransplant period, we built several models each examining a different exposure metric based on our prior work [13]: time-fixed: mean levels of magnesium or potassium calculated from Day 1 to Day 14 posttransplant; time-varying: all levels of magnesium or potassium from the day of transplant until the event of interest; and the rolling average: mean levels of magnesium or potassium calculated over a 3-month window to determine the risk of diabetes in the next 3 months and this process was continued until the event of interest or end of follow-up [15].
Covariates
Baseline characteristics included recipient sex, age at transplant and body mass index (BMI; closest BMI available from 60 days prior to until the day of transplant). Additional transplant-related confounders included the medications from the first day of transplant up to 15 days posttransplant [use of induction therapy, prednisone, CNIs, magnesium and potassium supplements (intravenous or oral), proton pump inhibitors and trough levels of tacrolimus and cyclosporine]. The prednisone schedule and taper varied by the organ transplant (Supplementary data, Table S1).
Statistical analysis
Continuous variables were reported using means standard deviations for normally distributed data and median [interquartile range (IQR)] for skewed data. Categorical variables were reported using frequencies and percentages. The Mann–Whitney U-test was used to compare continuous data and the chi-square or Fisher’s exact (if the value in any cell was <5) tests were used for categorical data. Due to data privacy issues, cell sizes <5 were not exactly reported. Children were followed until the development of hyperglycemia or diabetes or censored at the time of transfer to adult care at age 18 years, or 30 June 2012, whichever occurred first. In each organ group, the incidence rates of hyperglycemia and diabetes (per 100 person-years of follow-up) were calculated.
The Kaplan–Meier product limit method was used to graphically assess time to hyperglycemia or diabetes in different organ groups. Univariable and multivariable Cox proportional hazards models were fitted to examine the risk of hyperglycemia or diabetes in each organ group and to determine the independent association of hypomagnesemia or hypokalemia with hyperglycemia or diabetes posttransplant. The covariates in the multivariable model were considered a priori (age at transplant, sex, proton pump inhibitors, and magnesium or potassium supplements). Since almost all children were on prednisone and CNIs, these were not included in the models. Parsimonious models took into account the limited number of outcomes. The proportionality assumption was graphically examined using log–log plots and scaled Schöenfeld residuals. No important departures from the assumptions were observed. As a very few children died after transplant, we did not conduct any competing risk analysis.
Sensitivity analyses were performed to test some of the assumptions in the primary analysis. In the first sensitivity analysis, we determined the incidence of hyperglycemia or diabetes after excluding patients with cystic fibrosis and atypical hemolytic uremic syndrome who are at a high risk of developing diabetes. Second, we defined hyperglycemia or diabetes after 1 month of transplantation based on a recent consensus statement that suggests defining hyperglycemia or diabetes when the recipient is on a stable immunosuppression [16]. Finally, the definition of hyperglycemia was taken as random blood glucose levels ≥11.1 mmol/L on three consecutive days to reduce the likelihood of misclassifying the outcome. All analyses were performed using Stata/SE 13.0 (StataCorp, College Station, TX, USA). A two-sided P < 0.05 was considered statistically significant.
RESULTS
Of the 451 children included (57.4% boys), the majority received a liver (32.6%) transplant, followed by the kidney (32.1%) and heart (28.4%) transplants. In all, 66 (44.8%) kidney and 9 (6.1%) liver transplants recipients received a living donor organ. Table 1 displays the baseline characteristics of the recipients, both overall and across all organ groups. The median age at transplantation for the study cohort was 8.3 (IQR 1.3–14.1) years, with multiple organ recipients being the youngest (median 1.5 years; IQR 1.2–3) and those with kidney transplants being the oldest (median 13.3 years; IQR 8.3–15.6). In recent years, initial immunosuppression with prednisone, mycophenolate and tacrolimus were commonly prescribed across all organ groups except in the liver and heart recipients. The liver recipients received a dual immunosuppression protocol comprising of tacrolimus and prednisone followed by discontinuation of prednisone at 3 months after transplant and a majority of heart recipients received tacrolimus, mycophenolate and prednisone only for the first 5 days of transplant. The median follow-up after transplantation across all organ groups was 3.2 (IQR 1.4–5.9) years. The most common indication for transplantation across all organ groups was congenital/genetic diseases (Supplementary data, Table S2). Liver/kidney, liver/gut/pancreas, liver/gut and liver/pancreas were the common combinations in multiple organ transplant recipients.
Table 1.
Baseline recipient and medication characteristics of the pediatric transplant cohort, overall and by individual organ transplant groups
| Characteristics | Kidney | Heart | Liver | Lung | Multiple | Overall |
|---|---|---|---|---|---|---|
| Organ, n (%) | 145 (32.1) | 128 (28.4) | 147 (32.6) | 22 (4.9) | 9 (2) | 451 |
| Median age at transplantation (years), (IQR) | 13.3 | 3.7 | 2.9 | 12.7 | 1.5 | 8.3 |
| (8.3–15.6) | (0.5–13.2) | (0.8–10.3) | (10.5–15.2) | (1.2–3.0) | (1.3–14.1) | |
| Boys, n (%) | 90 (62.1) | 68 (53.1) | 82 (55.8) | 12 (54.5) | 7 (77.8) | 259 (57.4) |
| Median BMI at transplantation, Z scores (IQR) | 0.1 | −0.9 | 0.02 | −1.4 | −0.7 | −0.1 |
| (−0.4 to 1.2) | (−2 to 0.3) | (−0.9 to 0.8) | (−2.3 to − 0.1) | (−1.5 to 3.3) | (−1.2 to 0.9) | |
| Induction therapy, n (%) | 145 (100) | 128 (100) | 38 (25.8) | 9 (40.9) | 9 (100) | 329 (72.9) |
| Polyclonal antibodiesa | 47 (32.4) | 120 (93.7) | 23 (60.5) | <5 | 8 (88.9) | 199 (60.5) |
| IL-2 receptor antibodiesb | 98 (67.6) | 8 (6.2) | 15 (39.5) | 8 (88.9) | <5 | 130 (39.5) |
| Prednisone, n (%) | 145 (100) | 128 (100) | 145 (98.6) | 22 (100) | 9 (100) | 449 (99.5) |
| MMFc, n (%) | 143 (98.6) | 112 (87.5) | 20 (13.6) | 6 (27.3) | <5 | 282 (62.5) |
| CNI, n (%) | 144 (99.3) | 126 (98.4) | 147 (100) | 22 (100) | 9 (100) | 448 (99.3) |
| Tacrolimus | 143 (99.3) | 109 (86.5) | 145 (98.6) | <5 | 9 (100) | 410 (91.5) |
| Cyclosporine | <5 | 17 (13.5) | <5 | 18 (81.8) | 0 (0) | 38 (8.5) |
| Magnesium supplements, n (%) | 120 (82.7) | 66 (51.6) | 127 (86.4) | 21 (95.4) | 8 (88.9) | 342 (75.8) |
| Potassium supplements, n (%) | 60 (41.4) | 100 (78.1) | 86 (58.5) | 15 (68.2) | 7 (77.8) | 268 (59.4) |
| Proton pump inhibitors, n (%) | 27 (18.6) | 28 (21.9) | 98 (66.7) | 5 (22.7) | 6 (66.7) | 164 (36.4) |
| Median tacrolimus levels at Day 15 (ng/mL) (IQR) | 10.1 | 10.3 | 11.9 | 11.8 | 16.5 | 10.8 |
| (8.4–11.5) | (8.1–12.4) | (10.4–13.8) | (8.9–14.2) | (16.1–19) | (9.0–12.6) | |
| Median cyclosporine levels at Day 15 (ng/mL) (IQR) | 62 | 223 | 203 | 304.5 | 235.5 | |
| (151–268) | (96–203) | (229–323.5) | (151–309) |
Cell sizes <5 were collapsed due to privacy issues; medications data are presented for the first 15 days posttransplant.
Includes thymoglobulin; bincludes basiliximab and daclizumab; cMMF, mycophenolate mofetil.
Rejection episodes
There were 190 episodes of rejections among 451 children overall. Among the entire cohort, the median number of days to rejection after transplantation was 49 (22–189). Among those with hyperglycemia, 22 (32.8%) children had at least one episode of rejection prior to developing hyperglycemia. Lung recipients (eight) had the highest number of rejections followed by kidney (six), heart (five), liver (three) and multiple organ (zero) recipients prior to developing hyperglycemia.
Incidence of hyperglycemia and diabetes
During 1526 person-years at risk, 67 (14.8%) recipients met the study criteria for posttransplant hyperglycemia, and only 27 (6%) progressed to diabetes (Table 2). Hyperglycemia developed within a month after liver (median 26 days; IQR 18–422) and multiple organ transplantations (median 28 days; IQR 23–61), while occurring much later in heart transplant recipients (median 224.5 days; IQR 42–872). Among the 27 children with posttransplant diabetes, the majority 16 (59.2%) remained on insulin for at least 1 year or longer.
Table 2.
Incidence rates and HRs for hyperglycemia and diabetes after pediatric transplantation, overall and across individual organ transplant groups
| Organ type |
||||||
|---|---|---|---|---|---|---|
| Kidney | Heart | Liver | Lung | Multiple | Overall | |
| Hyperglycemia | ||||||
| Hyperglycemia, n (%) | 17 (11.7) | 16 (12.5) | 19 (12.9) | 10 (45.4) | 5 (55.6) | 67 (14.9) |
| Median days to hyperglycemia (IQR) | 48 (19–186) | 224.5 (42–872) | 26 (18–422) | 141 (35–482) | 28 (23–61) | 52 (22–422) |
| Incidence rate per 100 person-years (95% CI) | 4.4 (2.8–7.2) | 2.9 (1.8–4.8) | 3.5 (2.2–5.4) | 25.2 (13.6–46.9) | 32.3 (13.4–77.6) | 4.4 (3.4–5.6) |
| Unadjusted HR (95% CI) | Ref. | 0.9 (0.4–1.8) | 1.0 (0.5–1.9) | 4.3 (2.0–9.4) | 6.4 (2.4–17.5) | 1.5 (1.2–1.9) |
| Adjusted HRa (95% CI) | Ref. | 1.1 (0.5–2.3) | 1.3 (0.7–2.7) | 4.2 (1.9–9.2) | 8.9 (3.2–25.2) | 1.6 (1.3–2.0) |
| Diabetes | ||||||
| Diabetes, n (%) | 12 (8.3) | 2 (1.6) | 5 (3.4) | 8 (36.4) | 0 | 27 (6) |
| Incidence rate (per 100 person-years) | 3.1 (1.8–5.5) | 0.4 (0.1–1.5) | 0.9 (0.4–2.2) | 20.2 (10.1–40.4) | 1.8 (1.2–2.6) | |
| Unadjusted HR (95% CI) | Ref. | 0.2 (0.0–0.7) | 0.4 (0.1–1.1) | 4.6 (1.9–11.2) | 1.2 (0.8–1.8) | |
| Adjusted HRa (95% CI) | Ref. | 0.3 (0.1–1.4) | 1.0 (0.3–2.9) | 4.5 (1.8–11.1) | 1.5 (1.1–2.1) | |
Adjusted for age at transplant and sex. Ref., Reference Group
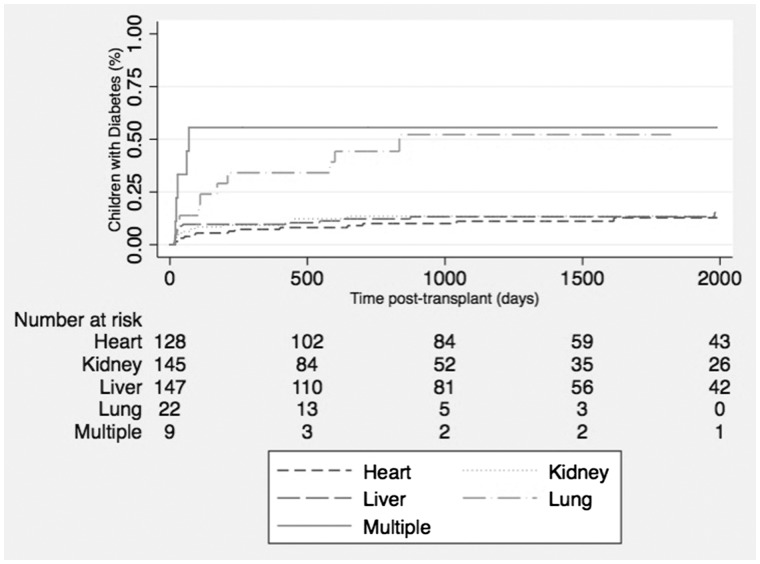
The incidence rate of hyperglycemia was lowest in the heart transplant recipients (2.9 per 100 person-years) and highest in the lung (25.2 per 100 person-years) and multiple organ (13.6 per 100 person-years) transplant recipients. Figure 1 shows the Kaplan–Meier failure curves for the risk of hyperglycemia across all organ groups. The unadjusted Cox proportional hazards model revealed that the highest risk of developing hyperglycemia occurred in the multiple organ transplant recipients [hazard ratio (HR) 6.4; 95% confidence interval (CI) 2.4–17.5] followed by lung transplant (HR 4.3; 95% CI 2.0–9.4) compared with the kidney transplant. After adjusting for age at transplantation and sex, multiple organ and lung transplant recipients had 9 and 4.2 times higher risks of developing hyperglycemia compared with kidney transplant recipients, respectively. The results were consistent with the outcome of diabetes in lung transplant recipients (Table 2).
FIGURE 1.
Cumulative incidence of hyperglycemia after transplantation in children across various organ groups.
After adjusting for age at transplantation, sex and presence of rejection prior to the event of interest, multiple organ recipients had 13.8 times (95% CI 4.7–40.9) higher risk of developing hyperglycemia compared with kidney transplant recipients. Interestingly, the effect in lung transplant recipients reduced to 0.9 (95% CI 0.4–2.1). For the outcome of diabetes, the relative hazard among lung transplant recipients was 1.3 (95% CI 0.5–3.9) after adjusting for confounders. (Supplementary data, Table S3).
After excluding patients with cystic fibrosis, the risk of hyperglycemia remained similar in most organ groups; however, in lung transplant recipients, the risk of hyperglycemia decreased to 1.3 (95% CI 0.3–5.5) after adjusting for other covariates (Supplementary data, Table S4). The incident rate (IR) of hyperglycemia did not differ in kidney transplant recipients after excluding those with atypical hemolytic uremic syndrome (IR 4.2 per 100 person-years compared with an overall incident rate 4.4 per 100 person-years) (Supplementary data, Table S5). After including individuals who developed hyperglycemia or diabetes on or after 30 days posttransplant, the incidence rate was lower, with now only 45 children having hyperglycemia; however, there was not a significant change in point estimates (Supplementary data, Table S6).
Association of cations with hyperglycemia and diabetes
The levels of magnesium and potassium were equally distributed among all organ recipients with no predilection to one organ excluding the effect of primary disease. The results of the time-fixed, time-varying and rolling average cation levels and association with outcomes are shown in Table 3. Every 0.1 mmol/L decrease in magnesium level was associated with a significantly decreased risk of hyperglycemia across all models and was statistically significant in the time-varying (HR 0.7; 95% CI 0.6–0.8) and rolling average (HR 0.6; 95% CI 0.5–0.7) models after adjusting for other clinically significant covariates. The results were similar when magnesium was analyzed as a categorical variable. On analyzing the outcome of diabetes, the association significantly attenuated and was no longer significant. With every 0.1 mmol/L decrease in potassium levels, the unadjusted time varying (HR 1.1; 95% CI 1.0–1.1) and rolling average (HR 1.1; 95% CI 1.1–1.2) models demonstrated an increased risk of hyperglycemia (Table 3). The results were consistent after adjusting for other covariates. On analyzing potassium as a categorical variable, an increased risk of hyperglycemia was noticed only in the time-varying model. The analysis with diabetes as the endpoint showed little or no effect of hypokalemia across all models. Similar results to the primary models were obtained in the sensitivity analysis when stricter definitions of hyperglycemia were used with random glucose levels ≥11.1 mmol/L after 14 days of transplant on three consecutive days (Supplementary data, Table S7).
Table 3.
Cox proportional hazards models showing association of low magnesium and potassium levels with hyperglycemia and diabetes
| Time-fixed |
Time-varying |
Rolling average |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariablea |
Univariable |
Multivariablea |
Univariable |
Multivariablea |
||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Magnesium Models | |||||||||||||
| Hyperglycemia (n = 67) | Hypomagnesemia (per 0.1 mmol/L decrease) | 0.9 | 0.6 | 0.9 | 0.4 | 0.7 | <0.001 | 0.7 | <0.001 | 0.6 | <0.001 | 0.6 | <0.001 |
| (0.7–1.2) | (0.7–1.1) | (0.6–0.8) | (0.6–0.8) | (0.6–0.7) | (0.5–0.7) | ||||||||
| Hypomagnesemia (<0.7 mmol/L) | 0.5 | 0.1 | 0.5 | 0.1 | 0.3 | <0.001 | 0.3 | <0.001 | 0.4 | <0.001 | 0.3 | <0.001 | |
| (0.2–1.1) | (0.2–1.1) | (0.2–0.5) | (0.2–0.5) | (0.2–0.6) | (0.2–0.6) | ||||||||
| Diabetes (n = 27) | Hypomagnesemia (per 0.1 mmol/L decrease) | 1.1 | 0.6 | 0.9 | 0.7 | 0.8 | 0.2 | 0.8 | 0.1 | 0.8 | 0.1 | 0.7 | 0.02 |
| (0.8–1.6) | (0.6–1.4) | (0.7–1.1) | (0.6–1.0) | (0.6–1.1) | (0.5–0.9) | ||||||||
| Hypomagnesemia (<0.7 mmol/L) | 0.8 | 0.7 | 0.7 | 0.4 | 0.5 | 0.1 | 0.4 | 0.03 | 1.1 | 0.8 | 0.9 | 0.9 | |
| (0.3–2.4) | (0.2–1.9) | (0.2–1.0) | (0.2–0.9) | (0.5–2.5) | (0.4–2.0) | ||||||||
| Potassium Models | |||||||||||||
| Hyperglycemia (n = 67) | Hypokalemia (per 0.1 mmol/L decrease) | 0.9 | 0.4 | 0.9 | 0.5 | 1.1 | 0.01 | 1.0 | 0.02 | 1.1 | <0.001 | 1.1 | <0.001 |
| (0.9–1.0) | (0.9–1.1) | (1.0–1.1) | (1.0–1.1) | (1.1–1.2) | (1.0–1.2) | ||||||||
| Hypokalemia (<3.5 mmol/L) | 0.6 | 0.6 | 0.6 | 0.6 | 2.3 | 0.04 | 2.3 | 0.04 | 2.3 | 0.2 | 1.9 | 0.4 | |
| (0.1–4.2) | (0.1–4.2) | (1.0–5.1) | (1.0–5.1) | (0.6–9.6) | (0.4–8.1) | ||||||||
| Diabetes (n = 27) | Hypokalemia (per 0.1 mmol/L decrease) | 0.9 | 0.6 | 1.0 | 0.7 | 1.0 | 0.9 | 0.9 | 0.6 | 1.0 | 0.4 | 1.0 | 0.9 |
| (0.8–1.1) | (0.9–1.1) | (0.9–1.1) | (0.9–1.0) | (0.9–1.1) | (0.9–1.1) | ||||||||
| Hypokalemia (<3.5 mmol/L) | 1.5 | 0.7 | 1.5 | 0.7 | – | – | – | – | – | – | – | – | |
| (0.2–11.5) | (0.2–11.5) | ||||||||||||
For magnesium: adjusted for age at transplant, use of proton pump inhibitors and magnesium supplements. For potassium: adjusted for age at transplant and potassium supplements.
DISCUSSION
Despite improvements in the survival of children after transplantation, there is still significant risk of developing chronic diseases, especially as children age. New-onset diabetes is a serious complication after transplantation and is associated with poor long-term graft and patient survival in adults [1]. Our study demonstrates that among transplant recipients under the age of 18 years, the absolute incidence of hyperglycemia and diabetes is 15 and 6%, respectively, with a median onset of 52 days after transplantation. Children with lung transplantation are at 4 times higher risk of developing hyperglycemia or diabetes compared with kidney transplant recipients, with similar risk in liver and heart transplant recipients. The increased risk is primarily among children with cystic fibrosis. We also demonstrate no significant association of hypomagnesemia and hypokalemia with development of hyperglycemia or diabetes in children.
The risk of posttransplant hyperglycemia is the strongest predictor for future development of diabetes as it may indicate pancreatic β-cell dysfunction [2]. Hence, it is imperative to screen for early posttransplant hyperglycemia but also to provide appropriate treatment to prevent the future risk of diabetes, as recently shown by a proof-of-concept clinical trial [17]. In the trial, adult kidney transplant recipients who received isophane insulin for blood glucose of ≥140 mg/dL (7.7 mmol/L) in the immediate postoperative period had 73% lower odds of developing diabetes throughout the follow-up as compared with those who received either short-acting insulin and/or oral hypoglycemic drugs for blood glucose ≥180–250 mg/dL (9.9–13.8 mmol/L). In our study, all the children that developed diabetes had evidence of hyperglycemia within the first 2 months from transplantation, which highlights the importance of screening during this critical period posttransplantation as it may identify individuals who are at risk of diabetes and warrant further work-up during the first-year posttransplant, especially in the lung recipients.
The proportion of children developing hyperglycemia or diabetes in our study is similar to previously reported rates; however, we are now able to provide a more precise estimate for patients and families. Previous reports widely differed with ranges from 3% to 20% in kidney [5, 18], 1.9–17.2% in liver [19–22], 4% in heart [23] and 38% in lung [24] pediatric transplant recipients. This large variation in the reported incidence is likely due to lack of standardized definitions of diabetes, and short follow-up or cross-sectional designs [5, 25]. The higher risk in lung transplant recipients of diabetes is consistent with earlier reports [26], and is likely due to the increased risk of diabetes from cystic fibrosis, the most common indication for lung transplantation. The lower risk in heart transplant recipients may reflect the lower burden of immunosuppression compared with other organ groups.
The role of hypomagnesemia as a potentially modifiable risk factor in the pathogenesis of diabetes has recently been investigated in adult liver and kidney transplant recipients but the results have been far from conclusive. A recent randomized controlled trial of magnesium supplementation in renal transplant recipients to prevent diabetes did not show a clear benefit [12]. A major limitation of prior observational studies on the relationship between hypomagnesemia and diabetes is that the time-varying nature of magnesium after transplantation was not considered. Except for one study [13], typically, levels were taken at a fixed time point (e.g. 1-month posttransplant) to predict the future development of diabetes. Recently, a study on 173 pediatric renal transplant recipients showed a significant association of 30-day moving average magnesium levels with diabetes (HR 4.6; 95% CI 1.4–14.6) [27]. However, the cohort was limited to kidney transplant recipients and the data on magnesium supplementation were not provided. Despite using robust analytical techniques, we found an inverse relationship with hyperglycemia and found no association with diabetes in children. Possible reasons explaining the modest protective association with hypomagnesemia could be related to the close monitoring and frequent electrolyte supplementation for magnesium (75% of children studied) and potassium (59% of children studied) early after transplantation in our pediatric cohort. This likely resulted in adequate magnesium and potassium body stores, which further prevented lowering of magnesium and potassium levels to be able to detect an association. Similarly, hypokalemia has been linked with diabetes in the general population both with [28] and without [29] diuretics use. In our cohort, there was an increased risk of hyperglycemia with decreasing levels of potassium but the results were not consistent due to the lack of power.
The strengths of our study include a relatively large pediatric transplant cohort with a considerable posttransplant follow-up and use of a standard definition of diabetes across all solid organ groups. Using a more conservative definition of hyperglycemia for three consecutive days compared with 2 days, did not change the results. Moreover, it improved precision compared with other studies using only a single elevated glucose level [30, 31]. We also took into consideration multiple repeated measurements of both magnesium and potassium, commonly not considered in other studies.
There are some limitations of our study that deserve note. Given the low incidence of diabetes in our cohort, we were underpowered to demonstrate a significant association of magnesium and potassium with hyperglycemia or diabetes. In addition, some children on prolonged parenteral nutrition could be at an increased risk of hyperglycemia that was not captured. Similarly, with a small number of events, we could not adjust for other potential risk factors, such as graft rejection, family history of diabetes, pretransplant use of diabetogenic medications, pretransplant magnesium levels and medications data beyond 2 weeks posttransplant, which could potentially be associated with diabetes after transplant. Although we did include pancreatic transplant recipients who are at a higher risk of diabetes, there were only two children in the entire cohort. Results from a single center also limit the generalizability of our findings. Despite these limitations, the findings demonstrate the risk of an important comorbid condition that impacts survival and quality of life of children after transplantation.
In summary, the overall incidence of hyperglycemia and diabetes in children after transplantation is relatively low; however, lung and multi-organ recipients are at greatest risk. Both hyperglycemia and diabetes develop quite early in children after transplantation, emphasizing the need for routine monitoring after transplant and considering closer screening for those with hyperglycemia. Future studies are needed to understand the risk as children age into adulthood and the effect on graft and patient survival.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Richard Child and Tony Pyle for their advice and insight into the electronic medical records at SickKids.
AUTHORS’ CONTRIBUTIONS
R.C. contributed to study design, analysis and interpretation of data, and drafted the manuscript and approved the final version. S.J.K. contributed to study design, analysis, interpretation of data and critical revision of the article, and approved the final version. E.K., T.B., K.B. and J.V.-R. contributed to collection of data, and critical revision of the article, and approved the final version. Y.L. contributed to analysis and interpretation of the data, and approved the final version. V.N., A.D., M.S. and D.H. contributed to collection of data and critical revision of the article, and approved the final version. R.S.P. contributed to study design, analysis and interpretation of data and drafting the article, and approved the final version.
FUNDING
We gratefully acknowledge funding from the Transplant & Regenerative Medicine Centre (TRMC) Catalyst Grant at The Hospital for Sick Children, and Canadian Institutes of Health Research (CIHR).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Kasiske BL, Snyder JJ, Gilbertson D. et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 2003; 3: 178–185 [DOI] [PubMed] [Google Scholar]
- 2. Cosio FG, Kudva Y, van der Velde M. et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int 2005; 67: 2415–2421 [DOI] [PubMed] [Google Scholar]
- 3. McAllister DA, Hughes KA, Lone N. et al. Stress hyperglycaemia in hospitalised patients and their 3-year risk of diabetes: a Scottish retrospective cohort study. PLoS Med 2014; 11: e1001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garro R, Warshaw B, Felner E.. New-onset diabetes after kidney transplant in children. Pediatr Nephrol 2015; 30: 405–416 [DOI] [PubMed] [Google Scholar]
- 5. Al-Uzri A, Stablein DM, A Cohn R. Posttransplant diabetes mellitus in pediatric renal transplant recipients: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Transplantation 2001; 72: 1020–1024 [DOI] [PubMed] [Google Scholar]
- 6. Pham PC, Pham PM, Pham PA. et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63: 429–436 [DOI] [PubMed] [Google Scholar]
- 7. McNair P, Christensen MS, Christiansen C. et al. Renal hypomagnesaemia in human diabetes mellitus: its relation to glucose homeostasis. Eur J Clin Invest 1982; 12: 81–85 [DOI] [PubMed] [Google Scholar]
- 8. Barzilay JI, Davis BR, Cutler JA. et al. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 2006; 166: 2191–2201 [DOI] [PubMed] [Google Scholar]
- 9. Osorio JM, Bravo J, Perez A. et al. Magnesemia in renal transplant recipients: relation with immunosuppression and posttransplant diabetes. Transplant Proc 2010; 42: 2910–2913 [DOI] [PubMed] [Google Scholar]
- 10. Van Laecke S, Desideri F, Geerts A. et al. Hypomagnesemia and the risk of new-onset diabetes after liver transplantation. Liver Transpl 2010; 16: 1278–1287 [DOI] [PubMed] [Google Scholar]
- 11. Van Laecke S, Van Biesen W, Verbeke F. et al. Posttransplantation hypomagnesemia and its relation with immunosuppression as predictors of new-onset diabetes after transplantation. Am J Transplant 2009; 9: 2140–2149 [DOI] [PubMed] [Google Scholar]
- 12. Van Laecke S, Nagler EV, Taes Y. et al. The effect of magnesium supplements on early post-transplantation glucose metabolism: a randomized controlled trial. Transpl Int 2014; 27: 895–902 [DOI] [PubMed] [Google Scholar]
- 13. Huang JW, Famure O, Li Y. et al. Hypomagnesemia and the risk of new-onset diabetes mellitus after kidney transplantation. J Am Soc Nephrol 2016; 27: 1793–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Standards of medical care in diabetes–2008. Diabetes Care 2008; 31 Suppl 1: S12–S54 [DOI] [PubMed] [Google Scholar]
- 15. Brunelli SM, Joffe MM, Israni RK. et al. History-adjusted marginal structural analysis of the association between hemoglobin variability and mortality among chronic hemodialysis patients. Clin J Am Soc Nephrol 2008; 3: 777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharif A, Hecking M, de Vries AP. et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant 2014; 14: 1992–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hecking M, Haidinger M, Doller D. et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol 2012; 23: 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prokai A, Fekete A, Kis E. et al. Post-transplant diabetes mellitus in children following renal transplantation. Pediatr Transplant 2008; 12: 643–649 [DOI] [PubMed] [Google Scholar]
- 19. Greig F, Rapaport R, Klein G. et al. Characteristics of diabetes after pediatric liver transplant. Pediatr Transplant 2013; 17: 27–33 [DOI] [PubMed] [Google Scholar]
- 20. Carroll PB, Rilo H, Reyes J. et al. FK 506-associated diabetes mellitus in the pediatric transplant population is a rare complication. Transplant Proc 1991; 23: 3171–3172 [PMC free article] [PubMed] [Google Scholar]
- 21. Romero R, Melde K, Pillen T. et al. Persistent hyperglycemia in pediatric liver transplant recipients. Transplant Proc 2001; 33: 3617–3618 [DOI] [PubMed] [Google Scholar]
- 22. Hathout E, Alonso E, Anand R. et al. Post-transplant diabetes mellitus in pediatric liver transplantation. Pediatr Transplant 2009; 13: 599–605 [DOI] [PubMed] [Google Scholar]
- 23. Wagner K, Webber SA, Kurland G. et al. New-onset diabetes mellitus in pediatric thoracic organ recipients receiving tacrolimus-based immunosuppression. J Heart Lung Transplant 1997; 16: 275–282 [PubMed] [Google Scholar]
- 24. Paolillo JA, Boyle GJ, Law YM. et al. Posttransplant diabetes mellitus in pediatric thoracic organ recipients receiving tacrolimus-based immunosuppression. Transplantation 2001; 71: 252–256 [DOI] [PubMed] [Google Scholar]
- 25. Greenspan LC, Gitelman SE, Leung MA. et al. Increased incidence in post-transplant diabetes mellitus in children: a case-control analysis. Pediatr Nephrol 2002; 17: 1–5 [DOI] [PubMed] [Google Scholar]
- 26. Alvarez A, Algar FJ, Santos F. et al. Pediatric lung transplantation. Transplant Proc 2005; 37: 1519–1522 [DOI] [PubMed] [Google Scholar]
- 27. Hayes W, Boyle S, Carroll A. et al. Hypomagnesemia and increased risk of new-onset diabetes mellitus after transplantation in pediatric renal transplant recipients. Pediatr Nephrol 2017; 32: 879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shafi T, Appel LJ, Miller ER 3rd. et al. Changes in serum potassium mediate thiazide-induced diabetes. Hypertension 2008; 52: 1022–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chatterjee R, Yeh HC, Shafi T. et al. Serum and dietary potassium and risk of incident type 2 diabetes mellitus: The Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med 2010; 170: 1745–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herskowitz-Dumont R, Wolfsdorf JI, Jackson RA. et al. Distinction between transient hyperglycemia and early insulin-dependent diabetes mellitus in childhood: a prospective study of incidence and prognostic factors. J Pediatr 1993; 123: 347–354 [DOI] [PubMed] [Google Scholar]
- 31. Chakkera HA, Knowler WC, Devarapalli Y. et al. Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin J Am Soc Nephrol 2010; 5: 1669–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.