Abstract
Aims
Blood biochemistry may provide information on associations between road traffic noise, air pollution, and cardiovascular disease risk. We evaluated this in two large European cohorts (HUNT3, Lifelines).
Methods and results
Road traffic noise exposure was modelled for 2009 using a simplified version of the Common Noise Assessment Methods in Europe (CNOSSOS-EU). Annual ambient air pollution (PM10, NO2) at residence was estimated for 2007 using a Land Use Regression model. The statistical platform DataSHIELD was used to pool data from 144 082 participants aged ≥20 years to enable individual-level analysis. Generalized linear models were fitted to assess cross-sectional associations between pollutants and high-sensitivity C-reactive protein (hsCRP), blood lipids and for (Lifelines only) fasting blood glucose, for samples taken during recruitment in 2006–2013. Pooling both cohorts, an inter-quartile range (IQR) higher day-time noise (5.1 dB(A)) was associated with 1.1% [95% confidence interval (95% CI: 0.02–2.2%)] higher hsCRP, 0.7% (95% CI: 0.3–1.1%) higher triglycerides, and 0.5% (95% CI: 0.3–0.7%) higher high-density lipoprotein (HDL); only the association with HDL was robust to adjustment for air pollution. An IQR higher PM10 (2.0 µg/m3) or NO2 (7.4 µg/m3) was associated with higher triglycerides (1.9%, 95% CI: 1.5–2.4% and 2.2%, 95% CI: 1.6–2.7%), independent of adjustment for noise. Additionally for NO2, a significant association with hsCRP (1.9%, 95% CI: 0.5–3.3%) was seen. In Lifelines, an IQR higher noise (4.2 dB(A)) and PM10 (2.4 µg/m3) was associated with 0.2% (95% CI: 0.1–0.3%) and 0.6% (95% CI: 0.4–0.7%) higher fasting glucose respectively, with both remaining robust to adjustment for air/noise pollution.
Conclusion
Long-term exposures to road traffic noise and ambient air pollution were associated with blood biochemistry, providing a possible link between road traffic noise/air pollution and cardio-metabolic disease risk.
Keywords: Traffic noise , Air pollution , Systemic inflammation , Blood lipids , Blood glucose
Introduction
Increasing numbers of studies have suggested that long-term exposure to road traffic noise1 and ambient air pollution2 have been linked to cardiovascular disease (CVD) morbidity and mortality via different but not mutually exclusive hypothesized mechanistic pathways.3
As proposed by Babisch,4 traffic noise as an environmental stressor exerts adverse health effects via both direct (e.g. sleep disturbance) and indirect (e.g. annoyance) pathways. Based on the stress reaction model, both pathways lead to a physiologic acute response, for example, elevated levels of stress hormones, through activations of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medulla axis.3 In the long-term, stress hormone such as cortisol is overly produced to restore homeostasis partly by increasing the supply of energy in the forms of glucose and fatty acids.5,6 Catecholamines are an important stress hormone to boost this supply by breaking down triacylglycerol.5 In addition, overproduction of cortisol is also believed to inhibit insulin secretion as well as to impair insulin sensitivity in both the liver and adipose tissue.6 For air pollution, oxidative stress is the principal hypothesized mechanism to explain the adverse health effects, with other pathways including impaired endothelial function, systemic inflammation, activation of autonomous nervous system (ANS), and direct transfer of particles from lung to blood circulation also contributing.2
It is biologically plausible to hypothesize that via these aforementioned mechanistic pathways both long-term noise and air pollution exposures may lead to adverse changes in CVD risk factors including systemic inflammation, blood lipids, and glucose,2–4 thus mediating the links between these exposures and manifest CVD. However, epidemiological evidence linking these exposures, especially noise, with CVD risk factors is rather limited.3,7 Noise could also be a potential confounder in any associations between air pollution and CVD risk factors, which merits further investigation.7
We evaluated cross-sectional associations between long-term road traffic noise, ambient air pollution, and high sensitivity C-reactive protein (hsCRP), blood lipids and glucose levels in two large European cohorts. Harmonized exposure and health data were analysed at the individual-level via DataSHIELD,8 a novel statistical platform that allows pooling of data via distributed data analysis.
Methods
Study populations
Two cohorts, HUNT39 and Lifelines,10 were included in this study as part of the Biobank Standardisation and Harmonisation for Research Excellence in the European Union-BioSHaRE-EU (BioSHaRE) project.
The Helseundersøkelsen i Nord-Trøndelag (HUNT) study is a population-based health survey conducted in the county of Nord-Trøndelag in central Norway, targeting all residents aged ≥ 20 years.9 We used data from the third survey (HUNT3) undertaken in 2006–2008, during which 50 805 residents participated and provided data, all of whom were included in our study.
Lifelines is a prospective multi-generational population-based cohort study to examine the health and health-related behaviours of people living in northern Netherlands.10 Compared with those of the HUNT study, study areas of Lifelines are more densely populated.11 During baseline recruitment (2006–2013), a random selection of residents aged 25–50 years and their family members registered in general practices were invited to participate. Quality-checked data from 93 277 participants were available for analysis by the time our study was conducted.
Ethics approvals for this study were obtained from the Regional Committee for Medical and Health Research Ethics in Norway for HUNT and from the Medical Ethics Committee of the University Medical Center Groningen for Lifelines.
Biochemistry data
Details of the collection, transport, storage, and analysis of the blood samples in each cohort have been published.9,10 In brief, for both cohorts, blood samples were collected and transported on the same day, and were stored at −80 °C for future analysis. Non-fasting blood samples were collected in HUNT3 whilst fasting blood samples were collected in Lifelines. Samples were analysed in a central laboratory for each cohort, following respective quality control measures.
Serum concentrations of hsCRP (mg/L), total cholesterol (mmol/L), triglycerides (mmol/L), and high-density lipoprotein (HDL) cholesterol (mmol/L) were measured in both cohorts. Additionally for Lifelines, fasting blood glucose (mmol/L) and glycated haemoglobin (HbA1c) concentrations (mmol/mol) were also measured.
Exposure assessment
A simplified version of the Common noise assessment methods in European Union (CNOSSOS-EU) noise modelling framework12 was developed and run for each cohort. Noise sound pressure level was estimated on all roads within 500 m of home address at recruitment. Noise propagation due to refraction and diffraction, absorption from buildings, distance and angle of view were considered in the model. Road network geography, calculated hourly vehicle flows using a daily average traffic profile, building heights, land cover, and meteorological data were obtained for the respective study areas. To account for participants living on minor roads that were not captured in the national level traffic datasets, a fixed low-level baseline traffic flow (600 vehicles per day) was assigned. Traffic data were for the year 2009 and land cover data were for the year 2006. Annual mean A-weighted sound pressure level in decibels (dB(A)) for day-time noise (average sound level from 07:00 to 19:00) and night-time noise (average sound level from 23:00 to 07:00) and weighted 24-h noise (Lden) were modelled at recruitment address of participants in both cohorts.
An European harmonized Land Use Regression (LUR) model at a resolution of 100 × 100 m was used to assign annual air pollution estimates, Particulate Matter with aerodynamic diameter ≤ 10 µm (PM10) and nitrogen dioxide (NO2), for year 2007 at participant’s recruitment address.13 The model used information from over 1500 monitoring sites across western Europe, satellite-based ground-level air pollution data and land use variables obtained from a Geographic Information System (GIS).13
Covariates
Age, sex, smoking status, smoking pack-years, education, paid employment, alcohol consumption, and ‘ever-had’ hypertension and diabetes were obtained from questionnaires at recruitment. Height (cm) and weight (kg) were measured after removal of heavy clothes and shoes and body mass index (BMI, kg/m2) was computed. Time at residence of recruitment in years was calculated for each participant. Season of blood draw was based on the calendar month the participant attended for clinical measurements. All these covariates were retrospectively harmonized across the two cohorts, following a validated protocol.14
Statistical analyses
Statistical analyses for hsCRP and blood lipids were performed using DataSHIELD v4.1.2.8 In brief, the DataSHIELD approach involves setting up secure servers hosting harmonized data in geographically dispersed research sites (e.g. Groningen for Lifelines and London for HUNT3) and allowing a central computer to conduct remote federated analyses of individual-level data without physically pooling this data. DataSHIELD offers an effective solution to overcome the complex ethico-legal issues associated with a physical sharing of the data. Analyses on fasting blood glucose and HbA1c were conducted in Lifelines only also using DataSHIELD in ‘single-site’ mode which provides, in effect, a privacy-protected analysis in an R statistical environment.
All outcomes were modelled as a log-transformed value to improve linearity and results were expressed as per cent change per inter-quartile range (IQR) higher exposure.
Spearman correlations between metrics of road traffic noise and ambient air pollution were calculated for each cohort. Associations between the noise, PM10, NO2, and each biochemical parameter were analysed using multivariate linear regression. Both noise and air pollution metrics were analysed on a continuous scale, assuming a linear effect. Additionally noise was categorised as <55, 55–60, ≥60 dB(A) for daytime and <45, 45–50, ≥50 dB(A) for night-time noise, respectively.
The covariates were chosen a priori based on current knowledge. The sequence of models was as follows: adjusted for study (in pooled analyses on hsCRP and blood lipids), age (continuous), and sex (Model1), further adjusted for season of blood draw, smoking status and pack-years, education, employment, and alcohol consumption (Model2). Based on Model2, ambient air pollution (or road traffic noise) were additionally added to the noise (or air pollution) model (mutually adjusted model).
Sensitivity analyses were conducted based on Model2: (i) further adjusting for BMI; (ii) further adjusting for ever-had hypertension and diabetes; (iii) restricting analyses to those living at current address ≥ 10 years; (iv) as with a previous study,15 excluding those with an hsCRP over 10 mg/L from the analysis as levels above this may indicate a current infection.
We also conducted study-specific analyses for hsCRP and blood lipids and then pooled estimates via meta-analysis using R ‘metafor’ packages v3.2.2.
Results
Overall there were 144 082 participants, mean age (SD) was 47.6 (13.7) years, and 56% (n = 82 574) were women (Table 1).
Table 1.
Characteristics of study populations in both cohorts
| Pooled data | HUNT3 | Lifelines | |
|---|---|---|---|
| Total N | 144 082 | 50 805 | 93 277 |
| Age, years, mean ± SD | 47.6 ± 13.7 | 52.7 ± 16.7 | 44.9 ± 12.3 |
| Sex, women, (%) | 56 | 55 | 59 |
| BMI, kg/m2, mean ± SD | 26.5 ± 4.4 | 27.2 ± 4.4 | 26.1 ± 4.3 |
| BMI categories, (%) | |||
| <25 kg/m2 | 41 | 33 | 45 |
| 25–30 kg/m2 | 41 | 44 | 39 |
| ≥30 kg/m2 | 18 | 23 | 16 |
| Smoking status, (%) | |||
| Never-smoker | 44 | 43 | 45 |
| Ex-smoker | 32 | 32 | 32 |
| Current-smoker | 24 | 25 | 23 |
| Current working status, (%) | |||
| Not in paid employment | 25 | 36 | 19 |
| In paid employment | 75 | 64 | 81 |
| Education level, (%) | |||
| Primary education or less | 11 | 31 | 2 |
| Secondary education | 62 | 47 | 68 |
| Post-secondary school or above | 28 | 22 | 30 |
| Ever-had diabetes, (%) | 3 | 4 | 2 |
| Ever-had hypertension, (%) | 24 | 22 | 25 |
| Alcohol consumption, gram per week, mean ± SD | 31.6 ± 88.8 | 28.0 ± 40.0 | 56.9 ± 71.5 |
| hsCRP, mg/L, median ± IQR | 1.2 ± 2.1 | 1.2 ± 2.1 | 1.2 ± 2.2 |
| Total cholesterol, mmol/L, mean ± SD | 5.2 ± 1.0 | 5.5 ± 1.1 | 5.0 ± 1.0 |
| HDL cholesterol, mmol/L, mean ± SD | 1.4 ± 0.4 | 1.4 ± 0.3 | 1.5 ± 0.4 |
| Triglycerides, mmol/L, mean ± SD | 1.3 ± 0.9 | 1.6 ± 1.0 | 1.1 ± 0.8 |
| Fasting glucose, mmol/L, mean ± SD | – | – | 5.0 ± 0.8 |
| HbA1c, mmol/mol, mean ± SD | – | – | 37.5 ± 4.8 |
Across the two cohorts, median Lday was 51.6 dB(A), with an IQR of 5.1 dB(A); median PM10 and NO2 were 18.8 and 17.2 µg/m3, with an IQR of 2.0 and 7.4 µg/m3, respectively (see Supplementary material online, SupplementS1). Spearman correlations between PM10 and Lday was 0.04 (HUNT3) and 0.38 (Lifelines); similar correlations were seen for NO2. Daytime and night-time noise or 24-h noise were almost perfectly correlated (r = 0.99). We therefore report only results for daytime noise here.
In the pooled analyses of both HUNT3 and Lifelines, an IQR increase in daytime noise, PM10 and NO2 was significantly associated with higher levels of hsCRP (Table 2) in the fully adjusted model (Model 2), but significance only remained for NO2 [1.7%, 95% confidence interval (CI): 0.2–3.2%] in the model further adjusted for noise (mutually adjusted model).
Table 2.
Cross-sectional associations between per IQR higher exposure and hsCRP: pooled analyses from HUNT3 and lifelines
|
%changes (95%CI) in hsCRP |
||||||
|---|---|---|---|---|---|---|
| Exposure | IQR | N | Model1 | N | Model2 | Model2* |
| Daytime noise | 5.1 dB(A) | 90 689 | 0.4 (0.1–0.8) | 55 930 | 1.1 (0.02–2.2) | 1.0 (−0.1 to 2.2)a |
| 0.9 (−0.3 to 2.0)b | ||||||
| PM10 | 2.0 µg/m3 | 87 622 | 1.3 (0.2–2.4) | 51 238 | 1.4 (0.1–2.7) | 0.9 (−0.4 to 2.3) |
| NO2 | 7.4 µg/m3 | 87 957 | 1.0 (−0.1 to 2.2) | 51 459 | 1.9 (0.5–3.3) | 1.7 (0.2–3.2) |
Model 1: adjusted for cohort, age, sex.
Model 2: further adjusted for season of blood draw, smoking status and pack-years, education, employment and alcohol consumption.
Model 2*: further adjusted for air pollution (a for PM10, b for NO2), or daytime noise, based on Model 2.
BOLD indicates where significance level <0.05.
An IQR increase in daytime noise, PM10 and NO2 was significantly associated with 0.7% (95% CI: 0.3–1.1%), 1.9% (95% CI: 1.5–2.4%), and 2.2% (95% CI: 1.6–2.7%) higher levels of triglycerides, respectively (Table 3). However, when Model 2 was mutually adjusted for both noise and air pollution, significance remained for both air pollutants, but not noise. We found no associations between any of the exposures and total cholesterol (see Supplementary material online, Supplement S2), but significant positive associations between noise or NO2 and HDL cholesterol were observed (Table 3).
Table 3.
Cross-sectional associations between per IQR higher exposure and triglycerides and HDL cholesterol: pooled analyses from HUNT3 and lifelines
| Exposure | IQR | N | Model1 | N | Model2 | Model2* |
|---|---|---|---|---|---|---|
| %changes (95% CI) in triglycerides | ||||||
| Daytime noise | 5.1 dB(A) | 119 464 | 0.7 (0.4–1.0) | 81 799 | 0.7 (0.3–1.1) | 0.2 (−0.2 to 0.7)a |
| 0.3 (−0.2 to 0.7)b | ||||||
| PM10 | 2.0 µg/m3 | 111 547 | 1.4 (1.0–1.8) | 72 794 | 1.9 (1.5–2.4) | 1.9 (1.4–2.4) |
| NO2 | 7.4 µg/m3 | 111 893 | 1.6 (1.1–2.0) | 73 026 | 2.2 (1.6–2.7) | 2.1 (1.6–2.7) |
| %changes (95%CI) in HDL cholesterol | ||||||
| Daytime noise | 5.1 dB(A) | 118 866 | 0.3 (0.2–0.5) | 81 590 | 0.5 (0.3–0.7) | 0.4 (0.1–0.6)a |
| 0.3 (0.04–0.5)b | ||||||
| PM10 | 2.0 µg/m3 | 110 834 | 0.1 (0.04–0.2) | 72 551 | 0.2 (−0.1 to 0.4) | 0.04 (−0.2 to 0.3) |
| NO2 | 7.4 µg/m3 | 111 178 | 0.4 (0.2–0.7) | 72 783 | 0.5 (0.3–0.8) | 0.4 (0.1–0.7) |
Model 1: adjusted for cohort, age, sex.
Model 2: further adjusted for season of blood draw, smoking status and pack-years, education, employment and alcohol consumption.
Model 2*: further adjusted for air pollution (a for PM10, b for NO2), or daytime noise, based on Model 2.
BOLD indicates where significance level <0.05.
In the analyses of fasting glucose in Lifelines only (Table 4), an IQR increase in daytime noise, PM10 and NO2 was significantly associated with 0.2% (95% CI: 0.1–0.3%), 0.6% (95% CI: 0.4–0.7%), and 0.6% (95% CI: 0.4–0.8%) higher levels of fasting glucose, respectively. These significant associations remained in the mutually adjusted models. Corresponding associations were not seen for HbA1c (see Supplementary material online, Supplement S2).
Table 4.
Cross-sectional associations between per IQR higher exposure and fasting glucose: analyses of Lifelines cohort
| % changes (95% CI) in fasting glucose |
||||||
|---|---|---|---|---|---|---|
| Exposure | IQR | N | Model1 | N | Model2 | Model2* |
| Daytime noise | 4.2 dB(A) | 72 401 | 0.2 (0.1–0.3) | 62 765 | 0.2 (0.1–0.3) | 0.2 (0.06–0.3)a |
| 0.1 (0.03–0.3)b | ||||||
| PM10 | 2.4 µg/m3 | 59 898 | 0.5 (0.4–0.7) | 52 234 | 0.6 (0.4–0.7) | 0.5 (0.3–0.6) |
| NO2 | 8.8 µg/m3 | 60 182 | 0.5 (0.4–0.7) | 52 543 | 0.6 (0.4–0.8) | 0.5 (0.3–0.7) |
Model 1: adjusted for age, sex.
Model 2: further adjusted for season of blood draw, smoking status and pack-years, education, employment and alcohol consumption.
Model 2*: further adjusted for air pollution (a for PM10, b for NO2), or daytime noise, based on Model 2.
BOLD indicates where significance level <0.05.
Sensitivity analyses only resulted in minor changes to the main findings described for each biochemistry parameter, except that the significant positive associations between air pollution and HDL cholesterol were no longer seen after further adjustment for BMI (see Supplementary material online, Supplement S3). Treating noise as a categorical variable in Model2 showed that for hsCRP, triglycerides and fasting glucose, significant associations were only observed among those with a day-time noise level ≥60 vs. <50 dB(A) (see Supplementary material online, Supplement S4).
Study-specific meta-analyses for hsCRP and blood lipids yielded similar results to the pooled analyses on DataSHIELD (see Supplementary material online, Supplement S5).
Discussion
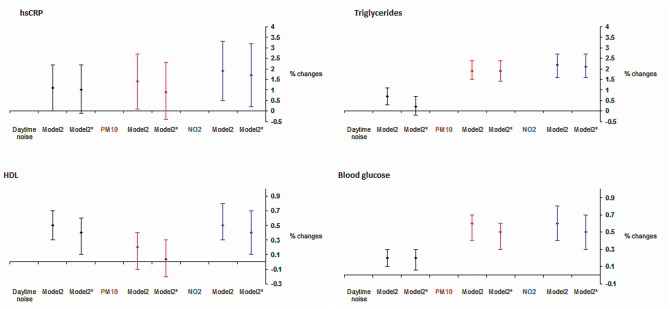
In this large population-based study, we found that higher long-term road traffic noise was significantly associated with higher levels of hsCRP, triglycerides, HDL cholesterol, and fasting glucose, with the latter two associations remaining after further adjustment for air pollution. Higher PM10 or NO2 exposure was significantly associated with higher levels of triglycerides and fasting glucose, independent of road traffic noise. Both air pollutants were also significantly associated with higher hsCRP levels, but the significance for PM10 was lost after adjustment for traffic noise (Figure 1).
Figure 1.
Cross-sectional associations between per IQR* higher exposure and per cent changes in each cardiovascular disease biochemical parameter. Model 2: adjusted for cohort (pooled analysis only), age, sex, season of blood draw, smoking status and pack-years, education, employment, and alcohol consumption. Model 2*: further adjusted for PM10 or daytime noise. *IQR, inter-quartile range, which is 5.1 dB(A) for daytime noise, 2.0 µg/m3 for PM10, 7.4 µg/m3 for NO2 for analyses on high-sensitivity C-reactive protein and lipids; 4.2 dB(A) for daytime noise, 2.4 µg/m3 for PM10, 8.8 µg/m3 for NO2 for analyses on blood glucose.
Road traffic noise effects
There have been very few studies investigating the effects of road traffic noise on cardiovascular risk factors to date. Chronic noise exposure may trigger the same responses (e.g. oxidative stress, systemic inflammation) as air pollutants6 and it has been suggested that the link between long-term psychological stress and CVD may operate via a chronic low-grade systemic inflammation.16 We are not aware of any other studies exploring the association between road traffic noise and hsCRP. We observed a small increase in hsCRP levels in relation to noise exposure when assessed as a continuous variable. Analysing noise as a categorical variable suggested that there may exist a threshold of 60 dB(A), above which a significant positive association on hsCRP was seen in our study. However, significance was lost after adjustment for air pollution.
The over-production of stress hormones (e.g. catecholamines) following long-term noise exposure is believed to increase the supply of fatty acids and glucose by breaking down triacylglycerol.5 In support of this hypothesized mechanism, we found significant positive associations between road traffic noise exposure and triglycerides. Only one population-based study was conducted previously and reported no association with total cholesterol.17 In our study, we found no associations with total cholesterol either, but a positive significant association was unexpectedly found with HDL cholesterol. There is no biological plausible explanation for this association and it is possible that this is a chance finding due to multiple testing. Findings across a small number of occupational studies were not consistent with regards to which lipids were adversely affected by noise exposures.18 The role of traffic noise in the perturbations in lipid levels is rarely investigated3 and our findings should be interpreted with caution until further studies have confirmed this result.
Our study provides novel evidence that increasing road traffic noise is associated with increasing fasting blood glucose levels, independent of air pollution effects. Only one study in the early 1990s reported a positive trend (P-value <0.05) between higher day-time noise level and increased glucose level in men.19 Another study in Stockholm found no associations between aircraft noise exposure and impaired fasting glucose.20 In a Danish prospective cohort, a 10 dB higher road traffic noise at diagnosis was associated with a 8% (95% CI: 2–14%) higher risk of incident diabetes, after adjustment for air pollution.21
Air pollution effects
An inflammatory response to ambient air pollution has been perceived as one of the key mechanisms that may explain air pollution effects on CVD.2 Our findings on elevated hsCRP for both air pollutants generally support this concept. An analysis of 22 561 adults from six European cohorts observed a significant association between elevated hsCRP levels and living close to traffic but not for modelled air pollutants.15 A longitudinal analysis with repeated measurements of hsCRP in Germany found that each IQR (2.4 µg/m3) higher PM2.5 was associated with 5.4% (95% CI: 0.6–10.5%) higher hsCRP, independent of short-term air pollution.22 Two other studies in England23 and Sweden24 reported no associations with hsCRP.
Inflammation-induced adverse lipid metabolism and lipid oxidation may be among the possible mechanisms underlying the associations between air pollution and perturbations in lipid levels.25 A study of nearly 40 000 adults aged 50–64 years in Copenhagen reported that both PM2.5 and NO2 were significantly associated with elevated non-fasting total cholesterol.17 Data on other lipid measures were not available. Similarly, a nationwide USA study of 11 623 participants (median age: 41 years) reported significant positive associations between annual PM10 exposure and both triglycerides and total cholesterol levels.25 Association with HDL cholesterol was positive but non-significant. Partly in line with this, we found both higher PM10 and NO2 were significantly associated not only with elevated triglycerides but also HDL cholesterol levels, although for the latter the association was non-significant after further adjustment for BMI.
Air pollution may also affect glucose homeostasis via pathways including increased adipose tissue inflammation and oxidative stress as well as impaired insulin responses in the liver and adipose tissue.3 Our findings of significant associations with fasting glucose for long-term PM10 and NO2 exposures were partly consistent with previous studies of various designs.26–29 However, we did not observe an association with HbA1c. Annual average of PM10 and NO2 was significantly associated with increased fasting glucose and HbA1c among elderly participants in Taiwan26 while in Germany higher regional PM10 levels were significantly associated with higher HbA1c levels among patients with diabetes.28 A study of 363 elderly German women reported that long-term exposure to NO2 may contribute to impaired glucose metabolism, although significance was lost after correction for multiple testing.27 Recently, a nationwide cross-sectional study of 11 847 adults in China observed that satellite-based modelled annual PM2.5 at residence was significantly associated with both increased fasting glucose and HbA1c.29
Strengths and limitations
Strengths include our ability to simultaneously examine the effects of noise and air pollution exposure on cardiovascular risk factors in this large sample. In addition, data across the two cohorts were harmonized and then individual-level analyses and data pooling were carried out via DataSHIELD, making this one of the largest such studies to date. The novel use of DataSHIELD was validated through meta-analysis of the two cohorts involved, with DataSHIELD giving similar effect estimates but (as expected) smaller confidence intervals as individual participant data were analysed together.
Limitations include the cross-sectional nature of the study, longitudinal studies are needed to strengthen these findings. Modelled noise and air pollution estimates at home address will inevitably have some misclassification (e.g. time spent away from home). However, this was likely non-differential with regard to biochemistry measurements. Correlations between PM10 and NO2 were high which precluded a co-pollutant analysis. Some simplified inputs were used in our noise model to enable a harmonized approach across the two cohorts investigated30; this may have resulted in non-differential misclassification of noise exposure that would be expected to bias results toward the null. The European harmonized LUR model for air pollution was likely less accurate for the HUNT cohort for two reasons. First, no Norwegian monitoring air pollution data were used in the model building and therefore the performance of this LUR model cannot be formally measured in Norway. However, the use of this LUR model for the HUNT cohort was reasonably justified based on the model’s moderate to good performance in other Scandinavian countries. Second, the road networks used in the air pollution/noise models building for the Netherlands had a more complete representation of both major and minor roads whilst for Norway the road networks only had major roads included. Therefore, the relatively low correlation between noise and air pollution seen for the HUNT cohort may be driven in part by the less detailed data inputs. While road traffic is a major source of both noise and air pollutants, these variables are not always closely correlated.31 Noise and air pollution estimates at recruitment address were modelled to a single year 2009 and 2007, respectively, which may differ by several years from when recruitment took place in both cohorts (2006–2013). We made a reasonable assumption that the spatial contrast in levels of both exposures will have been relatively stable over these years. Estimates may have residual confounding, as information about environmental tobacco smoke, diet, exercise, and area-level socioeconomic status were not harmonized and therefore were not included in our models. Finally, we used nominal P-values, so given the number of tests it is possible that some observed associations may be chance findings; replication in other studies is therefore recommended.
In our study, the observed effects of road traffic noise and air pollution on CVD risk factors are small and potentially within the precision of a laboratory measurement for an individual. The clinical impact of an increase in risk of that magnitude on any individual would be very small if not negligible, despite statistical significance. However, the importance of these population-based results is that they suggest that the entire distribution of these risk factors may be shifted towards a less favourable profile at population level, i.e. ‘at-risk’ populations may be increased. Our results contribute to the evidence base to identify potential mechanisms and quantify impacts of both road noise and air pollution exposures on cardiovascular disease at population level. Future work will explore potential for effect modification and whether effects are confined to a subset of the population.
In conclusion, we found significant associations of long-term exposure to road traffic noise and ambient air pollution with CVD biochemical parameters, providing a possible link between noise or air pollution exposure and cardiovascular disease.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The Nord-Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology NTNU), Nord-Trøndelag County Council, Central Norway Health Authority, and the Norwegian Institute of Public Health.
The authors wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centres delivering data to Lifelines, and all the study participants.
We thank all study members and data collection teams of each cohort.
P.E. is an NIHR Senior Investigator and acknowledges support from the Imperial College Healthcare NHS Trust and Imperial College London Biomedical Research Centre, the National Institute for Health Research (NIHR) Health Protection Research Unit on the Health Effects of Environmental Hazards, the Medical Research Council and Public Health England (MRC-PHE) Centre for Environment and Health. The views expressed are those of the authors and not necessarily those of the Department of Health, the NHS or the NIHR.
Lifelines is a multi-disciplinary prospective population-based cohort study examining in a unique three-generation design the health and health-related behaviours of 167 729 persons living in the North of The Netherlands and is open for all researchers. Information on application and data access procedure is summarized on www.lifelines.nl.
Funding
European Union Seventh Framework Programme (FP7/2007-2013) Biobank Standardisation and Harmonisation for Research Excellence in the European Union-BioSHaRE-EU (Grant Number 261433). DataSHIELD development is also partly funded under a strategic award from UK Medical Research Council (MRC) and Wellcome Trust underpinning the ALSPAC (Avon Longitudinal Study of Parents and Children) project; and the Welsh and Scottish Farr Institutes funded by MRC, BBMRI-LPC (EU-FP7, I3 grant). The Lifelines Cohort Study, and generation and management of GWAS genotype data for the Lifelines Cohort Study is supported by the Netherlands Organization of Scientific Research NWO (Grant Number 175.010.2007.006); the Ministry of Economic Affairs; the Ministry of Education, Culture and Science; the Ministry for Health, Welfare and Sports; the Northern Netherlands Collaboration of Provinces (SNN); the Province of Groningen; University Medical Center Groningen, the University of Groningen; Dutch Kidney Foundation; and Dutch Diabetes Research Foundation. ESCAPE project was supported by European Community’s Seventh Framework Program (FP7/2007-2011) (Grant Number 211250). The MRC-PHE centre for Environment and Health is funded by the UK Medical Research Council and Public Health England (Grant Number MR/L01341X/1). This work used the computing resources of the UK MEDical BIOinformatics partnership (UK MED-BIO) which is supported by the Medical Research Council (Grant Number MR/L01632X/1). Y.C. acknowledges support from the Early-Career Research Fellowship awarded by the UK Medical Research Council-Public Health England Centre for Environment and Health (Grant Number MR/M501669/1).
Conflict of interest: none declared.
References
- 1. Halonen JI, Hansell AL, Gulliver J, Morley D, Blangiardo M, Fecht D, Toledano MB, Beevers SD, Anderson HR, Kelly FJ, Tonne C.. Road traffic noise is associated with increased cardiovascular morbidity and mortality and all-cause mortality in London. Eur Heart J 2015;36:2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 3. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S.. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J 2017;38:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babisch W. Updated exposure-response relationship between road traffic noise and coronary heart diseases: a meta-analysis. Noise Health 2014;16:1–9. [DOI] [PubMed] [Google Scholar]
- 5. Brindley DN, McCann BS, Niaura R, Stoney CM, Suarez EC.. Stress and lipoprotein metabolism: modulators and mechanisms. Metabolism 1993;42:3–15. [DOI] [PubMed] [Google Scholar]
- 6. Recio A, Linares C, Banegas JR, Diaz J.. Road traffic noise effects on cardiovascular, respiratory, and metabolic health: an integrative model of biological mechanisms. Environ Res 2016;146:359–370. [DOI] [PubMed] [Google Scholar]
- 7. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook J, Chen LC, Brook RD, Rajagopalan S.. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 2017;38:550–556. [DOI] [PubMed] [Google Scholar]
- 8. Gaye A, Marcon Y, Isaeva J, LaFlamme P, Turner A, Jones EM, Minion J, Boyd AW, Newby CJ, Nuotio ML, Wilson R, Butters O, Murtagh B, Demir I, Doiron D, Giepmans L, Wallace SE, Budin-Ljosne I, Oliver Schmidt C, Boffetta P, Boniol M, Bota M, Carter KW, deKlerk N, Dibben C, Francis RW, Hiekkalinna T, Hveem K, Kvaloy K, Millar S, Perry IJ, Peters A, Phillips CM, Popham F, Raab G, Reischl E, Sheehan N, Waldenberger M, Perola M, van den Heuvel E, Macleod J, Knoppers BM, Stolk RP, Fortier I, Harris JR, Woffenbuttel BH, Murtagh MJ, Ferretti V, Burton PR.. DataSHIELD: taking the analysis to the data, not the data to the analysis. Int J Epidemiol 2014;43:1929–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krokstad S, Langhammer A, Hveem K, Holmen TL, Midthjell K, Stene TR, Bratberg G, Heggland J, Holmen J.. Cohort Profile: the HUNT Study, Norway. Int J Epidemiol 2013;42:968–977. [DOI] [PubMed] [Google Scholar]
- 10. Scholtens S, Smidt N, Swertz MA, Bakker SJ, Dotinga A, Vonk JM, van Dijk F, van Zon SK, Wijmenga C, Wolffenbuttel BH, Stolk RP.. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol 2015;44:1172–1180. [DOI] [PubMed] [Google Scholar]
- 11. Zijlema WL, Smidt N, Klijs B, Morley DW, Gulliver J, de Hoogh K, Scholtens S, Rosmalen JG, Stolk RP.. The LifeLines Cohort Study: a resource providing new opportunities for environmental epidemiology. Arch Public Health 2016;74:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kephalopoulos S, Paviotti M, Anfosso-Ledee F, Van Maercke D, Shilton S, Jones N.. Advances in the development of common noise assessment methods in Europe: the CNOSSOS-EU framework for strategic environmental noise mapping. Sci Total Environ 2014;482–483:400–410. [DOI] [PubMed] [Google Scholar]
- 13. Vienneau D, de Hoogh K, Bechle MJ, Beelen R, van Donkelaar A, Martin RV, Millet DB, Hoek G, Marshall JD.. Western European land use regression incorporating satellite- and ground-based measurements of NO2 and PM10. Environ Sci Technol 2013;47:13555–13564. [DOI] [PubMed] [Google Scholar]
- 14. Fortier I, Doiron D, Little J, Ferretti V, L'heureux F, Stolk RP, Knoppers BM, Hudson TJ, Burton PR.. International Harmonization Initiative. Is rigorous retrospective harmonization possible? Application of the DataSHaPER approach across 53 large studies. Int J Epidemiol 2011;40:1314–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lanki T, Hampel R, Tiittanen P, Andrich S, Beelen R, Brunekreef B, Dratva J, De Faire U, Fuks KB, Hoffmann B, Imboden M, Jousilahti P, Koenig W, Mahabadi AA, Kunzli N, Pedersen NL, Penell J, Pershagen G, Probst-Hensch NM, Schaffner E, Schindler C, Sugiri D, Swart WJ, Tsai MY, Turunen AW, Weinmayr G, Wolf K, Yli-Tuomi T, Peters A.. Air pollution from road traffic and systemic inflammation in adults: a cross-sectional analysis in the European ESCAPE Project. Environ Health Perspect 2015;123:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med 2014;76:181–189. [DOI] [PubMed] [Google Scholar]
- 17. Sorensen M, Hjortebjerg D, Eriksen KT, Ketzel M, Tjonneland A, Overvad K, Raaschou-Nielsen O.. Exposure to long-term air pollution and road traffic noise in relation to cholesterol: a cross-sectional study. Environ Int 2015;85:238–243. [DOI] [PubMed] [Google Scholar]
- 18. Arlien-Soborg MC, Schmedes AS, Stokholm ZA, Grynderup MB, Bonde JP, Jensen CS, Hansen AM, Frederiksen TW, Kristiansen J, Christensen KL, Vestergaard JM, Lund SP, Kolstad HA.. Ambient and at-the-ear occupational noise exposure and serum lipid levels. Int Arch Occup Environ Health 2016;89:1087–1093. [DOI] [PubMed] [Google Scholar]
- 19. Babisch W, Ising H, Gallacher JE, Sharp DS, Baker IA.. Traffic noise and cardiovascular risk: the Speedwell study, first phase. Outdoor noise levels and risk factors. Arch Environ Health 1993;48:401–405. [DOI] [PubMed] [Google Scholar]
- 20. Eriksson C, Hilding A, Pyko A, Bluhm G, Pershagen G, Ostenson CG.. Long-term aircraft noise exposure and body mass index, waist circumference, and type 2 diabetes: a prospective study. Environ Health Perspect 2014;122:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sorensen M, Andersen ZJ, Nordsborg RB, Becker T, Tjonneland A, Overvad K, Raaschou-Nielsen O.. Long-term exposure to road traffic noise and incident diabetes: a cohort study. Environ Health Perspect 2013;121:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viehmann A, Hertel S, Fuks K, Eisele L, Moebus S, Mohlenkamp S, Nonnemacher M, Jakobs H, Erbel R, Jockel KH, Hoffmann B; Heinz Nixdorf Recall Investigator Group. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup Environ Med 2015;72:656–663. [DOI] [PubMed] [Google Scholar]
- 23. Forbes LJ, Patel MD, Rudnicka AR, Cook DG, Bush T, Stedman JR, Whincup PH, Strachan DP, Anderson RH.. Chronic exposure to outdoor air pollution and markers of systemic inflammation. Epidemiology 2009;20:245–253. [DOI] [PubMed] [Google Scholar]
- 24. Panasevich S, Leander K, Rosenlund M, Ljungman P, Bellander T, de Faire U, Pershagen G, Nyberg F.. Associations of long- and short-term air pollution exposure with markers of inflammation and coagulation in a population sample. Occup Environ Med 2009;66:747–753. [DOI] [PubMed] [Google Scholar]
- 25. Shanley RP, Hayes RB, Cromar KR, Ito K, Gordon T, Ahn J.. Particulate air pollution and clinical cardiovascular disease risk factors. Epidemiology 2016;27:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chuang KJ, Yan YH, Chiu SY, Cheng TJ.. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med 2011;68:64–68. [DOI] [PubMed] [Google Scholar]
- 27. Teichert T, Vossoughi M, Vierkotter A, Sugiri D, Schikowski T, Schulte T, Roden M, Luckhaus C, Herder C, Kramer U.. Association between traffic-related air pollution, subclinical inflammation and impaired glucose metabolism: results from the SALIA study. PLoS One 2013;8:e83042.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tamayo T, Rathmann W, Kramer U, Sugiri D, Grabert M, Holl RW.. Is particle pollution in outdoor air associated with metabolic control in type 2 diabetes? PLoS One 2014;9:e91639.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu C, Yang C, Zhao Y, Ma Z, Bi J, Liu Y, Meng X, Wang Y, Cai J, Kan H, Chen R.. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in China. Environ Int 2016;92-93:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morley DW, de Hoogh K, Fecht D, Fabbri F, Bell M, Goodman PS, Elliott P, Hodgson S, Hansell A, Gulliver J.. International scale implementation of the CNOSSOS-EU road traffic noise prediction model for epidemiological studies. Environ Pollut 2015;206:332–341. [DOI] [PubMed] [Google Scholar]
- 31. Fecht D, Hansell AL, Morley D, Dajnak D, Vienneau D, Beevers S, Toledano MB, Kelly FJ, Anderson HR, Gulliver J.. Spatial and temporal associations of road traffic noise and air pollution in London: implications for epidemiological studies. Environ Int 2016;88:235–242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.