Abstract
The lung is constantly exposed to ambient pollutants such as ambient fine particulate matter (PM2.5), making it one of the most frequent locations of inflammation in the body. Given the establishment of crucial role of inflammation in the pathogenesis of cardiometabolic diseases, pulmonary inflammation is thus widely believed to be an important risk factor for cardiometabolic diseases. However, the causality between them has not yet been well established. To determine if pulmonary inflammation is sufficient to cause adverse cardiometabolic effects, SFTPC-rtTA+/–tetO-cre+/–pROSA-inhibitor κB kinase 2(IKK2)ca+/– (LungIKK2ca) and littermate SFTPC-rtTA+/–tetO-cre–/–pROSA-IKK2ca+/– wildtype (WT) mice were fed with doxycycline diet to induce constitutively active Ikk2 (Ikk2ca) overexpression in the lung and their pulmonary, systemic, adipose, and hypothalamic inflammations, vascular function, and glucose homeostasis were assessed. Feeding with doxycycline diet resulted in IKK2ca overexpression in the lungs of LungIKK2ca but not WT mice. This induction of IKK2ca was accompanied by marked pulmonary inflammation as evidenced by significant increases in bronchoalveolar lavage fluid leukocytes, pulmonary macrophage infiltration, and pulmonary mRNA expression of tumor necrosis factor α (Tnfα) and interleukin-6 (Il-6). This pulmonary inflammation due to lung-specific overexpression of IKK2ca was sufficient to increase circulating TNFα and IL-6 levels, adipose expression of Tnfα and Il-6 mRNA, aortic endothelial dysfunction, and systemic insulin resistance. Unexpectedly, no significant alteration in hypothalamic expression of Tnfα and Il-6 mRNA and glucose intolerance were observed in these mice. Pulmonary inflammation is sufficient to induce systemic inflammation, endothelial dysfunction, and insulin resistance, but not hypothalamic inflammation and glucose intolerance.
Keywords: air pollution, pulmonary inflammation, hypothalamic inflammation, insulin resistance, glucose intolerance
Exposure to ambient fine particulate matter (PM2.5) is associated with increased risk of cardiopulmonary mortality (Brook et al., 2010). This is attributed to various adverse cardiopulmonary effects of PM2.5 exposure such as endothelial dysfunction, hypertension, and insulin resistance (Brook et al., 2010). Inhaled PM2.5 has been shown to primarily deposit in the airway without access to the systemic circulation (U.S. Environmental Protection Agency’s Integrated Science Assessments-PM, available at www.epa.gov/ncea/isa/pm.htm). Although PM2.5 deposited in the upper respiratory tract is quickly cleaned by the mucociliary escalator, PM2.5 deposited in the lower respiratory tract is primarily cleaned by phagocytosis of macrophage. The latter is slow and may take several weeks, and thus PM2.5 in the lower respiratory tract is believed to be more relevant to the systemic effects of PM2.5 exposure.
Currently, how PM2.5 in the lower respiratory tract leads to the various extrapulmonary effects has not yet been fully understood. The putative mechanisms for this include (1) egress from the lung of PM2.5 components, (2) autonomic nervous system (ANS) dysfunction, and (3) systemic inflammation (Brook et al., 2010). Due to the inconsistent and usually very low concentration of inhaled nanoparticles in remote organs (Kreyling et al., 2009; Oberdorster et al., 2002; Oberdorster and Utell, 2002; Semmler-Behnke et al., 2008) and the well-known adaption of the nervous system, systemic inflammation is widely believed to play a major role in the development of adverse health effects due to chronic exposure to PM2.5. However, its genesis has not yet been fully understood. PM2.5 exposure causes pronounced pulmonary inflammation through toll-like receptors- and/or pattern recognition receptors-dependent mechanisms (Barton et al., 2014; Kampfrath et al., 2011; Levesque et al., 2011; Wood et al., 2014). Time course studies have revealed that PM2.5 exposure-induced pulmonary inflammation precedes extrapulmonary effects encompassing systemic inflammation (Brook et al., 2010), supporting a causality between them. However, we recently found that 5 weeks of withdrawal from exposure to PM2.5 resolved PM2.5 exposure-induced extrapulmonary inflammations, vascular dysfunction and hypertension, but not pulmonary inflammation (Ying et al., 2015), suggesting that pulmonary inflammation may be insufficient to cause PM2.5 exposure-induced systemic inflammation and/or abnormalities in glucose homeostasis. Determination of the explicit role of pulmonary inflammation in the development of adverse effects due to PM2.5 exposure is thus critical in our efforts to understand and control these adverse health effects.
Inflammation is a complex biological process. To determine the role of pulmonary inflammation in the development of adverse effects due to PM2.5 exposure, one of the major technical challenges is how to specifically manipulate its level. Inhibitor κB kinase 2(IKK2)/NF-κB pathway plays a pivotal role in inflammation and its activity is associated with local inflammation level (Pahl, 1999). PM2.5 exposure activates it in various tissues encompassing the lung (Dagher et al., 2007; Kafoury and Madden, 2005; Maciejczyk and Chen, 2005; Mantecca et al., 2010; Nam et al., 2004), and inhibition of IKK2 blocks PM2.5 exposure-induced expression of inflammatory cytokines in respiratory epithelial cells and alveolar macrophages (Kafoury and Madden, 2005; Liet al., 2013), indicating that pulmonary IKK2/NF-κB pathway mediates PM2.5 exposure-induced pulmonary and subsequent systemic inflammation. Given that gene targeting technique can enable us to tissue-specifically manipulate the expression of genes of interest, modulation of Ikk2 expression in the lung through gene targeting technique emerges as a rational method to manipulate PM2.5 exposure-related inflammation level. Supporting this, it has been shown that overexpression of Nf-κb in pulmonary epithelial cells enhances endotoxin-induced pulmonary inflammation, and disruption of Ikk2 in pulmonary epithelial cells inhibits endotoxin-induced pulmonary inflammation and injury (Lopez et al., 2015). In this report, Nkx2-1-cre was used, which causes gene deletion in several other tissues (http://jaxmice.jax.org/strain/ 008661.html). SFTPC-rtTA mice express the reverse tetracycline-controlled transactivator (rtTA) protein specifically in the lung epithelial cells.(Tichelaar et al., 2000) When crossed with tetracycline-responsive promoter element (tetO)-cre mice, the resultant SFTPC-rtTA+tetO-cre+ mice will allow for the inducible expression of Cre specifically in the lung epithelium.(Tichelaar et al., 2000).
In this study, to determine if pulmonary inflammation is sufficient to elicit PM2.5 exposure-related systemic inflammation and abnormalities in glucose homeostasis, we generated SFTPC-rtTA+/–tetO-cre+/–pROSA-IKK2ca+/– (LungIKK2ca) mice that allow us to induce lung-specific over-expression of constitutively active (IKK2ca) and subsequently cause pulmonary inflammation through doxycycline diet feeding (Tichelaar et al., 2000). Our assessments reveal that this overexpression of Ikk2ca was sufficient to induce marked pulmonary, systemic, and adipose inflammations and insulin resistance but not hypothalamic inflammation and glucose intolerance.
MATERIALS AND METHODS
Animals
University of Maryland, Baltimore is an AAALAC accredited institution. All procedures of this study were approved by the Institutional Animal Care and Use Committee at University of Maryland, Baltimore, and all the animals were treated humanely and with regard for alleviation of suffering. SFTPC-rtTA and tetO-cre transgenic mice were obtained from Jackson Laboratories. pROSA-IKK2ca mice were generated as previously described in Otero et al. (2012). SFTPC-rtTA+/–tetO-cre+/–pROSA-IKK2ca+/– (LungIKK2ca) and littermate control (SFTPC-rtTA+/–tetO-cre–/–pROSA-IKK2ca+/–, wildtype [WT]) mice were generated through crossing of SFTPC-rtTA+/+tetO-cre+/– and pROSA-IKK2ca+/+. Ikk2ca overexpression was induced through feeding with doxycycline diet (625 mg/kg diet, Envigo TD.01306) for one or 3 months. All mice were housed in standard cages (2–5 mice/cage) with free access to food and water, a 12-h light/12-h dark cycle, temperatures of 20°C–25 °C, and relative humidity of 40%–60%.
Bronchoalveolar lavage and lung histopathology: After euthanasia by overdose of isoflurane, the mouse trachea was cannulated and the right primary bronchus was closed off with a ligature. 0.5 ml sterile phosphate-buffered saline with 0.1 mM EDTA was instilled through the tracheal cannula and withdrawn to recover bronchoalveolar lavage fluid (BALF). This was repeated 3 times. Total number of cells in the collected BALF (around 1.5 ml) was estimated using a hemocytometer. Cytospin slides were prepared using Shandon Cytospin 3 and stained with Diff-Quick solution (EMS, Hatfield, PA). Differential cell counts for neutrophils, eosinophils, macrophages/monocytes, and lymphocytes were assessed by a pathologist who was blinded to the grouping.
Following BALF collection, the right lung was harvested and either fixed with 4% paraformaldehyde or snap-frozen in liquid nitrogen and then kept at −80 °C. To assess the inflammation in the lung, tissue blocks were embedded in paraffin and 5-μm thick sections were cut, and the sections were subjected to hematoxylin and eosin staining. Three consecutive sections per sample were used for histopathology. Images covering all the tissue area were taken by a laboratory technician who was blinded to the grouping, and all images were then sent to and quantitated by the pathologist (blinded to the grouping too).
Wet/Dry lung weight ratio: LungIKK2ca and littermate WT mice (12–15-weeks old, n = 5/group) were fed with doxycycline diet for 1 month. After necropsy, the lungs were dissected and weighed immediately after their excision (wet lung weight). The lungs were then dried in an oven at 60 °C for 5 days and re-weighed as dry weight. The Wet/Dry weight ratio was calculated by dividing the wet by the dry weight as described previously (Kitamura et al., 2001).
Intraperitoneal glucose tolerance test (IPGTT): Before testing, mice were fasted for 16 h. On the day of experiments, basal blood glucose level was determined using an automatic glucometer (Glucotrend 2, Roche Diagnostics), and then mice were intraperitoneally injected with glucose (2 g/kg body weight). Blood glucose levels at 15, 30, 60, and 120 min after injection were sequentially measured as described earlier.
Insulin tolerance test
Before testing, mice were fasted for 4 h. Basal blood glucose level was determined using an automatic glucometer (Glucotrend 2, Roche Diagnostics) and then mice were intraperitoneally injected with insulin (0.5 U/kg body weight). Blood glucose levels at 15, 30, 60, and 120 min after injection were sequentially measured as described above.
Plasma analysis
Plasma insulin (Ultra Sensitive Mouse Insulin ELISA Kit, Crystal Chemical), tumor necrosis factor-α (TNFα) (Mouse TNFα ELISA Ready-Set-Go!, eBioscience, Inc.), and IL-6 (Mouse IL-6 PicoKine ELISA Kit, Boster Biological Technology) levels were determined per manufacturer’s instructions.
Real-time RT-PCR
Total RNA was extracted and purified using the Trizol reagent (Invitrogen, USA). The quality of RNA was assessed by determination of the ratio of absorbance at 260 nm to absorbance at 280 nm by nanodrop. In total 2.0 μg of total DNase-treated RNA were reverse transcribed into cDNA using High Capacity cDNA Reverse Transcription Kits (Applied Biosystem) per manufacture’s instruction. Real-time PCR was performed using LightCycler 480 SYBR Green I Master in the LightCycler (Roche, German). Reactions were performed in a total volume of 10 μl containing 1 μl cDNA, 0.2 μM of each primer and 5 μl of the SYBR Green reaction mix. The amplification protocol was as follows: 95 °C/5 min (95 °C/10 s, 60 °C/20 s, and 72 °C/30 s) × 45. Following amplification, a dissociation curve analysis was performed to insure purity of PCR product. The specific sense and antisense primers were previously described in Chen et al. (2017).
Western blotting
Standard techniques as previously reported in Ying et al. (2013) were performed with primary antibodies: mouse antiIKK2 (Millipore catalog no.: 05-535), mouse anti-HA (Sigma catalog no.: H3663), and mouse anti-HPRT1 (BIO-RAD catalog no.: VMA00483). Signals were detected by chemiluminescence and analyzed by densitometry.
Vascular function assay
After euthanasia, mouse thoracic aorta was quickly removed and cleaned in physiological salt solution (PSS) containing (mM): NaCl, 130; NaHCO3, 14.9; KCl, 4.7; KH2PO4, 1.18; MgSO4·7H2O, 1.18; CaCl2·2H2O, 1.56; EDTA, 0.026; and glucose, 5.5. The aorta was cut into 2-mm rings. The aortic rings were then mounted in a muscle bath containing PSS at 37 °C and bubbled with 95% O2–5% CO2. Isometric force generation was recorded with a Multi Myograph System (Danish Myo Technology A/S, Aarhus N, Denmark). A resting tension of 4 mN was imposed on each ring, and the rings were allowed to equilibrate for 1 h. To test vascular function, the contractile responses of aortic rings to phenylephrine (PE) in the absence or presence of a NOS inhibitor ω-nitro-L-arginine methyl ester (L-NAME 100 μM) were assessed in an accumulative manner. To analyze the endothelial function, aortic rings were precontracted with PE (1 μM), and acetylcholine (ACh) or sodium nitroprusside (SNP) was added in an accumulative manner.
Statistics
All data are expressed as means ± SEMs unless noted otherwise. Statistical tests were performed using 1- or 2-way analysis of variance (ANOVA) or unpaired t test using GraphPad Prism (version 4.1.2; GraphPad Software, La Jolla, California). The significance level was set at p < .05.
RESULTS
Lung-Specific Overexpression of Constitutively Active Ikk2 (Ikk2ca) in Doxycycline-Fed LungIKK2ca Mice
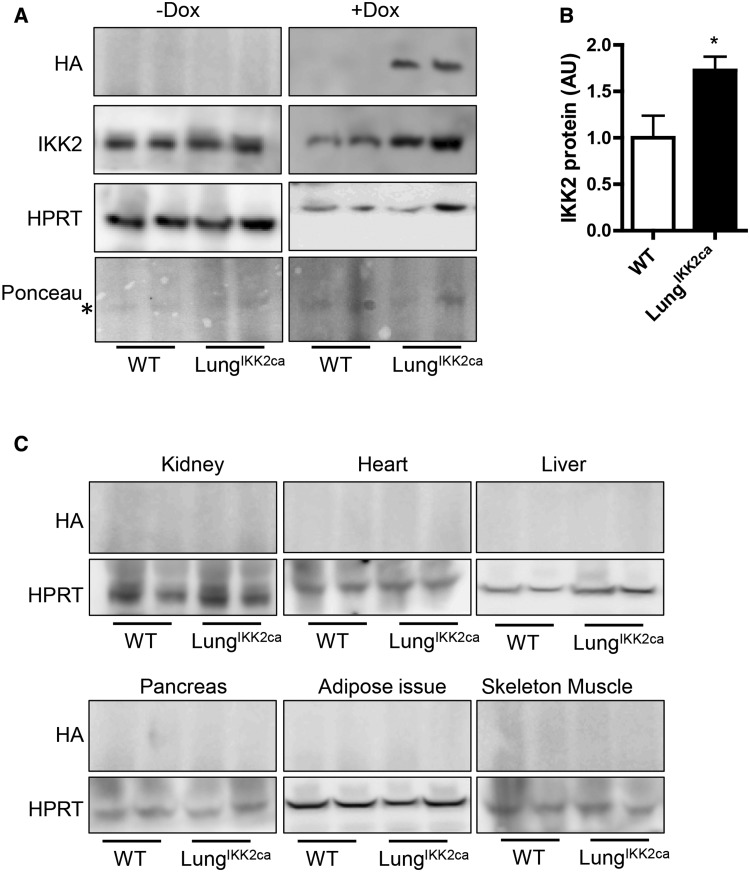
In order to specifically induce inflammation in the lung, we generated LungIKK2ca mice through outcrosses of 3 genetically modified mice (SFTPC-rtTA, tetO-cre, and pROSA-IKK2ca+/+). The ratio of LungIKK2ca mice in newborns was consistent with Mendelian inheritance (27 out of 52 newborns, p = .84 versus Mendelian inheritance, chi-square test.). No any macroscopic abnormality was observed in adult LungIKK2ca mice (up to 26-weeks old). Figure 1A reveals that no HA-tagged IKK2ca was observed in the lung of LungIKK2ca mice fed with normal diet. In contrast, there was marked overexpression of Ikk2ca in the lung of LungIKK2ca mice fed with doxycycline diet for one month. The concurrent western blot analyses did not observe Ikk2ca expression in kidney, heart, liver, pancreas, epididymal adipose tissue, and skeleton muscle of these doxycycline-fed WT and LungIKK2ca mice (Figure 1C).
Figure 1.
Lung-specific overexpression of IKK2ca induced by doxycycline diet. SFTPC-rtTA+tetO-cre+pROSA-IKK2ca+ (LungIKK2ca) and littermate SFTPC-rtTA+tetO-cre-pROSA-IKK2ca+ (WT) mice were fed with doxycycline (+DOX) or normal (−DOX) diet for one month. The expression of HA-tagged IKK2ca in the lung (A and B) and other major organs (C) were assessed by western blot. The representative images (A) and the summary (B) of IKK2ca expression in the lung are presented (n = 4–8/group). *p < .05 versus WT, student t test. C, The representative images of HA-tagged IKK2ca in the indicated major organs are presented.
Lung-Specific Overexpression of Ikk2ca Induces Pronounced Pulmonary Inflammation
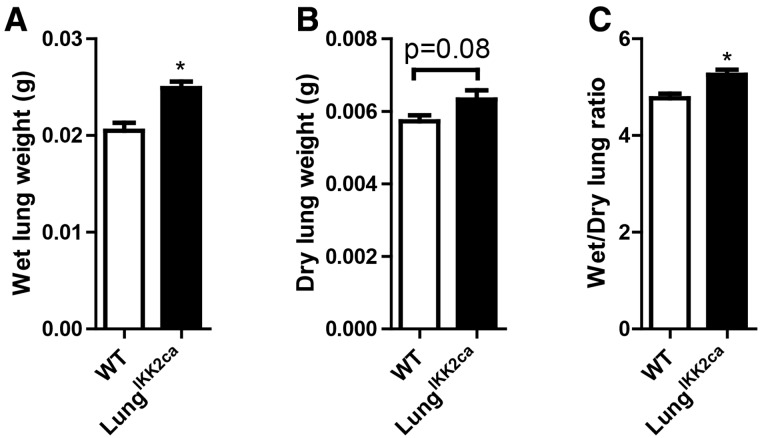
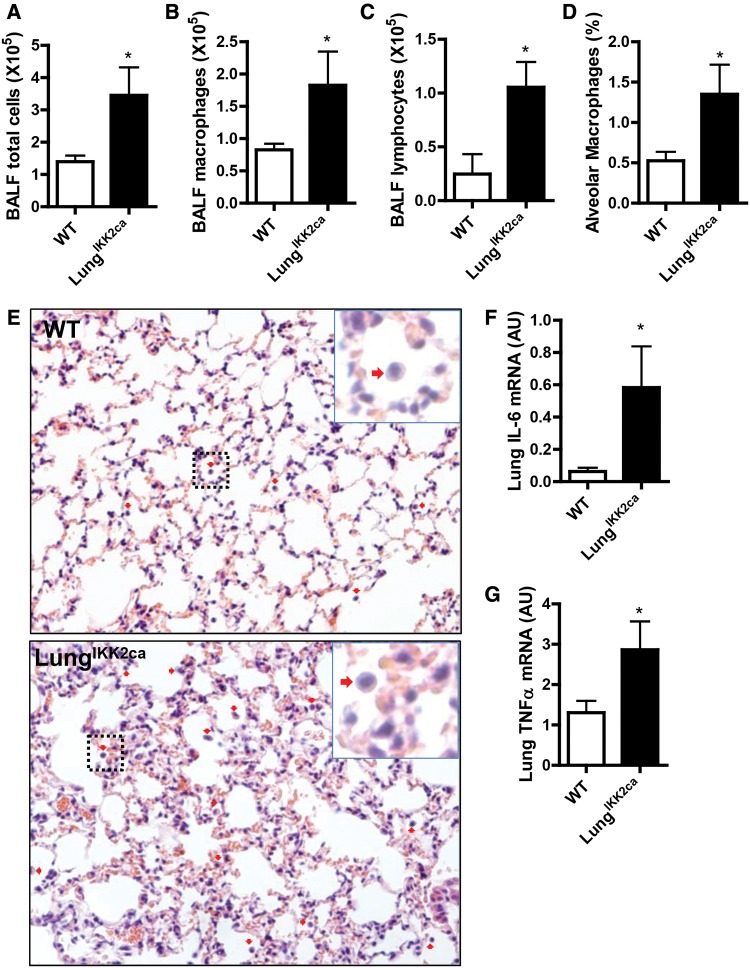
Consistent with the locus of Ikk2ca over-expression, Figure 2A and Table 1 reveal that compared with WT controls, doxycycline-fed LungIKK2ca mice had a significant increase in the weight of lung but not other major organs. This increase in wet lung weight appeared to be due to both edema and increase in dry lung weight (Figs. 2B and 2C). To determine whether there is pulmonary inflammation in doxycycline-fed LungIKK2ca mice, we performed BALF cell differentiation test. Figures 3A–C show that there were markedly increased total cells, macrophages, and lymphocytes in the BALF from doxycycline-fed LungIKK2ca mice, strongly suggesting that these mice had pulmonary inflammation. To further document this pulmonary inflammation, we performed pathological assessment of pulmonary inflammation. Figures 3D and 3E demonstrate markedly increased macrophage infiltration in the lung of LungIKK2ca but not WT mice. Consistent with BALF cell differentiation and pathological assessment, qPCR analyses showed marked increases in Tnfα and Il-6 mRNA expression levels in the lung of LungIKK2ca mice (Figs. 3F and 3G). Taken together, these data strongly support that over-expression of Ikk2ca in the lung induces marked pulmonary inflammation.
Figure 2.
Lung-specific overexpression of IKK2ca increases the weight of lung. LungIKK2ca and littermate WT mice were fed with doxycycline diet for one month. After necropsy, the lungs were harvested, and their wet weights (A), dry weights (B), and wet/dry ratio (C) were presented (n = 5/group). *p < .05 versusWT, student t test.
Table 1.
Organ Weights
| WT |
LungIKK2ca |
p Value | |||
|---|---|---|---|---|---|
| Mean (g) | SD | Mean (g) | SD | ||
| Body | 29.550 | 2.086 | 27.800 | 2.331 | 0.370 |
| Lung | 0.257 | 0.013 | 0.294 | 0.021 | 0.032 |
| Liver | 1.060 | 0.122 | 1.002 | 0.107 | 0.557 |
| Heart | 0.142 | 0.011 | 0.134 | 0.004 | 0.233 |
| Spleen | 0.085 | 0.009 | 0.081 | 0.015 | 0.696 |
| Kidney | 0.340 | 0.031 | 0.308 | 0.018 | 0.168 |
| Pancreas | 0.197 | 0.051 | 0.156 | 0.019 | 0.244 |
| Brain | 0.425 | 0.026 | 0.423 | 0.035 | 0.939 |
| Epididymal fat | 0.895 | 0.374 | 0.837 | 0.350 | 0.851 |
| Perirenal fat | 0.295 | 0.219 | 0.256 | 0.119 | 0.794 |
| Subcutaneous fat | 0.290 | 0.163 | 0.238 | 0.116 | 0.667 |
| Brown fat | 0.215 | 0.123 | 0.215 | 0.075 | 0.999 |
WT and LungIKK2ca mice were fed with doxycycline diet for 3 months and the indicated organs were harvested and weighed. 8 WT and 6 LungIKK2ca.
Figure 3.
Lung-specific overexpression of IKK2ca induces pulmonary inflammation. WT and LungIKK2ca mice were fed with doxycycline diet for 3 months. A–C, BALF cell differentiation. The right lungs were not subjected to lavage and thus used for histological assessments (E) and quantitation (D) of pulmonary inflammation. ↓Macrophages. Scale bar, 100 µm. F, G, qPCR analyses of proinflammatory cytokine expression in the lung (n = 6 or 8). *p < .05 versus WT, student t test.
Lung-Specific Overexpression of Ikk2ca Induces Systemic and Adipose Inflammations but Not Hypothalamic Inflammation
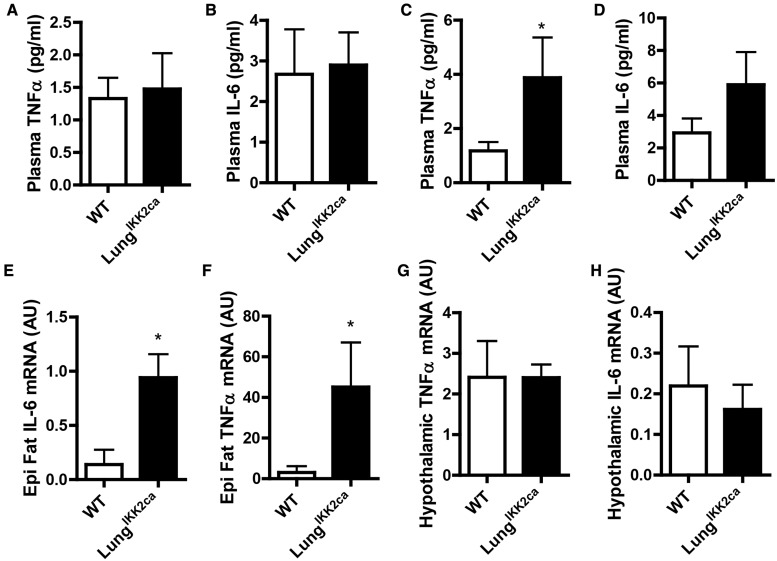
PM2.5 exposure causes cardiometabolic abnormalities putatively through various extrapulmonary inflammations which are widely believed to be subsequent to pulmonary inflammation (Brook et al., 2010). Given the pronounced pulmonary inflammation in doxycycline-fed LungIKK2ca mice, we next examined if they developed extrapulmonary inflammations. Figures 4A and 4B show that if fed with normal diet, WT and LungIKK2ca mice had comparable circulating TNFα and IL-6 levels. After 3-month-feeding with doxycycline, LungIKK2ca mice had significantly higher circulating TNFα and IL-6 levels than WT mice had (Figs. 4C and 4D). This increase in circulating TNFα and IL-6 was accompanied by increased mRNA expression of Tnfα and Il-6 in epididymal adipose tissue (Figs. 4E and 4F). We previously demonstrated that hypothalamic inflammation may play a crucial role in mediating PM2.5 exposure-related pathophysiology (Ying et al., 2014), which was also believed to be subsequent to pulmonary inflammation. We however found that lung-specific over-expression of Ikk2ca did not significantly change the mRNA expression of Tnfα and Il-6 in the hypothalamus (Figs. 4G and 4H).
Figure 4.
Lung-specific overexpression of IKK2ca induces systemic and adipose but not hypothalamic inflammation. A, B, Plasma were harvested from normal diet-fed WT and LungIKK2ca mice (10–18-weeks old, n = 5/group), and their TNFα and IL-6 were measured by ELISA. C-H, WT, and LungIKK2ca mice were fed with doxycycline diet for 3 months. C, D, Plasma TNFα and IL-6 were measured by ELISA. E, F, TNFα and IL-6 mRNA in the epididymal adipose tissue were measured by qPCR. G, H, TNFα and IL-6 mRNA in the hypothalamus were measured by qPCR (n = 6 or 8). *p < .05 versus WT, student t test.
Lung-Specific Overexpression of Ikk2ca Induces Endothelial Dysfunction
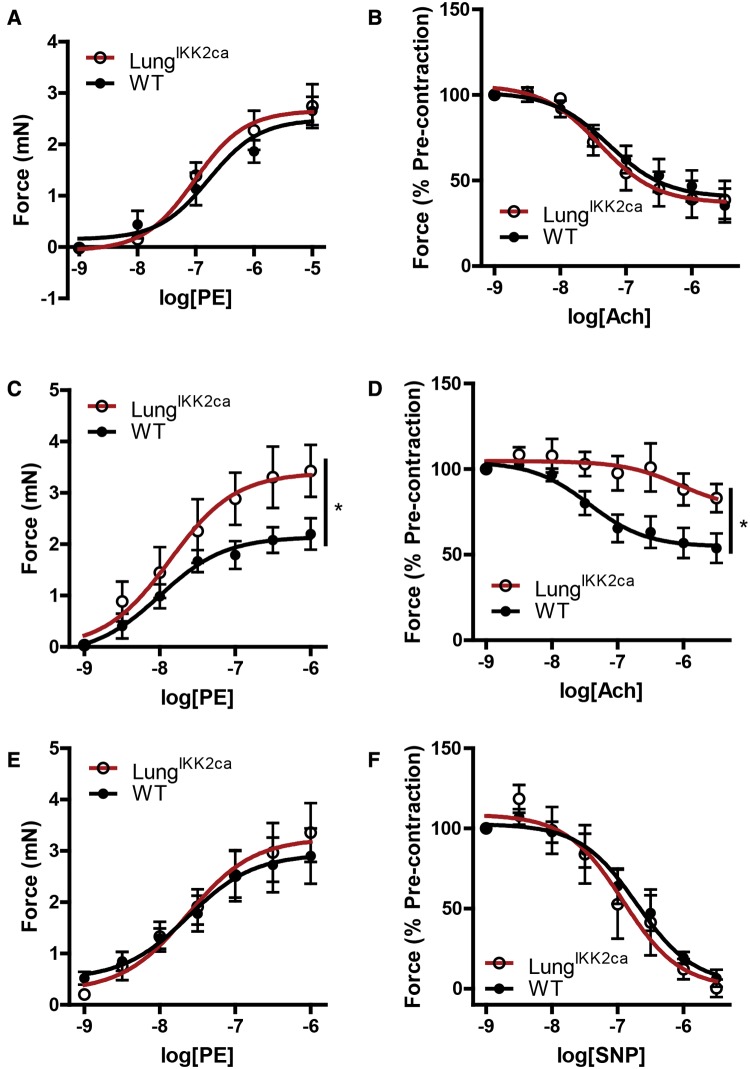
Endothelial dysfunction is one of the critical adverse effects induced by PM2.5 exposure that may be responsible for PM2.5 exposure-related various cardiometabolic abnormalities such as insulin resistance and hypertension, and it is also believed to be subsequent to systemic inflammation. Figures 5A and 5B reveal that if fed with normal diet, aorta from LungIKK2ca and WT littermates had comparable responses to PE, a vasoconstrictor, and Ach, an endothelium-dependent vasodilator. Consistent with study on PM2.5 exposure mouse models (Kampfrath et al., 2011), aortas from doxycycline-fed LungIKK2ca mice had a significantly increased contractile response to PE (Figure 5C), and this was paralleled by a significantly decreased relaxation by Ach (Figure 5D). NOS inhibitor L-NAME abolished the difference of aortic contractile response to PE between WT and LungIKK2ca mice (Figure 5E), and there was no difference in relaxation by endothelium-independent vasodilator SNP (Figure 5F), strongly suggesting that the vascular dysfunction of LungIKK2ca mice is primarily due to endothelial dysfunction.
Figure 5.
Lung-specific overexpression of IKK2ca induces endothelial dysfunction. A, B, Thoracic aorta were harvested from normal diet-fed WT and LungIKK2ca mice (10–18-weeks old, n = 5/group). A, Their contractile responses to PE. B, They were precontracted by PE (1 µM) and then relaxed by Ach. C–F, WT and LungIKK2ca mice were fed with doxycycline diet for 3 months. C, Aortic contractile response to PE. D, Aortic rings were precontracted by PE (1 µM) and then relaxed by Ach. E, Aortic contractile response to PE in the presence of NOS inhibitor L-NAME. F, Aortic rings were precontracted by PE and then relaxed by NO donor, SNP (n = 6 or 8). *p < .05 versus WT, 2-way ANOVA.
Lung-Specific Overexpression of Ikk2ca Induces Insulin Resistance but Not Glucose Intolerance
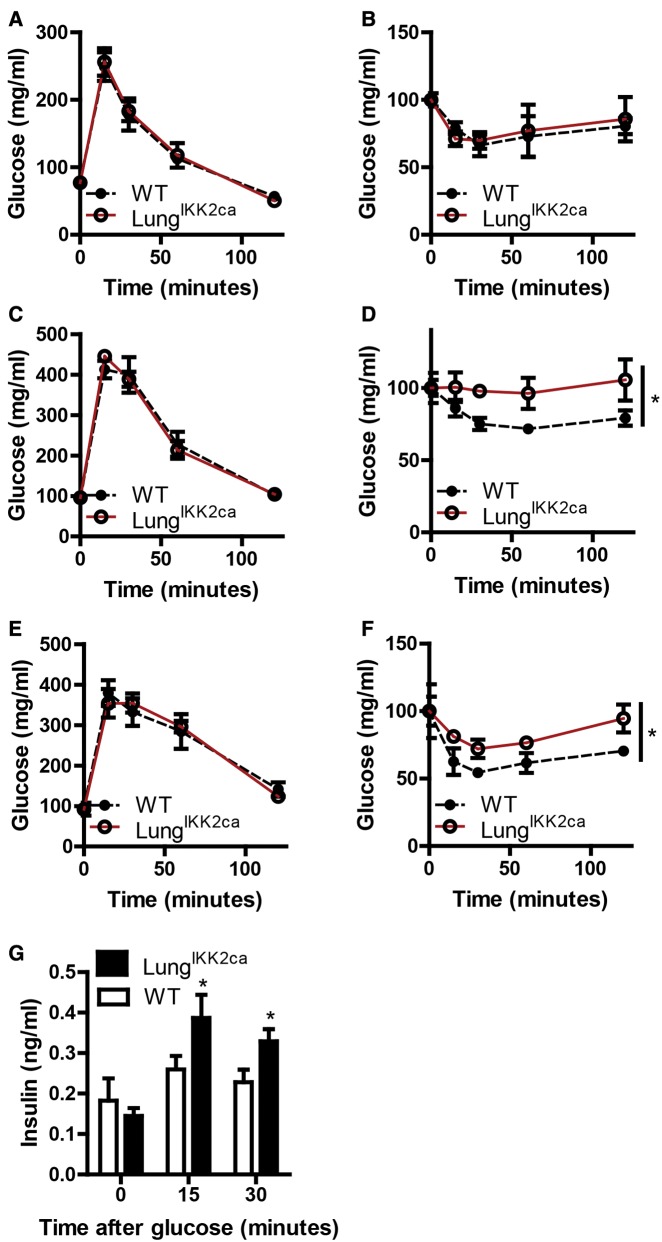
Increasing evidence has indicated that PM2.5 exposure is associated with abnormalities in glucose homeostasis (Liu et al., 2013). To test if pulmonary inflammation mediates PM2.5 exposure-induced abnormalities in glucose homeostasis, we examined glucose homeostasis of adult LungIKK2ca and WT mice. Figures 6A and 6B show that no any significant difference in glucose metabolism between LungIKK2ca and littermate WT mice was observed before the induction of IKK2ca overexpression in the lung. After 1-month-feeding with doxycycline diet, insulin tolerance test (ITT) revealed that compared with littermate WT, LungIKK2ca mice developed marked insulin resistance (Figure 6D). Surprisingly, IPGTT however did not show any impairment of glucose tolerance in LungIKK2ca mice (Figure 6D). We previously noted that it took about 12 weeks for exposure to PM2.5 to cause glucose intolerance in mouse models (Liu et al., 2014). To rule out the possibility that the lack of glucose intolerance in doxycycline-fed LungIKK2ca mice is due to not enough duration of feeding with doxycycline diet, these mice were fed with doxycycline diet for 2 more months, and IPGTT and ITT were repeated. Figures 6E and 6F reveal that after 3 months feeding with doxycycline diet, LungIKK2ca mice had insulin resistance but still normal glucose tolerance. As glucose tolerance is determined by both insulin sensitivity and glucose-induced insulin secretion (GIIS), we examined GIIS in these mice after 3 months feeding with doxycycline diet. Consistent with ITT and IPGTT results, Figure 6G shows that GIIS was significantly increased in LungIKK2ca mice.
Figure 6.
Lung-specific overexpression of IKK2ca induces insulin resistance but not glucose intolerance. IPGTT (A, C, and E) and ITT (B, D, and F) were performed before feeding with doxycycline (A and B), after 1-month feeding (C and D), and after 3 months feeding (E and F). G, When performing IPGTT after 3 months feeding with doxycycline diet, plasma was collected at the indicated time points, and insulin levels were assessed by ELISA. 8 WT and 6 LungIKK2ca. *p < .05 versus WT, 2-way ANOVA.
DISCUSSION
The airway is constantly exposed to environmental stimuli including various ambient microbes, particles, and toxicants, making it one of the most frequent locations for inflammation in the body. Pulmonary inflammation is not only well known to play a crucial role in the pathogenesis of pulmonary diseases such as asthma, chronic obstructive pulmonary disease (COPD), and sarcoidosis, but also widely believed to have the potential to initiate, aggravate and propagate extrapulmonary inflammation and thus cause various adverse extrapulmonary effects (Wahlund et al., 2017). However, whether pulmonary inflammation is sufficient to cause adverse cardiometabolic effects such as endothelial dysfunction and insulin resistance remains to be determined. In this study, we therefore investigated the extrapulmonary effects of pulmonary inflammation due to lung-specific overexpression of IKK2ca. The main findings include that: (1) lung-specific overexpression of Ikk2ca was sufficient to induce pulmonary inflammation that models various aspects of PM2.5 exposure-induced pulmonary pathology; (2) this pulmonary inflammation was accompanied by increases in circulating proinflammatory cytokines, adipose inflammation, endothelial dysfunction, and insulin resistance; (3) this pulmonary inflammation did not cause hypothalamic inflammation and glucose intolerance. To our best knowledge, this is the first study that experimentally examined the cardiometabolic effects of pulmonary inflammation. Our results strongly support that pulmonary inflammation is sufficient to cause systemic inflammation, endothelial dysfunction, and insulin resistance, but not hypothalamic inflammation.
IKK2/nuclear factor-κB (NF-κB) signaling pathway is well-known to play a central role in various inflammatory responses (Gamble et al., 2012). Consistent with this, our data reveal that overexpression of Ikk2ca was sufficient to induce pulmonary inflammation, as evidenced by increased BALF leukocytes, increased Tnfα and Il-6 mRNA expression, and marked inflammation-related pathological changes. These pathologies have also been observed in PM2.5-exposed humans and animal models (He et al., 2015; Li et al., 2015; Olsen et al., 2014; Riva et al., 2011). Given the compelling evidence that exposure to PM2.5 is sufficient to activate IKK2/NF-κB signaling pathway in the lung (Gu et al., 2017; Jeong et al., 2017; Li et al., 2016), these results have corroborated that the pulmonary activation of this signaling pathway is crucial in PM2.5 exposure-induced pulmonary inflammation. However, it should be noted that the present study does not rule out the possibility that other signaling pathways such as c-Jun N-terminal kinase/activator protein-1 may also play a role in the mediation of PM2.5 exposure-induced pulmonary inflammation.
Time course studies have demonstrated that pulmonary inflammation precedes elevation of circulating inflammatory markers in PM2.5 exposure-related pathophysiology (Brook et al., 2010), suggesting that there may be causality between pulmonary and systemic inflammations. However, their covariance has not yet been systemically investigated, despite that it is indispensable to establish the causality. Our present data show that while lung-specific Ikk2ca overexpression induced marked pulmonary inflammation, it was also sufficient to increase circulating TNFα and IL-6 levels. To our best knowledge, this is the first study showing evidence of covariance between pulmonary and systemic inflammations. These results are perfectly consistent with the time course studies (Brook et al., 2010), strongly supporting that pulmonary inflammation is sufficient to mediate PM2.5 exposure-related systemic inflammation.
In cardiometabolic research, it comes to be a consensus that systemic inflammation is one of the major risk factor for cardiometabolic diseases such as hypertension and diabetes (Maiorino et al., 2017). Given that exposure to PM2.5 not only causes various components of cardiometabolic diseases but also induced marked systemic inflammation, it has been widely believed that the development of adverse cardiometabolic effects due to PM2.5 exposure may be mediated by systemic inflammation and thus also subsequent to pulmonary inflammation (Brook et al., 2010). Consistent with this notion, our present data reveal that lung-specific overexpression of IKK2ca not only causes marked pulmonary inflammation and increases circulating TNFα and IL-6 levels but also induces marked endothelial dysfunction and insulin resistance, 2 well-known adverse effects of PM2.5 exposure (Esposito et al., 2016; Pope et al., 2016), strongly supporting a crucial role of pulmonary inflammation in the development of those adverse cardiometabolic effects due to PM2.5 exposure.
Rapidly increasing evidence has indicated that central, particularly hypothalamic, inflammation plays a critical role in the pathogenesis of various cardiometabolic diseases (Han et al., 2016). We recently demonstrated that hypothalamic inflammation may be crucial for PM2.5 exposure-induced hypertension, which was believed to be subsequent to systemic and thus pulmonary inflammation (Ying et al., 2014). In this study, our data however surprisingly show that lung-specific overexpression of Ikk2ca was not sufficient to induce hypothalamic inflammation. This is in direct contrast to marked adipose inflammation, another established adverse effects of exposure to PM2.5 (Sun et al., 2009). These data strongly suggest that hypothalamic inflammation has different genesis from that of peripheral inflammations, and Ikk2ca overexpression-induced pulmonary Notably, we recently also demonstrated that central inhibition of IKK2 markedly reduced PM2.5 exposure-induced pulmonary and systemic inflammations (Liu et al., 2014). Taken together, our data arouse the possibility that opposite to the putative pathophysiological cascade of PM2.5 exposure/pulmonary inflammation/systemic inflammation/hypothalamic inflammation, pulmonary and systemic inflammations may indeed be downstream from hypothalamic inflammation and thus partly mediate its effects. This is consistent with recent studies showing that hypothalamic inflammation is not a consequence but a cause of obesity in overnutrition-related pathophysiology (Jais and Bruning, 2017). As this study somehow disproved the putative genesis of PM2.5 exposure-induced hypothalamic inflammation, further studies are undergoing to test the other mechanisms that are recently proposed (Underwood, 2017).
One more important finding in the present study is that although lung-specific overexpression of IKK2ca is sufficient to induce insulin resistance, it does not cause glucose intolerance. This appears to be due to the counteraction between induction of insulin resistance and increased GIIS. Many studies have demonstrated that systemic inflammation is central in the pathophysiology of type 2 diabetes mellitus through induction of insulin resistance and injury of pancreatic beta cells (Keane et al., 2015). In addition to those mechanisms, the present study reveals that systemic inflammation may also impact glucose homeostasis through effects on GIIS. This is consistent with a recent study showing that postprandial macrophage-derived interleukine-1β (IL-1β) stimulates insulin secretion (Dror et al., 2017). In contrast, PM2.5 exposure causes both insulin resistance and glucose intolerance in mouse models (Liu et al., 2014). Given that glucose tolerance is determined by both insulin sensitivity and GIIS, this study additionally suggests that there may be pulmonary inflammation-independent mechanism(s) whereby exposure to PM2.5 inhibits GIIS. It is noteworthy that GIIS is regulated by ANS and PM2.5 exposure has been shown to induce ANS dysfunction likely through induction of hypothalamic inflammation (Ying et al., 2014). The present data thus raise a possibility that PM2.5 exposure inhibits GIIS through induction of hypothalamic inflammation and subsequently ANS dysfunction. Along with the demonstration of different genesis of hypothalamic inflammation from that of peripheral inflammation, these data highlight the role of hypothalamic inflammation in the mediation of PM2.5 exposure-related glucose intolerance.
CONCLUSION
This study demonstrates that pulmonary inflammation is sufficient to induce systemic inflammation, endothelial dysfunction, and insulin resistance, but not hypothalamic inflammation and glucose intolerance.
FUNDING
National Institutes of Health (R01ES024516 to Z.Y.); the American Heart Association (13SDG17070131 to Z.Y.); the National Natural Science Foundation of China (81270342 to Z.Y., 81500216 to M.C., and 81302452 to L.Q.); Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (SSF201008) and Shanghai 3-Year Public Health Action Plan (GWTD2015S04) to Z.Y.
REFERENCES
- Barton D. B., Betteridge B. C., Earley T. D., Curtis C. S., Robinson A. B., Reynolds P. R. (2014). Primary alveolar macrophages exposed to diesel particulate matter increase RAGE expression and activate RAGE signaling. Cell Tissue Res. 358, 229–238. [DOI] [PubMed] [Google Scholar]
- Brook R. D., Rajagopalan S., Pope C. A. 3rd, Brook J. R., Bhatnagar A., Diez-Roux A. V., Holguin F., Hong Y., Luepker R. V., Mittleman M. A., et al. (2010). Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378. [DOI] [PubMed] [Google Scholar]
- Chen M., Liang S., Zhou H., Xu Y., Qin X., Hu Z., Wang X., Qiu L., Wang W., Zhang Y., et al. (2017). Prenatal and postnatal mothering by diesel exhaust PM2.5-exposed dams differentially program mouse energy metabolism. Part Fibre Toxicol. 14, 3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher Z., Garcon G., Billet S., Verdin A., Ledoux F., Courcot D., Aboukais A., Shirali P. (2007). Role of nuclear factor-kappa B activation in the adverse effects induced by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. J. Appl. Toxicol. 27, 284–290. [DOI] [PubMed] [Google Scholar]
- Dror E., Dalmas E., Meier D. T., Wueest S., Thevenet J., Thienel C., Timper K., Nordmann T. M., Traub S., Schulze F., et al. (2017). Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 183, 283–292. [DOI] [PubMed] [Google Scholar]
- Esposito K., Petrizzo M., Maiorino M. I., Bellastella G., Giugliano D. (2016). Particulate matter pollutants and risk of type 2 diabetes: A time for concern?. Endocrine 51, 32–37. Review). [DOI] [PubMed] [Google Scholar]
- Gamble C., McIntosh K., Scott R., Ho K. H., Plevin R., Paul A. (2012). Inhibitory kappa B Kinases as targets for pharmacological regulation. Br. J. Pharmacol. 165, 802–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L. Z., Sun H., Chen J. H. (2017). Histone deacetylases 3 deletion restrains PM2.5-induced mice lung injury by regulating NF-kappaB and TGF-beta/Smad2/3 signaling pathways. Biomed. Pharmacother. 85, 756–762. [DOI] [PubMed] [Google Scholar]
- Han C., Rice M. W., Cai D. (2016). Neuroinflammatory and autonomic mechanisms in diabetes and hypertension. Am. J. Physiol. Endocrinol. Metab. 311, E32–E41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Ichinose T., Ren Y., Song Y., Yoshida Y., Arashidani K., Yoshida S., Nishikawa M., Takano H., Sun G. (2015). PM2.5-rich dust collected from the air in Fukuoka, Kyushu, Japan, can exacerbate murine lung eosinophilia. Inhal. Toxicol. 27, 287–299. [DOI] [PubMed] [Google Scholar]
- Jais A., Bruning J. C. (2017). Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Invest. 127, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S. C., Cho Y., Song M. K., Lee E., Ryu J. C. (2017). Epidermal growth factor receptor (EGFR)-MAPK-nuclear factor(NF)-kappaB-IL8: A possible mechanism of particulate matter(PM) 2.5-induced lung toxicity. Environ. Toxicol. 325, 1628–1636. [DOI] [PubMed] [Google Scholar]
- Kafoury R. M., Madden M. C. (2005). Diesel exhaust particles induce the over expression of tumor necrosis factor-alpha (TNF-alpha) gene in alveolar macrophages and failed to induce apoptosis through activation of nuclear factor-kappaB (NF-kappaB). Int. J. Environ. Res. Public Health 2, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfrath T., Maiseyeu A., Ying Z., Shah Z., Deiuliis J. A., Xu X., Kherada N., Brook R. D., Reddy K. M., Padture N. P., et al. (2011). Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 108, 716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane K. N., Cruzat V. F., Carlessi R., de Bittencourt P. I. Jr, Newsholme P. (2015). Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and beta-Cell Dysfunction. Oxid. Med. Cell. Longevity 2015, 181643.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Hashimoto S., Mizuta N., Kobayashi A., Kooguchi K., Fujiwara I., Nakajima H. (2001). Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am. J. Respir. Crit. Care Med. 163, 762–769. [DOI] [PubMed] [Google Scholar]
- Kreyling W. G., Semmler-Behnke M., Seitz J., Scymczak W., Wenk A., Mayer P., Takenaka S., Oberdorster G. (2009). Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal. Toxicol. 21(Suppl. 1), 55–60. [DOI] [PubMed] [Google Scholar]
- Levesque S., Taetzsch T., Lull M. E., Kodavanti U., Stadler K., Wagner A., Johnson J. A., Duke L., Kodavanti P., Surace M. J., et al. (2011). Diesel exhaust activates and primes microglia: Air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ. Health Perspect. 119, 1149–1155. Research Support, N.I.H., Extramural). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Kou X., Xie L., Cheng F., Geng H. (2015). Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environ. Sci. Pollut. Res. Int. 22, 20167–20176. [DOI] [PubMed] [Google Scholar]
- Li R., Zhao L., Tong J., Yan Y., Xu C. (2016). Fine Particulate Matter and Sulfur Dioxide Coexposures Induce Rat Lung Pathological Injury and Inflammatory Responses Via TLR4/p38/NF-kappaB Pathway. Int. J. Toxicol. 362, 165–173. [DOI] [PubMed] [Google Scholar]
- Li Y. J., Shimizu T., Hirata Y., Inagaki H., Takizawa H., Azuma A., Kawada T., Sugawara I., Kudoh S., Sunazuka T., et al. (2013). EM, EM703 inhibit NF-kB activation induced by oxidative stress from diesel exhaust particle in human bronchial epithelial cells: Importance in IL-8 transcription. Pulm. Pharmacol. Ther. 26, 318–324. [DOI] [PubMed] [Google Scholar]
- Liu C., Fonken L. K., Wang A., Maiseyeu A., Bai Y., Wang T. Y., Maurya S., Ko Y. A., Periasamy M., Dvonch T., et al. (2014). Central IKKbeta inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part. Fibre Toxicol. 11, 53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Ying Z., Harkema J., Sun Q., Rajagopalan S. (2013). Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol. Pathol. 41, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez B., Maisonet T. M., Londhe V. A. (2015). Alveolar NF-kappaB signaling regulates endotoxin-induced lung inflammation. Exp. Lung Res. 41, 103–114. [DOI] [PubMed] [Google Scholar]
- Maciejczyk P., Chen L. C. (2005). Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. VIII. Source-related daily variations in invitro responses to CAPs. Inhal. Toxicol. 17, 243–253. [DOI] [PubMed] [Google Scholar]
- Maiorino M. I., Bellastella G., Giugliano D., Esposito K. (2017). Cooling down inflammation in type 2 diabetes: How strong is the evidence for cardiometabolic benefit?. Endocrine 55, 360–365. [DOI] [PubMed] [Google Scholar]
- Mantecca P., Farina F., Moschini E., Gallinotti D., Gualtieri M., Rohr A., Sancini G., Palestini P., Camatini M. (2010). Comparative acute lung inflammation induced by atmospheric PM and size-fractionated tire particles. Toxicol. Lett. 198, 244–254. [DOI] [PubMed] [Google Scholar]
- Nam H. Y., Choi B. H., Lee J. Y., Lee S. G., Kim Y. H., Lee K. H., Yoon H. K., Song J. S., Kim H. J., Lim Y. (2004). The role of nitric oxide in the particulate matter (PM2.5)-induced NFkappaB activation in lung epithelial cells. Toxicol. Lett. 148, 95–102. [DOI] [PubMed] [Google Scholar]
- Oberdorster G., Sharp Z., Atudorei V., Elder A., Gelein R., Lunts A., Kreyling W., Cox C. (2002). Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J. Toxicol. Environ. Health A 65, 1531–1543. [DOI] [PubMed] [Google Scholar]
- Oberdorster G., Utell M. J. (2002). Ultrafine particles in the urban air: To the respiratory tract–and beyond? Environ. Health Perspect 110, A440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen Y., Karottki D. G., Jensen D. M., Beko G., Kjeldsen B. U., Clausen G., Hersoug L. G., Holst G. J., Wierzbicka A., Sigsgaard T., et al. (2014). Vascular and lung function related to ultrafine and fine particles exposure assessed by personal and indoor monitoring: A cross-sectional study. Environ. Health 13, 112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero J. E., Chen T., Zhang K., Abu-Amer Y. (2012). Constitutively active canonical NF-kappaB pathway induces severe bone loss in mice. PloS One 7, e38694.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl H. L. (1999). Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18, 6853–6866. [DOI] [PubMed] [Google Scholar]
- Pope C. A. 3rd, Bhatnagar A., McCracken J. P., Abplanalp W., Conklin D. J., O’Toole T. (2016). Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ. Res. 119, 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D. R., Magalhaes C. B., Lopes A. A., Lancas T., Mauad T., Malm O., Valenca S. S., Saldiva P. H., Faffe D. S., Zin W. A. (2011). Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal. Toxicol. 23, 257–267. [DOI] [PubMed] [Google Scholar]
- Semmler-Behnke M., Kreyling W. G., Lipka J., Fertsch S., Wenk A., Takenaka S., Schmid G., Brandau W. (2008). Biodistribution of 1.4- and 18-nm gold particles in rats. Small 4, 2108–2111. [DOI] [PubMed] [Google Scholar]
- Sun Q., Yue P., Deiuliis J. A., Lumeng C. N., Kampfrath T., Mikolaj M. B., Cai Y., Ostrowski M. C., Lu B., Parthasarathy S., et al. (2009). Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichelaar J. W., Lu W., Whitsett J. A. (2000). Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 275, 11858–11864. [DOI] [PubMed] [Google Scholar]
- Underwood E. (2017). The polluted brain. Science 355, 342–345. [DOI] [PubMed] [Google Scholar]
- Wahlund C. J. E., Eklund A., Grunewald J., Gabrielsson S. (2017). Pulmonary Extracellular Vesicles as Mediators of Local and Systemic Inflammation. Front. Cell Dev. Biol. 5, 39.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. T., Winden D. R., Marlor D. R., Wright A. J., Jones C. M., Chavarria M., Rogers G. D., Reynolds P. R. (2014). Acute secondhand smoke-induced pulmonary inflammation is diminished in RAGE knockout mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L758–L764. [DOI] [PubMed] [Google Scholar]
- Ying Z., do Carmo J. M., Xiang L., da Silva A. A., Chen M., Ryan M. J., Ostrowski M., Rajagopalan S., Hall J. E. (2013). Inhibitor kappaB kinase 2 is a myosin light chain kinase in vascular smooth muscle. Circ. Res. 113, 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z., Xie X., Bai Y., Chen M., Wang X., Zhang X., Morishita M., Sun Q., Rajagopalan S. (2015). Exposure to concentrated ambient particulate matter induces reversible increase of heart weight in spontaneously hypertensive rats. Part. Fibre Toxicol. 12, 15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z., Xu X., Bai Y., Zhong J., Chen M., Liang Y., Zhao J., Liu D., Morishita M., Sun Q., et al. (2014). Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: A role for hypothalamic inflammation. Environ. Health Perspect. 122, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]