Abstract
APOL1 nephropathies comprise a range of clinical and pathologic syndromes, which can be summarized as focal segmental glomerulosclerosis, in various guises, and arterionephrosclerosis, otherwise known as hypertensive kidney diseases. Current therapies for these conditions may achieve therapeutic targets, reduction in proteinuria and control of blood pressure, respectively, but often fail to halt the progressive decline in kidney function. It appears that current therapies fail to address certain underlying critical pathologic processes that are driven, particularly in podocytes and microvascular cells, by the APOL1 renal risk genetic variants. Mechanisms hypothesized to be responsible for APOL1 variant-associated cell injury can be summarized in five domains: increased APOL1 gene expression, activation of inflammasomes, activation of protein kinase R, electrolyte flux across plasma or intracellular membranes, and altered endolysosomal trafficking associated with endoplasmic reticulum stress. We briefly review the available evidence for these five mechanisms and suggest possible novel therapeutic approaches.
Keywords: chronic kidney disease, focal segmental glomerulosclerosis, glomerulosclerosis, inflammation, podocytes
INTRODUCTION
APOL1 genetic variants, uniquely found among individuals of sub-Saharan African decent, are responsible for a substantial proportion of the disparity in chronic kidney disease, and end-stage kidney disease in particular, that characterizes this population [1]. APOL1 is expressed in multiple tissues and cell types, but to date disease manifestations associated with the genetic variants have been confined to the podocyte and possibly microvascular cells. APOL1 nephropathy has diverse clinical manifestations, and the reasons for this diversity are not well understood. One model would be that APOL1 confers susceptibility to podocyte and/or microvascular injury, so that among individuals with two APOL1 risk alleles, additional diverse cellular stressors would be more likely to lead to cell dysfunction, injury and perhaps loss.
The hypothesized cellular stressors are summarized in Table 1, aligned with six kidney syndromes; doubtless there are many more stressors yet to be described. First, ‘primary FSGS’ can manifest as various histologic forms, with the collapsing variant being particularly typical for APOL1 disease [2]. A plasma factor has been long postulated to cause FSGS recurrence following kidney transplant, and by inference to cause primary FSGS; whether this factor interacts with APOL1 risk alleles is not known. Second, ‘adaptive FSGS’ is due to an imbalance resulting from increased glomerular load (a concept difficult to define, but an increase in which is manifested as glomerular hyperfiltration) and/or diminished glomerular capacity (e.g. reduced glomerular number or available filtration surface). APOL1 genetic variants increase the risk for at least one form of adaptive FSGS, associated with sickle cell anemia [3]. Third, APOL1 variants are associated with HIV infection [5], in which elevated levels of interferon may play a role as interferon drives APOL1 gene expression. Fourth, APOL1 variants are also associated with exposure to elevated levels interferon in other clinical settings, when it is administered as therapy [4], and in the context of lupus nephritis [6]. Fifth, APOL1 variants are associated with ‘arterionephrosclerosis’, particularly with a progressive form of that disorder [7], and manifest a characteristic glomerular and tubular histopathology [8]. Sixth, APOL1 risk variants in the donor kidney are associated with ‘reduced renal allograft survival’, although the mechanisms are unknown [9].
Table 1.
Clinical manifestations and initiators of APOL1 nephropathies
| Clinical pathologic syndrome | Proposed initiating factors |
|---|---|
| Primary FSGS, manifesting as various histologic subtypes | Presumed plasma factor(s) associated with recurrent FSGS; possible role of chronic microvascular inflammation (e.g. chronic HIV infection with effective anti-retroviral therapy) |
| Adaptive FSGS, the most characteristic histologic manifestation being perihilar FSGS | Imbalance between glomerular load and glomerular capacity |
| Sickle cell disease | |
| Medication-associated FSGS, particularly collapsing variant | Interferon therapy |
| HIV-associated nephropathy, particularly FSGS collapsing variant with uncontrolled HIV infection | Interferon |
| Podocyte infection with HIV | |
| HIV accessory proteins Tat, Vpr and Nef | |
| Collapsing glomerulopathy associated with lupus nephritis | Possibly interferon |
| Arterionephrosclerosis | Chronic microvascular inflammation, driven by smoking, obesity, hyperlipidemia, chronic viral infection (e.g. HIV) |
| Accelerated kidney allograft loss | Possibly drivers of chronic allograft nephropathy |
The links between stressors and cell injury and cell loss in the APOL1 risk individuals are more clearly appreciated in some settings (e.g. HIV-1 infection of podocytes in vivo; interferon therapy stimulating APOL1 gene expression in podocytes; and case reports of recurrent FSGS following transplant, suggesting a role for a circulating factor), while the mechanisms by which HIV proteins might interact with APOL1 variant proteins, the role of chronic inflammation in driving arterionephrosclerosis and mechanisms that APOL1 variants increase renal allograft loss are unknown (in these settings, systematic examination of allograft biopsies, particularly early in the disease process, might be informative).
Current therapies for the APOL1 nephropathies sometimes achieve a particular surrogate endpoint, but often fail to prevent progressive loss of kidney function. Thus in FSGS, use of glucocorticoids [5] or mycophenolate or cyclosporine [2] reduced proteinuria in individuals with two APOL1 risk alleles. Similarly, in arterionephrosclerosis (hypertension-associated kidney disease) control of blood pressure was equivalent, but these therapies were much less effective at preventing end-stage kidney disease in individuals with two APOL1 risk alleles compared with other individuals [7, 10]. Thus, it appears that available therapies do not successfully address the ongoing pathologic processes that ARE driven by the APOL1 variant proteins. This suggests that a better understanding of APOL1-induced glomerular and microvascular injury is required in order to devise more effective therapies.
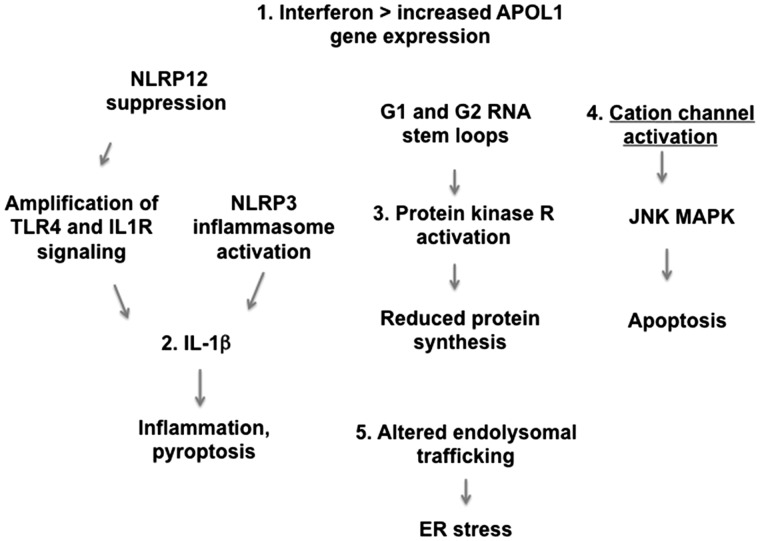
The mechanisms by which APOL1 variants disturb cellular phenotype and cellular function are being delineated in various laboratories, and much work has been published only in abstract form. Our purpose is to review five proposed pathways by which APOL1 variants might damage cells, and suggest possible therapeutic approaches. Figure 1 summarizes these five pathways.
FIGURE 1.
Proposed mechanisms of cell injury induced by APOL1 variants. Shown are five postulated mechanisms by which APOL1 damages podocytes and microvascular cells. Pathway 1 involves increased APOL1 gene expression. The other pathways involve activation of cation channels, altered endolysosomal trafficking, increased IL-1β driven inflammation and PKR activation, which leads to reduced protein synthesis.
APOL1 GENE EXPRESSION
Increased APOL1 expression in podocytes is associated with cellular injury and glomerular damage. As Pollak and colleagues have shown, APOL1 gene expression is driven by two double-stranded RNA recognition pathways: one that is toll-like receptor (TLR)-dependent and interferon-independent, and the other that is due to pattern recognition receptors that induce interferon [4]. In terms of potency of promoter stimulation, TLR3 > TLR4 and interferon γ > β > α, with interferons enhancing STAT3 occupancy of binding sites in the promoter. Thus, therapeutic approaches to antagonize these pathways might reduce glomerular and microvascular injury.
At least two TLR4 antagonists have been tested in clinical trials for sepsis, eritoran (a lipid A derivative) and resatorvid (TAK-242); whether these would suitable for chronic use remains to be seen [11]. An effective antagonist for TLR3 has not been reported. Signaling downstream of TLR3 may be a suitable approach, including targeting TANK binding kinase 1 (TBK1) and Janus kinase 3 (JAK3) inhibitors. A TBK1 inhibitor has been used in mice [12], but there appear to be no human studies. With regard to JAK3 inhibitors, the situation is more promising. Tofacitinib has been approved for rheumatoid arthritis, and decernotinib and peficitinib are under development for the same indication [13].
The interferon antagonists, rontazilumab and sifalimumab (anti-interferon-α monoclonal antibodies) and anifrolimunab (a monoclonal antibody targeting the interferon-α/β receptor), have been used in lupus [14, 15]. Interferon-γ is targeted by fontolizumab and has shown efficacy in three trials for Crohn disease [16]. Interferon signaling is mediated by JAK1 and JAK2 and the JAK1/2 inhibitor baricitinib has been shown to be effective in lowering proteinuria in a phase 2 trial in diabetic nephropathy [17]. Given the evidence for a prominent role of interferon in APOL1-mediated kidney disease, it might be fruitful to consider testing these JAK1/2 inhibitors for APOL1 nephropathy, though the side-effect profile of JAK inhibitors might limit their use in this patient population.
INFLAMMASOME ACTIVATION
The inflammasome is a molecular platform composed of three elements: a sensing molecule, e.g. NOD-like receptor (NLR) pyrin domain-containing 3 (NLRP3); an adaptor protein, e.g. apoptosis-associated speck-like protein containing a CARD (ASC); and caspase-1. Upon activation, the inflammasome processes pro-IL-1β and pro-IL-18 into active forms and induces cell death pathways.
The APOL1 RNA splice variant B3, and specifically the G2 risk variant compared with the G0 variant, promotes intracellular pro-inflammatory pathways in podocytes by several pathways [18]. First, it inhibits the repressive effect of NLRP12 on TLR4 signaling. Second, it promotes inflammasome activation by binding NLRP3, resulting in more caspase-1 release and more production of active IL-1β. These data suggest that podocyte production of active IL-1β may compromise normal podocyte function, and that IL-1 antagonists might benefit APOL1 nephropathy.
There are three Food and Drug Administration (FDA)-approved IL-1 antagonists available for the treatment of auto-inflammatory disorders [19]. Anakinra (Kineret) is a recombinant form of the soluble IL-1 receptor antagonist and was approved by the FDA in 2001 for treatment of rheumatoid arthritis. Canakinumab (Ilaris) is a fully human monoclonal antibody directed against the IL-1 receptor and was approved in 2009 for cryopyrin-associated periodic fever syndrome (CAPS). Rilonacept (IL-1 Trap; Arcalyst) is a fully human dimeric fusion protein consisting of the extracellular domains of both components of the IL-1 receptor and in 2012 this agent was also approved by the FDA for treatment of CAPS.
Gevokizumab is a fully humanized monoclonal antibody directed against IL-1β with high affinity for IL-1β. A trial in Behcet disease was unsuccessful, and there are no clinical trials at present. With regard to the three approved therapies, long-term therapy with these agents is generally well tolerated in individuals with systemic inflammatory disorders.
PKR ACTIVATION
Protein kinase R (PKR) is activated by double-stranded RNA (which is absent from normal eukaryotic cells), and PKR activation leads to suppressed protein synthesis. Double-stranded RNA is present in cells during viral infection, and thus PKR inhibition of cellular protein synthesis serves to limit viral replication but at the cost of cell stress or death. APOL1 risk variant (G1, G2) RNAs have a greater propensity to form regions with double-stranded RNA compared with the common variant G0 [20] and TLR3 binds double-stranded RNA. APOL1, and particularly the risk variants G1 and G2, activate PKR in cultured human podocytes and transgenic mice, and this results in reduced protein synthesis and in the mice, proteinuria [20].
Activated PKR also activates the inflammasome by binding NLRP3 and other related proteins, resulting in activation of caspase-1 and generation of active IL-1β. This effect does not require phosphorylation of eukaryotic initiation factor 2-α (eIF2-a). Thus, there is the potential for at least three APOL1 interactions, namely binding NLRP12, binding NLRP3 and activating PKR, to converge on the inflammasome. At present, it is not clear that PKR inhibitors are available for clinical studies but therapies directed against inflammasome-generated activated IL-1β are available, as discussed above.
CATION FLUX
There has been controversy about the cellular location (lyososome versus plasma membrane) and nature (anionic versus cationic) of the electrolyte flux associated with expression of APOL1 risk variants in cultured cells. However, most studies agree that APOL1 is a pore-forming protein whose activity is dependent on at least a transient exposure to a low-pH environment. Recently, Olabisi and colleagues have suggested that APOL1 activates plasma membrane K+ channels, leading to loss of cellular K+ stores [21]. This imposes cellular stress and activates JNK and p38 mitogen-activated mitogen kinase (MAPK). MAPK promotes synthesis and release of tumor necrosis factor (TNF) and IL-1β, and thus amplifies the inflammasome activation pathway described above.
Therapeutic options are available at several points along this proposed pathway. There are many distinct kinds of K+ channels, most or all of which have pharmacologic agents available to modulate activity; some of these agents have been used in human trials [22]. MAPK inhibitors have been developed and at least 12 have been tested in phase 1 or phase 2 trials [23]. The ClinicalTrials.gov website currently lists 150 studies using MAPK inhibitors for a wide range of diseases, suggesting that these agents may find one more niches for treatment of disease. As our understanding of the APOL1 pore matures, it may also be possible to develop approaches to directly inhibit the assembly of the pore or alternatively to inhibit ion flux through the pore.
ALTERATIONS IN ENDO LYSOSOMAL TRAFFICKING LEADING TO ER STRESS
Lan and colleagues have demonstrated that expression of APOL1 variants in cells is associated with increased lysosomal permeability [24]. Expression of APOL1 risk variants in cultured cells [25] and in transgenic mice compromises endolysosomal trafficking and impairs autophagic flux [26]. The accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER) activates the unfolded protein response (UPR), which alleviates stress, but if unsuccessful, will promote programmed cell death [27]. Over-expression of the APOL1 renal risk variants, compared with the G0 variants, induces ER stress in some cell lines (HeLa [25] cells) but not in other cell types (HEK293). Importantly, it is not clear how the APOL1 variants induce ER stress, and whether protein misfolding contributes.
Podocyte ER stress has been linked to other glomerular diseases, most notably diabetic nephropathy [28]. More work is required to define whether ER stress and the UPR associated with the APOL1 renal risk variant proteins occur at expression levels and in model systems that are relevant to human disease, and in particular to determine whether these findings have relevance to human kidney disease. Lipid and glucose toxicity may contribute to ER stress, suggesting these and other co-factors that might synergize with APOL1-mediated effects. ER stress induces activities that appear above as downstream of the APOL1 variants, including increased PERK activity, eIF2a phosphorylation and JNK activation [29].
If these studies are supportive, there are number of therapeutic approaches that could be considered, as this injury pathway has been implicated in diverse human diseases, including cancer (where the therapeutic goal is to amplify the stress and cause cancer cell death, rather than ameliorate the stress), neurodegenerative diseases [30], heart disease, diabetes [31], other metabolic diseases and inflammation [32]. Interestingly, in the case of another apolipoprotein, APOLE, the E4 isoform (which is a genetic risk for atherosclerosis, diabetes [31] and Alzheimer disease) induces ER stress and promotes autophagy [33].
ER stress in podocytes, associated with increased autophagy, has been proposed to contribute to podocyte injury in progressive glomerular disease [34]. Other factors can contribute to ER stress, including tissue hypoxia, oxidative stress and chronic inflammation, and thus synergize to promote progressive kidney disease [35].
THERAPEUTIC APPROACHES TO ER STRESS
Various therapies have been proposed to address ER stress, although more work is required to develop effective therapies. Palmitic acid, a saturated fatty acid, induces ER stress and apoptsis in cultured podocytes, whereas the mono-unsaturated palmitoleic and oleic acids attenuate this effect; there is no evidence that the effect of unsaturated fatty acids extends to other settings of ER stress [32]. Glucocortocoids are commonly used to treat FSGS, and subjects with APOL1-associated FSGS respond equally as well as others to these therapies, although progression to end-stage kidney disease may subsequently occur [5]. Glucose deprivation induces ER stress in cultured podocytes, resulting in impaired glycosylation of nephrin and consequent retention of nephrin, complexed with [36] calreticulin and the chaperone calnexin, in the ER; exposure of the cells to dexamethasone restored nephrin trafficking [37]. It is possible that the glucocorticoid-induced remissions, albeit often transient, that are seen in APOL1 nephropathy are due to a similar benefit on APOL1 trafficking or the consequences of abnormal trafficking.
Downstream of the UPR lie distinct pathways, including PERK signaling, IRE1a and ATF6; small molecule antagonists have been identified that act on each of these pathways [38–40]. Other approaches include ER chaperones (such as 4-phenyl butyrate, approved for primary biliary cirrhosis; tauroursodeoxycholic acid, approved for urea cycle disorders; and trehalose, a food preservative) and chaperone modulators [39].
EXTRACELLULAR APOL1
APOL1 was originally identified as a component of high-density lipoprotein and a significant proportion of APOL1 is found in the circulation where it reaches concentrations as high as 2 μM [41]. Available data from transplant studies and a lack of association between circulating APOL1 and renal function tend to argue against a primary role for circulating APOL1 in disease pathogenesis, but it is difficult to definitively rule out a role for the circulating material. Uptake of extracellular APOL1 has been reported in cultured podocytes [42], and data from a recently published Genentech patent suggest that liver-expressed variant APOL1 worsens experimental doxirubicin-induced nephropathy, a mouse mode of toxin-induced FSGS [43]. If APOL1 in the circulation contributes to manifestations of renal disease, depletion or interference with the uptake of this circulating material could represent another therapeutic approach.
CONCLUSION
APOL1 renal risk variants are associated with cellular injury when gene expression is increased, and they are associated with cellular injury in association with inflammasome activation, PKR activation, altered cation flux and altered endolysomal trafficking resulting in ER stress. The five pathways listed here have been demonstrated to be active in cell culture, and in some case to be active in mouse models, but only in the case of PKR activation have they been demonstrated in kidney samples obtained from human subjects.
We need a better understanding of the relative importance of each of these pathways in particular cells, in vitro, and cells and organs (e.g. kidney, microvasculature) in vivo. This would allow us to select which of the five pathways are critical to organ damage. We also need a better understanding of what causes APOL1 variants to activate certain pathways in some individuals with the high-risk genotype and not in other individuals with the same genetic predisposition. Plausible provocative factors include physiologic stressors (low birth weight, hypertension and obesity), acute or chronic viral infections or viral carriage, and environmental toxins or other chemicals.
More work is required to confirm that these cellular pathways are active and relevant to kidney injury in patients with the APOL1 nephropathies. Further, the possible therapeutic agents listed above will need to be tested in the various cell culture models and transgenic models that are available for the APOL1 nephropathies. Supportive data from preclinical work and samples obtained from human subjects will be required to justify clinical trials.
ACKNOWLEDGEMENTS
This work was supported by NIDDK, NIH, including the NIDDK Intramural Research Program under ZO1 DK043308 and RO1 DK105821 to K.S. The work was also supported by Intramural Research Program of the NCI, Center for Cancer Research, in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. The authors appreciate the willingness of members of our laboratories to allow us to reference data that have only been published in abstract form.
CONFLICT OF INTEREST STATEMENT
M.H. is an employee of Merck Laboratories. This article presents no previously unpublished results.
REFERENCES
- 1. Genovese G. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329: 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kopp JB. et al. Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol 2015; 26: 1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashley-Koch AE. et al. MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol 2011; 155: 386–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nichols B. et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kopp JB. et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011; 22: 2129–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Larsen CP. et al. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lipkowitz MS. et al. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 2013; 83: 114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larsen CP. et al. Histopathologic findings associated with APOL1 risk variants in chronic kidney disease. Mod Pathol 2015; 28: 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freedman BI. et al. APOL1 genotype and kidney transplantation outcomes from deceased African American donors. Transplantation 2016; 100: 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parsa A. et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 2013; 369: 2183–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Savva A, Roger T. Targeting toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol 2013; 4: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reilly SM. et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med 2013; 19: 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs 2014; 23: 1067–1077 [DOI] [PubMed] [Google Scholar]
- 14. Mathian A. et al. Targeting interferons in systemic lupus erythematosus: current and future prospects. Drugs 2015; 75: 835–846 [DOI] [PubMed] [Google Scholar]
- 15. Rovin BH, Parikh SV. Lupus nephritis: the evolving role of novel therapeutics. Am J Kidney Dis 2014; 63: 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cui D. et al. Efficacy and safety of interferon-gamma-targeted therapy in Crohn's disease: a systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol 2013; 37: 507–513 [DOI] [PubMed] [Google Scholar]
- 17. Perez-Gomez MV. et al. Targeting inflammation in diabetic kidney disease: early clinical trials. Expert Opin Investig Drugs 2016; 25: 1045–1058 [DOI] [PubMed] [Google Scholar]
- 18. Wakashin H, KJB. Apolipoprotein L1 has diverse RNA and protein isoforms and APOL1-B3 activates pro-inflammatory signaling. In: American Society of Nephrology Annual Meeting, San Diego, CA: 2015 [Google Scholar]
- 19. Moll M, Kuemmerle-Deschner JB. Inflammasome and cytokine blocking strategies in autoinflammatory disorders. Clin Immunol 2013; 147: 242–275 [DOI] [PubMed] [Google Scholar]
- 20. Okamoto K, Kopp J. Biological mechanisms underlying African American kidney disease risk: APOL1 mRNA renal risk variants activate protein kinase R (PKR) and reduce cell protein synthesis. In: 11th International Podocyte Conference, Haifa, Israel, 2016 [Google Scholar]
- 21. Olabisi OA. et al. APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci USA 2016; 113: 830–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li GR, Dong MQ. Pharmacology of cardiac potassium channels. Adv Pharmacol 2010; 59: 93–134 [DOI] [PubMed] [Google Scholar]
- 23. Kong TT, Zhang CM, Liu ZP. Recent developments of p38alpha MAP kinase inhibitors as antiinflammatory agents based on the imidazole scaffolds. Curr Med Chem 2013; 20: 1997–2016 [DOI] [PubMed] [Google Scholar]
- 24. Lan X. et al. APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am J Physiol Renal Physiol 2014; 307: F326–F336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dummer P. et al. Increased toxicity of APOL1 kidney risk variants is not due to decreased VAMP8 binding. Am Soc Nephrol 2015 [Google Scholar]
- 26. Susztak K. Podocytes and APOL1 associated kidney disease. In: 11th International Podocyte Conference, Haifa, Israel, 2016
- 27. Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab 2010; 12 (Suppl 2): 108–115 [DOI] [PubMed] [Google Scholar]
- 28. Zhuang A, Forbes JM. Stress in the kidney is the road to pERdition: is endoplasmic reticulum stress a pathogenic mediator of diabetic nephropathy? J Endocrinol 2014; 222: R97–R111 [DOI] [PubMed] [Google Scholar]
- 29. Ozcan U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306: 457–461 [DOI] [PubMed] [Google Scholar]
- 30. Garcia-Huerta P. et al. The intersection between growth factors, autophagy and ER stress: A new target to treat neurodegenerative diseases? Brain Res. 2016 doi: 10.1016/j.brainres.2016.02.052. [DOI] [PubMed] [Google Scholar]
- 31. Prattichizzo F. et al. ‘Inflammaging’ as a druggable target: a senescence-associated secretory phenotype-centered view of type 2 diabetes. Oxid Med Cell Longev 2016; 2016: 1810327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016; 529: 326–335 [DOI] [PubMed] [Google Scholar]
- 33. Cash JG. et al. Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J Biol Chem 2012; 287: 27876–27884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cybulsky AV. The intersecting roles of endoplasmic reticulum stress, ubiquitin- proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int 2013; 84: 25–33 [DOI] [PubMed] [Google Scholar]
- 35. Inagi R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr Opin Pharmacol 2010; 10: 156–165 [DOI] [PubMed] [Google Scholar]
- 36. Sieber J. et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol 2010; 299: F821–F829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujii Y. et al. The effect of dexamethasone on defective nephrin transport caused by ER stress: a potential mechanism for the therapeutic action of glucocorticoids in the acquired glomerular diseases. Kidney Int 2006; 69: 1350–1359 [DOI] [PubMed] [Google Scholar]
- 38. Jiang D, Niwa M, Koong AC. Targeting the IRE1alpha-XBP1 branch of the unfolded protein response in human diseases. Semin Cancer Biol 2015; 33: 48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rivas A, Vidal RL, Hetz C. Targeting the unfolded protein response for disease intervention. Expert Opin Ther Targets 2015; 19: 1203–1218 [DOI] [PubMed] [Google Scholar]
- 40. Rozpedek W. et al. Unfolded protein response and PERK kinase as a new therapeutic target in the pathogenesis of Alzheimer’s disease. Curr Med Chem 2015; 22: 3169–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kozlitina J, Zhou H, Brown PN, Rohm RJ, Pan Y, Ayanoglu G, Du X, Rimmer E, Reilly DF, Roddy TP, Cully DF, Vogt TF, Blom D, Hoek M. Plasma Levels of Risk-Variant APOL1 Do Not Associate with Renal Disease in a Population-Based Cohort. J Am Soc Nephrol 2016; 27: 3204–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma L. et al. Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol 2015; 26: 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wiilson DG. et al. Animal Model for Nephropathy and Agents for Treating the Same. San Francisco, CA: Genetech, 2014 [Google Scholar]