Abstract
Background: Associations of reproductive history with breast cancer risk differ by oestrogen receptor (ER±) status and possibly by the joint expression of ER and the human epidermal growth factor receptor-2 (ER±/HER2±). However, large sample sizes are needed to establish ER-specific risks by HER2± expression.
Methods: We linked a cancer registry covering nearly 95% of the primary breast cancer diagnoses in Denmark with a research parity database to assess associations for parity, number of live births and age at first live birth (AFLB) with receptor-specific risk. Relative risks (RRs) for associations were estimated with Poisson regression models.
Results: With nearly 31 million women-years of follow-up, 45 786 Danish women aged 20–84 years developed invasive breast cancer during 1992–2011. ER± expression was available for the entire study period and HER2± after 2006. Of the breast cancers with known ER expression, 79% were ER+. Most breast cancers with known ER and HER2 were HER2– (90% of ER+ cancers and 65% of ER– cancers). RRs differed by ER± expression for all reproductive variables (p-homogeneity < 0.001). Associations were stronger for ER+ than ER– cancers and for those diagnosed before age 50. Parity and early [not later] AFLB showed a protective association with ER+/HER2– and risk association with ER–/HER2– cancers.
Conclusion: Associations of reproductive history with breast cancer risk varied among Danish women by ER± and ER±/HER2± expression and age-at-diagnosis, consistent with receptor-specific and age-related etiological heterogeneity. Further stratification by HER2 status demonstrated dual (or opposite) effects for ER+/HER2– and ER–/HER2– cancers.
Keywords: breast cancer aetiology, breast cancer subtype, reproductive history, oestrogen receptor, human epidermal growth factor receptor-2
Key Messages
1. In this large-scale and population-based analysis of nearly 95% of all primary breast cancer cases in Denmark, we assessed the association of reproductive history with receptor-specific breast cancer risk.
Parity-related relative risks differed by receptor-specific expression.
Risk associations were stronger for ER+ than ER– cancers and for women diagnosed with breast cancer before age 50 years.
The addition of HER2 expression showed opposite effects for ER+/HER2– and ER–/HER2– cancers, especially among women with early AFLB and multiple pregnancies.
Epidemiological studies have long-established a dual (or opposite) pregnancy-related effect for breast cancer overall with early risk followed by long term protection. Viewed in the context of receptor-specific breast cancer heterogeneity, the dual parity-related effect for breast cancer overall might simply reflect opposite associations for receptor-specific breast cancers with protection for ER+ breast cancers and risk for ER- cancers, especially the ER-HER2- subtype.
Introduction
Associations of reproductive factors with breast cancer risk differ between oestrogen receptor (ER±) and progesterone receptor (PR±) breast cancers,4 and possibly between the four ‘intrinsic’ molecular or genomic subtypes.5,6 There are two ER+ and two ER– intrinsic subtypes that can be approximated with various immunohistochemical staining algorithms,7,8 including the joint expression of ER± and HER2 ± (human epidermal growth factor receptor-2)9; i.e. ER+/HER2– (a.k.a. luminal A), ER+/HER2 + (luminal B), ER–/HER2 + (HER2+ or enriched) and ER–HER2– [basal-like and the related triple negative cancer (ER–/PR-/HER2–)].
Reproductive history has been shown to be more strongly associated with the ER+ than ER– types of breast cancer,10 e.g. early age at first live birth (AFLB) is a protective factor for ER+ breast cancers. On the other hand, parity and/or pregnancy may increase the risk for ER– and ER–/HER2– cancers, as shown in some6,11,12 but not all studies.13,14 Early AFLB in the absence of lactation appears to be an especially strong risk factor for the ER–/HER2– subtypes.6,11,12,15
Given the inconsistency between some studies regarding the associations between reproductive histories and risk of receptor-specific breast cancer subtypes (especially ER– and ER–/HER2– cancers), we linked two national registries in Denmark to further assess parity-related risk factors for ER± and ER±/HER2± breast cancers. To our knowledge, this is one of the largest population-based studies to ever examine the impact of parity upon receptor-specific breast cancer risk.
Material and methods
National registries in Denmark
The Civil Registration System (CRS) in Denmark has assigned a unique registration number to all Danish residents since 1968 and incorporates a linkage of mothers and children. Using the CRS, we merged reproductive information from a research parity database with invasive primary breast cancer cases from the Danish Breast Cancer Group (DBCG). Our project was approved by the DBCG Institutional Review Board and the Danish Data Protection Agency (J.nr. 2013–41–2321). It was exempt from review by the National Institutes of Health (NIH) Office of Human Subject Research, since it did not involve interaction with human subjects or use personal identifiers (OHSR #12098).
Denmark’s research parity database includes dates of registered live births, and thereby information on parity and AFLB.16,17 Most births are known completely among women born in 1935 on later. The DBCG has conducted national prospective studies to assess impact of breast cancer treatment since the late 1970s and has registered nearly 95% of primary breast cancer diagnoses in Denmark.18 The DBCG collects detailed information on age at breast cancer diagnosis, date of diagnosis, tumour size, lymph nodal status, tumour grade, ER status since 1977 and HER2 status since 2007.
Biochemical assay for the ER was introduced in Denmark in the late 1970s and replaced with immunohistochemistry (IHC) staining during the early 1990s,19,20 with all participating pathology laboratories encouraged to use an external quality-control programme for IHC assessment. For this study, we specified 10–100% IHC staining for ER+ status and 0–9% for ER– expression. For HER2 status, IHC stains were scored as 0, 1+, 2+ or 3+. Tumours with a 0 or 1+ IHC score were considered HER2– and those with 3+ were HER2+. Tumours with 2+ IHC score were further assessed using insitu hybridization and scored HER2+ if the HER2-to-centromere 17 copy number ratio was >2.0. HER2 results were obtained from specialized laboratories dealing with breast pathology, all of which participated in external quality-assurance programmes.21
Analytic cohort
We merged population data from Denmark’s research parity database and breast cancer case data from the DBCG to assemble an anonymized cohort of first primary invasive breast cancers. We restricted our analysis to breast cancers diagnosed from 1992 to 2011 among women born in 1935 or later to ensure complete reproductive history and ER assessment by IHC. The analytic dataset included information on reproductive history, age at breast cancer diagnosis (20–84 years), time since last live birth (≥10 years compared with <10 years), calendar year of diagnosis (in single years), ER expression and HER2 expression (2007 through 2011). Receptor data were grouped as ER+ vs ER– and by the joint expression for ER± and HER2 ± (ER+/HER2–, ER+/HER2+, ER–/HER2+ and ER–/HER2–). Women-years for the analytic cohort were calculated from the entire female population in Denmark at risk for developing a first primary invasive breast cancer according to age, calendar year and reproductive history. Follow-up began on 1 January 1992 and continued until death, emigration or 31 December 2011, whichever occurred first.
Statistical analysis
The associations of parity and AFLB with breast cancer incidence overall and by known receptor status were estimated using Poisson regression models. Incidence rate ratios were expressed as relative risks (RRs) with 95% confidence intervals (CIs). We restricted our main analyses to breast cancer cases with known receptor data. Models based on data where missing receptor status was imputed gave similar results (data not shown). The Poisson models included attained age and calendar year modelled with splines (with smoothing parameters chosen by generalized cross validation), the number of live births (in categories 0, 1, 2, 3 and 4+ births) and AFLB (ages 12–19, 20–24, 25–29, 30–34 and 35+ years) (PROC GAM, SAS 9.2). Models that assessed parity (nulliparous/parous) were only adjusted for age and calendar year. We also analysed the effect of time since last live birth among women with two or more pregnancies. These models were adjusted for AFLB, number of live births (fitted with a trend) and calendar year fitted with a spline. Tests for linear trend were evaluated by entering a categorical variable into the model based on integer categories. The log-transformed woman-years were included as an offset in all models.
To assess effect modification by age at breast cancer diagnosis, we conducted analyses stratified by age of diagnosis 50± years. Interactions were tested by including an interaction term of the risk factor with an indicator variable for age <50 years in the regression models. To compare trend associations between subtypes, we combined the datasets used for the separate subtype-specific Poisson regression models and included an interaction term of the exposure with subtype. All P-values were obtained using Wald tests and were two-sided.
Results
There were 45 786 first primary breast cancers diagnosed among Danish women born in 1935 or later and between the ages 20 and 84 years during the study period 1992–2011. These breast cancers were detected during 30 976 395 women-years of follow-up (Table 1); 5317 (12%) of the cancers occurred among nulliparous women, 7951 (17%) among women with one live birth, 21 404 (47%) with two live births and 8710 (19%) and 2404 (5%) with three and four or more live births, respectively.
Table 1.
First primary invasive breast cancer cases (n = 45 786) among Danish women between the ages of 20 and 84 years in 1992–2011 and born from 1935 onwards
| Exposure | Cases (n) | % of total | Person-years |
|---|---|---|---|
| Parity | |||
| Nulliparous | 5317 | 12% | 8 135 014 |
| Parous | 40 469 | 88% | 22 841 381 |
| Number of live births | Parous cases (n = 40 469) | ||
| 1 | 7951 | 17% | 5 351 202 |
| 2 | 21 404 | 47% | 11 541 003 |
| 3 | 8710 | 19% | 4 598 474 |
| 4+ | 2404 | 5% | 1 350 702 |
| AFLB (years) | Parous cases (n = 40 469) | ||
| 12–19 | 6004 | 13% | 3 020 822 |
| 20–24 | 17 699 | 39% | 9 744 343 |
| 25–29 | 11 524 | 25% | 7 194 321 |
| 30–34 | 4009 | 9% | 2 303 162 |
| 35+ | 1233 | 3% | 578 733 |
Table 2 shows the number of breast cancers by receptor status and reproductive factors. ER status was known for 93% of cancer diagnoses in 1992–2011. HER2 status was known for 87% of cancer diagnoses from 2007 to 2011. Among the breast cancers with known ER expression, 79% were ER+. Among the breast cancers with known ER and HER2 expression, most of the ER+ cancers were HER2– (90%), as were most of the ER– cancers (65%). Only 14% of the breast cancers with known ER and HER2 expression were HER2+, with 9% ER+/HER2+ and 5% ER–/HER2+.
Table 2.
Descriptive statistics for first primary invasive breast cancer cases among Danish women between the ages of 20 and 84 years and born in 1935 or later
| Total (column %) | Number of live births (column %) |
Age at first live birth (column %) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | NP | 1 | 2 | 3 | 4+ | 12–19 | 20–24 | 25–29 | 30–34 | 35+ | |
| Total 1992–2011 | 45 786 (100%) | 5317 (100%) | 7951 (100%) | 21 404 (100%) | 8710 (100%) | 2404 (100%) | 6004 (100%) | 17 699 (100%) | 11 524 (100%) | 4009 (100%) | 1233 (100%) |
| ER + | 33 631 (73%) | 3893 (73%) | 5941 (75%) | 15 648 (73%) | 6446 (74%) | 1703 (71%) | 4350 (72%) | 12 961 (73%) | 8501 (74%) | 2982 (74%) | 944 (77%) |
| ER– | 9123 (20%) | 1009 (19%) | 1489 (19%) | 4389 (21%) | 1720 (20%) | 516 (21%) | 1277 (21%) | 3577 (20%) | 2289 (20%) | 765 (19%) | 206 (17%) |
| Unknown | 3032 (7%) | 415 (8%) | 521 (7%) | 1367 (6%) | 544 (6%) | 185 (8%) | 377 (6%) | 1161 (7%) | 734 (6%) | 262 (7%) | 83 (7%) |
| Total (2007–11) | 17 564 (100%) | 1967 (100%) | 3011 (100%) | 8263 (100%) | 3424 (100%) | 899 (100%) | 2249 (100%) | 6608 (100%) | 4502 (100%) | 1710 (100%) | 528 (100%) |
| ER+/HER2– | 11 446 (65%) | 1253 (64%) | 1998 (66%) | 5376 (65%) | 2242 (65%) | 577 (64%) | 1488 (66%) | 4340 (66%) | 2883 (64%) | 1123 (66%) | 359 (68%) |
| ER+/HER2+ | 1321 (8%) | 155 (8%) | 230 (8%) | 646 (8%) | 252 (7%) | 38 (4%) | 128 (6%) | 471 (7%) | 386 (9%) | 137 (8%) | 44 (8%) |
| ER–/HER2+ | 882 (5%) | 97 (5%) | 138 (5%) | 413 (5%) | 183 (5%) | 51 (6%) | 102 (5%) | 338 (5%) | 238 (5%) | 83 (5%) | 2 (5%) |
| ER–/HER2– | 1611 (9%) | 172 (9%) | 262 (9%) | 776 (9%) | 300 (9%) | 101 (11%) | 226 (10%) | 577 (9%) | 424 (9%) | 165 (10%) | 47 (9%) |
| Unknown | 2304 (13%) | 290 (15%) | 383 (13%) | 1052 (13%) | 447 (13%) | 132 (15%) | 305 (14%) | 882 (13%) | 571 (13%) | 202 (12%) | 54 (10%) |
Total, total number of breast cancer cases; ER, oestrogen receptor; ER±/HER2±, joint oestrogen receptor ER± and Human Epidermal Growth Factor Receptor-2 (HER2±) expression; unknown or missing receptor status; NP, nulliparous women.
Associations of reproductive factors and ER ±
Compared with nulliparous women, parous women had a reduced risk for ER+ cancer (RR = 0.88; 95% CI: 0.85, 0.91) with no association for ER– cancers (Table 3a). Among parous women, RRs declined with each additional live birth for both ER+ and ER– cancers, p-trend and p-heterogeneity < 0.001. RRs increased with later AFLB for ER+ cancers (RR = 1.22 for AFLB 35+ years compared with 20–24 years, p-trend < 0.001). RRs were slightly elevated for both earlier and later AFLB for ER– cancers compared with the reference group 20–24 years (p-heterogeneity) = 0.015, e.g. age group 12–19 (RR = 1.07; 95% CI: 1.00, 1.14) and age group 30–34 (RR = 1.13; 95% CI: 1.04, 1.22). Associations for all three reproductive variables were stronger for ER+ than ER– breast cancers (p-homogeneity < 0.001).
Table 3a.
(1992–2011) Relative risks (RRs) with 95% confidence intervals for invasive breast cancer overall, oestrogen receptor positive (ER+) and ER negative (ER–) breast cancers by reproductive variables among nulliparous and parous women born in 1935 or later
| Exposure | Overall | ER + | ER– | P-value for homogeneity ER ± |
|---|---|---|---|---|
| Nulliparous cases (n = 5317) and parous cases (n = 40 469) | ||||
| Number of cases | n = 45 786 | n = 33 631 | n = 9123 | |
| Parity | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Nulliparous | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |
| Parous | 0.90 (0.88, 0.93) | 0.88 (0.85, 0.91) | 1.04 (0.98, 1.11) | |
| p-heterogeneity | <0.001 | <0.001 | 0.22 | <0.001 |
| Parous cases (n = 40 469) | ||||
| Number of cases | n = 40 469 | n = 29 738 | n = 8114 | |
| Number of live births | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| 1 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |
| 2 | 0.97 (0.94, 0.99) | 0.94 (0.91, 0.97) | 1.07 (1.01, 1.14) | |
| 3 | 0.88 (0.85, 0.91) | 0.87 (0.84, 0.90) | 0.95 (0.88, 1.02) | |
| 4+ | 0.75 (0.71, 0.78) | 0.71 (0.67, 0.75) | 0.87 (0.79, 0.97) | |
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 |
| p-heterogeneity | <0.001 | <0.001 | <0.001 | |
| AFLB (years) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| 12–19 | 1.01 (0.98, 1.04) | 1.00 (0.97, 1.04) | 1.07 (1.00, 1.14) | |
| 20–24 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |
| 25–29 | 1.09 (1.06, 1.11) | 1.10 (1.07, 1.13) | 1.04 (0.98, 1.09) | |
| 30–34 | 1.22 (1.18, 1.27) | 1.25 (1.20, 1.30) | 1.13 (1.04, 1.22) | |
| 35+ | 1.18 (1.11, 1.26) | 1.22 (1.14, 1.31) | 0.99 (0.85, 1.16) | |
| p-trend | <0.001 | <0.001 | 0.52 | <0.001 |
| p-heterogeneity | <0.001 | <0.001 | 0.015 | |
| Nulliparous cases (n = 5317) and uniparous cases (n = 7951) | ||||
| Number of cases | n = 13 268.00 | n = 9834 | n = 2498 | |
| AFLB (years) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| 12–19 | 0.90 (0.83 0.98) | 0.88 (0.80, 0.97) | 1.03 (0.85, 1.24) | |
| 20–24 | 0.92 (0.88, 0.96) | 0.92 (0.87, 0.98) | 0.95 (0.85, 1.07) | |
| 25–29 | 0.98 (0.94, 1.03) | 1.00 (0.94, 1.05) | 1.00 (0.89, 1.11) | |
| 30–34 | 1.09 (1.03, 1.15) | 1.08 (1.02, 1.15) | 1.18 (1.04, 1.33) | |
| 35+ | 1.05 (0.97, 1.14) | 1.08 (0.99, 1.18) | 0.95 (0.79, 1.16) | |
| Nulliparous | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |
| p-trend | <0.001 | 0.003 | 0.87 | 0.65 |
| p-heterogeneity | <0.001 | <0.001 | 0.35 | |
Cases with unknown ER status (n = 3032) were excluded from the ER-specific analyses. AFLB, age at first live birth; P-value for homogeneity is based on comparison ER± subtypes.
To further evaluate the impact of AFLB apart from multiple live births, we compared uniparous to nulliparous women. RRs increased with advancing AFLB for ER+ cancers (p-trend = 0.003) but not for ER– cancers (p-trend = 0.87), with nulliparous women treated as the oldest group in the trend test. Nonetheless, there was no difference for AFLB by ER± status among uniparous women (p-heterogeneity = 0.65).
When stratified by age (<50 years and 50+ years, Table 3b), associations for AFLB were stronger for ER+ cancers before than after age 50 years among all parous women (p-homogeneity = 0.014 for 50± years) but not for ER– cancers (p-homogeneity = 0.14). There was no difference in the associations for parity or number of live births by age 50± years for either ER+ or ER– breast cancers.
Table 3b.
(1992–2011) Relative risks (RRs) with 95% confidence intervals for invasive breast cancers among Danish women with complete reproductive information by reproductive variables (women born in 1935 or later), known ER± expression and aged 50± years at the time of breast cancer diagnosis
| Exposure | ER + <50 years | ER + 50+ years | P-value for homogeneity ER + 50± | ER– <50 years | ER– 50+ years | P-value for homogeneity ER–50± |
|---|---|---|---|---|---|---|
| Nulliparous cases (n = 5317) and parous cases (n = 40 469) | ||||||
| Number of cases | n = 8573 | n = 25 058 | n = 3314 | n = 5809 | ||
| Parity | RR | RR | RR | RR | ||
| Nulliparous | 1 | 1 | 1 | 1 | ||
| Parous | 0.91 (0.86, 0.97) | 0.87 (0.84, 0.91) | P = 0.14 | 1.02 (0.93, 1.13) | 1.05 (0.96, 1.15) | P = 0.72 |
| p-heterogeneity | 0.003 | <0.001 | 0.68 | 0.26 | ||
| Parous cases (n = 40 469) | ||||||
| Number of cases | n = 7314 | n = 22 424 | n = 2819 | n = 5295 | ||
| Number of live births | RR | RR | RR | RR | ||
| 1 | 1 | 1 | 1 | 1 | ||
| 2 | 1.00 (0.94, 1.06) | 0.93 (0.90, 0.97) | 1.06 (0.96, 1.16) | 1.09 (1.01, 1.18) | ||
| 3 | 0.90 (0.84, 0.97) | 0.87 (0.83, 0.91) | 0.85 (0.75, 0.97) | 1.00 (0.91, 1.10) | ||
| 4+ | 0.77 (0.68, 0.88) | 0.70 (0.66, 0.75) | P = 0.69 | 0.84 (0.69, 1.03) | 0.90 (0.79, 1.02) | P = 0.11 |
| p-trend | <0.001 | <0.001 | 0.004 | 0.035 | ||
| AFLB (years) | RR | RR | RR | RR | ||
| 12–19 | 0.94 (0.87, 1.03) | 1.02 (0.98, 1.06) | 1.09 (0.96, 1.23) | 1.07 (0.99, 1.15) | ||
| 20–24 | 1 | 1 | 1 | 1 | ||
| 25–29 | 1.14 (1.08, 1.21) | 1.10 (1.06, 1.13) | 1.05 (0.96, 1.15) | 1.04 (0.97, 1.11) | ||
| 30–34 | 1.36 (1.26, 1.46) | 1.21 (1.15, 1.28) | 1.23 (1.09, 1.39) | 1.02 (0.90, 1.14) | ||
| 35+ | 1.34 (1.19, 1.50) | 1.19 (1.09, 1.30) | P = 0.014 | 0.98 (0.79, 1.22) | 1.09 (0.89, 1.32) | P = 0.14 |
| p-trend | <0.001 | <0.001 | 0.27 | 0.70 | ||
| Nulliparous cases (n = 5317) and uniparous cases (n = 7951) | ||||||
| Number of cases | n = 2895 | n = 6939 | n = 1123 | n = 1375 | ||
| AFLB (years) | RR | RR | RR | RR | ||
| 12–19 | 0.82 (0.67, 1.01) | 0.91 (0.81, 1.01) | 1.04 (0.76, 1.43) | 1.05 (0.83, 1.32) | ||
| 20–24 | 0.87 (0.78, 0.98) | 0.94 (0.88, 1.00) | 0.91 (0.75, 1.10) | 0.97 (0.84, 1.12) | ||
| 25–29 | 1.05 (0.95, 1.16) | 0.98 (0.91, 1.04) | 0.93 (0.79, 1.11) | 1.05 (0.90, 1.21) | ||
| 30–34 | 1.03 (0.92, 1.16) | 1.11 (1.03, 1.20) | 1.36 (1.15, 1.61) | 1.02 (0.85, 1.23) | ||
| 35+ | 1.08 (0.94, 1.25) | 1.13 (1.02, 1.25) | P = 0.78 | 0.95 (0.73, 1.22) | 1.09 (0.86, 1.39) | P = 0.81 |
| Nulliparous | 1 | 1 | 1 | 1 | ||
| p-trend | 0.06 | 0.009 | 0.72 | 0.98 | ||
Cases with unknown ER status (n = 3032) were excluded from the ER-specific analyses. AFLB, age at first live birth.
Associations of reproductive factors by ER+/HER2± and ER–/HER2±
Parity reduced risk for ER+/HER2– breast cancers (RR = 0.92; 95% CI: 0.87, 0.98) but not for ER+/HER2+ cancers (Table 4a). Similar to ER+ cancers in Table 3a, RRs for both ER+/HER2– and ER+/HER2+ cancers declined with each additional live birth and increased with advancing age at AFLB. Associations for AFLB were slightly stronger between ER+/HER2– and ER+/HER2+ cancers among all parous women (p-homogeneity < 0.001, data not shown in Table 4a), but there were no differences between ER+/HER2– and ER+/HER2+ for parity or number of live births.
Table 4a.
(2007–11) Relative risks (RRs) with 95% confidence intervals for overall and ER±/HER2± first primary invasive breast cancers diagnosed in 2007 or later by reproductive variables among nulliparous and parous women born in 1935 or later
| Exposure | Overall | ER+/HER2– | ER+/HER2+ | ER–/HER2+ | ER–/HER2– |
|---|---|---|---|---|---|
| Number of cases | n = 17 564 | n = 11 446 | n = 1321 | n = 882 | n = 1611 |
| Parity | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) |
| Nulliparous | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Parous | 0.94 (0.89, 0.98) | 0.92 (0.87, 0.98) | 1.02 (0.86, 1.02) | 1.03 (0.83, 1.27) | 1.16 (0.99, 1.36) |
| p-heterogeneity | 0.006 | 0.008 | 0.98 | 0.76 | 0.09 |
| Parous cases (n = 15 597) | |||||
| Number of cases | n = 15 597 | n = 10 193 | n = 1166 | n = 785 | n = 1439 |
| Number of live births | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) |
| 1 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 2 | 0.97 (0.93, 1.02) | 0.95 (0.90, 1.00) | 1.02 (0.88, 1.20) | 1.08 (0.88, 1.32) | 1.10 (0.95, 1.27) |
| 3 | 0.93 (0.89, 0.98) | 0.92 (0.86, 0.98) | 0.97 (0.80, 1.17) | 1.14 (0.90, 1.44) | 0.99 (0.83, 1.18) |
| 4+ | 0.79 (0.73, 0.85) | 0.76 (0.69, 0.84) | 0.50 (0.35, 0.71) | 1.06 (0.75, 1.48) | 1.07 (0.84, 1.37) |
| p-trend | <0.001 | <0.001 | 0.006 | 0.43 | 0.97 |
| p-heterogeneity | <0.001 | <0.001 | <0.001 | 0.66 | 0.27 |
| AFLB (years) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) |
| 12–19 | 1.02 (0.97, 1.07) | 1.01 (0.95, 1.07) | 0.87 (0.72, 1.06) | 0.89 (0.71, 1.12) | 1.20 (1.02, 1.40) |
| 20–24 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 25–29 | 1.07 (1.03, 1.12) | 1.02 (1.02, 1.13) | 1.18 (1.03, 1.36) | 1.06 (0.89, 1.25) | 1.04 (0.96, 1.38) |
| 30–34 | 1.24 (1.17, 1.31) | 1.29 (1.20, 1.38) | 1.19 (0.97, 1.45) | 1.09 (0.85, 1.40) | 1.15 (1.01, 1.40) |
| 35+ | 1.16 (1.05, 1.27) | 1.22 (1.09, 1.37) | 1.17 (0.85. 1.61) | 1.03 (0.67, 1.59) | 1.10 (0.81, 1.50) |
| p-trend | <0.001 | <0.001 | 0.002 | 0.18 | 0.59 |
| p-heterogeneity | <0.001 | <0.001 | 0.024 | 0.68 | 0.12 |
| Nulliparous cases (n = 1967) and uniparous cases (n = 3011) | |||||
| Number of cases | n = 4978 | n = 3251 | n = 385 | n = 235 | n = 434 |
| AFLB (years) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) |
| 12–19 | 0.93 (0.81, 1.06) | 0.91 (0.77, 1.08) | 0.85 (0.49, 1.47) | 1.36 (0.79, 2.35) | 0.99 (0.60, 1.64) |
| 20–24 | 0.93 (0.86, 1.01) | 0.91 (0.83, 1.01) | 1.20 (0.90, 1.59) | 0.90 (0.62, 1.32) | 0.98 (0.73, 1.31) |
| 25–29 | 0.99 (0.92, 1.08) | 1.03 (0.94, 1.13) | 1.07 (0.81, 1.41) | 0.97 (0.68, 1.38) | 1.11 (0.86, 1.45) |
| 30–34 | 1.15 (1.05, 1.25) | 1.18 (1.06, 1.32) | 0.96 (0.69, 1.35) | 0.94 (0.62, 1.43) | 1.32 (1.00, 1.75) |
| 35+ | 1.05 (0.93, 1.17) | 1.10 (0.95, 1.26) | 1.05 (0.70, 1.59) | 0.87 (0.49, 1.52) | 1.05 (0.71, 1.55) |
| Nulliparous | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| p-trend | 0.14 | 0.18 | 0.57 | 0.77 | 0.80 |
| p-heterogeneity | <0.001 | 0.001 | 0.72 | 0.85 | 0.11 |
Cases with unknown ER/HER2 status (n = 2304) were excluded from the ER/HER2 specific analyses. AFLB, age at first live birth.
There was the suggestion that parity increased risk of ER–/HER2– cancers (RR = 1.16; 95% CI: 0.99, 1.36) but not of ER–/HER2+. Similar to ER– cancers in Table 3a, RRs were slightly elevated for both earlier and later AFLB for ER–/HER2– cancers compared with the reference group aged 20–24 years, e.g. age group 12–19 (RR = 1.20; 95% CI: 1.02, 1.40) and age group 30–34 (RR = 1.15; 95% CI: 1.01, 1.40).
When stratified by age (<50 years and 50+ years, Table 4b), associations for AFLB were stronger for women before the age 50 years for ER+/HER2– cancers among all parous women (p-homogeneity < 0.001 for age 50± years) but not among uniparous women (p-homogeneity = 0.44). We also observed a stronger effect for parity among women with ER–HER2– cancers aged <50 years (RR = 1.36; 95% CI: 1.04, 1.77) than 50+ years (RR = 1.03; 95% CI: 0.84, 1.26).
Table 4b.
(2007–11) Relative risks (RRs) with 95% confidence intervals for invasive breast cancers among Danish women with complete reproductive information by reproductive variables (women born in 1935 or later), known ER±/HER2- expression and aged 50± years at the time of breast cancer diagnosis
| Exposure | ER+/HER2– <50 years | ER+/HER2– 50+ years | P-value for homogeneity ER+/HER2– 50± | ER–/HER2– <50 years | ER–/HER2– 50+ years | P-value for homogeneity ER–/HER2– 50± |
|---|---|---|---|---|---|---|
| Nulliparous cases (n = 1967) and parous cases (n = 15 597) | ||||||
| Number of cases | n = 1776 | n = 9670 | n = 468 | n = 1143 | ||
| Parity | RR | RR | RR | RR | ||
| Nulliparous | 1 | 1 | 1 | 1 | ||
| Parous | 1.03 (0.90, 1.18) | 0.90 (0.84, 0.96) | p = 0.10 | 1.36 (1.04, 1.77) | 1.03 (0.84, 1.26) | p = 0.09 |
| p-heterogeneity | 0.70 | 0.002 | 0.023 | 0.78 | ||
| Parous cases (n = 15 597) | ||||||
| Number of cases | n = 1527 | n = 8666 | n = 402 | n = 1037 | ||
| Live births | RR | RR | RR | RR | ||
| 1 | 1 | 1 | 1 | 1 | ||
| 2 | 1.01 (0.89, 1.15) | 0.94 (0.88, 0.99) | 1.03 (0.80, 1.32) | 1.13 (0.95, 1.36) | ||
| 3 | 0.96 (0.82, 1.14) | 0.91 (0.85, 0.97) | 0.79 (0.56, 1.11) | 1.08 (0.87, 1.33) | ||
| 4+ | 0.78 (0.58, 1.03) | 0.76 (0.68, 0.84) | P = 0.78 | 1.14 (0.70, 1.87) | 1.07 (0.80, 1.42) | P = 0.24 |
| p-trend | 0.27 | <0.001 | 0.52 | 0.79 | ||
| AFLB (years) | RR | RR | RR | RR | ||
| 12–19 | 0.95 (0.74, 1.21) | 1.01 (0.95, 1.08) | 1.14 (0.73, 1.78) | 1.21 (1.02, 1.42) | ||
| 20–24 | 1 | 1 | 1 | 1 | ||
| 25–29 | 1.16 (1.02, 1.32) | 1.06 (1.01, 1.12) | 0.99 (0.77, 1.27) | 1.07 (0.92, 1.24) | ||
| 30–34 | 1.48 (1.28, 1.72) | 1.25 (1.15, 1.35) | 1.22 (0.91, 1.63) | 1.04 (0.81, 1.34) | ||
| 35+ | 1.52 (1.22, 1.89) | 1.14 (1.00, 1.31) | <0.001 | 1.19 (0.75, 1.88) | 0.98 (0.63, 1.51) | P = 0.06 |
| p-trend | <0.001 | <0.001 | 0.42 | 0.21 | ||
| Nulliparous cases (n = 1967) and uniparous cases (n = 3011) | ||||||
| Number of cases | n = 579 | n = 2672 | n = 164 | n = 270 | ||
| AFLB (years) | RR | RR | RR | RR | ||
| 12–19 | 0.80 (0.41, 1.56) | 0.92 (0.77, 1.10) | 1.50 (0.47, 4.78) | 0.87 (0.50, 1.52) | ||
| 20–24 | 0.90 (0.66, 1.23) | 0.91 (0.82, 1.01) | 0.83 (0.41, 1.67) | 0.93 (0.67, 1.29) | ||
| 25–29 | 1.16 (0.92, 1.46) | 1.01 (0.91, 1.12) | 1.45 (0.95, 2.21) | 0.94 (0.67, 1.30) | ||
| 30–34 | 1.23 (0.98, 1.54) | 1.18 (1.04, 1.33) | 1.83 (1.23, 2.71) | 0.96 (0.64, 1.45) | ||
| 35+ | 1.17 (0.90, 1.53) | 1.08 (0.91, 1.27) | 1.14 (0.64, 2.06) | 0.98 (0.58, 1.67) | ||
| Nulliparous | 1 | 1 | P = 0.44 | 1 | 1 | P = 0.11 |
| p-trend | 0.86 | 0.13 | 0.15 | 0.52 | ||
Cases with unknown ER/HER2 status (n = 2304) were excluded from the ER/HER2-specific analyses. AFLB, age at first live birth.
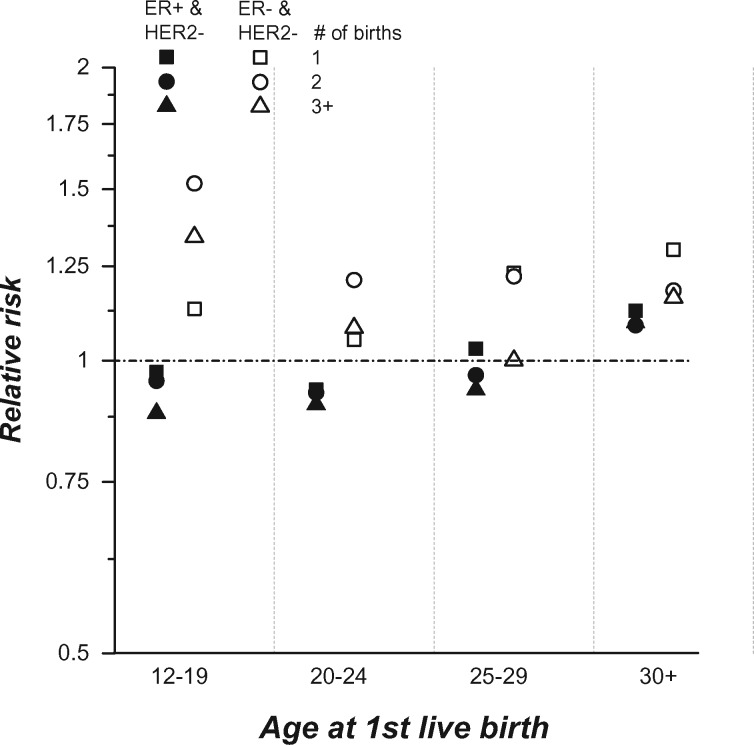
Figure 1 illustrates the combined effects of AFLB and number of live births among women with ER+/HER2– and ER–/HER2– breast cancers compared with nulliparous women (see Supplementary Table 1, available as Supplementary data at IJE online, for point estimates and 95% CIs). Parous women generally had lower risks for ER+/HER2– cancers with AFLB less than 30 years and higher risks for ER–/HER2– cancers. Early AFLB was associated with the lowest risks for ER+/HER2– cancers and the highest risks for ER–/HER2– cancers. RRs tended to decrease with increasing number of live births for ER+/HER2– cancers and to increase for ER-/HER2-.
Figure 1.
Relative risks (RRs) for first primary invasive ER+/HER2– and ER–HER2– breast cancers.
Parous women by age at first live birth and number (#) of live births are compared with nulliparous women with an assigned RR of 1.0. The y-axis is displayed on the log scale. See Supplementary Table 1 (available as Supplementary data at IJE online) for point estimates and 95% confidence intervals.
Among women with two or more live births, time since last live birth (>10 years compared with <10 years) showed a long-term protective effect with similar effect sizes for all breast cancer subtypes and age groups (Supplementary Tables 2 and 3, available as Supplementary data at IJE online). The one exception was ER–/HER2–, for which there was no association with time since last birth [RR 1.00 (0.96, 1.04); Supplementary Table 3, available as Supplementary data at IJE online].
Discussion
Relative risk estimates for the association of reproductive factors and breast cancer risk varied by receptor-specific subtype and age of diagnosis among Danish women born in 1935 or later and with cancers diagnosed during the time period 1992–2011. In an earlier Danish study using breast cancer cases diagnosed from 1978 to 1994,22 breast cancer risks were associated with nulliparity and later AFLB for ER+ cancers only. Our current analysis confirms and extends these prior results with a contemporary and larger dataset that also assesses the impact of HER2± expression.
Almost 80% of the breast cancers in Denmark with known ER expression were ER+. Most of the ER± cancers with known HER2 expression were HER2–. Consequently, there were relatively few HER2+ cancers (14% in total).
Our study has three main findings. (i) Parity-related reproductive histories differed by ER± and ER±/HER2± expression, consistently with receptor-specific etiologic heterogeneity. (ii) The associations with reproductive history and breast cancer risk were generally stronger for ER+ than ER– cancers and greater among women age <50 years at breast cancer diagnosis. (iii) The addition of HER2– expression modified the risk estimates for ER± subgroups (ER+/HER2– vs ER–HER2–), with the greatest contrasts occurring among women with early AFLB and multiple live births. There were too few HER2+ cancers to reliably assess the HER2+ subtypes (ER+/HER2+ and ER–HER2+).
The associations for ER+ and ER+/HER2– cancers in Denmark were similar in magnitude to well-established associations for breast cancer overall,23,24 as might be expected, since most breast cancers were ER+ and most ER+ cancers were HER2–. However, risk estimates for individual reproductive factors for the ER– subtypes are generally less well established. Different results among studies could be related to many factors including but not limited to missing data, small sample size, different populations with varying age and racial distributions. Among the largest studies with case–control data and a full age range to assess both early and late effects (ages 20–70+ years), associations for parity and ER– and ER–/HER2– cancers have ranged from no effect13,14 to increased risk.6,11,12 However, the combination of parity and breastfeeding has shown a relatively consistent protective effect for the ER– subtypes.6,11,12,15 For example, in the AMBER consortium study in the USA,11 RRs for ER– cancers were nearly 70% greater among women who did not breast feed and had 4+ live births compared with uniparous women who breast fed [odds ratio = 1.68 (1.15–2.44)]. A recent systematic review and meta-analysis concluded that lactation was associated with 10% reduction in risk of ER– cancers and 20% reduction in triple negative cancers.25
Though RRs for ER– cancer were modest in Denmark, our results suggest that parity increased risk for ER– breast cancer subtypes, especially for the ER–/HER2– cancers among women with early AFLB and multiple births (Figure 1). Unfortunately, Denmark’s research parity database did not have data on breastfeeding. However, ecological data from the Organisation for Economic Co-operation and Development (OECD) show that nearly 100% of mothers in Denmark initiate breastfeeding.26 The Copenhagen cohort study on infant nutrition and growth also confirms that breastfeeding is initiated by 99.5% of Denmark mothers,27 with 71%, 52% and 33% still breastfeeding at 3, 6 and 9 months after birth, respectively. We speculate that the associations for ER– cancers in Denmark might have been attenuated by the high prevalence of lactation.
Our study has the usual caveats of retrospective registry analyses including missing data and changing screening practice patterns over time. Another limitation was the lack of well-established risk factors (other than the three provided by Denmark’s research parity database). However, elsewhere, we have found that age at menarche (<12, 13+), BMI (<25, 25–30, 30–35, >35) and HRT use (none, <10 years, 10+ years) had very little correlation with parity and AFLB when assessed in two large US cohorts,28 i.e. all Spearman rank correlations were less than 0.05 in the large-scale National Cancer Institute (NCI)-AARP cohort (about 190 000 women) and the NCI PLCO cohort (64 000). Thus, residual confounding by those factors likely did not impact our findings. An additional limitation is that, though this is one of the largest studies to ever assess receptor-specific associations for reproductive history in a homogenous Caucasian population, we still had low power to detect differences among the relatively uncommon ER+/HER2+ and ER–/HER2+ cancers. Very large sample sizes will be needed to ever completely quantify the impact of HER2± status upon breast cancer aetiology. Finally, PR data were incomplete from the Danish Breast Cancer Group, so we approximated the four main breast cancer tumour subtypes without PR expression, as reviewed.9
In sum, though HER2 expression is a well-established modifier of breast cancer prognosis and prediction,29 its impact upon breast cancer aetiology is difficult to determine. In Denmark, the addition of HER2– expression suggested an opposite or dual effect between ER+/HER2– and ER–/HER2– cancers, especially among women with early AFLB and multiple births. Epidemiologic studies have long established a dual parity-related effect for breast cancer overall,1–3 with an early at risk period of 7–10 years that is followed by long-term protection. Viewed in the context of receptor-specific breast cancer heterogeneity, the dual effect for breast cancer overall might simply reflect an opposite association with protection for ER+ breast cancers and risk for ER– cancers, especially the ER–HER2– subtype.
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI).
Conflict of interest: The authors have no conflicts of interest to declare.
Supplementary Material
References
- 1. MacMahon B, Cole P, Lin TM. et al. Age at first birth and breast cancer risk. Bull World Health Organ 1970;43(2):209–21. [PMC free article] [PubMed] [Google Scholar]
- 2. Lambe M, Hsieh C, Trichopoulos D. et al. Transient increase in the risk of breast cancer after giving birth. N Engl J Med 1994;331(1):5–9. [DOI] [PubMed] [Google Scholar]
- 3. Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer 2006;6(4):281–91. [DOI] [PubMed] [Google Scholar]
- 4. Colditz GA, Rosner BA, Chen WY. et al. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst 2004;96(3):218–28. [DOI] [PubMed] [Google Scholar]
- 5. Perou CM, Sorlie T, Eisen MB. et al. Molecular portraits of human breast tumours. Nature 2000;406(6797):747–52. [DOI] [PubMed] [Google Scholar]
- 6. Millikan RC, Newman B, Tse CK. et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 2008;123(1):123–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldhirsch A, Wood WC, Coates AS. et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22(8):1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howlader N, Altekruse SF, Li CI. et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014; doi: 10:1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson WF, Rosenberg PS, Prat A. et al. How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst 2014;106(8):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Althuis MD, Fergenbaum JH, Garcia-Closas M. et al. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev 2004;13(10):1558–68. [PubMed] [Google Scholar]
- 11. Palmer JR, Viscidi E, Troester MA. et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst 2014;106(10):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ambrosone CB, Zirpoli G, Hong CC. et al. Important role of menarche in development of estrogen receptor-negative breast cancer in African American women. J Natl Cancer Inst 2015;107(9):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang XR, Chang-Claude J, Goode EL. et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium Studies. J Natl Cancer Inst 2011;103(3):250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang XR, Sherman ME, Rimm DL. et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev 2007;18:439–47. [DOI] [PubMed] [Google Scholar]
- 15. Li CI, Beaber EF, Tang MT. et al. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2–neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat 2013;137(2):579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melbye M, Wohlfahrt J, Olsen JH. et al. Induced abortion and the risk of breast cancer. N Engl J Med 1997;336(2):81–5. [DOI] [PubMed] [Google Scholar]
- 17. Westergaard T, Wohlfahrt J, Aaby P. et al. Population based study of rates of multiple pregnancies in Denmark, 1980–94. BMJ 1997;314(7083):775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The NORCAN project. 2016. http://www-dep.iarc.fr/NORDCAN/english/frame.asp (20 July 2016, date last accessed).
- 19. Talman ML, Rasmussen BB, Andersen J. et al. Estrogen receptor analyses in the Danish Breast Cancer Cooperative Group: history, methods, prognosis and clinical implications. Acta Oncol 2008;47(4):789–94. [DOI] [PubMed] [Google Scholar]
- 20. Laenkholm AV, Knoop A, Ejlertsen B. et al. ESR1 gene status correlates with estrogen receptor protein levels measured by ligand binding assay and immunohistochemistry. Mol Oncol 2012;6(4):428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rasmussen BB, Andersson M, Christensen IJ. et al. Evaluation of and quality assurance in HER2 analysis in breast carcinomas from patients registered in Danish Breast Cancer Group (DBCG) in the period of 2002–2006: a nationwide study including correlation between HER–2 status and other prognostic variables. Acta Oncol 2008;47(4):784–8. [DOI] [PubMed] [Google Scholar]
- 22. Wohlfahrt J, Mouridsen H, Andersen PK. et al. Reproductive risk factors for breast cancer by receptor status, histology, laterality and location. Int J Cancer 1999;81(1):49–55. [DOI] [PubMed] [Google Scholar]
- 23. Colditz GA,, Baer HJ,, Tamimi RM.. Breast cancer In: Schottenfeld D, Fraumeni JF Jr (eds) Cancer Epidemiology and Prevention, 3rd edn Oxford University Press, 2006, pp. 995–1012. [Google Scholar]
- 24. Anderson KN, Schwab RB, Martinez ME.. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat 2014;144(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status-a systematic review and meta-analysis. Ann Oncol 2015;26:2398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. OECD. C01.5: Breastfeeding Rates 2009 http://www.oecd.org/els/family/43136964.pdf (3 October 2016, date last accessed).
- 27. Michaelsen KF, Larsen PS, Thomsen BL. et al. The Copenhagen cohort study on infant nutrition and growth: duration of breast feeding and influencing factors. Acta Paediatr 1994; 83(6):565–71. [DOI] [PubMed] [Google Scholar]
- 28. Pfeiffer RM, Park Y, Kreimer AR. et al. Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS Medicine 2013;10(7):e1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Slamon DJ, Leyland-Jones B, Shak S. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344(11):783–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.