Abstract
Aims
To investigate if recent technical and procedural developments in percutaneous coronary intervention (PCI) significantly influence outcomes in appropriately selected patients with three-vessel (3VD) coronary artery disease.
Methods and results
The SYNTAX II study is a multicenter, all-comers, open-label, single arm study that investigated the impact of a contemporary PCI strategy on clinical outcomes in patients with 3VD in 22 centres from four European countries. The SYNTAX-II strategy includes: heart team decision-making utilizing the SYNTAX Score II (a clinical tool combining anatomical and clinical factors), coronary physiology guided revascularisation, implantation of thin strut bioresorbable-polymer drug-eluting stents, intravascular ultrasound (IVUS) guided stent implantation, contemporary chronic total occlusion revascularisation techniques and guideline-directed medical therapy. The rate of major adverse cardiac and cerebrovascular events (MACCE [composite of all-cause death, cerebrovascular event, any myocardial infarction and any revascularisation]) at one year was compared to a predefined PCI cohort from the original SYNTAX-I trial selected on the basis of equipoise 4-year mortality between CABG and PCI. As an exploratory endpoint, comparisons were made with the historical CABG cohort of the original SYNTAX-I trial. Overall 708 patients were screened and discussed within the heart team; 454 patients were deemed appropriate to undergo PCI. At one year, the SYNTAX-II strategy was superior to the equipoise-derived SYNTAX-I PCI cohort (MACCE SYNTAX-II 10.6% vs. SYNTAX-I 17.4%; HR 0.58, 95% CI 0.39–0.85, P = 0.006). This difference was driven by a significant reduction in the incidence of MI (HR 0.27, 95% CI 0.11–0.70, P = 0.007) and revascularisation (HR 0.57, 95% CI 0.37–0.9, P = 0.015). Rates of all-cause death (HR 0.69, 95% CI 0.27–1.73, P = 0.43) and stroke (HR 0.69, 95% CI 0.10–4.89, P = 0.71) were similar. The rate of definite stent thrombosis was significantly lower in SYNTAX-II (HR 0.26, 95% CI 0.07–0.97, P = 0.045).
Conclusion
At one year, clinical outcomes with the SYNTAX-II strategy were associated with improved clinical results compared to the PCI performed in comparable patients from the original SYNTAX-I trial. Longer term follow-up is awaited and a randomized clinical trial with contemporary CABG is warranted.
ClinicalTrials.gov Identifier
Keywords: PCI, Drug-eluting stents, Multivessel disease, Coronary artery bypass graft
Introduction
Percutaneous coronary interventions (PCI) were introduced 40 years ago and became the standard of care for patients with non-complex coronary artery disease (CAD) not responding to optimal medical therapy.1 Despite the improved efficacy of PCI with drug eluting stents (DES), in patients with three-vessel (3VD) CAD, surgery remains the recommended revascularization modality in patients with intermediate or high anatomical complexity.1 Among several trials comparing coronary artery bypass graft (CABG) surgery with PCI in patients with 3VD, the pivotal SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) trial identified CABG as the preferred revascularization strategy, compared with PCI with first-generation drug-eluting stents (DES). In the 3VD cohort, PCI was associated with a higher rate of all cause death, myocardial infarction, and repeat revascularization at 5 years.2 Subsequently, the superiority of CABG over PCI, with first-generation DES, was confirmed in patients with diabetes in the FREEDOM trial.3 Recently, in the BEST trial, comparing CABG with PCI in 3VD using newer generation DES, the 5-year clinical outcomes were inferior amongst patients who underwent PCI.4
Outcomes of patients with complex CAD are dependent on the anatomical complexity and patient’s individual clinical characteristics and comorbidities.5 In appropriately selected patients with less complex 3VD or high surgical risk, PCI may be considered by the heart team as an acceptable alternative. The SYNTAX Score II is a clinical tool that combines anatomical and clinical factors to aid the heart team to undertake objective decision-making between CABG and PCI based on 4-year mortality.6 Accordingly, patients with anatomical complexities, including those exceeding the low SYNTAX score group, are potentially appropriate for either therapy provided there is equipoise for projected 4-year mortality between CABG and PCI. Furthermore, advances in PCI [including intracoronary physiology to assess the appropriateness of revascularization, the advent of thin strut DES, intravascular imaging guided stent implantation and optimization, new techniques for revascularization of chronic total occlusions (CTO), and potent dual antiplatelet therapy] have been shown to improve patients’ outcomes.7–11 We hypothesized that the use of these PCI technologies, coupled with refined patient selection, could lead to a marked improvement in outcomes of 3VD patients treated with PCI.
The present study reports the 1-year clinical follow-up of the SYNTAX II trial, which was initiated to explore how the integration of new developments in PCI practice may potentially improve patient outcomes, compared with the results obtained in the original SYNTAX-I trial.
Methods
Study design
SYNTAX II is a multicentre, all-comers, open label, single-arm study which included patients with de novo 3VD. Patients were enrolled in 22 interventional cardiology centres from four European countries between February 2014 and November 2015 (Supplementary material online, Table S1). The study design has been described previously.12 Briefly, patients with de novo 3VD with no left main involvement were screened by the local heart team (interventional cardiologist and cardiac surgeon). All site reported, anatomical SYNTAX scores, were eligible for initial screening.13 Eligible patients had a SYNTAX score II (site-reported) with an equipoise recommendation between CABG and PCI based on 4-year mortality. In addition, patients were asked to volunteer only if the local heart team judged that an ‘equivalent anatomical revascularisation’ based on a vessel size of 1.5 mm could potentially be achieved.14
SYNTAX II was an investigator-initiated study, sponsored by the European Cardiovascular Research Institute (ECRI, Rotterdam, the Netherlands) with unrestricted research grants from Volcano and Boston Scientific. The grant givers were not involved in data collection, data interpretation or writing the manuscript. The local Ethics committee approved the study in all participating sites.
Procedural characteristics
Target lesions were assessed using a hybrid coronary physiology approach [Instantaneous wave-free ratio (Volcano Corporation) and fractional flow reserve (iFR/FFR)] to define the appropriateness of revascularization based on the presence of ischaemia. An iFR <0.86 indicated need for revascularization, an iFR between 0.86 and 0.93 required decision making based on FFR, and an iFR >0.93 indicated deferral of PCI.12 The SYNERGY DES (Boston Scientific, Natick, MA, USA) was implanted according to routine local clinical practice. Pre-PCI intravascular ultrasound (IVUS) was used at the discretion of the operator. Post-PCI IVUS assessment (site-reported) was mandatory to optimize stent expansion and apposition following the modified MUSIC criteria (Supplementary material online, Table S2).15 Treatment of bifurcation lesions was consistent with the European Bifurcation Club’s consensus.16 Revascularization of CTOs, by a dedicated CTO operator in all participating centres, was recommended. Chronic total occlusion recanalization techniques were left to the discretion of the operator. Staged procedures were permitted and encouraged for more complex cases to increase the likelihood of complete revascularization and to decrease the risk of contrast-induced nephropathy. Guideline-directed medical therapy included mandatory dual antiplatelet therapy (aspirin and clopidogrel or ticagrelor or prasugrel) for at least 6 months, while aspirin was recommended indefinitely as per current ESC/AHA/ACC guidelines.1,17 Strict control of LDL-C using high-intensity statin treatment following the current guidelines and control of risk factors was recommended.1,17 The patient’s clinical status was assessed at discharge. Hospital visits occurred at 1 month (±7 days), 6 months (±14 days), and 1 year (±30 days) post-procedure. Extended follow-up is planned up to 5 years.
Endpoints
The primary endpoint was a composite of major adverse (patient-oriented) cardiac and cerebrovascular events (MACCE) at 1-year follow-up. Major adverse cardiac and cerebrovascular event was defined as all-cause death, stroke, any myocardial infarction (MI) or any revascularization. One-year MACCE was compared with a predefined PCI cohort from the original SYNTAX-I trial. To allow for comparison, MACCE was adjudicated using the same trial definitions, periprocedural MI was defined as CK-MB ≥5xULN and new pathological Q-waves in the ECG within 7 days after PCI. Reflecting contemporary practice the definition of MI was expanded to allow a troponin ≥35 ULN (if the CK-MB was not available) with a new pathological Q-waves in the ECG.12 Spontaneous MI was defined as new Q-waves or one plasma level of CK-MB 5x ULN (or Tn ≥35 ULN if CK-MB not available) in the context of clinical syndrome consistent with ACS. Secondary endpoints included (i) composite of all-cause death, stroke, any MI at 1-year follow-up compared with the equipoise-derived SYNTAX-I PCI cohort (safety endpoint); (ii) incidence of the individual components of MACCE at 1-, 2-, and 5-year follow-up; and (iii) definite stent thrombosis—according to ARC definitions at all time points.18 As an additional exploratory endpoint the composite of MACCE was compared with the equipoise-derived SYNTAX-I CABG cohort of the original SYNTAX-I trial. Adverse events were adjudicated by an independent clinical event committee.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median [interquartile range (IQR)] and compared with the Student’s t-test or Mann–Whitney test as appropriate. Categorical variables are presented as counts and percentages and compared with the χ2 test. The SYNTAX-I control groups were predefined by selecting all patients from the 3VD cohort who showed equipoise between CABG and PCI on the basis of 4-year mortality using the SYNTAX score II calculator, resulting in a population of 315 patients from the PCI and 334 patients from the CABG arm. The SYNTAX score II combines age, sex, creatinine clearance (ml/min), left ventricular ejection fraction (%), peripheral vascular disease, chronic obstructive pulmonary disease, the presence of left main disease, and the anatomical SYNTAX score. The primary endpoint was superiority (two-sided alpha of 5%) in MACCE of the SYNTAX II group compared with predefined patients from the original SYNTAX-I trial. Assuming an expected rate of MACCE of 11.5% at 360 days for the EES arm, a sample size of 450 patients was calculated to obtain a power of at least 90% with a 5% of significance.12 The outcome analyses were performed according to the intention-to treat principle and are presented as Kaplan–Meier estimates and compared with Cox proportional hazards models. For the primary endpoint, a sensitivity analysis using inverse propensity score weighting (IPTW) and multivariate Cox proportional hazard regression model were performed. As an exploratory endpoint, non-inferiority in MACCE to the equipoise-derived SYNTAX-I CABG cohort was investigated using a non-inferiority margin of 5% with a one-sided alpha of 5%. The primary analysis was based on the intention-to-treat principle. A P-value <0.05 was considered significant. The statistical analyses were performed using the SAS System software, version 9.2 (SAS Institute Inc., SAS Campus Drive, Cary, NC, USA).
Results
The SYNTAX II strategy
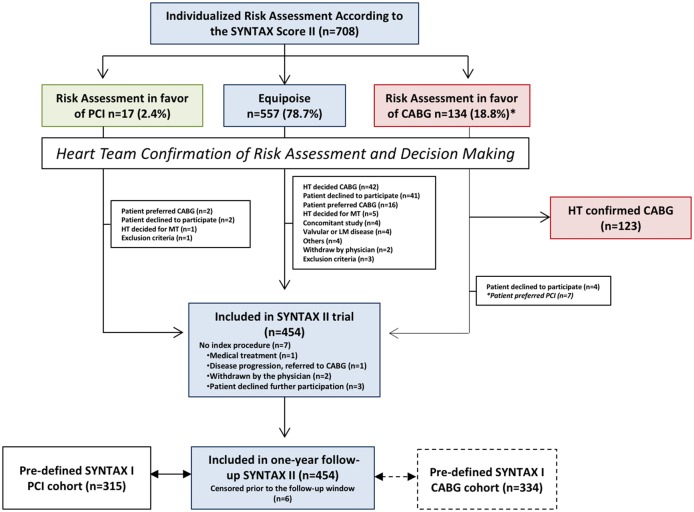
Figure 1 shows the patient flow in this study. Overall, 708 patients with de novo 3VD were screened and discussed within the heart team. Based on the SYNTAX score II recommendation, 557 (78.7%) patients demonstrated equipoise between CABG and PCI. After the heart team assessment of the appropriateness of percutaneous coronary revascularization, 454 patients signed written informed consent and were included in the study. In SYNTAX I, 643 (58.8%) patients with 3VD (without left main disease) had an equipoise recommendation for CABG or PCI based on the SYNTAX score II.
Figure 1.
Flowchart of the study. Patients were screened on the basis of the SYNTAX score II and discussed within the heart team to assess the appropriateness of percutaneous based revascularization; 708 patients with three-vessel (3VD) demonstrated on angiography were screened using the SYNTAX score II by the local study coordinator and submitted to the assessment of the Heart Team who had to confirm the treatment recommendation based on the SYNTAX score II. Despite equipoise in the treatment recommendation, the Heart Team recommended CABG in 42 patients; the other reasons for non-compliance to the treatment recommendation of the SYNTAX score II were: patient preference or decline participation (n = 57) exclusion criteria (n = 11) and others (see flowchart).The 1-year clinical outcomes of patients undergoing percutaneous coronary intervention (PCI) using the SYNTAX II strategy were compared with predefined cohorts of the PCI and CABG arm of the original SYNTAX trial. CABG, coronary-artery bypass grafting.
Baseline characteristics
Baseline characteristics are shown in Table 1. The mean anatomic SYNTAX score was lower in the SYNTAX-II group compared with equipoise-derived SYNTAX-I PCI cohort whereas the SYNTAX score II was similar between groups (Supplementary material online, Figure S1). In the SYNTAX II group, 298 patients (65.6%) were in the low (0–22), 140 (30.8%) in the intermediate (23–32) and 16 (3.5%) in the high (>32) anatomic SYNTAX score group. Staged procedures were performed in 138 (30%) patients with a median of 17 days (interquartile range 6.0–29.8).
Table 1.
Baseline clinical characteristics
| SYNTAX II | SYNTAX I PCI arm | P-value | |
|---|---|---|---|
| (n = 454) | (n = 315) | ||
| Age (years) | 66.7±9.7 (454) | 66.7±9.1 (315) | 0.99 |
| Male | 93.2% (423/454) | 93.0% (293/315) | 0.93 |
| Body mass index (kg/m2) | 28.9±4.7 (449) | 28.2±4.4 (315) | 0.032 |
| Diabetes mellitus type I or II | 30.3% (135/446) | 29.2% (92/315) | 0.75 |
| Insulin treated | 8.5% (38/446) | 10.5% (33/315) | 0.36 |
| Oral medication | 19.5% (87/446) | 16.8% (53/315) | 0.35 |
| Diet only | 2.0% (9/446) | 1.9% (6/315) | 0.91 |
| Current smoker | 14.7% (64/435) | 17.8% (56/315) | 0.26 |
| Previous MI | 12.5% (56/447) | 28.7% (89/310) | <0.001 |
| Previous stroke | 5.6% (25/449) | 1.9% (6/315) | 0.010 |
| Hypertension | 77.0% (344/447) | 73.4% (229/312) | 0.26 |
| Hyperlipidaemia | 77.3% (341/441) | 74.4% (232/312) | 0.35 |
| Creatinine clearance (ml/min) | 82.0±26.9 (454) | 87.3±28.5 (315) | 0.008 |
| Ejection fraction (%) | 58.1±8.3 (454) | 61.8±11.3 (315) | <0.001 |
| Peripheral vascular disease | 7.7% (35/454) | 9.5% (30/315) | 0.37 |
| COPD | 10.8% (49/454) | 12.7% (40/315) | 0.42 |
| Clinical presentation | <0.001 | ||
| Silent ischaemia | 5.5% (30/449) | 13.3% (42/315) | |
| Stable angina | 68.8% (309/449) | 61.6% (194/315) | |
| Unstable angina | 25.6% (115/449) | 25.1% (79/315) | |
| Anatomic SYNTAX Score | 20.3±6.4 (454) | 22.8±8.7 (315) | <0.001 |
| SYNTAX Score II PCI | 30.2±8.6 (454) | 30.6±8.7 (315) | 0.528 |
| Predicted 4-year mortality PCI (%) | 8.9±8.8% (454) | 9.2±8.7% (315) | 0.64 |
| SYNTAX Score II CABG | 29.1±10.4 (454) | 29.1±9.6 (315) | 1.0 |
| Predicted 4-year mortality CABG (%) | 9.0±9.3 (454) | 8.5±8.1 (315) | 0.44 |
MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease.
Procedural characteristics
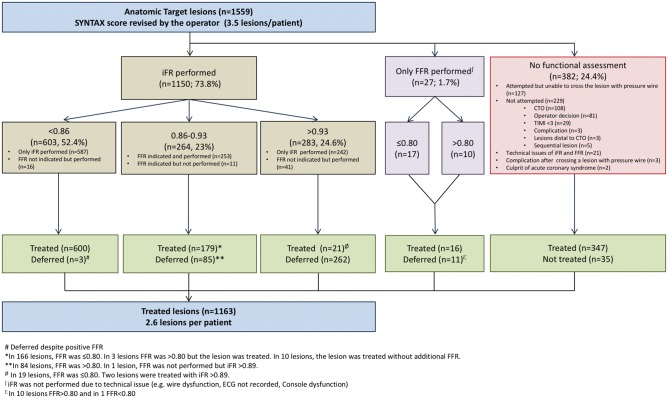
Physiological assessment was performed in 75.5% of the lesions (Figure 2). The mean iFR value was 0.77 ± 0.21. Out of 1559 lesions (3.49 ± 0.97 lesions per patient) initially intended to be treated based on the angiographic findings, only 74.6% (2.64 ± 1.12 lesions per patient) were found to be functionally significant. The use of coronary physiology deferred stenting in a quarter of the lesions (25.0%, n = 396). The iFR results based on the current cut-off of 0.89 and the reclassification according to the FFR measurements are presented in the Supplementary material online, Figure S2.
Figure 2.
Flowchart depicting ischaemia-driven revascularization in the SYNTAX II study. Physiologic evaluation of target lesions was performed using an hybrid iFR/FFR strategy. An iFR <0.86 indicated need for revascularization, an iFR between 0.86–0.93 required decision making based on FFR (0.80 cut-off), and an iFR >0.93 indicated deferral of PCI. iFR, instantaneous wave-free-ratio; FFR, fractional flow reserve.
Post-implantation IVUS was performed in 84.1% of the patients (76.4% of the lesions) leading to a further optimization (i.e. balloon post-dilatation) of the stented lesion in 30.2%. The minimum stent area post-procedure was 6.17 ± 2.31 mm2. Overall, 108 CTO’s were attempted with a procedural success of 87%. Procedural characteristics are shown in Table 2.
Table 2.
Lesion and procedural characteristics and medication
| SYNTAX II | SYNTAX I PCI arm | P-value | |
|---|---|---|---|
| Lesions anatomical syntax score per patient | 4.16±1.17 (454) | 4.31±1.34 (315) | 0.10 |
| Lesions intended to be treated per patient | 3.49±0.97 (447) | 4.60±1.55 (311) | <0.001 |
| Lesions treated per patient | 2.64±1.11 (440) | 4.02±1.34 (311) | <0.001 |
| Stents per patient | 3.78±1.92 (440) | 5.19±2.04 (308) | <0.001 |
| Stents per lesion | 1.43±0.76 (1165) | 1.28±0.65 (1251) | <0.001 |
| Vessel assessed by physiology (iFR/FFR) | |||
| Left main | 0.9% (4/447) | N/A | |
| RCA | 86.4% (386/447) | N/A | |
| LAD | 98.9% (442/447) | N/A | |
| LCX | 96.0% (429/447) | N/A | |
| Assessment in three vessels | 82.8% (370/447) | ||
| Vessel treated | |||
| Left main | 0.9% (4/441) | 2.3% (7/311) | 0.22 |
| RCA | 60.5% (267/441) | 87.1% (271/311) | <0.001 |
| LAD | 92.5% (408/441) | 99.0% (308/311) | <0.001 |
| LCX | 67.1% (296/441) | 96.5% (300/311) | <0.001 |
| Treatment in three vessels | 37.2% (164/441) | 83.3% (259/311) | <0.001 |
| Mean stent length (per stent, mm) | 24.43±9.18 (1663) | 18.82±7.04 (1599) | <0.001 |
| Total stent length (per patient, mm) | 92.32±52.78 (440) | 97.71±43.66 (308) | 0.13 |
| Bifurcation treated (%) | 35.0% (159/454) | 60.6% (191/315) | <0.001 |
| Total occlusion treated (%) | 27.8% (126/453) | 28.3% (89/315) | 0.88 |
| Post-implantation IVUS MLA (mm2) | 6.17±2.31 (1094) | N/A | |
| Medications | |||
| Aspirin | |||
| At discharge | 99.8% (448/449) | 96.2% (302/314) | <0.001 |
| At 1 month | 99.6% (443/445) | 93.9% (292/311) | <0.001 |
| At 1 year | 95.6% (413/432) | 92.1% (278/302) | 0.046 |
| P2Y12 inhibitor | |||
| At discharge | 99.3% (446/449) | 98.4% (309/314) | 0.234 |
| Clopidogrel | 66.8% (298/446) | N/A | |
| Prasugrel | 4.5% (20/446) | N/A | |
| Ticagrelor | 28.7% (128/446) | N/A | |
| At 1 month | 99.6% (443/445) | 97.1% (302/311) | 0.004 |
| Clopidogrel | 66.8% (298/446) | N/A | |
| Prasugrel | 4.5% (20/446) | N/A | |
| Ticagrelor | 28.7% (128/446) | N/A | |
| At 1 year DAPT | 61.8% (267/432) | 72.2% (218/302) | 0.0034 |
| Beta-blocker at discharge | 75.7% (339/448) | 77.1% (242/314) | 0.66 |
| Statin at discharge | 97.3% (437/449) | 85.4% (268/314) | <0.001 |
DAPT, dual antiplatelet therapy; IVUS, intravascular ultrasound; FFR, fractional flow reserve; iFR, Instantaneous wave-free ratio; MLA, minimum lumen area; LAD, left anterior descending artery; LCX, left circumflex; RCA, right coronary artery.
Optimal medical therapy
Although not specifically mandated by the protocol, ticagrelor, and prasugrel were used in approximately one-third (33.2%) of patients; clopidogrel therapy was used in the remaining patients. At 1 year, 60.0% of patients were on dual antiplatelet therapy (DAPT). Statins were used in 97.3% of the patients and high-intensity statin therapy (atorvastatin 80 mg or rosuvastatin 40 mg) was used in 80% of patients in SYNTAX-II.
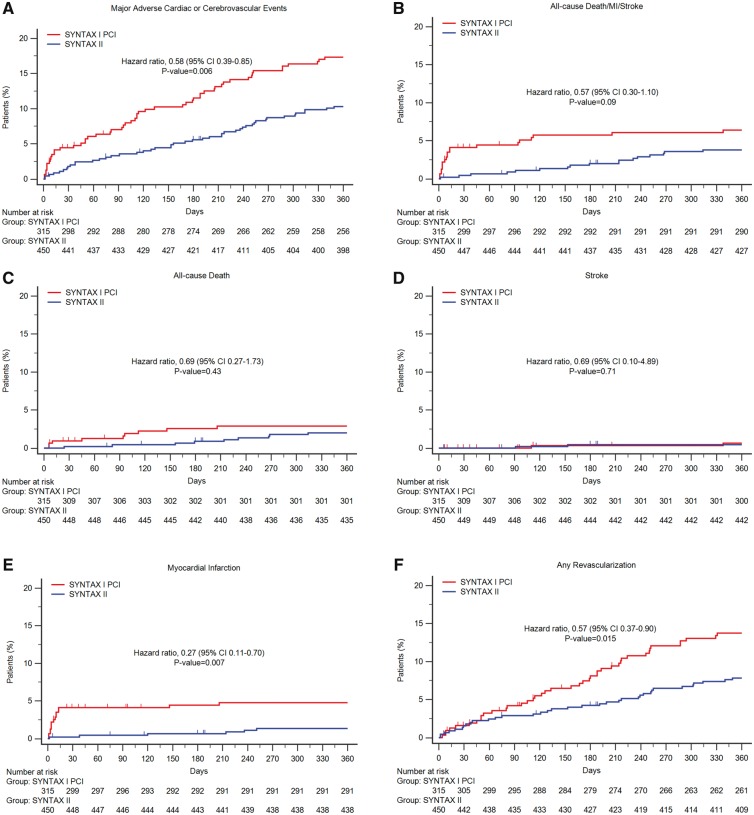
Primary endpoint and components
Table 3 and Figure 3 (panels A–F) show the MACCE and its components at 1-year follow-up. The incidence of MACCE was significantly lower with the SYNTAX II strategy (10.6% SYNTAX-II vs. 17.4% equipoise-derived SYNTAX-I PCI cohort; unadjusted HR 0.58, [95% CI 0.39–0.85], P = 0.006; adjusted HR 0.59 [95% CI 0.59–0.89], P = 0.01; Figure 3, panel A). This difference was driven by a reduction of 73% in the incidence of any MI (Figure 3, panel E) and 43% in any revascularization (Figure 3, panel F). The lower rate of MI in the SYNTAX-II group was driven by a significant reduction in periprocedural MI. No significant difference was found in all-cause death (Figure 3, panel C) and stroke (Figure 3, panel E) between the SYNTAX II and the equipoise-derived SYNTAX-I PCI cohort.
Table 3.
One year clinical outcomes between SYNTAX II cohort and the equipoise-derived SYNTAX-I PCI
| Outcome | SYNTAX II (n = 454) | SYNTAX I PCI arm (n = 315) | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|
| MACCE, % (n) | 10.6% (47) | 17.4% (54) | 0.58 (0.39–0.85) | 0.006 |
| All-cause death, stroke and any MI, % (n) | 3.8% (17) | 6.4% (20) | 0.58 (0.30–1.10) | 0.09 |
| All-cause death, % (n) | 2.0% (9) | 2.9% (9) | 0.69 (0.27–1.73) | 0.43 |
| Cardiac death, % (n) | 1.1% (5) | 2.6% (8) | — | 0.13 |
| Vascular death, % (n) | 0.2% (1) | 0.0% (0) | — | 0.41 |
| Non-cardiovascular death, % (n) | 0.7% (3) | 0.3% (1) | — | 0.52 |
| Stroke, % (n) | 0.4% (2) | 0.7% (2) | 0.69 (0.10–4.89) | 0.71 |
| Ischaemic, % (n) | 0.4% (2) | 0.3% (1) | — | 0.79 |
| Haemorrhagic, % (n) | 0.2% (1) | 0.3% (1) | — | 0.80 |
| Any MI, % (n) | 1.4% (6) | 4.8% (15) | 0.27 (0.11–0.70) | 0.007 |
| Periprocedural MI, % (n) | 0.2% (1) | 3.8% (12) | — | <0.001 |
| Spontaneous MI, % (n) | 1.1% (5) | 1.0% (3) | — | 0.880 |
| Any revascularization, % (n) | 8.2% (36) | 13.7% (42) | 0.57 (0.37–0.90) | 0.015 |
| CABG, % (n) | 0.7% (3) | 1.6% (5) | — | 0.21 |
| PCI, % (n) | 7.5% (33) | 12.5% (38) | — | 0.022 |
| Definite stent thrombosis, % (n) | 0.7% (3) | 2.6% (8) | 0.26 (0.07–0.97) | 0.045 |
| Acute, % (n) | 0.2% (1) | 1.6% (5) | — | 0.40 |
| Sub-acute, % (n) | 0.0% (0) | 1.6% (5) | — | 0.007 |
| Late, % (n) | 0.5% (2) | 1.0% (3) | — | 0.37 |
| Probable stent thrombosis, % (n) | 0.2% (1) | NA | — |
The event rates are based on Kaplan–Meier estimates.
MACCE, major adverse cardiac and cerebrovascular events (any death, any stroke, any MI and any revascularization); MI, myocardial infarction; CABG, coronary artery bypass graft.
Figure 3.
One-year clinical outcomes among the study patients, compared with the equipoise-derived SYNTAX-I PCI cohort. Kaplan–Meier curves are shown for the SYNTAX-II group (blue) and the percutaneous coronary intervention (PCI) arm of the original SYNTAX-I trial (red) for the composite primary endpoint of major adverse cardiac or cerebrovascular events (MACCE, panel A), all-cause death/stroke/MI (panel B), all cause death (panel C); stroke (panel D); any myocardial infarction (panel E); any revascularization (panel F).
There was no difference in MACCE between SYNTAX II patients with low (<22) vs. intermediate (22–32) anatomical SYNTAX score or in patients with or without diabetes mellitus (Supplementary material online, Figures S3, S4). Non-hierarchical clinical outcomes are shown in the Supplementary material online, Table S3 and the description of the revascularization procedures in SYNTAX II is presented in the (Supplementary material online, Table S4). In the complete PCI cohort of SYNTAX I trial the rate of MACCE was 17.8% at 1-year follow-up. No difference in MACCE was found between SYNTAX II and the equipoise-derived SYNTAX-I PCI cohort with an anatomical SYNTAX Score >22 [HR 0.66 (95% CI 0.36 to 1.21), P = 0.179; Supplementary material online, Figure S5]. The rate of definite stent thrombosis was significantly lower in SYNTAX-II (HR 0.26, 95% CI 0.07–0.97, P = 0.045). A sensitivity analyses for MACCE adjusted for confounding factors using IPTW and multivariate Cox regression analysis confirmed these findings (Supplementary material online, Tables S5, S6). Patients with staged procedures were associated with a higher incidence of MACCE compared with single-PCI [HR 2.16 (95% CI 1.15–4.05), P = 0.0071] and similar incidence of all-cause death/stroke and MI [HR 0.96 (95% CI 0.34 to 2.70, P = 0.93); Supplementary material online, Figure S6].
The exploratory short-term comparison with the equipoise-derived SYNTAX-I CABG cohort suggests non-significant differences in the occurrence of MACCE at 1 year (SYNTAX-II 10.6% vs. equipoise-derived SYNTAX-I CABG cohort 11.2%; HR 0.91 [95% CI 0.59 to 1.14], P = 0.684) (Supplementary material online, Tables S7, S8, and Figure S7).
Discussion
The main findings of this study can be summarized as follows: (i) PCI undertaken using the SYNTAX II strategy was associated with superior outcomes compared with the PCI arm of the original SYNTAX-I trial, with a lower incidence of MACCE driven by a reduction in MI, revascularization and definite stent thrombosis at 1-year follow-up; (ii) the short term outcomes of patients with intermediate anatomical risk (SYNTAX score 23–32), treated with PCI using the SYNTAX score II risk stratification algorithm, were similar to those observed in patients with low anatomical risk (SYNTAX score ≤22); (iii) physiological assessment, which was feasible in 75.5% of lesions, contributed to deferring treatment in 25% of the interrogated stenoses; (iv) the systematic use of IVUS post-stent implantation resulted in further stent optimization (predominantly further post-dilatation) in 30.2% of the lesions; (v) contemporary CTO revascularization techniques were associated with significantly improved procedural outcomes; and (vi) in an exploratory analysis at 1-year, PCI with the SYNTAX II strategy was associated with similar clinical outcomes to the equipoise-derived SYNTAX-I CABG cohort.
Translation of scientific progress to clinical practice can be hampered by simultaneous developments in different domains. Clinical trials are typically designed to test the impact of a single or a reduced number of variables on patient outcomes, using outcomes associated with standard care as a reference. However, this approach can fail to depict the cumulative benefit of incremental technological advances and the application of Guideline recommended practice. The SYNTAX II strategy was developed as representative of contemporary state-of-the art of coronary revascularization. Of note, the observed differential outcomes associated with its use cannot be attributed to a single diagnostic or therapeutic approach but rather to this integrated approach.
The first step of this strategy was optimizing the decision-making process between CABG and PCI in patients with 3VD. Currently, the European guidelines recommend CABG as the preferred revascularization strategy in patients with multivessel disease (IA recommendation).1 In patients with low anatomical complexity (i.e. anatomic SYNTAX Score < 22) percutaneous based revascularization can be an alternative treatment (IB recommendation).1 However, beyond the anatomical complexity, objectively quantified by the anatomic SYNTAX score, patient‘s characteristic and comorbidities should be taken into consideration to individualize the decision-making process between CABG and PCI based on the long-term vital prognosis.6 In the present study, patients were screened on the basis of the SYNTAX score II, a clinical tool that objectively allows decision making between CABG and PCI based on 4-year mortality. Whenever the SYNTAX score II recommended PCI treatment or equipoise with CABG, the patient was considered for inclusion in the trial, patients with a recommendation for CABG were enrolled in a registry. By utilizing the SYNTAX Score II, 156 patients (45%) had an anatomical SYNTAX score above 22, of which 140 patients had an anatomical SYNTAX Score 23–32. The 1-year results demonstrated that this approach was not associated with an increase in event rates.
Within the recent EXCEL trial, investigating left main disease, the protocol imposed an anatomic SYNTAX score >32 as a key exclusion criteria.19 By utilizing the SYNTAX Score II, the present study has allowed us to investigate patients with anatomically more complex disease who, based on the current guidelines, might not be considered initially for PCI.1 Furthermore, similar outcomes were found in patients with low and intermediate-high anatomical SYNTAX scores (site reported) when selected with the SYNTAX Score II.
The use of physiology-guided revascularization has been shown to reduce the rate of MACCE in patients with multivessel disease.11 In the SYNTAX II study, and in line with the FAME trial, physiological assessment was required in all stenoses suitable for pressure guidewire interrogation, irrespective of angiographic severity.20 Accordingly, mean iFR in SYNTAX II (iFR 0.77 ± 0.21) was lower than in recent studies focused on intermediate severity stenoses (0.91 ± 0.09 in DEFINE FLAIR).21 Physiological assessment was successfully performed in 88% (1177/1338) of attempted stenoses (Figure 3). In most cases the proposed hybrid iFR/FFR approach was followed, with iFR-alone used in 829 (70.4%) cases resulting in a reduction of the number of treated lesions from 3.49 ± 0.97 to 2.64 ± 1.12. Moreover, the physiologic interrogation of the three coronary vessels performed in 82.8% of the patients decreased the number of three-vessel interventions to 37.2%. Instantaneous wave-free ratio contributed to a simpler physiological interrogation by avoiding the use of adenosine in 73% of the lesions assessed by coronary physiology. It is quite likely that, compared with FFR-alone guidance, this resulted in shorter procedural times.21,22 As shown in the FAME trial, FFR-guided PCI strategy proved to be cost-effective in reducing the number of stented lesions.23 In addition, iFR spares the use of adenosine and is time saving which may also result in cost saving.
Together with optimized implant technique, the thin-strut bioresorbable polymer DES used in the present study is probably responsible for the observed reduction in stent thrombosis, MI, and revascularization. In a network meta-analysis by Palmerini et al.,9 contemporary metallic DES were found to be superior to the thick strut permanent polymer paclitaxel-eluting stent (PES) used in SYNTAX (Taxus, Boston Scientific). Paclitaxel-eluting stent was associated with sustained vessel wall toxicity, and greater on-going risk for very late stent thrombosis compared with newer generation DES.9,24 Also, in agreement with previous studies, post-procedural IVUS guidance may have contributed to a improved stent implantation and a reduction in device-related adverse events such as revascularization and stent thrombosis.10 The use of second-generation DES in the context of patients with 3VD was investigated in the BEST trial. Patients were randomized to CABG or PCI with a thin-strut durable polymer DES (Xience, Abbott Vascular, USA).4 At 1 year, the MACCE rate in the PCI arm of BEST was 11.4% which is similar to the 10.6% observed in the present study. The number and the length of the stents placed were similar between BEST and SYNTAX II, and the proportion of IVUS used was also comparable (71% in BEST and 84.1% in SYNTAX II). Notably, in the BEST trial no difference in MACCE between CABG and PCI was observed at 1 year; however, at 5 years CABG was associated with a significant reduction in the cumulative incidence of adverse cardiac events (21.1% PCI vs. 14.6% CABG, P = 0.01). In concordance with the BEST trial, we found no difference in the rates of MACCE at 1 year between CABG and PCI but we recognize the superiority of CABG over PCI might become apparent over a longer period of follow-up.25,26
In SYNTAX II the procedural success rate of CTO recanalization was 87%, similar to that recently reported by dedicated European CTO operators.27 The difference in procedural success compared with that observed in CTOs in SYNTAX-I trial (i.e. 53%) can be explained by the evolution in CTO revascularization techniques, coronary wires and devices, and the increased systematization of the procedure.
Overall, the initial results of SYNTAX II provide evidence of the impact of the technological developments in the field of PCI in clinical outcomes. The plan is to follow this cohort for 5 years to facilitate understanding of whether lesion deferral in patients with 3VD is safe in the long-term; and whether the non-inferiority of PCI using the SYNTAX II strategy compared with CABG is maintained.
Limitations
The present study has several limitations. First, this is a non-randomized study comparing a contemporary strategy with a historical control group (SYNTAX-I). This study was designed to investigate a novel approach for selecting and treating patients, based on long-term (4 years) prognosis and suggests that treatment of selected patients with an anatomical SYNTAX score >22 is not associated with a higher risk of MACCE at 1 year. Because of the observed advantage of CABG in females and young patients, the SYNTAX score II as a screening tool resulted in a low proportion of these subgroups of patients in the current study. Second, the exclusion of patients with previous PCI, who represent a large proportion of current clinical practice, might impair the generalizability of these findings. Third, for the comparison with the original SYNTAX-I trial a temporal bias should be acknowledged as surgical techniques may have improved together with adjunctive medical therapy. Fourth, 6 out of the 30 adjudicated revascularization events were due to reintervention at the CTO location (i.e. post-dilatation and additional stent implantation) to adapt the stent to the vessel vasodilation seen after the restoration of coronary flow at the index PCI procedure.28 Although these non-ischaemic driven reinterventions may not be perceived as true revascularization events, they were adjudicated as revascularization events to respect the protocol definition of all cause revascularization (Supplementary material online, Figure S4). Fifth, the definition of spontaneous MI used in SYNTAX-II did not result in lower rates of MI compared with the third universal definition of MI; however, we have to acknowledge that the SYNTAX definition of spontaneous MI might be less sensitive compared with the third universal definition of MI.29 Sixth, although we have used several statistical methods to attempt to ensure balance between the groups we acknowledge that it is not possible to adjust for every potential confounder. Seventh, although the use of coronary physiology was mandatory in lesions intended to be treated, mild stenoses not included in the anatomic SYNTAX score which could potentially be associated with reduced FFR were not systematically assessed by iFR/FFR.11 Finally, regarding the IVUS analysis, we only provided the site-reported minimum stent area post-procedure; an ongoing core lab analysis will provide a further insight into the IVUS data within this study.
Conclusion
At 1 year, clinical outcomes with the SYNTAX-II strategy were associated with improved clinical results compared with the PCI performed in comparable patients from the original SYNTAX-I trial. Longer term follow-up is awaited and a randomized clinical trial with contemporary CABG is warranted.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was sponsored by the European Cardiovascular Research Institute (ECRI) with unrestricted research grants from Volcano and Boston Scientific. Prof Adrian Banning is partially supported by the NIHR Biomedical Research Centre, Oxford.
Conflict of interest: J.E.: Consultant Philips/Volcano, Boston Scientific; C.C.: Research grant from Heart Flow Inc.; J.D.: Consultant and research grant from Volcano. Several patents licensed to Volcano Corporation; J.J.P.: Consultant Volcano. Member advisory board Abbott Vascular; N.V.M.: grant support from Boston Scientific, Abbott Vascular, Medtronic, and Claret Medical; Y.O.: Member advisory board Abbott Vascular; P.W.S.: Consultant Volcano. Member advisory board Abbott Vascular; A.P.B.: lecture fees from Abbott Vascular, Medtronic, and Boston Scientific, and grant support from Boston Scientific. All other authors declare that they have no conflict to declare.
Supplementary Material
References
- 1. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A.. 2014 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention 2015;10:1024–1094. [DOI] [PubMed] [Google Scholar]
- 2. Mohr FW, Morice MC, Kappetein AP, Feldman TE, Stahle E, Colombo A, Mack MJ, Holmes DR Jr., Morel MA, Van Dyck N, Houle VM, Dawkins KD, Serruys PW.. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629–638. [DOI] [PubMed] [Google Scholar]
- 3. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S 3rd, Bertrand M, Fuster V.. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 4. Park SJ, Ahn JM, Kim YH, Park DW, Yun SC, Lee JY, Kang SJ, Lee SW, Lee CW, Park SW, Choo SJ, Chung CH, Lee JW, Cohen DJ, Yeung AC, Hur SH, Seung KB, Ahn TH, Kwon HM, Lim DS, Rha SW, Jeong MH, Lee BK, Tresukosol D, Fu GS, Ong TK.. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204–1212. [DOI] [PubMed] [Google Scholar]
- 5. Serruys PW, Farooq V, Vranckx P, Girasis C, Brugaletta S, Garcia-Garcia HM, Holmes DR Jr., Kappetein AP, Mack MJ, Feldman T, Morice MC, Stahle E, James S, Colombo A, Pereda P, Huang J, Morel MA, Van Es GA, Dawkins KD, Mohr FW, Steyerberg EW.. A global risk approach to identify patients with left main or 3-vessel disease who could safely and efficaciously be treated with percutaneous coronary intervention: the SYNTAX Trial at 3 years. JACC Cardiovasc Interv 2012;5:606–617. [DOI] [PubMed] [Google Scholar]
- 6. Farooq V, van Klaveren D, Steyerberg EW, Meliga E, Vergouwe Y, Chieffo A, Kappetein AP, Colombo A, Holmes DR Jr, Mack M, Feldman T, Morice MC, Stahle E, Onuma Y, Morel MA, Garcia-Garcia HM, van Es GA, Dawkins KD, Mohr FW, Serruys PW.. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013;381:639–650. [DOI] [PubMed] [Google Scholar]
- 7. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M.. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM.. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 9. Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, Vlachojannis GJ, Jensen LO, Christiansen EH, Berencsi K, Valgimigli M, Orlandi C, Petrou M, Rapezzi C, Stone GW.. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2015;65:2496–2507. [DOI] [PubMed] [Google Scholar]
- 10. Zhang YJ, Pang S, Chen XY, Bourantas CV, Pan DR, Dong SJ, Wu W, Ren XM, Zhu H, Shi SY, Iqbal J, Gogas BD, Xu B, Chen SL.. Comparison of intravascular ultrasound guided versus angiography guided drug eluting stent implantation: a systematic review and meta-analysis. BMC Cardiovasc Disord 2015;15:153.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Bruyne B, Fearon WF, Pijls NHJ, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius-Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nüesch E, Jüni P; for the FAME 2 Trial Investigators. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 12. Escaned J, Banning A, Farooq V, Echavarria-Pinto M, Onuma Y, Ryan N, Cavalcante R, Campos CM, Stanetic BM, Ishibashi Y, Suwannasom P, Kappetein AP, Taggart D, Morel MA, van Es GA, Serruys PW.. Rationale and design of the SYNTAX II trial evaluating the short to long-term outcomes of state-of-the-art percutaneous coronary revascularisation in patients with de novo three-vessel disease. EuroIntervention 2016;12:e224–e234. [DOI] [PubMed] [Google Scholar]
- 13. Serruys PW, Onuma Y, Garg S, Sarno G, van den Brand M, Kappetein AP, Van Dyck N, Mack M, Holmes D, Feldman T, Morice MC, Colombo A, Bass E, Leadley K, Dawkins KD, van Es GA, Morel MA, Mohr FW.. Assessment of the SYNTAX score in the Syntax study. EuroIntervention 2009;5:50–56. [DOI] [PubMed] [Google Scholar]
- 14. Ong AT, Serruys PW, Mohr FW, Morice MC, Kappetein AP, Holmes DR Jr., Mack MJ, van den Brand M, Morel MA, van Es GA, Kleijne J, Koglin J, Russell ME.. The SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study: design, rationale, and run-in phase. Am Heart J 2006;151:1194–1204. [DOI] [PubMed] [Google Scholar]
- 15. de Jaegere P, Mudra H, Figulla H, Almagor Y, Doucet S, Penn I, Colombo A, Hamm C, Bartorelli A, Rothman M, Nobuyoshi M, Yamaguchi T, Voudris V, DiMario C, Makovski S, Hausmann D, Rowe S, Rabinovich S, Sunamura M, van Es GA.. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC Study). Eur Heart J 1998; 19: 1214–1223. [DOI] [PubMed] [Google Scholar]
- 16. Lassen JF, Holm NR, Banning A, Burzotta F, Lefevre T, Chieffo A, Hildick-Smith D, Louvard Y, Stankovic G.. Percutaneous coronary intervention for coronary bifurcation disease: 11th consensus document from the European Bifurcation Club. EuroIntervention 2016; 12: 38–46. [DOI] [PubMed] [Google Scholar]
- 17. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK.. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2014;130:1749–1767. [DOI] [PubMed] [Google Scholar]
- 18. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW.. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 19. Kappetein AP, Serruys PW, Sabik JF, Leon MB, Taggart DP, Morice MC, Gersh BJ, Pocock SJ, Cohen DJ, Wallentin L, Ben-Yehuda O, van Es GA, Simonton CA, Stone GW.. Design and rationale for a randomised comparison of everolimus-eluting stents and coronary artery bypass graft surgery in selected patients with left main coronary artery disease: the EXCEL trial. EuroIntervention 2016;12:861–872. [DOI] [PubMed] [Google Scholar]
- 20. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, Van' T Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF.. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–224. [DOI] [PubMed] [Google Scholar]
- 21. Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, Janssens L, Vrints CJ, Khashaba A, Laine M, Van Belle E, Krackhardt F, Bojara W, Going O, Harle T, Indolfi C, Niccoli G, Ribichini F, Tanaka N, Yokoi H, Takashima H, Kikuta Y, Erglis A, Vinhas H, Canas Silva P, Baptista SB, Alghamdi A, Hellig F, Koo BK, Nam CW, Shin ES, Doh JH, Brugaletta S, Alegria-Barrero E, Meuwissen M, Piek JJ, van Royen N, Sezer M, Di Mario C, Gerber RT, Malik IS, Sharp ASP, Talwar S, Tang K, Samady H, Altman J, Seto AH, Singh J, Jeremias A, Matsuo H, Kharbanda RK, Patel MR, Serruys P, Escaned J.. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med 2017;376:1824–1834. [DOI] [PubMed] [Google Scholar]
- 22. Gotberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Ohagen P, Olsson H, Omerovic E, Calais F, Lindroos P, Maeng M, Todt T, Venetsanos D, James SK, Karegren A, Nilsson M, Carlsson J, Hauer D, Jensen J, Karlsson AC, Panayi G, Erlinge D, Frobert O.. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med 2017;376:1813–1823. [DOI] [PubMed] [Google Scholar]
- 23. Fearon WF, Bornschein B, Tonino PA, Gothe RM, Bruyne BD, Pijls NH, Siebert U.. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation 2010;122:2545–2550. [DOI] [PubMed] [Google Scholar]
- 24. Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kutys R, Ladich E, Finn AV, Kolodgie FD, Virmani R.. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 2014;129:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang JC, Normand SL, Mauri L, Kuntz RE.. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation 2004;110:278–284. [DOI] [PubMed] [Google Scholar]
- 26. Taggart DP, Thomas B.. Ferguson Lecture. Coronary artery bypass grafting is still the best treatment for multivessel and left main disease, but patients need to know. Ann Thorac Surg 2006;82:1966–1975. [DOI] [PubMed] [Google Scholar]
- 27. Maeremans J, Walsh S, Knaapen P, Spratt JC, Avran A, Hanratty CG, Faurie B, Agostoni P, Bressollette E, Kayaert P, Bagnall AJ, Egred M, Smith D, Chase A, McEntegart MB, Smith WH, Harcombe A, Kelly P, Irving J, Smith EJ, Strange JW, Dens J.. The hybrid algorithm for treating chronic total occlusions in Europe: the RECHARGE Registry. J Am Coll Cardiol 2016;68:1958–1970. [DOI] [PubMed] [Google Scholar]
- 28. Gomez-Lara J, Teruel L, Homs S, Ferreiro JL, Romaguera R, Roura G, Sanchez-Elvira G, Jara F, Brugaletta S, Gomez-Hospital JA, Cequier A.. Lumen enlargement of the coronary segments located distal to chronic total occlusions successfully treated with drug-eluting stents at follow-up. EuroIntervention 2014;9:1181–1188. [DOI] [PubMed] [Google Scholar]
- 29. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR.. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.