Abstract
Glutathione (GSH) pathways play a central role in methylmercury (MeHg) metabolism and elimination, largely due to formation of a more readily transported MeHg-GSH conjugate. Glutathione S-transferases (GSTs) have therefore been proposed to facilitate MeHg elimination by catalyzing MeHg-GSH conjugation. A role for human GSTP1 in MeHg disposition is suggested by the association of two common polymorphisms in the coding region (Ile105Val and Ala114Val) with Hg levels in either blood or hair. In this study, we investigated a functional role for GSTs in modulating MeHg toxicity during development. Using the Drosophila model to execute targeted manipulations of both endogenous GSTs and introduced human GSTP1 variants we correlate gene and protein expression levels with GST activity and also with MeHg body burden and developmental outcomes. RNAi knockdown of endogenous GSTD1, GSTE1, or GSTS1, individually, increased susceptibility to MeHg during pupal development resulting in a reduced rate of adult eclosion. Exogenous expression of human GSTP1 in developing flies resulted in increased MeHg tolerance relative to control flies as seen with elevated eclosion rates when reared on MeHg containing food. Furthermore, the GSTP1105 and GSTP1114 variants showed a reduced enzyme activity relative to wild-type GSTP1 (GSTP1wt). Finally, we observed a trend whereby Hg body burden was inversely related to the levels of GST activity. However, in some instances GSTP1 expression resulted in increased eclosion rates without reducing Hg body burden suggesting that GSTs interact with MeHg via both toxicokinetic and toxicodynamic mechanisms. These findings indicate that GSTs moderate MeHg toxicity during development in our experimental model.
Keywords: methylmercury, glutathione S-transferase, GSTP1, Drosophila.
Methylmercury (MeHg) is an environmental toxicant and a naturally occurring contaminant in dietary fish. The risks of MeHg exposure are greatest during early life, with the developing nervous system being a major target. A number of human population studies have demonstrated a wide variation in neurological outcomes associated with early life MeHg exposure, ranging from none at all to measureable motor and cognitive symptoms that appear to persist in children up to 14 years (Davidson et al., 2006, 2008; Grandjean et al., 1997; Murata et al., 2004). This variation in outcomes has led to hypotheses for a genetic basis of susceptibility to MeHg toxicity (Llop et al., 2015).
In general, the degree of toxicity is presumed to correlate with the mercury body burden that results from MeHg exposure. Several studies in rodents and in humans support the hypothesis that systemic levels of MeHg are controlled by glutathione (GSH)-dependent pathways (Ballatori and Clarkson, 1982, 1983). Emerging evidence from studies on genes predicted to be involved in MeHg metabolism [e.g. glutathione-S-transferases (GSTs) and glutamyl-cysteine ligase (GCL)], and transport [e.g., ATP-binding cassette transporters (ABC transporters)] are beginning reveal underlying sources of variation in MeHg-related outcomes during development (Custodio et al., 2004; Engstrom et al., 2016; Llop et al., 2014, 2015; Prince et al., 2014; Schlawicke Engstrom et al., 2008).
GSTs constitute a large family of enzymes present in bacteria, plants, and animals that are required for essential cellular functions, notably in detoxification. Among the wide variety of reactions for normal cellular homeostasis that GSTs engage in, they are best known for conjugating GSH to xenobiotics, which commonly results in more efficient transport and elimination of toxicants (Hayes et al., 2005). A given organism is likely to harbor multiple GST genes (e.g., 21 human GSTs and 39 Drosophila GSTs) (Flybase, 2016; Nebert and Vasiliou, 2004), which afford both diversity and redundancy in their tissue expression and substrate specificity. Classification of GSTs is based on sequence homologies that range from ∼25 to 40% amino acid similarity and has resulted in 13 classes, of which a subset are harbored in a given species (e.g., six human GST subfamilies: Alpha, Kappa, Mu, Omega, Pi, Theta; six Drosophila subfamilies: Delta, Epsilon, Omega, Sigma, Tau, Zeta) (Flybase, 2016; Nebert and Vasiliou, 2004).
The notion that GSTs can mediate MeHg-GSH conjugation has prompted several studies into the relationship of polymorphic variants of GSTs and MeHg disposition in humans. Findings from such studies, while varied, suggest a role for GSTs in moderating Hg body burden. Two common polymorphisms in the coding region of the human GSTP1, Ile105Val (GSTP1105) and Ala114Val (GSTP1114), have received considerable attention. Biochemical studies indicate that the GSTP1105 and GSTP1114 enzymes are catalytically less active than the wild-type GSTP1wt (Ali-Osman et al., 1997; Goodrich and Basu, 2012). In population studies, it has been shown that carriers of the GSTP1114 allele are more likely to exhibit higher Hg levels as seen in biomarkers of blood or hair (Custodio et al., 2004; Gundacker et al., 2009). Conflicting results are also reported whereby GSTP1105 and GSTP1114 carriers showed association with lower Hg retention (Goodrich et al., 2011; Schlawicke Engstrom et al., 2008). Despite inconsistencies, these prior association studies point to a potential for GSTs to affect Hg body burden.
While direct functional studies are few, a role for GSTs in moderating MeHg transport and toxicity is implicated by experiments in animal models. Early studies in rats, using pharmacological modulation of GST activity with the inhibitors azathioprine or benziodarone showed a decreased biliary transport of MeHg (Magos et al., 1985). However, pretreatment with trans-stilbene oxide, an inducer of GSTs, showed little to no effect on MeHg biliary transport (Gregus and Varga, 1985; Magos et al., 1985). In a screen for MeHg tolerance genes in Drosophila, three GSTs, GSTD3, GSTE3, and GSTE7, showed higher expression in strains of flies that exhibited greater tolerance to MeHg (Mahapatra et al., 2010).
In this study, we tested for a functional role of GSTs in modulating MeHg toxicity during development. Using the Drosophila model to execute targeted manipulations of both endogenous GSTs and introduced human GSTP1 variants we correlate gene and protein expression levels with GST transferase activity and also with MeHg body burden and developmental outcomes.
MATERIALS AND METHODS
Fly Strains and Transgene Expression
Transgene expression was accomplished with the standard methods of the GAL4 > UAS heterologous transgene expression system (Brand et al., 1994) using transgenic flies obtained from various Drosophila stock centers, other investigators or generated de novo in the lab. Flies were maintained in a humidified incubator at 25 °C on a standard preparation of cornmeal, molasses, and agar medium with yeast. The fat body GAL4 line (FB-GAL4 (c754-GAL4, #6984)) and the UAS-GFP reporter fly (#5431) were obtained from the Bloomington Drosophila Stock Center at Indiana University (Bloomington, Indiana). The gut specific NP1 GAL4 line was a gift from Benoit Biteau (Department of Biomedical Genetics, University of Rochester, New York). Fly lines carrying UAS constructs encoding short hairpin RNAi for the specific gene targets of GSTD1 (#103246), GSTE1 (#110529), and GSTS1 (#50140), and the corresponding parent line for these transformants (#60100), were obtained from the Vienna Drosophila RNAi Center (VDRC, http://stockcenter.vdrc.at/control/main) (Dietzl et al., 2007). UAS-human GSTP1 flies were created as described below.
RNAi knockdown of Drosophila GSTs was achieved in progeny derived from crosses between virgin females of the FB> and NP1> driver lines and males of each of the respective UAS-GST RNAi lines. Similarly, human GSTP1 expression in fat body or gut tissue was accomplished with crosses of FB > and NP1> driver lines and males of each of the respective UAS-GSTP1 wild-type and mutant fly lines.
Human GSTP1 Mutant Constructs and Transformed Flies
UAS-GSTP1 flies were generated with constructs carrying human GSTP1 coding sequences inserted in the pUAST-attB transformation vector [(Bischof et al., 2007) gift from Johannes Bischof and Konrad Basler, University of Zurich, Switzerland]. The cDNA for human GSTP1 was purchased from Origene (clone NM_000852 Cat. #RG203086). For purposes of this study, the reference sequence containing Ile105 and Ala114 was termed the “wild type” sequence. Mutants were constructed to generate the Ile105Val and the Ala114Val coding changes by changing the respective codons: ATC to GTC (Ile105Val) and GCG to GTG (Ala114Val). Point mutations were executed using the Quick Change Site Directed Mutagenesis kit (Agilent Technologies) and were sequence verified. The resulting pUAST-GSTP1 wild type and mutant constructs were used to create transgenic flies utilizing the phiC31 integrase system (Bischof et al., 2007) via the embryo injection services of Rainbow Transgenic Flies, Inc. (Camarillo, California). In brief, the phiC31 integrase system allows for targeted insertion of the transgene of interest (e.g., UAS-GSTP1) at a defined site in the fly chromosome. This strategy was developed to minimize variation in expression levels that can result from positional effects due to random chromosomal insertion seen with conventional P-element methods (Bischof et al., 2007). The acceptor site at 86Fa on the third chromosome was used for insertion of each of the three UAS-GSTP1 constructs. The parent strain carrying the acceptor site at 86Fa (RTF #24486, Rainbow Transgenic Flies, Inc.) was used to create these transformants was therefore used as the appropriate control strain (cont) for the GAL4 > UAS crosses with GSTP1 constructs.
Quantitative PCR for GST gene expression
GST expression, under the influence of various GAL4 > UAS combinations, was analyzed using RNA extracted from third instar wandering larvae (approximately day 4 of larval growth). Total RNA was extracted from a pooled sample of 20 larvae disrupted in Trizol reagent (Invitrogen) using a Kontes Pellet Pestle cordless motor (Fisher # NC0493674). Quantitative real-time PCR (qPCR) was performed using iScript One-Step RT-PCR Kit with SYBR Green (Bio-Rad) according to manufacturer’s protocol. cDNA template was synthesized from 40 ng of RNA for each sample and samples were run in duplicate. Samples were run on a BioRad CFX Connect Real-time System (Biorad). Fold-change in expression was calculated using ribosomal protein 49 (RP49) as a housekeeping gene, by the ΔΔCT method (Livak and Schmittgen, 2001). The following primers were used: GSTD1: For: 5′-TCAGCAAGTACGCCAATGT and Rev 5′-TTCTTGAACTCCAGGCATCC; GSTE1: For: 5′-GTGGACATAGATCCTGCTACATATC and Rev: 5′-GAGCTTCGTTGATCTCCTTGT; GSTS1: For: 5′-GCGGAGGTCATTCCATTCT and Rev: 5′-TGTAGTTCATGTAGTCGGTGATG; GSTP1: For: 5′-CTGCCCGGGCAACTGAAG-3′, Rev: 5′-CAGGAAGGCCTTGAGCTTG-3′ and RP49-AGTATCTGATGCCCAACATCG/TTCCGACCAGGTTACAAGAAC.
MeHg tolerance assays
MeHg tolerance in fly development was determined with a previously described eclosion behavior assay (Rand et al., 2014). Eclosion is the first behavior performed by the newly-formed adult fly after metamorphosis and constitutes coordinated pulsatile contractions to execute emergence (extrication) from the pupal case (Denlinger and Zdarek, 1994). Our recent findings indicate MeHg targets neuromuscular development that is likely required for eclosion behavior (Montgomery et al., 2014). Assays are performed by placing 50 first instar larvae on vials of fly food (Jazz Mix, Fischer Scientific #AS153) containing MeHg ranging from 0 to 20 μM (methylmercury chloride, Aldrich #442534), concentrations previously defined to span the inhibition of eclosion behavior in a variety of fly strains (Rand et al., 2014). Replicates of three vials, totaling n = 150 larvae, were performed for each MeHg concentration. Eclosion (emergence of adult flies from the pupal case) was scored on day 13 after larvae were placed on food. Rates of eclosion for indicated strains at each MeHg concentration are measured as a proportion and expressed as percent eclosion, whereby a higher rate of eclosion is indicative of greater MeHg tolerance. Technical variation is reflected by the standard deviation of the three replicates, and is represented by error bars for each data point.
GST Activity Assays
GST activity was determined using the Glutathione-S-transferase Activity Kit (Cayman Chemical, Cat. # 703302) according to the manufacturer’s protocol. This assay measures total GST in a DTNB (5,5′-dithio-bis-2-(nitrobenzoic acid), Ellman’s reagent) GSH reductase recycling reaction where 5-thio-2-nitrobenzoic acid (TNB) product is measured kinetically at absorbance of 405 nm. Thirty 3rd-instar larvae were collected for each sample and suspended with 250 µl protein lysis buffer [50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% IGEPAL CA 630, 1% Complete EDTA-free protease inhibitor cocktail (Roche Diagnostic)]. The larvae were homogenized using Kontes Pellet Pestle cordless motor (Fisher # NC0493674), frozen and thawed on ice four times. The sample was clarified by centrifugation (13 000 rpm × 15 min, 4°C) and supernatant collected for the enzyme assay. Protein concentration was measured using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) according to manufacturer’s protocol. All samples were diluted with protein lysis buffer to final total protein concentration 2 mg/ml.
Western Blotting
SDS-PAGE separation of the above larval lysates was done in 10% gels, transferred to PVDF membrane (Millipore) and Western blotted using standard procedures. Membranes were probed with rabbit anti-human GSTP1 (LifeSpan BioSciences, LS-B2376, 1:5000 dilution) or mouse anti-actin (Developmental Studies Hybridoma Bank, JLA-20, 1:100 dilution) antibodies in AquaBlock (EastCoast Bio) diluted 1:1 in PBS. Secondary antibodies used were peroxidase-conjugated rabbit anti-goat (Jackson ImmunoResearch #305-035-003, 1:5000 dilution) and peroxidase-conjugated goat anti-mouse (Jackson ImmunoResearch #115-036-146, 1:5000 dilution). Blots were developed using the Clarity Western ECL Substrate (Bio-Rad Cat. #170-5060) and imaged using a ChemiDoc station (Biorad). Band densities were quantified using the Image Lab Software (Bio-Rad, Version 4.1 on ChemiDoc MP Imaging System).
Mercury determinations
Total mercury (Hg) levels were measured directly in whole pupa by a thermal decomposition-Hg amalgamation-atomic adsorption method using a DMA80 Direct Mercury Analyzer (Milestone, Sorisole, Italy). MeHg treatments were performed on pupae derived from first instar larvae that had been reared on various concentrations of MeHg food at 25°C. For each treatment and GAL4 > UAS combination 10 pupae were harvested directly from the vial wall, transferred to a nickel sample boat and weighed directly on a Mettler MX5 microbalance (Mettler, Columbus, Ohio). Total Hg determinations were performed with the DMA80 analyzer that was previously calibrated to a reference sample (SRM# IAEA-086 and IAEA-085 Certified Reference Hair, IAEA, Vienna, Austria). Hg values were determined on a weight basis in µg/g (ppm).
Statistical analysis
For MeHg tolerance (eclosion) assays, statistical analyses were done by comparing each of the experimental cross combinations (e.g., FB > GSTD1, FB > GSTE1, or FB > GSTS1) to the control cross (FB > cont.) using a pairwise 2-tailed z-test and by treating each concentration of MeHg categorically. P-values of less than .05 were considered significant. z-test analysis was selected since eclosion rate is determined as a proportion value (number of flies eclosed out of n = 150, reported as percent) and proportion values are restricted at the edges (i.e., near 100%). Categorical treatment of MeHg concentrations was seen as more straightforward and interpretable for statistical purposes due to the narrow concentration range of the dose response in this model. Additional analyses were performed using ANOVA with trigonometrically transformed values and posthoc contrasts (data not shown). Posthoc comparisons were found to match between the ANOVAs and the z-tests for differences in proportions.
For mercury body burden, values were from a single Hg determination performed on a pooled sample of 10 pupae of each GAL4 > UAS cross combination. Thus, replicate values for each MeHg concentration were not available. Differences in mercury levels were therefore determined by using three measurements, at 2, 5, and 10 µM MeHg treatment levels, and evaluating the change in percentage of Hg level between a given experimental cross and its respective control cross (where the control cross Hg level at each MeHg treatment represents 100%). A one-sample t test for a mean change in percentage was performed for each experimental cross relative to the control cross. Results are expressed in terms of a confidence interval (C.I.) and two-sided P-values where less than .05 was considered significant. Bonferroni corrected P-values are also reported to account for the three experimental cross combinations (i.e., >GSTD1, >GSTE1, and >GSTS1 RNAi or >GSTP1wt, >GSTP105, and >GSTP1114).
RESULTS
Knockdown of Drosophila GST Expression Induces MeHg Susceptibility
In previous studies, we demonstrated the effectiveness of the Drosophila model for assaying MeHg developmental toxicity (Rand et al., 2014) and for executing RNAi knockdown of the MRP1 (ABCC1) transporter and subsequent effects on MeHg susceptibility (Prince et al., 2014). We therefore aimed to reduce GST activity using this model and approach. Of the 39 Drosophila GST genes we chose to target knockdown of GSTD1, GSTE1, and GSTS1, which represent three of the six GST classes in Drosophila and which are shown to be among the more catalytically active GST isoforms (Saisawang et al., 2012) that are also highly expressed during larval and pupal development (see modEncode Temporal Expression Data at FlyBase 2009). We elected to target knockdown to the fat body and the gut where, in general, most GST isoforms are more highly expressed relative to other tissues, such as the nervous system (Flybase, 2016). It should be noted that the insect fat body harbors conserved xenobiotic metabolizing enzymes and thus performs functions akin to the mammalian liver (Yang et al., 2007). Individual knockdown was accomplished with UAS-RNAi constructs expressed under a GAL4 driver with expression localized to the fat body (FB>) or to the gut (NP1>). The distribution of expression under the respective FB > and NP1> GAL4 drivers at the larval stage can be seen by the expression of a GFP reporter (UAS-GFP, Figs. 1A–C). The efficacy of the RNAi was determined by measuring the respective mRNA levels for each of the GSTs when RNAi was expressed with the FB > driver. With RNAi, GSTD1, GSTE1, and GSTS1 expression was reduced to 46%, 24%, and 17% of the control level (FB > cont.), respectively (Figure 1D). The effect of GST knockdown on MeHg tolerance was assayed with eclosion behavior of larva carrying each of the three GST knockdown combinations in either fat body or gut tissues. A decrease in tolerance to MeHg was observed with knockdown of each GST isoform as seen by a reduced rate of eclosion on MeHg food (Figs. 2A and B). Knockdown of GSTS1 (FB > GSTS1 RNAi) in fat body showed the greatest effect with 3% eclosion on 10 µM MeHg food compared with 29% and 42% eclosion of the GSTE1 (FB > GSTE1 RNAi) and GSTD1 (FB > GSTD1 RNAi) RNAi knockdown flies and 57% eclosion of control flies, respectively (Figure 2A). Targeted knockdown in the gut for each of the three GSTs similarly showed a decreased rate of eclosion with 7%, 17%, and 23% eclosion with GSTS1, GSTE1, and GSTD1 knockdown, respectively, compared with 42% for control flies on 15 µM MeHg food (Figure 2B).
FIG. 1.
GAL4 > UAS targeted RNAi knockdown of GSTs. The GAL4 > UAS transgene expression system was used to direct RNAi to fat body (FB) and gut (NP1) tissues. (A) Larval anatomy illustrating the fat body and gut (Dorsal view, anterior to the left; brain, trachea and other organs not represented for clarity). (B, C) a GFP reporter gene reveals expression in fat body and gut, respectively (anterior to the left). Live larvae carrying the GFP reporter were visualized under epifluorescence microscopy. (D) Quantitative rt-PCR determination of relative mRNA expression levels of GSTD1, GSTE1, and GSTS1 in control (FB > Cont.) and RNAi knockdown larva (single determinations of pooled samples of 20 larva).
FIG. 2.
Eclosion behavior with GST knockdown. Eclosion of flies from larvae reared on indicated MeHg concentrations in the food is shown for control and RNAi knockdown of GSTD1, GSTE1, and GSTS1 in (A) fat body and (B) gut. A total of n = 150 larvae (three trials of 50 larvae) are assayed for each data point. (*, z-statistic with P < .01 (see Methods section); error bars = Std. Dev.).
Effect of GST Knockdown on MeHg Body Burden
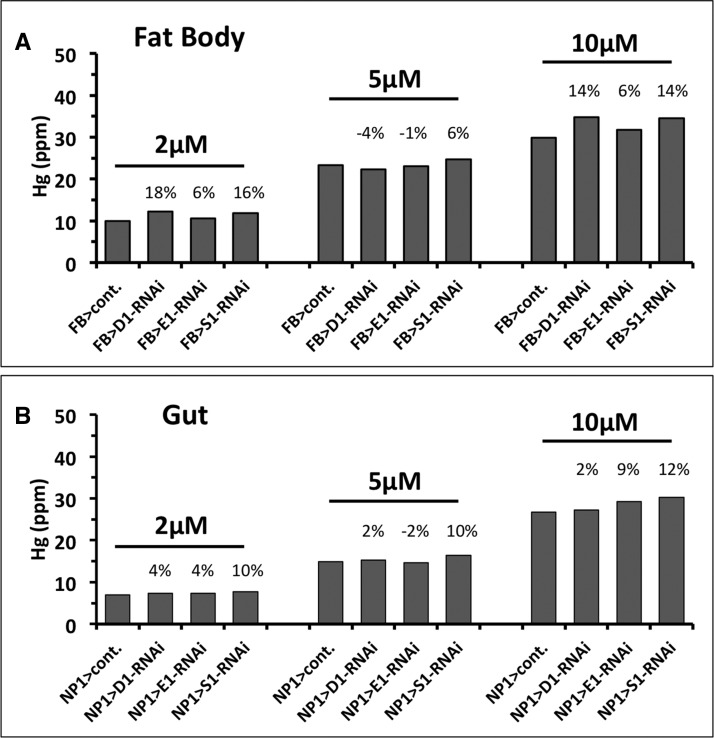
Since polymorphic variation in GSTs has been associated with variation in blood mercury concentrations in humans, we next sought to examine directly if GST expression level could alter the body burden of MeHg in our controlled assay system. In this system, MeHg feeding occurs throughout the larval life stage and reaches a steady state of MeHg accumulation in the third instar stage prior to formation of the pupa (pupariation) (M.R., D.V., unpublished observation). The body burden of MeHg can be conveniently assayed by direct total mercury analysis of pupae once they have formed. Since feeding and excretion cease within the pupa, total Hg measurement at this stage not only represents what is accumulated at steady state during the larval stage but what the animal will be exposed to for the duration of metamorphosis until eclosion. Subsequent to larval feeding on MeHg food spanning sub-lethal to semi-lethal concentrations (2–10 µM MeHg), total Hg was measured in a pooled sample of 10 pupae from each of the control and GST RNAi crosses with the FB > and NP1> drivers. First, we observed an overall strain difference in Hg accumulation between the FB > crosses versus the NP1> crosses. FB > cont. pupae accumulated 10.0, 23.3, and 29.7 ppm Hg with 2, 5, and 10 µM MeHg exposure, respectively (Figure 3A). Whereas, NP1 > cont. pupae showed less accumulation of Hg with 7.0, 14.9, and 26.8 ppm Hg resulting from 2, 5, and 10 µM MeHg exposures, respectively (Figure 3B). This lower overall Hg accumulation in NP1> crosses relative to FB > crosses corresponds with an overall higher MeHg tolerance of NP1> crosses in eclosion assays (compare FB > cont and NP1 > cont in Figs. 2A and B, also in Figs. 6 and 7 and data not shown). A trend toward higher Hg body burden was seen with GST knockdown among the various crosses and the different MeHg concentrations (Figure 3). This trend was most consistent with GSTS1 knockdown, where 6%–16% higher levels of Hg were seen with FB > knockdown (95% CI (−2% to 25%), P = .065, P = .195 multiple comparison protected) and 10%–12% higher levels of Hg seen with NP1> knockdown (95% CI 8%–13%, P = .004, P = .012 multiple comparison protected) compared with control crosses (Figs. 3A and B). GST knockdown in fat body showed higher Hg body burdens with 2 and 10 µM MeHg treatments, yet slightly lower Hg with 5 µM MeHg treatment [range −4% to 18%, 95% CI (−20.6% to −39.4%), P = .309, P = .927 multiple comparison protected]. However, with knockdown in the gut GSTD1 gave slightly higher Hg body burden levels at all concentrations [range 2%–4%, 95% CI (0.3% to −5%), P = .041, P = .123 multiple comparison protected] compared with the control cross (Figure 3B). Knockdown of GSTE1 did not consistently yield higher Hg body burden with both the FB > [range −1% to 6%, 95% CI (−6.4% to −13.4%), P = .268, P = .804 multiple comparison protected] and NP1> [−%2 to 9%, 95% CI (−9.0% to −16.3%), P = .339, P = 1 multiple comparison protected] combinations (Figs. 3A and B).
FIG. 4.
mRNA expression level of GSTP1 variants. Quantitative rt-PCR was used to determine GSTP1 mRNA expression for each of four independent UAS-GSTP1105 and UAS-GSTP1114 transformant lines and the single UAS-GSTP1wt line in combination with the FB > driver. Expression is relative to the GSTP1wt line. (single determinations of pooled samples of 20 larva; ✓ indicates lines selected for characterization in this study).
FIG. 3.
Mercury body burden with GST knockdown. Larvae carrying the GSTD1, GSTE1 or GSTS1 RNAi, or control larva, were reared on the indicated concentrations of MeHg food. For each treatment a single determination of a pooled sample of 10 pupae was conducted for total mercury (Hg in parts per million (ppm)). Numbers above the bars indicate the percent change in Hg in the GST RNAi pupae compared with the respective control pupae for each MeHg treatment. Significant increase in Hg accumulation across MeHg treatments was seen for NP1 > GSTS1-RNAi (95% CI (8%–13% above control), P = .004, P = .012 multiple comparison protected) and NP1 > GSTD1-RNAi (95% CI (0.3%–5% above control), P = .041, P = .123 multiple comparison protected). See Results text for additional statistics.
FIG. 6.
Eclosion behavior with GSTP1 expression. Eclosion of larvae reared on indicated MeHg concentrations in food is shown for control and GSTP1wt, GSTP1105, GSTP1114 expression in (A) fat body (FB>) and (B) gut (NP1>). A total of n = 150 larvae (three trials of 50 larvae) were assayed for each data point. (*, z-statistic with P < .01(see Methods section); error bars = Std. Dev.).
FIG. 7.
Mercury body burden with GSTP1 expression. Larvae carrying GSTP1wt, GSTP1105, GSTP1114 expression, or control larvae, were reared on the indicated concentrations of MeHg food. For each treatment a single determination of a pooled sample of 10 pupae was analyzed for total mercury (Hg (ppm)). Numbers above bars indicate the percent change in Hg in each of the GSTP1 variant pupae compared with the respective control pupae for each MeHg treatment. Significant decrease in Hg body burden across MeHg treatments was seen for FB > GSTP1114 [95% CI (−34% to − 2% below control), P = .041, P = .123 multiple comparison protected], NP1 > GSTP1114 [95% CI (−11% to − 6% below control), P = .004, P = .012 multiple comparison protected] and NP1 > GSTP1105 [95% CI (−3% to − 0.2% below control), P = .038, P = .114 multiple comparison protected]. See Results section text for additional statistics.
Expression and Activity of Human GSTP1 in Developing Flies
We next sought to induce elevated expression of GST activity to see if tolerance to MeHg could be enhanced during development. With UAS constructs for the endogenous Drosophila GSTD1, GSTE1, or GSTS1 being unavailable we took the opportunity to explore the activity of human GST isoforms in the fly model. We focused on GSTP1 for the reason that the common polymorphic variants GSTP1105 and GSTP1114, both of which have clinical relevance in several diseases (Cai et al., 2013; Kim et al., 2015), have previously been associated with MeHg disposition in human studies (Custodio et al., 2004; Schlawicke Engstrom et al., 2008). UAS expression constructs were generated for the human GSTP1 wild type isoform (GSTP1wt) and variants Ile105Val (GSTP1105) and Ala114Val (GSTP1114). Several lines of transformed flies carrying the UAS-GSTP1wt, UAS-GSTP1105, and UAS-GSTP1114 mutants were generated. The mRNA expression levels of the respective wild type and mutant GSTP1 were examined under the expression of the FB > driver. Unexpectedly, a large variation in mRNA expression levels was observed among the transformed lines including a more than 25-fold difference in expression between the GSTP1wt and the highest expressing UAS-GSTP1105 transformants (Figure 6). Only one transformed line of the UAS-GSTP1wt construct was obtained, which showed expression at a much lower level than the mutant transformants. Since one goal of the study was to compare the relative activities of the GSTP1 variants we chose to characterize the GSTP1105-3 line and the GSTP1114-3 line due to their similar expression levels. For the GSTP1wt line we resorted to the single transformant that was obtained.
To characterize the relative activity of the GSTP1 variants expressed in vivo we determined the level of GSTP1 protein expression and compared it with the GST enzyme activity attributable to the respective GSTP1 variant. Quantitative Western blotting of larval extracts showed that GSTP1 protein levels followed a similar overall trend as seen with mRNA, where GSTP1105 and GSTP1114 gave higher protein expression (5.5- and 4.5-fold, respectively) than GSTP1wt (Figure 5A). As expected, GSTP1 protein was not detected in the larval extracts of the FB > cont. larvae (Figure 5A). GST enzyme activity in the same larval extracts was assayed for GSH conjugation with DTNB. GST activity in extracts prepared from FB > GSTP1wt, FB > GSTP1105, and FB > GSTP1114 larva were thus compared with the basal GST activity in extract of the FB > cont. larva. Expression of each of the three GSTP1 constructs demonstrated an increase over the basal GST activity showing a 39% increase with GSTP1wt expression and 51% and 117% increase with GSTP1105 and GSTP1114 expression, respectively (Figure 5B). Compared with GSTP1wt, the GSTP1105 and GSTP1114 expression resulted in 1.3- and 3.0-fold greater GST activity, respectively, that was attributed to GSTP1 (Figure 5B). The specific activity (enzyme activity/protein level) of GSTP1105 and GSTP1114 enzymes was thereby estimated to be 24% and 67%, respectively, relative to GSTP1wt (100%).
FIG. 5.
Protein expression and enzyme activity of GSTP1 variants. (A) Total protein extracts of 30 pooled larvae for each indicated GSTP1-expressing or control larvae were separated on SDS-PAGE and Western blotted with anti-human GSTP1 (top panel) and anti-actin (bottom panel) antibodies. Relative GSTP1 protein levels were determined by densitometry with normalization to the actin levels and expressed relative to the GSTP1wt. (B) GST activity in GSTP1 expressing larvae was determined with the same protein extracts of GSTP1-expressing or control larvae used for Western blotting above. GSH S-transferase activity was determined in a DTNB-GSH reductase recycling reaction assay (see Methods section). Numbers in parentheses indicate % increase in GSH S-transferase activity relative to the control (FB > cont.), representing the activity attributed to GSTP1.
Expression of hGSTP1 and MeHg Tolerance
We next tested the effects of GSTP1 activity on MeHg tolerance in eclosion behavior. FB > GSTP1wt expression resulted in a marked increase in eclosion with 79% eclosion on 10 µM MeHg food seen compared with 19% for the FB > cont. strain (Figure 6A). The GSTP1105 and GSTP1114 mutants also induced tolerance with 53% and 73% eclosion on 10 µM MeHg, respectively. All three GSTP1 variants similarly induced MeHg tolerance when expressed under the control of the NP1> driver, where ∼66% eclosion was seen for GSTP1105 and 59% for both GSTP1wt and GSTP1114 on 10 µM MeHg, respectively, compared with 40% for the NP1 > cont. strain (Figure 6B).
Effect of GSTP1 Expression on MeHg Body Burden
Since previous studies have shown an association of GSTP1 variants with mercury levels in erythrocytes in a human population (Custodio et al., 2004; Schlawicke Engstrom et al., 2008) we tested whether or not inducing GSTP1 expression would alter MeHg accumulation, presumably by lowering MeHg levels. At all of the MeHg exposure levels FB > GSTP1wt [range −10% to −23%, 95% CI (−32% to 1%), P = .059, P = .177 multiple comparison protected] and FB > GSTP1114 [range −11% to −24%, 95% CI (−34% to −2%), P = .041, P = .123 multiple comparison protected] pupa were seen to accumulate lower levels of Hg relative to the FB > cont. (Figure 7A). FB > GSTP1105 pupa showed a similar pattern of reduced Hg body burden with 5 and 10 µM MeHg exposures, but not with 2-µM MeHg [range 0% to −23%, 95% CI (−41% to 17%), P = .197, P = .591 multiple comparison protected] (Figure 7A). In contrast, NP1> GSTP1wt pupa showed no consistent difference in Hg body burden compared to NP1 > cont. pupa [range −3% to 1%, 95% CI (−7% to 7%), P = .972, P = 1 multiple comparison protected] (Figure 7B). However, NP1 > GSTP1114 [range −8% to −10%, 95% CI (−11% to 6%), P = .004, P = .012 multiple comparison protected] and NP1> GSTP1105 [range −2% to −1%, 95% CI (−3% to −0.2%), P = .038, P = .114 multiple comparison protected] pupa showed slightly lower Hg levels at all MeHg treatment concentrations (Figure 7B).
DISCUSSION
Previous genetic association studies relating GST polymorphisms to Hg levels in humans have supported the hypothesis that GSTs play a role in MeHg toxicokinetics and can potentially moderate MeHg toxicity. This study is the first attempt to associate Hg body burden and developmental outcomes of MeHg exposure with GST enzyme activity in the context of controlled genetic manipulation of GST expression. Using an established functional assay of MeHg toxicity in Drosophila we show that modulation of GST expression can indeed influence tolerance and susceptibility to MeHg toxicity during development. Consistent with an overall protective role for GSTs, we find that knockdown of endogenous GSTD1, GSTE1, or GSTS1, individually, is sufficient to make flies more susceptible to MeHg toxicity. In addition, we demonstrate that exogenous expression of human GSTP1 in flies yields more tolerance to MeHg. We also show evidence for a reduced specific activity of the GSTP1105 and GSTP1114 variant enzymes relative to GSTP1wt. Finally, we observe a trend whereby Hg accumulation increases slightly with knockdown of endogenous GSTs and decreases slightly when human GSTP1 activity is introduced to developing flies. These findings indicate GSTs play a role in moderating the toxic insult of MeHg.
The mode of action of GSTs in moderating MeHg toxicity remains uncertain. A previously proposed mechanism suggested that GST-catalyzed conjugation of GSH to MeHg might enhance excretion since MeHg-GSH is a more readily transported species (Custodio et al., 2004). Consistent with this mechanism, we see a trend whereby elevated GST enzyme activity in GSTP1 expressing flies results in a lower Hg body burden in pupae; whereas GSTD1, GSTE1, or GSTS1 knockdown, in some instances, gives elevated Hg levels. It is unlikely that GST activity is required for this conjugation since the spontaneous formation of MeHg-GSH from MeHg and GSH in solution occurs with exceedingly high efficiency [formation constant ∼1015 M−1 (Rabenstein, 1978)]. Nonetheless, GST activity may facilitate overall MeHg-GSH formation and support subsequent elimination (Gregus and Varga, 1985; Magos et al., 1985). However, we see that GST-induced changes in Hg body burden do not correspond strictly with changes in MeHg tolerance in eclosion behavior. For example, with 10-µM MeHg exposure NP1 > GSTP1wt and NP1 > GSTP1105 give a significantly increased eclosion rate compared to NP1 > cont, yet they exhibit no difference in Hg body burden. We conclude that while it is possible GSTs facilitate MeHg-GSH conjugation, alternative mechanisms for GSTs in moderating MeHg toxicity are likely. One possibility is that GSTs act to conjugate GSH to toxic byproducts stemming from a MeHg insult. MeHg is known to induce oxidative stress in a number of systems, which predicts propagation of several possible downstream toxic metabolites, including lipid peroxidation products. In this regard, GST-mediated GSH conjugation of 4-hydroxy-2-nonenal (4-HNE), a particularly reactive lipid peroxidation metabolite that forms DNA and protein adducts (Yamashita et al., 2004), might serve as a first line of defense to MeHg induced oxidative stress (Ayyadevara et al., 2007). It is of interest that GSTS1 has been characterized as the major enzyme responsible for 4-HNE-GSH conjugation in Drosophila (Singh et al., 2001).
Our findings suggest that carriers of polymorphic GSTs with lower catalytic activity (e.g., GSTP1105 and GSTP1114) might experience a greater risk of MeHg toxicity, which may also accompany an elevated Hg body burden. In our attempt to compare the activity of human GSTP1105 and GSTP1114 to GSTP1wt we observed that expression of all three variants yielded a similar magnitude of effect despite the 4.5–5.5-fold higher levels of GSTP1105 and GSTP1114 protein expression that occurred relative to GSTP1wt. This apparent reduced specific activity of GSTP1105 and GSTP1114 is consistent with prior biochemical studies with these enzymes. Ali-Osman et al. (1997) showed, using bacterial-expressed and purified recombinant enzymes, that the GSTP1105 or GSTP1114 enzymes have ∼3–4-fold lower catalytic efficiency (kcat/Km) for the DTNB substrate compared to GSTP1wt. A similar degree of reduced catalytic efficiency of recombinant GSTP1105 and GSTP1114 (2.7- and 1.8-fold less active toward DTNB, respectively) was reported by Goodrich and Basu (2012). Here, we estimate GSTP1105 and GSTP1114, expressed in a eukaryotic in vivo system, to be ∼6- and 2-fold less active than GSTP1wt, respectively. Thus, our results are consistent with previous studies and further validate that the variant GSTP1105 and GSTP1114 alleles indeed produce less active enzymes in an in vivo context. These functional data therefore reinforce the notion that GSTs, and human GSTP1 in particular, play an enzymatic role in moderating MeHg toxicity, and possibly MeHg metabolism and excretion.
Our results, albeit in a small organism experimental model, are consistent with several existing epidemiologic studies that show a correlation of predicted GST activity and Hg body burden (Barcelos et al., 2013; Custodio et al., 2004; Gundacker et al., 2007; Mazzaron Barcelos et al., 2012). Custodio et al. (2004) demonstrated that carriers of the GSTP1114 allele in a Swedish cohort exhibited higher erythrocyte levels of Hg. The GSTP1114 allele also showed association with higher hair Hg levels in a cohort of Austrian medical students with relatively low mercury exposure (Gundacker et al., 2009). In this latter study, while the GSTP1105 allele alone showed no association with Hg levels, combined genotypes of GSTP1105 with loss of function alleles of GCLC and GSTM1 showed association with elevated Hg in hair (Gundacker et al., 2009). However, GSTP1105 and GSTP1114 carriers showed association with lower Hg retention in erythrocytes in a second sampling of a subset of the Swedish cohort that focused on individuals who exhibited higher levels of fish consumption, and therefore, higher mercury exposure (Schlawicke Engstrom et al., 2008). Similarly, GSTP1105 and GSTP1114 association with lower Hg levels in hair has been reported in a U.S. dental worker cohort (Goodrich et al., 2011). These apparent inconsistencies remain unexplained, however, may reflect a compensatory activity of other GST family members in the genetic background of certain carriers of the GSTP1 variants.
One limitation of this study is that, by design, the interpretation of outcomes is with respect to single gene manipulations. We have not attempted to account for expression levels of the numerous GST family members in the fly. Thus, one possibility not controlled for is off-target effects of the RNAi, possibly causing reduced expression of more than one GST. It is conceivable that the effects we have attributed solely to knockdown, for example, of GSTD1 are the consequence of a composite effect of reduced expression of a panel of endogenous GSTs. Future studies will therefore be strengthened by a comprehensive survey of all the GST family members under a MeHg exposure paradigm, which is beyond the scope of the present study. An additional limitation stems from the ability to obtain only one transformed line of the GSTP1wt construct, which in turn demonstrated a markedly lower GAL4-induced expression level than the GSTP1105 and GSTP1114 variants. Nonetheless, by accounting for protein expression and enzyme activity we were able to infer functional differences that could be attributed to the polymorphisms.
In summary, we demonstrate a functional role for GSTs in moderating MeHg toxicity. These findings support the over-arching concept that GSH-dependent pathways are central to moderating MeHg metabolism and toxicity. Whether or not GSTs act in MeHg-GSH conjugation in support of a putative elimination pathway awaits further biochemical characterization. Nonetheless, the data indicate that GST polymorphisms can potentially contribute to variation in MeHg susceptibility and are thus relevant to consider in human population studies of MeHg exposures and outcomes.
ACKNOWLEDGMENTS
We thank Lisa Prince and Gary Myers for a critical review of the manuscript. We thank Thomas Scrimale for assistance with mercury measurements. We thank Benoit Biteau for generous sharing of flies and the Vienna Drosophila Resource Center for supplies of Drosophila RNAi stocks. Flies obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were also used in this study. The JLA-20 hybridoma (anti-actin antibody) developed by J.J. Lin was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
FUNDING
This study was supported by National Institute of Environmental Health Sciences [R01 ES01219 to P.W.D. and E.vW. (mP.I.s) (M.D.R., K.B. and TL (Co-I.s)); and the University of Rochester Environmental Health Center [P30 ES001247].
REFERENCES
- Ali-Osman F., Akande O., Antoun G., Mao J. X., Buolamwini J. (1997). Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J. Biol. Chem. 272, 10004–10012. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S., Dandapat A., Singh S. P., Siegel E. R., Shmookler Reis R. J., Zimniak L., Zimniak P. (2007). Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal. Mech. Age. Dev. 128, 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatori N., Clarkson T. W. (1982). Developmental changes in the biliary excretion of methylmercury and glutathione. Science 216, 61–63. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Clarkson T. W. (1983). Biliary transport of glutathione and methylmercury. Am. J. Physiol. 244, G435–G441. [DOI] [PubMed] [Google Scholar]
- Barcelos G. R., Grotto D., de Marco K. C., Valentini J., Lengert A., de Oliveira A. A., Garcia S. C., Braga G. U., Schlawicke Engstrom K., Colus I. M., et al. (2013). Polymorphisms in glutathione-related genes modify mercury concentrations and antioxidant status in subjects environmentally exposed to methylmercury. Sci. Tot. Environ. 463–464, 319–325. [DOI] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K. (2007). An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl Acad. Sci. U. S. A. 104, 3312–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Manoukian A. S., Perrimon N. (1994). Ectopic expression in Drosophila. Methods Cell Biol. 44, 635–654. [DOI] [PubMed] [Google Scholar]
- Cai Q., Wu T., Zhang W., Guo X., Shang Z., Jiang N., Tian J., Niu Y. (2013). Genetic polymorphisms in glutathione S-transferases P1 (GSTP1) Ile105Val and prostate cancer risk: a systematic review and meta-analysis. Tumour Biol. 34, 3913–3922. [DOI] [PubMed] [Google Scholar]
- Custodio H. M., Broberg K., Wennberg M., Jansson J. H., Vessby B., Hallmans G., Stegmayr B., Skerfving S. (2004). Polymorphisms in glutathione-related genes affect methylmercury retention. Arch. Environ. Health 59, 588–595. [DOI] [PubMed] [Google Scholar]
- Davidson P. W., Myers G. J., Weiss B., Shamlaye C. F., Cox C. (2006). Prenatal methyl mercury exposure from fish consumption and child development: a review of evidence and perspectives from the Seychelles Child Development Study. Neurotoxicology 27, 1106–1109. [DOI] [PubMed] [Google Scholar]
- Davidson P. W., Strain J. J., Myers G. J., Thurston S. W., Bonham M. P., Shamlaye C. F., Stokes-Riner A., Wallace J. M., Robson P. J., Duffy E. M., et al. (2008). Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology 29, 767–775. [CrossRef]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger D., Zdarek J. (1994). Metamorphosis behavior in flies. Annu. Rev. Entomol. 39, 243–266. [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156. [DOI] [PubMed] [Google Scholar]
- Engstrom K., Love T. M., Watson G. E., Zareba G., Yeates A., Wahlberg K., Alhamdow A., Thurston S. W., Mulhern M., McSorley E. M., et al. (2016). Polymorphisms in ATP-binding cassette transporters associated with maternal methylmercury disposition and infant neurodevelopment in mother-infant pairs in the Seychelles Child Development Study. Environ. Int. 94, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FlyBase (2009). FlyBase: enhancing Drosophila Gene Ontology annotations. Available at: http://flybase.org/. Accessed December 20, 2016. [DOI] [PMC free article] [PubMed]
- Flybase (2016). Gene Group: CYTOPLASMIC S-GLUTATHIONE TRANSFERASES. Available at: http://flybase.org/reports/FBgg0000155.html. Accessed December 20, 2016.
- Goodrich J. M., Basu N. (2012). Variants of glutathione s-transferase pi 1 exhibit differential enzymatic activity and inhibition by heavy metals. Toxicol. In Vitro 26, 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J. M., Wang Y., Gillespie B., Werner R., Franzblau A., Basu N. (2011). Glutathione enzyme and selenoprotein polymorphisms associate with mercury biomarker levels in Michigan Dental Professionals. Toxicol. Appl. Pharmacol. 257, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Weihe P., White R. F., Debes F., Araki S., Yokoyama K., Murata K., Sorensen N., Dahl R., Jorgensen P. J. (1997). Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 19, 417–428. [DOI] [PubMed] [Google Scholar]
- Gregus Z., Varga F. (1985). Role of glutathione and hepatic glutathione S-transferase in the biliary excretion of methyl mercury, cadmium and zinc: a study with enzyme inducers and glutathione depletors. Acta Pharmacol. Toxicol. 56, 398–403. [DOI] [PubMed] [Google Scholar]
- Gundacker C., Komarnicki G., Jagiello P., Gencikova A., Dahmen N., Wittmann K. J., Gencik M. (2007). Glutathione-S-transferase polymorphism, metallothionein expression, and mercury levels among students in Austria. Sci. Tot. Environ. 385, 37–47. [DOI] [PubMed] [Google Scholar]
- Gundacker C., Wittmann K. J., Kukuckova M., Komarnicki G., Hikkel I., Gencik M. (2009). Genetic background of lead and mercury metabolism in a group of medical students in Austria. Environ. Res. 109, 786–796. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Flanagan J. U., Jowsey I. R. (2005). Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 45, 51–88. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Kang S. W., Chung J. H., Park H. J., Cho K. B., Park M. S. (2015). Genetic polymorphisms of glutathione-related enzymes (GSTM1, GSTT1, and GSTP1) and schizophrenia risk: a meta-analysis. Int. J. Mol. Sci. 16, 19602–19611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Llop S., Ballester F., Broberg K. (2015). Effect of gene-mercury interactions on mercury toxicokinetics and neurotoxicity. Curr. Environ. Health Rep. 2, 179–194. [DOI] [PubMed] [Google Scholar]
- Llop S., Engstrom K., Ballester F., Franforte E., Alhamdow A., Pisa F., Tratnik J. S., Mazej D., Murcia M., Rebagliato M., et al. (2014). Polymorphisms in ABC transporter genes and concentrations of mercury in newborns—evidence from two Mediterranean birth cohorts. PLoS One 9, e97172.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magos L., Cikrt M., Snowden R. (1985). The dependence of biliary methylmercury secretion on liver GSH and ligandin. Biochem. Pharmacol. 34, 301–305. [DOI] [PubMed] [Google Scholar]
- Mahapatra C. T., Bond J., Rand D. M., Rand M. D. (2010). Identification of methylmercury tolerance gene candidates in Drosophila. Toxicol. Sci. 116, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaron Barcelos G. R., de Marco K. C., Grotto D., Valentini J., Garcia S. C., Leite Braga G. U., Barbosa F. Jr. (2012). Evaluation of glutathione S-transferase GSTM1 and GSTT1 polymorphisms and methylmercury metabolism in an exposed Amazon population. J. Toxicol. Environ. Health 75, 960–970. [DOI] [PubMed] [Google Scholar]
- Montgomery S. L., Vorojeikina D., Huang W., MacKay T. F. C., Anholt R. R. H., Rand M. D. (2014). Genome-wide association analysis of tolerance to methylmercury toxicity in Drosophila implicates myogenic and neuromuscular developmental pathways. PLoS One 9, e110375.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K., Weihe P., Budtz-Jorgensen E., Jorgensen P. J., Grandjean P. (2004). Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. J. Pediatr. 144, 177–183. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Vasiliou V. (2004). Analysis of the glutathione S-transferase (GST) gene family. Hum. Genomics 1, 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince L., Korbas M., Davidson P., Broberg K., Rand M. D. (2014). Target organ specific activity of drosophila MRP (ABCC1) moderates developmental toxicity of methylmercury. Toxicol. Sci. 140, 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein D. L. (1978). The aqueous solution chemistry of methylmercury and its complexes. Acc. Chem. Res. 11, 100–107. [Google Scholar]
- Rand M. D., Montgomery S. L., Prince L., Vorojeikina D. (2014). Developmental toxicity assays using the Drosophila model. Curr. Protoc. Toxicol. 59, 1.12.1–1.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisawang C., Wongsantichon J., Ketterman A. J. (2012). A preliminary characterization of the cytosolic glutathione transferase proteome from Drosophila melanogaster. Biochem. J. 442, 181–190. [DOI] [PubMed] [Google Scholar]
- Schlawicke Engstrom K., Stromberg U., Lundh T., Johansson I., Vessby B., Hallmans G., Skerfving S., Broberg K. (2008). Genetic variation in glutathione-related genes and body burden of methylmercury. Environ. Health Perspect. 116, 734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. P., Coronella J. A., Benes H., Cochrane B. J., Zimniak P. (2001). Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1-1 (GST-2) in conjugation of lipid peroxidation end products. Eur. J. Biochem. /FEBS 268, 2912–2923. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Ando Y., Nakamura M., Obayashi K., Terazaki H., Haraoka K., Guo S. X., Ueda M., Uchino M. (2004). Inhibitory effect of alpha-tocopherol on methylmercury-induced oxidative stress. Environ. Health Prev. Med. 9, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., McCart C., Woods D. J., Terhzaz S., Greenwood K. G., ffrench-Constant R. H., Dow J. A. (2007). A Drosophila systems approach to xenobiotic metabolism. Physiol. Genomics 30, 223–231. [DOI] [PubMed] [Google Scholar]