Abstract
Background: Individuals of African ancestry harboring two variant alleles within apolipoprotein L1 (APOL1) are classified with a high-risk (HR) genotype. Adults with an HR genotype have increased risk of focal segmental glomerulosclerosis and chronic kidney disease compared with those with a low-risk (LR) genotype (0 or 1 variants). The role of APOL1 risk genotypes in children with glomerular disease is less well known.
Methods: This study characterized 104 African-American children with a glomerular disease by APOL1 genotype in two cohorts: the Chronic Kidney Disease in Children (CKiD) and Nephrotic Syndrome Study Network (NEPTUNE).
Results: Among these subjects, 46% had an HR genotype with a similar age at cohort enrollment. For APOL1 HR children, the median age of disease onset was older (CKiD: 4.5 versus 11.5 years for LR versus HR; NEPTUNE: 11 versus 14 years for LR versus HR, respectively) and preterm birth was more common [CKiD: 27 versus 4%; NEPTUNE: 26 versus 12%; combined odds ratio 4.6 (95% confidence interval: 1.4, 15.5)]. Within studies, HR children had lower initial estimated glomerular filtration rate (eGFR) (CKiD: 53 versus 69 mL/min/1.73 m2; NEPTUNE: 74 versus 94 mL/min/1.73 m2). Longitudinal eGFR decline was faster among HR children versus LR (CKiD: −18 versus −8% per year; NEPTUNE: −13 versus −3% per year).
Conclusions: Children with an HR genotype in CKiD and NEPTUNE seem to have a more aggressive form of glomerular disease, in part due to a higher prevalence of focal segmental glomerulosclerosis. These consistent findings across independent cohorts suggest a common natural history for children with APOL1-associated glomerular disease. Further study is needed to determine the generalizability of these findings.
Keywords: APOL1, epidemiology, FSGS, nephrotic syndrome, pediatrics
INTRODUCTION
African-Americans harboring a high-risk (HR) genotype of apolipoprotein L1 (APOL1) (homozygous for ‘G1’ or ‘G2’, or G1G2 compound heterozygosity) have substantially increased risk of development of diverse glomerular diseases, particularly focal segmental glomerulosclerosis (FSGS), as compared with those with 0 or 1 risk alleles [a low-risk (LR) genotype] [1–4]. In diverse renal diseases, those with an HR genotype have a lower estimated glomerular filtration rate (eGFR) at presentation [5, 6], more severe histologic damage on renal biopsy [3, 5, 6], and more rapid decline in eGFR [3, 7, 8]. In the general population, an HR genotype is associated with increased odds of development of chronic kidney disease (CKD) [9], decreased glomerular number and increased glomerular volume with advancing age [10].
Similar patterns of APOL1 risk alleles in teens and young adults with kidney disease have been reported [11], although the natural history and associated phenotypes are less clear. To increase our understanding and improve precision of care for pediatric APOL1-associated glomerular disease, we studied 104 APOL1 genotyped African-American children from the Chronic Kidney Disease in Children (CKiD) cohort [12] and the Nephrotic Syndrome Study Network (NEPTUNE) [13]. CKiD and NEPTUNE are both prospective, longitudinal cohorts enrolling pediatric patients with glomerular disease, and have detailed phenotyping and repeated biosample collection. Using the rich data present in these independent cohorts, the present study aimed to: (i) present glomerular disease diagnoses by APOL1 genotype status, (ii) describe baseline characteristics, particularly birth history, by APOL1 genotype and (iii) characterize the putative effects of APOL1 genotype on longitudinal measures of eGFR.
MATERIALS AND METHODS
CKiD
The CKiD study is a prospective observational cohort study initiated in 2003 to describe the natural history of CKD in children [12]. Eligibility criteria were age between 1 and 16 years and eGFR 30–90 mL/min/1.73 m2. Blood and urine samples were collected at each annual visit. Inclusion criteria for the present analysis were having a glomerular diagnosis, self-reported African-American race and available APOL1 genotypes.
NEPTUNE
NEPTUNE is a prospective cohort study of individuals presenting with proteinuria (≥500 mg/24 h), with a suspected diagnosis of primary nephrotic syndrome [13]. Participants underwent a clinically indicated kidney biopsy to establish a glomerular diagnosis and were followed longitudinally. Inclusion criteria for this study were onset of disease prior to 18 years of age, self-reported African-American race and available APOL1 genotypes.
No participants were enrolled in both CKiD and NEPTUNE. Institutional review boards at each participating center approved these studies.
Genotyping
CKiD:APOL1 G1 single nucleotide polymorphisms (SNPs) (rs73885319 and rs60910145) and the G2 indel (rs71785313) were genotyped by TaqMan assays (ABI, Foster City, CA, USA). NEPTUNE: G1 and G2 were genotyped using Sanger sequencing of the last 250 bases of the terminal exon of APOL1.
Risk classification and model of inheritance
Participants were categorized with zero (G0G0), one (G1G0 or G2G0) or two (G1G1, G2G2, G1G2) risk alleles, and further classified as ‘high-risk (HR)’ if they harbored any combination of two risk alleles and ‘low-risk (LR)’ if they had 0 or 1 risk alleles. All analyses were performed using a recessive model of inheritance (HR versus LR).
Birth history and family history of disease
Parent-reported birth history variables included weeks gestation and birthweight. These variables were categorized as premature birth (<36 weeks gestation) and being small for gestational age (SGA; birth weight <10th percentile for gestational age) [14]. Family history of kidney disease, end-stage renal disease, hypertension and diabetes were parent-reported.
Renal function
Central biochemistry laboratories measured serum creatinine and urinalysis for each study. eGFR was estimated using serum creatinine by the CKiD pediatric equation [15]. Proteinuria was quantified as a protein to creatinine ratio (mg/dL:mg/dL).
Statistical methods
Differences of baseline demographic and clinical characteristics by APOL1 status, within each study, were compared using the Wilcoxon rank sum test for continuous variables and Fisher's exact test for categorical variables.
Odds ratios for prematurity and SGA by APOL1 risk genotype were derived from a logistic regression model with birth history variables as the outcome and APOL1 HR genotype as the exposure of interest (APOL1 LR as reference), stratified by study and combined with adjustment for study.
To characterize longitudinal changes in eGFR, the annual percent change in eGFR was estimated by a linear mixed model with random intercepts and slopes with time and APOL1 genotype, and an interaction between the two as the independent variables, with adjustment for study (for more details, see Supplemental Note #1).
Since CKiD had a longer follow-up time than NEPTUNE, follow-up time was restricted to 5 years or less for comparable study duration between the two studies. This time restriction excluded 19 eGFR observations from nine participants in CKiD.
RESULTS
Of 891 CKiD participants, 56 had an underlying glomerular diagnosis, self-reported as African-American and had DNA available for APOL1 genotyping. Of the 311 NEPTUNE participants enrolled and with available DNA at the time of this study, 48 had childhood-onset proteinuric disease, self-reported as African-American and had APOL1 genotyping. None of these NEPTUNE participants had disease-causing mutations in any of the 20 genes previously implicated in monogenic forms of nephrotic syndrome [16]. Sequencing of these genes was not performed in CKiD participants.
The clinical diagnoses for children in each study, stratified by risk genotype, are presented in Table 1. In both studies HR genotype children were predominantly diagnosed with FSGS [CKiD (89%; 25/28) and NEPTUNE (67%; 14/21)]. Among those with an LR genotype, the prevalence of FSGS was 25% (7/28) in CKiD and was 26% (7/27) in NEPTUNE. Table 2 provides clinical and demographic characteristics for the two cohorts, stratified by risk genotype. Within each study, there was no significant difference in gender, height or distribution of body mass index category between HR and LR children. There were no differences between HR and LR children for two indicators of socioeconomic status: maternal age <21 years at participant's birth and maternal education less than college. There were few differences in family history of disease with the exception of a higher prevalence of family kidney disease among HR NEPTUNE children.
Table 1.
Distribution of kidney disease diagnoses in CKiD and NEPTUNE among African-American children by APOL1 risk genotype
| CKiD |
NEPTUNE |
|||
|---|---|---|---|---|
|
APOL1 Low risk n = 28 |
APOL1 High risk n = 28 |
APOL1 Low risk n = 27 |
APOL1 High risk n = 21 |
|
| Focal segmental glomerulosclerosis | 25% (7) | 89% (25) | 26% (7) | 67% (14) |
| Minimal change disease | 0% (0) | 0% (0) | 52% (14) | 19% (4) |
| Hemolytic uremic syndrome | 18% (5) | 0% (0) | 0% (0) | 0% (0) |
| Immune complex glomerulonephritis | 0% (0) | 0% (0) | 15% (4) | 5% (1) |
| Alport syndrome (familial nephritis) | 14% (4) | 0% (0) | 0% (0) | 0% (0) |
| Systemic immunological disease | 11% (3) | 0% (0) | 0% (0) | 0% (0) |
| Chronic glomerulonephritis | 7% (2) | 4% (1) | 0% (0) | 0% (0) |
| IgA nephropathy | 4% (1) | 4% (1) | 0% (0) | 5% (1) |
| C1Q nephropathy | 0% (0) | 0% (0) | 0% (0) | 5% (1) |
| Membranous nephropathy | 0% (0) | 0% (0) | 4% (1) | 0% (0) |
| Nephrocalcinosis | 0% (0) | 0% (0) | 4% (1) | 0% (0) |
| Congenital nephrotic syndrome | 0% (0) | 4% (1) | 0% (0) | 0% (0) |
| Idiopathic crescentic glomerulonephritis | 4% (1) | 0% (0) | 0% (0) | 0% (0) |
| Henoch–Schönlein nephritis | 4% (1) | 0% (0) | 0% (0) | 0% (0) |
| Membranoproliferative glomerulonephritis type II | 4% (1) | 0% (0) | 0% (0) | 0% (0) |
| Sickle cell nephropathy | 4% (1) | 0% (0) | 0% (0) | 0% (0) |
| Diffuse proliferative glomerulonephritis | 4% (1) | 0% (0) | 0% (0) | 0% (0) |
| Other glomerular disease | 4% (1) | 0% (0) | 0% (0) | 0% (0) |
Data are presented as % (n).
Table 2.
Baseline characteristics of African-American children in CKiD (n = 56) and NEPTUNE (n = 48) studies, by APOL1 risk genotype status
| Variable | CKiD |
NEPTUNE |
||||
|---|---|---|---|---|---|---|
|
APOL1 low risk n = 28 |
APOL1 high risk n = 28 |
P-valuea |
APOL1 low risk n = 27 |
APOL1 high risk n = 21 |
P-valuea | |
| Male | 57% (16) | 50% (14) | 0.789 | 63% (17) | 57% (12) | 0.770 |
| Age at study entry | 14.6 [11.0, 16.3] | 14.7 [12.9, 15.7] | 0.922 | 12.2 [6.1, 15.0] | 15.1 [12.1, 16.1] | 0.029 |
| Height, cm | 159.3 [140.7, 166.6] | 163.5 [150.5, 170.3] | 0.204 | 156.5 [121, 170.8] | 168 [155, 172.8] | 0.111 |
| Weight, kg | 59.95 [37.75, 74] | 70.6 [55.25, 90.5] | 0.066 | 53.5 [27, 73.8] | 77 [62, 96] | 0.016 |
| Body mass index category | 0.417 | 0.373 | ||||
| Overweight | 18% (5) | 21% (6) | 11% (3) | 10% (2) | ||
| Obese | 29% (8) | 43% (12) | 33% (9) | 52% (11) | ||
| Socioeconomic characteristics | ||||||
| Maternal age at birth ≤21 years | 33% (9) | 24% (6) | 0.548 | 44% (12) | 43% (9) | 1.000 |
| Maternal education less than college | 75% (21) | 67% (18) | 0.562 | 63% (17) | 67% (14) | 1.000 |
| Birth history | ||||||
| Birth weight, g | 3176 [2608, 3515] | 3175 [2126, 3544] | 0.468 | 3218 [2807, 3487] | 2948 [2353, 3317] | 0.135 |
| Premature birth | 4% (1) | 27% (7) | 0.024 | 12% (3) | 26% (5) | 0.253 |
| Gestational age | 40 [39, 40] | 39 [35, 40] | 0.137 | 39 [38, 40] | 39 [33, 40] | 0.483 |
| Small for gestational age | 36% (9) | 29% (7) | 0.762 | 28% (5) | 7% (1) | 0.186 |
| Disease characteristics | ||||||
| Age at glomerular disease onset | 4.5 [1.5, 12.5] | 11.5 [9.5, 12.5] | 0.020 | 11 [4, 14] | 14 [10.5, 16.5] | 0.023 |
| Duration of glomerular disease, years | 3.71 [1.18, 12.45] | 3.31 [1.19, 5.08] | 0.250 | 0.60 [0.09, 1.19] | 0.08 [0.03, 1.06] | 0.110 |
| Proportion of life with glomerular disease | 0.45 [0.09, 0.89] | 0.21 [0.09, 0.35] | 0.095 | 0.06 [0.01, 0.18] | 0.01 [0, 0.07] | 0.045 |
| eGFR at entry, mL/min/1.73 m2 | 69 [46, 87] | 53 [44, 68] | 0.112 | 94 [78, 114] | 74 [66, 90] | 0.010 |
| UPC ratio at entry (mg/mg) | 0.46 [0.18, 1.69] | 0.81 [0.24, 1.36] | 0.589 | 3.1 [1.32, 7.8] | 2.9 [1.37, 7.64] | 0.568 |
| Therapy use | ||||||
| Diuretic use | 25% (7) | 18% (5) | 0.746 | 30% (8) | 29% (6) | 1.000 |
| ACE inhibitor and/or ARB use | 89% (25) | 86% (24) | 1.000 | 19% (5) | 14% (3) | 1.000 |
| Calcium channel blocker | 18% (5) | 18% (5) | 1.000 | 11% (3) | 10% (2) | 1.000 |
| Family history | ||||||
| Family kidney disease | 23% (6) | 39% (9) | 0.352 | 19% (5) | 57% (12) | 0.007 |
| Parent kidney disease | 17% (4) | 10% (2) | 0.669 | 7% (2) | 24% (5) | 0.215 |
| Sibling kidney disease | 13% (3) | 10% (2) | 1.000 | 7% (2) | 5% (1) | 1.000 |
| Family ESRD | 26% (7) | 38% (9) | 0.546 | 0% (0) | 14% (3) | 0.077 |
| Family diabetes mellitus | 62% (16) | 67% (16) | 0.774 | 19% (5) | 48% (10) | 0.058 |
| Family hypertension | 82% (23) | 75% (21) | 0.746 | 33% (9) | 48% (10) | 0.380 |
Data are presented as % (n) or median [95% confidence interval]. eGFR, estimated glomerular filtration rate; UPC, urine protein to creatinine; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; ESRD, end-stage renal disease.
aFor continuous variables, P-values based on the Wilcoxon rank-sum test. For categorical variables, P-values based on Fisher's exact test. All tests are two-sided comparing APOL1 high risk and low risk within each study; bold indicates statistically significant (P < 0.05).
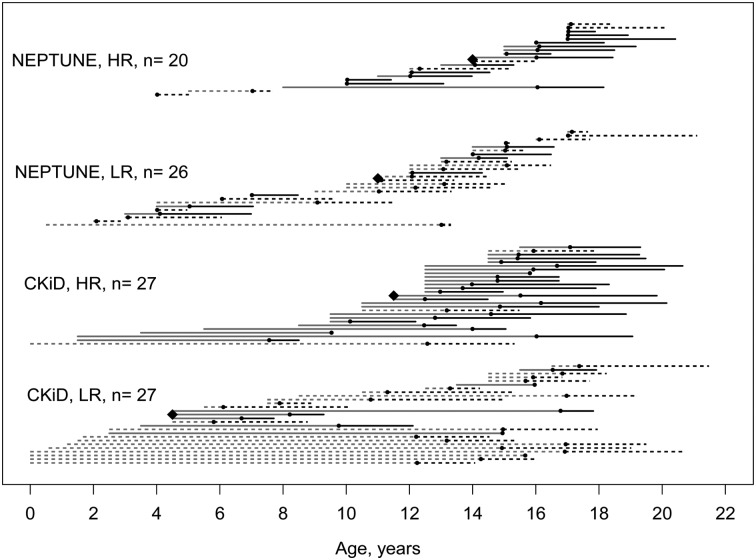
Age at study entry did not differ between HR and LR children in CKiD (14.7 versus 14.6 years); however, HR NEPTUNE children were significantly older at study entry (15.1 versus 12.2 years). The age of disease onset was significantly older among HR children in both studies. Figure 1 graphically depicts the disease and observational study time course for each participant among those with a known age at disease onset. In CKiD, HR children had a median age of disease onset 7 years older than that of LR children (11.5 versus 4.5 years; P = 0.02). In NEPTUNE, HR children had an age of onset 3 years older than LR children (14 versus 11 years; P = 0.02).
FIGURE 1.
Cascade plot describing age at disease onset (orange line), study entry (black dot) and time under study observation (black line). Each line represents one child, with the orange line displaying the age at disease onset to study entry, represented by a black point. Children with an FSGS diagnosis are represented by the solid line; children with non-FSGS diagnoses are represented by the discontinuous lines. The time under study observation is represented by the black line to the last study visit date. The median age at disease onset for each group is represented by the black diamond and corresponds to data presented in Table 2. CKiD, Chronic Kidney Disease in Children; NEPTUNE, Nephrotic Syndrome Study Network; LR, low risk; HR, high risk; FSGS, focal segmental glomerulosclerosis.
The prevalence of preterm birth was higher in children with the HR genotype (CKiD: 27 versus 4%; NEPTUNE: 26 versus 12%). Table 3 presents the results of logistic models estimating the association of genotype with preterm birth and SGA. For CKiD and NEPTUNE children with an HR genotype, the odds of being preterm were 9.6 and 2.7 times higher than those of LR children, respectively. When pooling the cohorts and adjusting for study, the odds of being preterm for a child with glomerular disease and an HR genotype were 4.6 [95% confidence interval (CI): 1.4, 15.5] times higher than the odds for participants with an LR genotype. APOL1 risk genotype status was not associated with being SGA. There was no significant difference in median birthweight by risk genotype status in either study (Table 1).
Table 3.
Relative odds of prematurity and small for gestational age associated with high-risk APOL1 compared with low-risk APOL1 from logistic regression models
| High-risk versus low-risk genotype | Prematurity |
Small for gestational age |
||
|---|---|---|---|---|
| Odds ratio | (95% CI) | Odds ratio | (95% CI) | |
| CKiD only | 9.6 | (1.1, 84.5) | 0.7 | (0.2, 2.4) |
| NEPTUNE only | 2.7 | (0.6, 13.3) | 0.2 | (0.02, 1.8) |
| CKiD and NEPTUNE combineda | 4.6 | (1.4, 15.5) | 0.5 | (0.2, 1.4) |
aCombined analysis was adjusted for study (i.e. indicator for CKiD and NEPTUNE included as covariate). Birth outcome data were missing for six individuals.
Despite differences in duration of disease (>3 years for CKiD versus <1 year for NEPTUNE) and spectrum of glomerular diagnoses (particularly in LR children), the disparities in baseline eGFR by risk genotype were remarkably similar between studies. In CKiD, the median eGFR at study entry was lower among HR children (53 mL/min/1.73 m2) compared with LR children (69 mL/min/1.73 m2). This difference was notable given that the HR group had a shorter duration of disease. In NEPTUNE, HR and LR children had a median eGFR of 74 and 94 mL/min/1.73 m2, respectively. As in CKiD, the HR participants had disease duration of approximately 1 month.
Nearly all CKiD participants were treated with an angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB). The prevalence was 86 and 89% for HR and LR participants, respectively. In contrast, in the NEPTUNE study, only 14% of HR children and 19% of LR children were treated with this therapy at baseline.
The duration of follow-up time was similar between studies and between HR and LR children (median follow-up between 1.93 and 2.53 years). The vast majority of the children in both studies had at least three serum creatinine-based eGFR observations (in CKiD: 61 and 79% among LR and HR children; in NEPTUNE: 78 and 81% among LR and HR children, respectively). This allowed formal comparisons of eGFR changes over time, pooling the data and adjusting for study. This was evaluated using a linear mixed effects model with random intercepts and slopes.
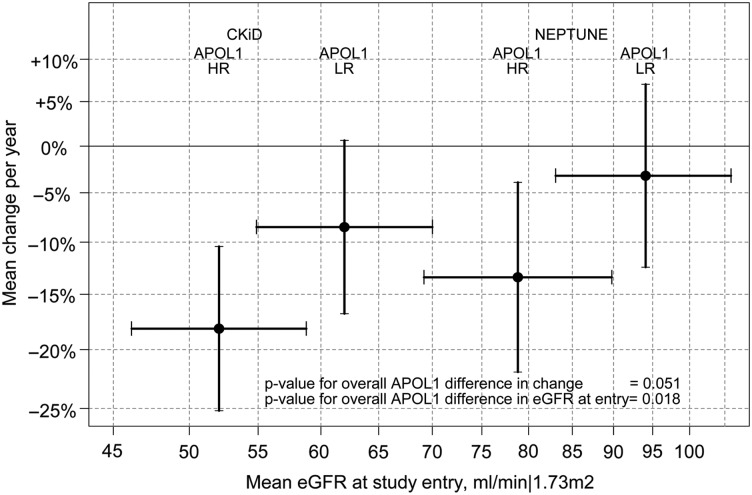
Figure 2 presents a graphical description of this model summarizing the relationship of eGFR level at study entry (x-axis) and percent change per year (y-axis) for the four groups. Overall, eGFR at study entry was 16% lower for those with HR APOL1 genotype (95% CI: −27%, −3%; P = 0.018). Specifically, in CKiD, among those with LR genotype, the estimated mean eGFR at entry was 62 mL/min/1.73 m2 (95% CI: 55, 70 mL/min/1.73 m2); for those with HR genotype, the mean eGFR at entry was 52 mL/min/1.73 m2 (95% CI: 46, 59 mL/min/1.73 m2). In NEPTUNE, among LR children, the estimated mean eGFR at entry was 94 mL/min/1.73 m2 (95% CI: 83, 106 mL/min/1.73 m2) and for those with HR genotype, the mean eGFR at entry was 79 mL/min/1.73 m2 (95% CI: 69, 90 mL/min/1.73 m2).
FIGURE 2.
Graphical depiction of eGFR level at study entry (x-axis) and percent change per year (y-axis) by study and APOL1 risk status from linear mixed effects model (random intercepts and slopes). The black circles represent the point estimates with bars corresponding to the associated 95% confidence intervals. CKiD, Chronic Kidney Disease in Children; NEPTUNE, Nephrotic Syndrome Study Network; LR, low risk; HR, high risk; APOL1, apolipoprotein L1; eGFR, estimated glomerular filtration rate.
In this model, the estimated eGFR decline per year was faster in HR versus LR children (P = 0.051). Specifically, HR CKiD participants had an average decline of −18.1% per year (95% CI: −25.1%, −10.4%) versus −8.4% per year (95% CI: −16.7%, +0.6%) in LR participants. Similar patterns were observed in NEPTUNE: −13.4% per year (95% CI: −22.0%, −3.9%) for HR, −3.2% (95% CI: −12.4%, +7.0%) for LR.
To further explore these patterns, individual linear regressions of longitudinal eGFR data for each participant with eGFR as the dependent variable and years from baseline as the independent variable were fit. Detailed results of this analysis are presented in Supplemental Note #2. Overall, this analysis supported our initial model and indicated that HR participants had a more rapid decline in eGFR by percent change, and had higher proportions with declines greater than 10% per year and 5 mL/min per year.
Similar analyses of proteinuria at baseline and over time in both cohorts, stratified by study, showed no significant effect of APOL1 genotype (data not presented).
DISCUSSION
Through the study of African-American adults affected by diverse kidney conditions, as well as unaffected members of the general population, APOL1 risk alleles have been consistently associated with the development of certain renal conditions (e.g. FSGS, HIV-associated nephropathy) [1–3], while also increasing loss of kidney function across renal diseases [7]. Yet, since glomerular conditions are rare overall, and even more rare when restricting study to African-American children, there have been a paucity of studies examining pediatric APOL1-mediated glomerular disease. Thus, this collaboration between CKiD and NEPTUNE provided a unique opportunity to study the epidemiology and natural history of APOL1-associated glomerular disease in an African-American pediatric population. In this way, we found that children with glomerular disease who have an APOL1 HR genotype were more likely to have a later age of onset of their condition, and increased prevalence of preterm birth. Similar to adults with established kidney disease, children with an HR genotype had lower eGFR at study entry and experienced a steeper decline in renal function [7].
A strength of this study was the longitudinal and standardized phenotypic data collected on these children. This allowed us to jointly analyze data from both studies, maximizing the ability to detect meaningful baseline and longitudinal characteristics associated with an HR genotype. The differences in enrollment criteria were also beneficial: CKiD was eGFR based (30 to 90 mL/min/1.73 m2 over two recruitments), and NEPTUNE enrollment was based on a suspicion of primary nephrotic syndrome in need of a biopsy. Thus, associations common to both studies, despite their heterogeneity, may provide validity to these inferences and suggest more generalizability to other children with glomerular disease harboring an HR APOL1 genotype.
Among all participants with an FSGS diagnosis, the estimated prevalence of harboring an APOL1 HR genotype was 74% (CKiD: 78%; NEPTUNE: 67%). This was similar to previous studies across ages, which reported a 70% HR genotype frequency in African-American FSGS patients [1, 3, 17]. Our research question was interested in the overall effect of APOL1 HR among those with glomerular disease, and therefore the type of diagnosis was considered on the causal pathway between genetic risk and changes in renal function. Thus, it is possible that the accelerated eGFR decline observed among those with APOL1 HR was mediated through the high prevalence of FSGS. In a subset analysis among those with FSGS only, there were no significant differences by APOL1 in eGFR at entry and change over time. However, only seven children from each cohort were LR with FSGS, and these results should be interpreted cautiously due to the low numbers available for analysis. Overall, our results provide further confirmation of the strong relationship between APOL1 HR and FSGS that is present in pediatric populations.
A second consistent feature seen in both CKiD and NEPTUNE was an onset of glomerular disease outside the early childhood range. Interestingly, in a recent study of black NEPTUNE participants (including adults), age of onset of disease among those with the HR genotype showed a significantly restricted age range [interquartile range (IQR) 14–28] compared with those with LR genotypes (IQR 10–41). This observation was consistent with an adult study by Kopp et al. [3].
A potential explanation for not observing APOL1-mediated disease in early childhood may be that a second hit (e.g. HIV [18], other viral infection, obesity [19], prematurity [17]) is required to potentiate glomerular damage due to the HR genotype. Recent work has reported accelerated age-related nephron loss in adults with an HR APOL1 risk genotype [10], as well as exaggerated activation of intrarenal inflammatory pathways. This conceptual two-hit model would suggest that disease onset does not occur in early childhood since it takes time for the effects of a ‘second hit’ to accumulate and potentiate APOL1-associated damage. An alternative hypothesis is that the absence of children with early childhood forms of disease carrying APOL1 risk alleles in the CKiD and NEPTUNE cohorts is due to selection bias. Specifically, it may be that children with congenital or early-onset glomerular disease and HR APOL1 genotype progressed more rapidly and thus more quickly became ineligible for recruitment into the CKiD or NEPTUNE studies.
We observed that children with an HR genotype had significantly increased history of premature birth (odds ratio 4.6). In addition, the overall proportion of preterm birth among those with APOL1 HR was remarkably similar between the two cohorts (27% in CKiD and 26% in NEPTUNE), while the prevalence of prematurity among non-black children with glomerular disease was 7% in both cohorts (13/188 in CKiD and 29/390 in NEPTUNE). An increased prevalence of prematurity and low birth weight was previously reported in CKiD overall [21] and a recent NEPTUNE study of 90 black participants, including the 48 from this study, identified a three times increased odds of prematurity in participants of all ages with the HR genotype (P = 0.08). Interestingly, in the present study, APOL1 HR was not associated with SGA, indicating that in utero growth was normal, despite preterm birth. However, we are cautious about this inference, given our small sample size for analysis of low birth weight.
Previous work has demonstrated a higher prevalence of prematurity among children with CKD in general [20] and among a small group of adults with FSGS [17]. Prematurity and decreased nephron endowment from birth, typically conceptualized as related to low birth weight, are known to be associated with increased glomerular stress and higher rates of CKD [21–23]. Through experimental studies in model species as well as observational studies in humans, decreased podocyte density per glomerulus is a major determinant of glomerulosclerosis [17, 24–26]. It is possible that prematurity is a second hit that potentiates the glomerular damage attributed to APOL1 risk genotypes, given the higher prevalence of prematurity among the HR children with glomerular disease.
It is also possible that preterm birth itself is a phenotypic consequence of an HR APOL1 genotype in the mother, fetus or both. Support for this hypothesis comes from a recent study reporting that pregnant female mice expressing transgenic APOL1 (the G2 risk variant and wildtype), or carrying the offspring of transgenic APOL1 males, developed preeclampsia and fetal and neonatal demise [27]. This pregnancy phenotype was worse with expression of the G2 APOL1 variants. Convergence of this experimental and human data provides substantial motivation for additional epidemiologic and functional studies to clarify the associations between glomerular disease, an HR APOL1 genotype and prematurity. For instance, we do not know whether infants born preterm and with an HR genotype have increased risk of glomerular disease in their lifetime. Future studies that address this question may lead to insights whether there should be closer monitoring of preterm African-American children with an HR genotype for development of glomerular disease.
Similar patterns of eGFR at entry and longitudinal trajectories were observed between both studies: eGFR was, on average, lower among those with the HR genotype at entry and these children declined faster than their LR peers. This is consistent with previous findings in adults [3, 6, 7] and suggests that similar mechanisms may operate in these pediatric populations. Given the lower eGFR at entry and the faster renal function decline associated with this genotype, as well as reports that the HR genotype may be associated with lower rates of complete remission, genotyping African-American pediatric patients with glomerular disease for the APOL1 risk alleles may eventually be clinically warranted.
There are several limitations to this study. First, this study combined two cohorts that were not designed together and did not share the same standardized protocol. To address this, all analyses were stratified by or adjusted for study. Serum creatinine and urinalysis data were measured according to protocol within each study, but were not measured centrally at the same biochemistry laboratory. However, for eGFR the same estimating equation was used [15] and other variables were harmonized as much as possible. Second, the enrollment criteria between two populations differed (eGFR <90 mL/min/1.73 m2 in CKiD and nephrotic syndrome in NEPTUNE) and inferences should be appropriately contextualized. For example, given the disparate proteinuria levels and ACE inhibitor/ARB use between CKiD and NEPTUNE, we could not evaluate the impact of proteinuria as a factor in eGFR decline by APOL1 genotype. Despite these differences, it is important to note that the association between preterm birth and the APOL1 HR genotype was likely more comparable between studies since birth history was completely independent of disease stage, treatment or study enrollment processes and was parent-reported. Third, this study does not answer the question of whether prematurity itself is associated with increased loss of eGFR. However a previous CKiD study of 332 children, 85% of whom were not black, found that abnormal birth history did not affect progression of CKD [28]. Finally, the study sample was relatively small and may have limited statistical power to detect differences associated with HR APOL1. However, glomerular disease is rare, and, to our knowledge, this pooled NEPTUNE-CKiD data set represents the largest cohort of black children with glomerular disease to date.
In summary, through the study of African-American children with glomerular disease enrolled in two independent pediatric cohorts, we observed that the APOL1 HR genotype was associated with an increased prevalence of premature birth, a later age of onset, FSGS histology, a lower eGFR at study entry and a faster renal function decline compared with LR participants. These findings suggest that African ancestry, APOL1 genotype status and birth history are important clinical considerations in the management of pediatric glomerular disease. Given the strong likelihood of the presence of an APOL1 HR genotype for an African-American child presenting with FSGS, more frequent monitoring of these children, and attention to adherence to standard therapies, may be warranted, even in the absence of genetic testing. While previous studies in adults have not found that higher doses of ACE inhibition or more strict blood pressure control improved outcomes in those with an HR genotype, it is unclear how generalizable these results are to a pediatric population. Future interventional research in this pediatric population should investigate the effect of clinical knowledge of APOL1 status in modifying treatment course and improving disease outcomes.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
M.G.S. is a Carl Gottschalk Research Scholar of the American Society of Nephrology and is supported by the Charles Woodson Clinical Research Fund and NIDDK Grant 1K08-DK100662-01. The Nephrotic Syndrome Study Network Consortium (NEPTUNE), U54-DK-083912, is a part of the National Center for Advancing Translational Sciences (NCATS) Rare Disease Clinical Research Network (RDCRN), supported through a collaboration between the Office of Rare Diseases Research (ORDR), NCATS, and the National Institute of Diabetes, Digestive, and Kidney Diseases. RDCRN is an initiative of ORDR, NCATS. Additional funding and/or programmatic support for this project has also been provided by the University of Michigan, NephCure Kidney International and the Halpin Foundation. Data in this manuscript were collected by the Chronic Kidney Disease in Children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children's Mercy Hospital and the University of Missouri – Kansas City (B.A.W.) and Children's Hospital of Philadelphia (S.L.F.), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Genovese G, Friedman DJ, Ross MD et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329: 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tzur S, Rosset S, Shemer R et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 2010; 128: 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kopp JB, Nelson GW, Sampath K et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011; 22: 2129–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kruzel-Davila E, Wasser WG, Aviram S et al. APOL1 nephropathy: from gene to mechanisms of kidney injury. Nephrol Dial Transplant 2016; 31: 349–358 [DOI] [PubMed] [Google Scholar]
- 5. Sampson MG, Robertson CC, Martini S et al. Integrative genomics identifies novel associations with APOL1 risk genotype in African American NEPTUNE subjects. J Am Soc Nephrol 2016; 27: 814–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kopp JB, Winkler CA, Zhao X et al. Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol 2015; 26: 1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parsa A, Kao WH, Xie D et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 2013; 369: 2183–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freedman BI, Langefeld CD, Andringa KK et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol 2014; 66: 390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foster MC, Coresh J, Fornage M et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 2013; 24: 1484–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoy WE, Hughson MD, Kopp JB et al. APOL1 risk alleles are associated with exaggerated age-related changes in glomerular number and volume in African-American adults: an autopsy study. J Am Soc Nephrol 2015; 26: 3179–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anyaegbu EI, Shaw AS, Hruska KA et al. Clinical phenotype of APOL1 nephropathy in young relatives of patients with end-stage renal disease. Pediatr Nephrol 2015; 30: 983–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furth SL, Cole SR, Moxey-Mims M et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 2006; 1: 1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gadegbeku CA, Gipson DS, Holzman LB et al. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 2013; 83: 749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alkalay AL, Graham JM Jr, Pomerance JJ. Evaluation of neonates born with intrauterine growth retardation: review and practice guidelines. J Perinatol 1998; 18: 142–151 [PubMed] [Google Scholar]
- 15. Schwartz GJ, Munoz A, Schneider MF et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20: 629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sampson MG, Gillies CE, Robertson CC et al. Using population genetics to interrogate the monogenic nephrotic syndrome diagnosis in a case cohort. J Am Soc Nephrol 2015; (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodgin JB, Rasoulpour M, Markowitz GS et al. Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2009; 4: 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papeta N, Kiryluk K, Patel A et al. APOL1 variants increase risk for FSGS and HIVAN but not IgA nephropathy. J Am Soc Nephrol 2011; 22: 1991–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kambham N, Markowitz GS, Valeri AM et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001; 59: 1498–1509 [DOI] [PubMed] [Google Scholar]
- 20. Greenbaum LA, Munoz A, Schneider MF et al. The association between abnormal birth history and growth in children with CKD. Clin J Am Soc Nephrol 2011; 6: 14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hughson M, Farris AB III, Douglas-Denton R et al. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 200363: 2113–2122 [DOI] [PubMed] [Google Scholar]
- 22. Lackland DT, Egan BM, Fan ZJ et al. Low birth weight contributes to the excess prevalence of end-stage renal disease in African Americans. J Clin Hypertens 2001; 3: 29–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vikse BE, Irgens LM, Leivestad T et al. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 2008; 19: 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ikezumi Y, Suzuki T, Karasawa T et al. Low birthweight and premature birth are risk factors for podocytopenia and focal segmental glomerulosclerosis. Am J Nephrol 2013; 38: 149–157 [DOI] [PubMed] [Google Scholar]
- 25. Rodriguez MM, Gomez A, Abitol C et al. Comparative renal histomorphometry: a case study of oligonephropathy of prematurity. Pediatr Nephrol 2005; 20: 945–949 [DOI] [PubMed] [Google Scholar]
- 26. Rodriguez MM, et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 2004; 7: 17–25 [DOI] [PubMed] [Google Scholar]
- 27. Bruggeman LA, Wu Z, Luo L et al. APOL1-G0 or APOL1-G2 transgenic models develop preeclampsia but not kidney disease. J Am Soc Nephrol doi:10.1681/ASN.2015111220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flynn JT, Ng D, Chan GJ et al. The effect of abnormal birth history on ambulatory blood pressure and disease progression in children with chronic kidney disease. J Pediatr 2014; 165: 154–162.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.