Why was the cohort set up?
The Madagascar Health and Environmental Research (MAHERY) study cohort was set up in 2004 to understand the human health impacts of environmental change (e.g. deforestation, unsustainable hunting, biodiversity loss, climate change etc.) in Madagascar. There was a particular focus on the role of local people in rainforested areas of north-eastern Madagascar (near the city of Maroantsetra) in depleting the stocks of wild foods and how that may affect nutritional status.1,2 Nutritional status is viewed as the interaction of dietary intake, disease status and physiological state (for example, rapid growth or lactation). We selected this site for our cohort because it is nearly a fully autarchic food production system where local people are heavily reliant on wild harvest plants and animals (including wild meats like lemurs, bats, carnivores, tenrecs, etc.) for food. Local people (predominantly of Betsimisaraka and Tsimihety ethnicity) are agriculturalists with a heavy focus on rice production. In addition to intensive agricultural labour, they also hunt and gather wild harvest foods that the forests and rivers provide. Market access is nearly non-existent and domesticated meats are a rare luxury in this region. The consumption of wild harvest meats (often known as ‘bushmeat’) thus can provide crucial micronutrients that are otherwise unavailable in the diet.
We began a prospective cohort study in a rural, remote rainforest region of north-eastern Madagascar to understand the contribution of wild foods (specifically wild meats) in protecting the nutritional well-being of local people. The cohort also provides an opportunity to understand the interactive dynamics between wildlife hunting, dietary intake, nutritional status, the human faecal microbiome and the incidence of intestinal parasites, zoonotic pathogens and malaria. It is notoriously difficult to characterize the diet of individuals over long time scales because food frequency questionnaires and 24-h recall can be inaccurate for capturing seasonally variable diets. In the context of the very high prevalence of stunting (linear growth retardation) in Madagascar (over 50% of children aged under 5 years), it is critically important to understand what people are eating and how it is related to physical, nutritional and developmental outcomes across the life course. It is of the utmost importance, particularly in low-income countries, where micronutrient deficiencies such as iron, zinc and vitamin As and B12 can cause a range of poor health outcomes.
Who is in the cohort?
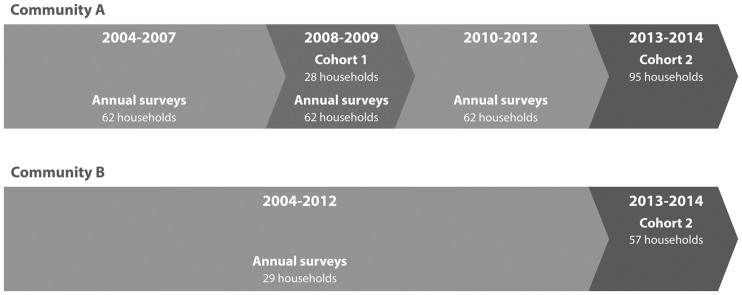
For Cohort 1, systematic random sampling was used to select 48 households from a census list of the 105 households in the one selected study community (Community A) near Makira Natural Park. These 48 households were enrolled in an environmental resource use study examining rates of wildlife and other non-timber forest product extraction.3 In all, 29 of the 48 households enrolled in the environmental cohort had pre-adolescent children (12 years of age or younger) at baseline and were subsequently invited to join the MAHERY health study in 2008, forming Cohort 14 (Figure 1). We restricted the study base to pre-adolescent children to exclude girls who had reached menarche (a factor known to affect haemoglobin concentrations and nutritional status). After screening, we individually spoke to the females in the study group to assure that they had not yet reached menarche. Informed consent or assent was obtained for all study participants (Protocol # 2007–2‐3, issued by the Office for the Protection of Human Subjects at the University of California, Berkeley, CA).
Figure 1.
This flow chart shows the timing and follow-up of Cohort 1 and Cohort 2 in Communities A and B.
In 2013, for Cohort 2, two communities adjacent to the Makira Natural Park were selected: one was the original community (Community A) enrolled in 2008–09, and the other community (Community B) was approximately 10 km west of Community A, more embedded within the rainforest. Cohort 1 was focused on pre-adolescent health and Cohort 2 was expanded to all individuals within households. The objective in selecting these cohorts was to understand the year-to-year and seasonal variation in income generation, natural resource use, forest reliance, food security and human nutrition. Community A provided an ideal foundation to situate our in-depth case studies concerning the role of wildlife consumption in human nutrition. There were a total of 160 households total in Community A and 157 households total in Community B. We assigned each household a number and created a public lottery for people to observe the random sampling. We did not assign numbers to households where the head of household was older than 75 years of age. We recruited 95 households in Community A. Two households were suggested for withdrawal because the heads of households had such a heavy alcohol dependency that they would be unable to complete the diet records and the blood draw may have been detrimental to health. We then randomly selected an additional two households to reach a total enrolment of 95 households. In Community B, we used the same public random sample process and recruited 57 households. This household random sample process allowed the enrolment of 719 individuals of both sexes of ages 73 years and younger in both communities. These numbers of households were selected in each community due to varying levels of capacity and research staff to oversee the research. During Cohort 2, households received salt and soap once per month in compensation for their efforts in the study. At the end of the study, those households who were still enrolled were allowed to vote on a gift to improve the community (up to a total of USD $500). The gift was designed to benefit the entire community, enrolled or not in the study, and thus was not seen as coercive. All households were recruited and enrolled, and each individual consented or assented following our IRB approved methods (Protocol #22826, Harvard T. H. Chan School of Public Health).
How often have they been followed up?
In the initial pilot study in Community A, all 28 households in Cohort 1 were enrolled in March 2008, including 77 pre-adolescent children. Before the end of the study in February 2009, four children withdrew from the study (one after 6 months of follow-up and three after 9 months of follow-up). From 2008–09, one household was not included in the analysis because a detailed diet calendar was not maintained.
The cohort in Community A was maintained between 2009 and 2013 by annual environmental resource use surveys and periodic visits by the principal investigator (PI) and the MAHERY team. In January 2013, we enrolled 152 households and 719 individuals in communities A and B. Throughout the 17-month study (January 2013 until May 2014), two entire households and a total of 45 individuals withdrew (approximately 6% of the overall enrolment). The two households that withdrew did so because they found the daily diet calendars overly burdensome. The individuals who withdrew outside these two households did so because they did not want to donate blood or faeces. The PI withdrew one household because their house burned down along with their diet calendars, and withdrew two individuals because of a prolonged illness and fear that the blood draw would be too burdensome. By the end of the study, 47 individuals had left the village to go to school and 57 individuals left either their household or the community for other reasons and were withdrawn. Two enrolled individuals died during the course of the study.
What has been measured?
Cohort 1
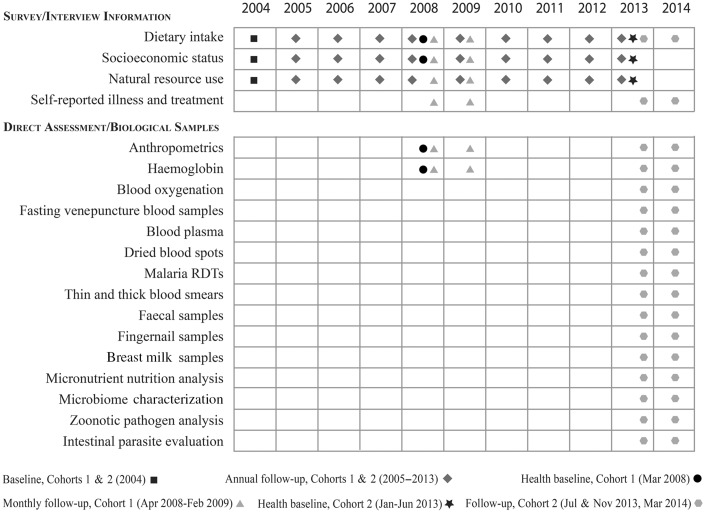
The focus of Cohort 1 was on pre-adolescent children (12 years of age and younger). Every household included in our sample was visited each month for anthropometric measurements, illness recalls and haemoglobin sampling using a HemoCue Hb 201+ Analyzer, between March 2008 and February 20094 (Figure 2). For children who were too young to answer their own health questions (typically under age 5 years), surrogate responses from mothers were accepted. The female head of household maintained a daily diet calendar throughout the duration of the study, recording the type (i.e. chicken, duck, fish, beef, pork or species of wildlife) and weight of all meat consumed by the household. Meat weights were measured through the use of digital kitchen scales and recorded daily in diet calendars over the course of 1 year. Dressed meat weight (after hair removal, feather plucking etc.) was used, before cooking.
Figure 2.
Inventory of Data and Sample Collection for the MAHERY Cohort Studies.
Fourteen of the 28 households in the study, also randomly selected at the beginning of the study, were visited without warning once a month and observed during dinner to determine patterns of intra-household food allocation.5 Because the daily diet calendars measure the amount of food consumed at the level of the household, it was also necessary to develop estimates of how this food was then shared between individuals within households. The 14 households were visited each month during the study, and 40 of the 77 children in the study sample were observed. A research assistant counted the number of spoonfuls of stew consumed by each household member from a communal stew bowl. These stews occasionally comprised meat, or vegetables or a mixture of both. These observations permitted the calculation of a mean proportion of stew typically consumed by individuals by summing all spoonfuls and then calculating an individual’s allotment. Determining the proportion of stew consumed by individuals based on age and sex permitted modelling of individual-level dietary intake.
Cohort 2
Cohort 2 included Community A where Cohort 1 was conducted but also expanded to Community B. The initial 28 households enrolled in Cohort 1 were expanded to 95 households in Community A and an additional 57 households were enrolled in Community B.
Dietary intake
We also measured the amount of all foods consumed at the household level on a daily basis in this cohort, beginning in February 2013 and ending in March 2014. Each household enrolled in the study measured the mass (to the nearest gram) of all foods (grains, vegetables, meats etc.) consumed during mealtime using an EatSmart Precision Pro Digital Kitchen Scale before cooking them. All meat weights were dressed weights after skinning, feathering and cleaning had taken place. Local research assistants lived in these communities and trained households to use these scales, so that every head of household was able to competently complete the work. Because the local research assistants lived in the communities, any questions that arose could be answered immediately. Photo guides were used to cross-reference food names in semi-literate households. These same local research assistants visited all households several times per week to monitor their work, assure adherence and answer all questions. A Chrome 100-g calibration weight was carried during these visits to ensure proper scale functioning.
Biological samples
Each month during follow-up, fingernails of each individual in the study were clipped and weighed on American Weigh Signature Series Digital Pocket Scales to obtain approximately 30 mg of fingernails. These fingernails were then placed in a Staples #1 Coin Envelope and folded closed without using the glue seal, to avoid contamination. These fingernails will be tested for their carbon and nitrogen isotopic signature as well as for mercury and arsenic content.
At the beginning of the study, end of the study and occasionally during diarrhoeal episodes during the study, faecal samples were obtained from each individual enrolled in the study. Each subject was given a sterile polypropylene screw cap faeces collection tube from Sarstedt (ref. 80.623). The subject was instructed to defaecate on top of the waxy side of a banana leaf, to collect three small spoonfuls of faeces and to drop them into the collection tube. Once the samples were returned to our local research team (typically 10 min to 5 h after collection), we added 3 ml of 97% ethanol. All samples were stored in a −23°C freezer within 14 days of collection, and then shipped on dry ice before being stored at −80°C after shipment to the USA.
Clinical visits
We conducted the same protocols for three clinical visits, each separated by 4 months (July/August 2013, November/December 2013 and March/April 2014) to account for the three distinct seasons (cold/wet, hot/dry and hot/wet, respectively) in north-eastern Madagascar. Every individual in the study received three clinical visits over the course of the study. Clinical visits were defined as a consultation with a doctor and the collection of blood and/or other biological samples. The household was notified approximately 1 week in advance of their scheduled date. Every household visited our health centre in their respective community. On the evening before the subject’s blood draw, each individual scheduled for the following morning came to the health centre etablished for this study to have their height and weight measured and to answer all of the questions in our health survey. We did this to streamline activities the following morning and to remind all individuals that they needed to fast before their blood draw. The health survey comprised several questions concerning morbidity recalls, bed net usage, vitamin intake and medication usage (including deworming medicine). Women of reproductive age were asked about pregnancy and breastfeeding. For anthropometry, we used a Health-o-Meter HDL 626‐05 digital scale to measure body weight. For small children, we measured them in their mother’s arms and then subtracted the mother’s independent weight. We measured height using a Seca Road Rod and infant length using a Quick Medical Starters Measure Mat. We calibrated all instruments daily by measuring a standard weight on the scale to ensure accuracy. Measurement error by the observer is inherent in anthropometric measurement, and detailed training before the project began and repeated re-training and observation were provided throughout the project.
Haemoglobin and blood oxygenation were measured using a fingercuff from a Pronto photospectrometer. Breastfeeding women were asked to express breast milk for nutritional analysis. The women were instructed to wash their hands and use alcohol swabs to wipe their nipples. After drying, they would put the infant on the breast for 1 min and then switch the baby to the other breast, collecting milk by hand expressing up to 60 ml from the breast where the infant was not feeding into a collection bag. The bag contents were then mixed and transferred into a falcon tube. The tubes were then dropped directly into liquid nitrogen and shipped frozen on dry ice to the Western Human Nutrition Research Center (WHNRC/USDA) in California, USA.
The following morning, approximately 24 individuals (usually members of between 2and 6 households) arrived each day at approximately 4:30–5:15 am, a time chosen to ensure fasted subjects. When subjects arrived, we applied a 5% lidocaine topical anaesthetic cream to the area where the needle would be injected, to minimize the amount of discomfort from blood collection. We drew blood via venepuncture using 21G x 1.5” safety needles, collecting into S-Monovette® venous blood collection tubes (7.5 ml 15 x 92 mm, lithium heparin). Smaller children and infants would have their blood drawn using 23G x 1.5” safety needles. All blood collection materials were designed for trace metal analysis. Once blood was collected, we inverted tubes three times to properly activate the lithium heparin, and attached a Haemo-Diff® with a smear edge so that we could apply a drop of blood onto a slide to create a thin blood smear for microscopy. Another blood drop was applied to a rapid diagnosis test (RDT) for immediate malaria diagnosis. Finally, several blood drops were applied to Whatman filter paper Flinders Technology Associates (FTA®) cards (two spots per individual). Two nurses were working simultaneously on blood draws. All blood tubes were stored in portable refrigerators and kept at 5°C before centrifugation. Within 25 min of drawing, all tubes were spun in the centrifuge.
Centrifugation of the lithium heparin tubes permitted the separation of plasma from pelleted red blood cells following centrifugation. We centrifuged tubes at 3300 rpm for 5–10 min using the Block Scientific Octafuge Plus Centrifuge. Following centrifugation, all plasma was pipetted into 1.8-ml cryo-tubes (also trace metal free). These cryo-tubes were then placed in groups of 2–4 inside a section of nylon pantyhose (which is resistant to degradation in liquid nitrogen) and dropped into a liquid nitrogen tank for flash freezing. We obtained plasma samples from 593 individuals over the course of the study. We were unable to obtain venous blood samples from some of the individuals due to unwillingness (n = 19) and technical difficulties (n = 87) primarily from unsusccessful attempts to find a vein.
All frozen plasma was shipped on dry ice from Madagascar to the Western Human Nutrition Research Center (WHNRC/USDA) via World Courier. At the WHNRC, aliquots of plasma were sampled to test levels of zinc, copper, iron, ferritin, transferrin receptor, retinol, beta carotene, alpha tocopherol, vitamin B12, cholesterol, glucose, triglycerides and inflammation markers (C-reactive protein and alpha-glycolic protein).
What have we found? Key findings and publications
In Cohort 1 we found that, in the market-limited, autarchic production systems in remote rainforest regions in north-eastern Madagascar, wild foods (particularly wild meats) are important in providing crucial micronutrients to the dietary supply. If wildlife became locally depleted and local people lost access to wild meats, we predicted anaemia to increase by 29%.4 This was calculated through a generalized linear mixed model controlling for age, sex, income and infectious disease status, and assuming that wild meat was replaced by a vegetarian substitute. Key findings and publications from Cohort 2 are still in progress. Baseline data are shown in Table 1 above (n = 719).
Table 1.
Baseline statistics of the MAHERY cohort study population
| Sex (% female) | 51.1% |
| Age (median years, minimum–maximum) | 15.8 (0.1–73.8) |
| Household monthly mean income in USD (PPPa) | 23.17 (99.12) |
| Highest educational attainment | |
| None | 17.1% |
| Elementary school | 67.9% |
| Middle school | 14.0% |
| High school | 1.0% |
| Age of first marriage (years) | 21.3 |
| Stunting (severe; total)b | |
| Both sexes | 14.2%; 36.8% |
| Females | 9.6%; 38.4% |
| Males | 11.9%; 35.6% |
| Underweight (severe; total)b | |
| Both sexes | 4.9%; 14.6% |
| Females | 7.1%; 19.6% |
| Males | 3.0%; 10.4% |
| Wasting (severe; total)b | |
| Both sexes | 2.4%; 5.3% |
| Females | 0.9%; 4.5% |
| Males | 3.7%; 5.9% |
| Reproductive-aged womenc | 31.1% |
| Pregnant womend | 19.0% |
| Lactating womene | 39.3% |
aUS dollars (purchasing power parity).
bStunting, underweight and wasting were all defined for children under 5 years of age by the World Health Organization’s standardized distributions, and the percentages here represent those falling under -2 z scores.6
cPercentage of females 15–49 years old.
dPercentage of reproductive-aged women reporting a pregnancy.
ePercentage of reproductive-aged women lactating.
What are the main strengths and weaknesses?
The main strength of these two cohorts are the detailed dietary data that correspond to the suite of nutritional biomarkers, and the diseases characterized. Another strength is that we sample a population of both sexes and all ages that was randomized within communities. Both a strength and a weakness of the study is the unique population examined. Those enrolled in the cohort live in a remote region of Madagascar and are part of a nearly entirely autarchic agricultural system. Thus, generalization of study findings can only reasonably be done to other similar populations. In Cohort 1, a key weakness of the study was the reliance on self-reported illnesses rather than direct analysis.
Can I get hold of the data? Where can I find out more?
The data for these cohorts are not open access, but collaboration is welcomed. Please contact Dr Christopher Golden [golden@hsph.harvard.edu)] for more information.
Profile in a nutshell
Individuals of all ages from two communities were recruited from a remote rainforest region in north-eastern Madagascar for two separate but overlapping cohorts from 2008–09 and 2013–14.
Follow-up in cohort 1 from 2008–09 has included daily dietary intake records, 12 questionnaires (monthly) and 12 clinical assessment visits (for 0–12 years of age).
Follow-up in cohort 2 from 2013–14 has included daily dietary intake records, 16 questionnaires (monthly), and three clinical assessment visits (for 0–75 years of age). The dataset comprises a wide range of environmental measures, biological samples, nutritional measures, disease metrics and genetic information. Approximately 6% of individuals withdrew from the study.
The MAHERY cohort is not actively collecting new health data from cohorts 1 and 2.
The MAHERY cohort data are not open access, but collaboration and enquiries are welcome.
Funding
Cohort 1, with 28 households and 77 pre-adolescent children followed from 2008/09, was funded by the National Geographic Society-Conservation Trust (C135‐08), the Margot Marsh Biodiversity Fund (023815) and the National Science Foundation GRFP and DDIG (1011714). Cohort 2, with 142 households and 719 children and adults of both sexes and all ages, was funded by the Rockefeller Foundation. In addition to the primary funding for nutritional analyses conducted by the Western Human Nutrition Research Center (USDA), laboratory analyses of: (i) malaria genetics and genomics were supported by the Harvard Malaria Initiative and funded by the Bill and Melinda Gates Foundation and the National Institutes of Health (AI106734 and AI099105); (ii) human faecal microbiome characterization was supported by the Curtis Huttenhower laboratory at the Harvard T. H. Chan School of Public Health; (iii) intestinal parasite microscopy was supported by the Milner laboratory at Brigham and Women’s Hospital; and (iv) zoonotic pathogen investigation was supported by supported by Columbia University and benefited from funding by USAID (PREDICT: GHN-A-OO‐09‐00010‐00).
Acknowledgements
We would like to extend our deepest gratitude to many of the Malagasy research assistants from the MAHERY team. Without their assistance, and the tremendous support we received from local communities, this work would never have been possible. We would also like to thank Madagascar’s Ministry of Public Health and the Maroantsetra District Public Health Service for their logistical and intellectual support.
Conflict of interest: None declared.
References
- 1. Golden CD. Bushmeat hunting and use in the Makira Forest, north-eastern Madagascar: a conservation and livelihoods issue. Oryx 2009;43:386–92. [Google Scholar]
- 2. Farris ZJ, Golden CD, Karpanty S. et al. Hunting, exotic carnivores, and habitat loss: anthropogenic effects on a native carnivore community, Madagascar. PloS One 2015;10:e0136456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Golden CD, Bonds M, Brashares J, Rasolofoniaina BJR, Kremen C. Economic valuation of subsistence harvest of wildlife in Madagascar. Conserv Biol 2014;28:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Golden CD, Fernald LCH, Brashares JS, Rasolofoniaina BJR, Kremen C. Benefits of wildlife consumption to child nutrition in a biodiversity hotspot. Proc Natl Acad Sci U S A 2011;108:19653–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golden CD, Gupta AC, Vaitla B, Myers SS. Ecosystem services and food security: assessing inequality at community, household and individual scales. Environ Conserv 2016;43:381–88. [Google Scholar]
- 6. World Health Organization. WHO Anthro version 3.2.2 and macros: World Health Organization; 2011http://www.who.int/childgrowth/software/en/ (15 September 2014, date last accessed).