Abstract
The number of mesenchymal stem cell (MSC) therapeutic modalities has grown in recent years. Adipose-derived mesenchymal stem/stromal cells (ASCs) can be isolated and expanded relatively easily as compared with their bone-marrow counterparts, making them a particularly promising source of MSCs. And although the biological mechanisms surrounding ASCs are actively being investigated, little is known about the effects that in vivo environmental exposures might have on their ability to properly differentiate. Therefore, we hypothesized that ASCs isolated from mice exposed to inorganic arsenic (iAs) would have an altered response towards adipogenic, osteogenic, and/or chondrogenic differentiation. To test this hypothesis, C57BL/6J male mice were provided drinking water containing 0, 300, or 1000 ppb iAs. ASCs were then isolated and differentiated, which was assessed by immunocytochemistry and real-time quantitative PCR (RT-qPCR). Our results showed that total urinary arsenic equilibrated within 1 week of exposure to iAs and was maintained throughout the study. ASCs isolated from each exposure group maintained differentiation capabilities for each lineage. The magnitude of differentiation-specific gene expression, however, appeared to be concentration dependent. For osteogenesis and chondrogenesis, differentiation-specific gene expression decreased, whereas adipogenesis showed a biphasic response with an initial decrease followed by an increase in adipogenic-related gene expression following iAs exposure. These results suggest that the level in which differentiation-specific genes are induced within these stromal cells might be sensitive to environmental contaminants. These findings highlight the need to take into account potential environmental exposures prior to selecting stromal cell donors, so ASCs can achieve optimal efficiency in regenerative therapy applications.
Keywords: metals; agents; exposure, environmental; environmental toxicology; stem cells.
Mesenchymal stem cells (MSCs) and particularly adipose-derived mesenchymal stem/stromal cells (ASCs) have garnered a lot of attention as a promising therapeutic strategy for a variety of conditions including cancer (Shah, 2012), osteoarthritis (Koh et al., 2015), and regenerative medicine (Gimble et al., 2007). This is in part due to their ease of isolation and culture, immunotolerance, and tropism towards areas of inflammation (Dai et al., 2016). Although the characterization of ASCs can vary slightly depending on the tissue source there are 3 major hallmarks of MSCs that have been suggested by the International Society for Cellular Therapy (Dominici et al., 2006). They include the ability of these cells to be cultured on plastic, possess a particular immunophenotype including positive markers such as CD105, CD73, and CD90 as well as being negative for CD45 and CD34, and most notably, having the ability to undergo adipogenesis, osteogenesis, and chondrogenesis. The plasticity of these cells is important because MSCs are generally recruited into inflammatory environments where they can receive cues from the microenvironment to initiate differentiation in order to promote healing and return the area to a homeostatic state (Hocking, 2015).
The plastic nature of these cells is one of the major reasons ASCs have been utilized for osteoarthritis and regenerative medicine. However, the wide heterogeneity described among human MSC donors (Siegel et al., 2013) makes the biology of these cells all the more important to unravel. Understanding the factors that influence how effective ASCs may be as a therapeutic prior to use will be vital if they are to be used as a mainstream therapy. One understudied factor relates to the susceptibility of these stromal cells to environmental exposures prior to therapeutic use. The consequences of such a potential alteration need to be further understood in order to maximize the utility of these cells and mitigate the chances of using less effective donors, ie, those with impaired regenerative or differentiation potential.
One environmental contaminant of particular concern is inorganic arsenic (iAs), which we have demonstrated previously can adversely affect the secretome associated with ASCs in vitro following as little as 1 week of exposure (Shearer et al., 2016). iAs is a ubiquitously distributed environmental contaminant found in food, the air, and many sources of drinking water. The United States Environmental Protection Agency (EPA) has mandated a maximum safe exposure limit of 10 ppb iAs in the drinking water in the U.S. (EPA, 2001). This was partly due to a host of evidence cumulating in arsenic being designated as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC, 2012), which suggested that exposure was directly linked to the risk of developing cancer within humans. However, iAs also has been shown to play a role in the dysfunction of regenerative capacity in muscles (Ambrosio et al., 2014) and bone (Wu et al., 2014), suggesting detrimental effects on stem/progenitor cell populations in these tissues.
Exposure to iAs in vitro has been shown to influence the differentiation potential of MSCs (Hou et al., 2013; Yadav et al., 2013), but there is little evidence on the specific consequences of in vivo iAs exposure on subsequent ASC plasticity. Particularly lacking is the role that iAs may play in osteogenesis or chondrogenesis. Our hypothesis was that in vivo exposure to iAs would alter the response to differentiation cues of isolated ASCs. We aimed to test this hypothesis by exposing cohorts of male C57BL/6J mice to a range of iAs concentrations through drinking water and subsequently isolating ASCs to investigate their adipogenic, osteogenic, and chondrogenic potential. Our results suggest that while phenotypic changes were not readily evident, it appears as though a 1 week in vivo exposure can alter the level of differentiation-specific gene expression of these cells in a sustained manner. These results may have important ramifications when screening for suitable ASC donors for therapeutic modalities and also suggests a potential role that environmental exposures may play in the differentiation stromal-based cell populations.
MATERIALS AND METHODS
Animals and Arsenic Exposure
Animal care and procedures were performed in accordance with the Purdue Animal Care and Use Committee institutional review board guidelines and the Laboratory Animal Program. Five- to six-week-old C57BL/6J male mice were purchased from Taconic (Hudson, NY). The mice (N = 6, per exposure group) were provided low-arsenic chow, AIN76A from Envigo Teklad (Madison, WI), and Milli-Q H2O for 2 weeks in an attempt to remove levels of background arsenic. AIN-76A chow has been shown to contain trace (< 20 ppb) iAs, which is much lower than conventional chow (Kozul et al., 2008). After flushing of residual iAs was complete, low-arsenic chow and Milli-Q H2O containing specific concentrations of arsenic were provided ab libitum for the remainder of the study with fresh water being provided twice weekly. H2O provided included 0, 300, and 1000 ppb iAs (Sodium Arsenite; RICCA, Arlington, TX) and were continuously maintained throughout the study. After 1 week of exposure 3 mice from each cohort were sacrificed for ASC isolation. One week was chosen as an important time point because prior evidence suggests that urinary arsenical compound levels stabilize in humans following a single week of exposure (Buchet et al., 1981). The remaining mice (N = 3, per exposure) continuously received iAs H2O for 3 more weeks in order to monitor the stability of urinary arsenic levels over time. We chose these concentrations by mimicking the exposure required to induce a similar induction in heme-oxygenase 1 gene expression in mouse ASCs (300 ppb) relative to human ASCs (75 ppb) in vitro (data not shown), which we previously have suggested plays a role in altering ASC signaling (Shearer et al., 2016). Exposure to 75 ppb iAs in humans represents a high level of arsenic that exceeds the safety limit set by the EPA but is not uncommon in certain portions of the world including Bangladesh who are at times exposed to levels of iAs exceeding 300 ppb (Smith et al., 2000).
Urine Collection and Digestion
Once a week, mice (N = 3, per exposure group) were individually placed inside metabolic cages with food and iAs-containing H2O provided ab libitum. After 24 h, urine was collected and centrifuged at 1250 rpm for 5 min to remove particulates and stored at −80 °C until future digestion. Mice were then returned to their home cages for the remainder of the week. Urine samples were digested by incubating 20 µl of urine in a minimal volume (135 µl) of trace metal grade concentrated nitric acid (Fisher Scientific, Waltham, MA) in a 70 °C H2O bath for 24 h. These temperature and incubation times were critical for detection of arsenical compounds during inductively coupled argon plasma spectrometry (ICP-MS) analysis. After digestion, samples were diluted to a final concentration of 2% nitric acid with Milli-Q H2O.
ICP-MS Analysis
Analysis was performed by the Campus-wide Mass Spectrometry Center at Purdue University. Briefly, the 75As ICP-MS results were obtained using an ELEMENT-2 (ThermoFinnigan, Bremen, Germany) mass spectrometer in the medium resolution mode. The samples were introduced into the plasma using an Aridus desolvating system with a T1H nebulizer (Teledyne Cetac Technologies, Omaha, NE), which is used to enhance sensitivity and reduce oxide and hydride interferences. The argon sweep gas and nitrogen of the Aridus is adjusted for maximum peak height and stability using 7Li, 115In, and 238U peaks obtained from a diluted Exaxol multi-element standard (10 ppm, Exaxol Chemical Corporation, Clearwater, FL).
Creatinine Determination
Urinary creatinine levels for each urine sample was examined using a colorimetric Mouse Creatinine Kit (Crystal Chem, Downers Grove, IL). The urine samples were diluted 10× with 0.9% saline solution prior to assay, as per manufacturer’s protocols. Reaction vessels were scaled down to a 270 µl reaction volume, and ΔA550 nm values for each sample were determined by subtracting baseline absorbance values from the absorbance of the same sample following a 5 min incubation period at 37 °C.
ASC Isolation
A cell isolation protocol was adapted from previous work (Yu et al., 2011). Briefly, mice were humanely euthanized by CO2 after 1 week of exposure and immediately following euthanasia white inguinal/gonadal fat deposits were excised and placed in sterile phosphate buffered saline (PBS) solution. Fat from each of the 0, 300, or 1000 ppb cohorts were then pooled together (N = 3 per exposure group). PBS was removed and replaced with Krebs-Ringer bicarbonate buffer, in which fat tissue was minced finely with a scalpel. Minced tissue was digested in a 1:1 (v/v) with ‘Collagenase Solution” (comprised of 0.1 g type I collagenase [Sigma, St. Louis, MO] and 1 g Bovine Serum Albumin fraction V [BSA, Fisher] per 100 ml PBS supplemented with 2 mM CaCl2 [Mallinckrodt, St. Louis, MO]) in a 37 °C shaking incubator for ∼90 min. Samples were centrifuged at 1250 rpm for 5 min at room temperature and subsequently shaken to mix cells completely. This was followed by another centrifugation step at 1250 rpm for 5 min at room temperature where the supernatant was aspirated off. The remaining pellet or stromal vascular fraction was resuspended in 1% BSA in PBS and centrifuged again at 1250 rpm for 5 min. Supernatant was aspirated and cell pellet was resuspended in 10 ml ‘stromal media’ (DMEM:F12 [Corning, Corning, NY] with 10% fetal bovine serum [Fisher] and 1× antibiotic-antimycotic [ThermoFisher, Waltham, MA]), transferred to a flask containing an additional 10 ml of stromal media, and designated as passage 0.
ASC Culture and Differentiation
Differentiation protocols were as described in the Mouse Mesenchymal Stem Cell Functional Identification Kit (R&D Systems, Minneapolis, MN). Briefly, for adipogenesis, ASCs were seeded at a cell density of 2.1 × 104 cells/cm2 in a 24-well format. Once the cells were 100% confluent, media was changed to adipogenic media, and then switched every 2–3 days for a total of 14 days. For osteogenesis, ASCs were seeded at a concentration of 4.2 × 103 cells/cm2 in a 24-well format and grown to ∼70% confluency before changing media to osteogenic media. Media was changed then every 2–3 days for a total of 21 days. For chondrogenesis, 2.5 × 105 ASCs were placed into a 15-ml conical tube and centrifuged at 1250 rpm for 5 min. Cells were then resuspended in DMEM/F-12 medium and pelleted again. Media was aspirated and chondrogenic medium added without disturbing the cell pellet. Chondrogenic medium was then replaced every 2–3 days for a total of 21 days.
Immunostaining and Imaging of ASCs
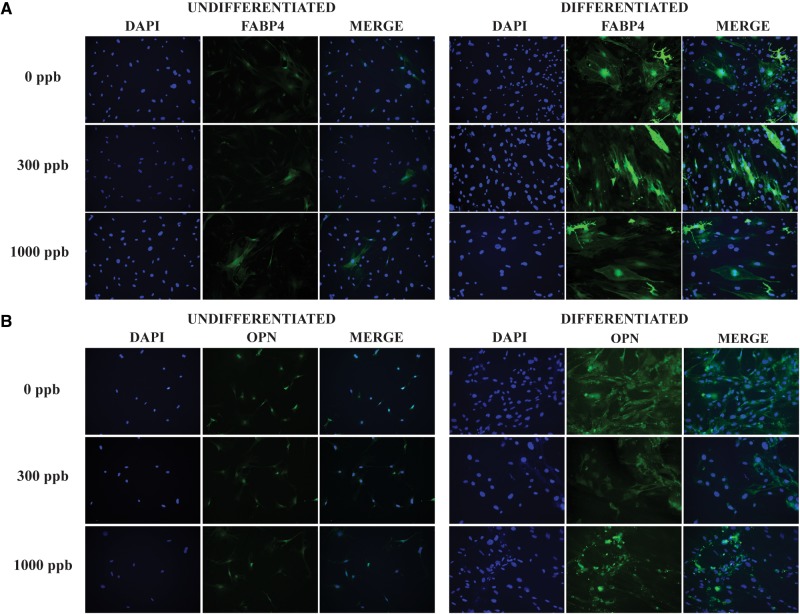
Differentiated or non-differentiated control ASCs were stained for the presence of either Fatty-Acid Binding Protein 4 (FABP4) or Osteopontin (OPN) following the protocol outlined in the Mouse Mesenchymal Stem Cell Functional Identification Kit. Briefly, following differentiation or control conditions ASCs were washed twice with PBS before being fixed with 2% paraformaldehyde (Fisher) in PBS for 30 min at room temperature. ASCs were then washed with 1% BSA in PBS 3 times for 5 min. Cells were then incubated in a blocking solution consisting of 0.3% Triton X-100 (Alfa Aesar, Ward Hill, MA), 10% Donkey Serum (Sigma) in 1% BSA for 45 min at room temperature. Then cells were incubated for 3 h at room temperature with antibodies provided in the kit, either a Goat Anti-mouse FABP4 (adipogenic samples) or a Goat Anti-mouse OPN (osteogenic sample) at a final antibody concentration of 10 µg/ml diluted in 1% BSA, 10% Donkey Serum in PBS. Cells were then washed 3× for 5 min with 1% BSA in PBS. ASCs were incubated with a Donkey anti-goat secondary (NL-557, R&D Systems) diluted 1:200 in 1% BSA in PBS for 1 h at room temperature. ASCs were then washed 3 times for 5 min with 1% BSA in PBS prior to being mounted using ProLong® Diamond Antifade Mountant with DAPI (Molecular Probes, Eugene, OR). Imaging was done on an Olympus DP72 Camera with exposure times of 20 ms for DAPI, and 75 ms for the secondary antibody, using a ×20 objective. Four fields were taken for each image with a representative image displayed.
RT-qPCR
Cell pellets were collected both from ASCs undergoing the aforementioned differentiation protocols as well as for undifferentiated control ASCs. For each pellet, total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA) and 500 ng (adipogenic and osteogenic samples) or 70 ng (chondrogenic samples) total RNA were reverse transcribed subsequently using the amfiRivert cDNA synthesis master mix (GenDEPOT, Katy, TX). Real-time reaction vessels contained 1 μl cDNA template, 2× SYBR Green Master Mix (Applied Biosystems, Foster City, CA), and 20 μM (adipogenic and osteogenic samples) or 40 µM (chondrogenic samples) forward and reverse primers for both experimental and β-actin controls. Primer sequences are provided in Supplementary Table 1. RT-qPCR was performed on a ViiA7 (Applied Biosystems) instrument, using: 95 °C for 3 min; 40 cycles of (95 °C for 3s; 60 °C 30s; 72 °C 19s), and analyzed using Quant Studio Version 1.2 (Applied Biosystems).
Western Blots
Western blot analysis was performed as previously described (Shearer et al, 2016), with minor modifications. Thirty micrograms total protein per isolated ASC group pre- and post-differentiation following iAs exposure in vivo (N = 1) was assessed as follows, Smad 2/3 (catalog no. AF3795, R&D Systems, 1:2000), phospho-Smad 2 (catalog no. 3108, Cell Signaling Technology, Danvers, MA; 1:1000), β-Actin (catolog no. ab8227, Abcam, Cambridge, MA; 1:1000) followed by secondary detection (catalog nos. 926-32214 or 926-68021, LICOR, Lincoln, NE; 1:15000). The LICOR Odyssey system was used for detection with quantifications done using Image Studio Lite Ver. 5.2 (LICOR).
Statistical Analysis
Results are shown in triplicate as the mean ± SD. A P-value of < 0.05 was considered to be statistically significant. A Two-way ANOVA analysis was used when comparing either weight or total urinary arsenic levels for each exposure cohort across time including determining the significance of any changes in urinary arsenic levels within each group over the course of exposure. One-Way ANOVA and Tukey’s test for multiple comparisons was used to assess statistical significance between exposure groups regarding fold changes in gene expression of differentiated versus undifferentiated ASCs. GraphPad Prism 6 was used for all statistical calculations.
RESULTS
Overall Mouse Health and Water Consumption
Each mouse was weighed twice weekly throughout the 6 weeks of the study (Figure 1A). No statistically significant differences were observed between any of the groups at any point during the study. No changes in grooming tendencies, activity level, or general appearance were observed between the groups. Water consumption was also measured starting on the week that arsenic exposure had begun. Over the course of the 4 weeks water consumption was changed by an average of 9% ± 12% and 35% ± 15% as arsenic concentrations increased to 300 and 1000 ppb, respectively, compared with their 0 ppb controls (Figure 1B).
FIG. 1.
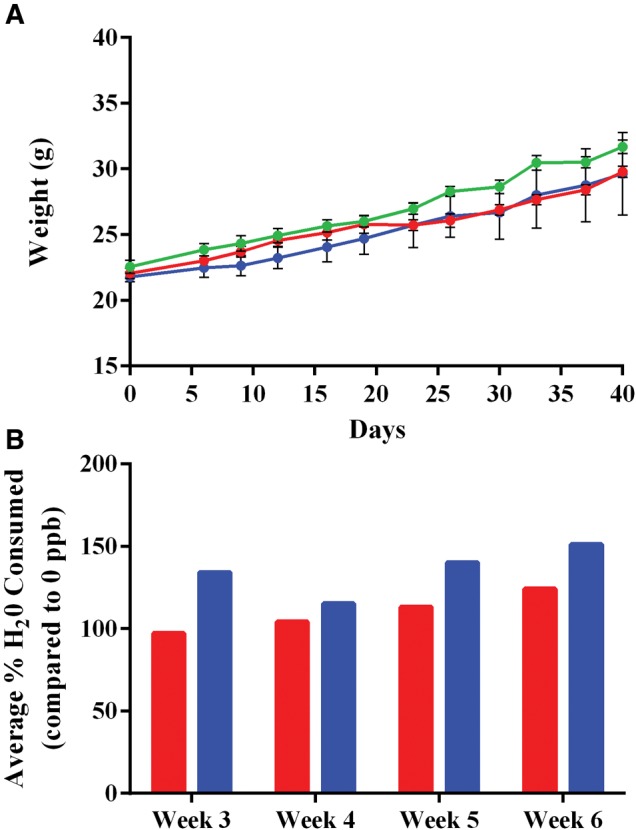
Health status and water consumption of mice during in vivo exposure. Concentrations include 0 (green), 300 (blue), or 1000 (red) ppb inorganic arsenic. A, Weight was measured twice weekly for each mouse, shown as mean ± SD with no statistical significance as determined by Two-Way ANOVA (P-value < 0.05). B, Average weekly % H2O consumed compared with control 0 ppb group for each week following inorganic arsenic exposure shown for 300 (red/left bars) and 1000 ppb (blue/right bars). Following 1 week of exposure N for each exposure group went from 6 to 3 due to animal sacrifice.
Total Urinary iAs Concentrations
Digested urine samples were analyzed for total arsenic levels by ICP-MS for each week of the study. Following the introduction of iAs into the drinking water at week 2, raw total urinary iAs showed a clear separation of exposure groups from weeks 2 through 6. The exposure groups showed total urinary iAs ranging from 19 to 47 ppb in the 0 ppb, 295 to 406 ppb in the 300 ppb, and 918 to 1190 ppb in the 1000 ppb groups (Figure 2A). Prior to introduction of iAs, no statistically significant differences in total urinary iAs levels were detected between groups. After iAs addition, the urine samples from animals exposed to 300 and 1000 ppb of iAs had significantly (P < 0.05) higher levels of arsenic relative to the 0 ppb group for each of the 4 exposure weeks. The 1000 ppb group was statistically distinct from the 300 ppb group for weeks 2–5. No statistically significant differences were detected over the exposure window within each exposure cohort.
FIG. 2.
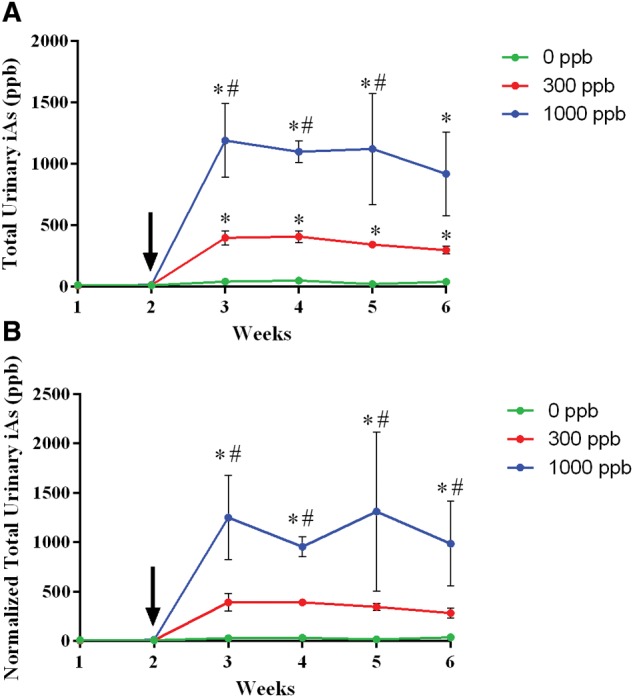
Total urinary arsenic levels. Urine samples from the same mice were collected weekly for the entire 6-week study (N = 3 per exposure group). Arrow indicates when water was replaced with either 0 (green/bottom), 300 (red/middle), and 1000 (blue/top) ppb iAs. Raw (A) or creatinine normalized (B) Values as determined by ICP-MS are shown as mean ± SD with a P-value of < 0.05 being considered statistically significant result by Two-Way ANOVA. *, indicates statistically significance with relation to the 0 ppb cohort. #, indicates statistical significance with relation to the 300 ppb cohort.
Because of the changes observed in water consumption these values were also normalized to urinary creatinine (Figure 2B). A similar pattern was observed with an apparent rapid separation of data groups in the chart upon introduction of iAs into the drinking water, although there were no statistically differences within either cohort across the 4 weeks of exposure. One observation was that in the normalized values the 300 and 0 ppb groups were not statistically significant from one another. However, despite the lack of significance, these trends in cohort differences may still bear biological relevance.
ASC Differentiation
Adipose-derived mesenchymal stem/stromal cells were isolated from mice after 1 week of in vivo exposure to iAs. ASCs were passaged in culture and did not receive any further iAs contact. These cultured ASCs were subsequently subjected to adipogenesis, osteogenesis, or chondrogenesis protocols to investigate their innate differentiation potential following in vivo exposure. Figure 3 shows adipogenic-related staining of FABP4 or osteogenic-related OPN staining overlaid by 4',6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI) nuclei staining following differentiation. Undifferentiated controls were also stained to show background or baseline expression of differentiation markers. These data show that ASCs isolated from all 3 groups possess multipotent differentiation capabilities characteristic of MSCs. Due to the relatively small cell number associated with the chondrogenesis protocol, differentiation assessment was only performed by RT-qPCR.
FIG. 3.
mASC differentiation. mASC isolated after 1 week of arsenic exposure were stained for the A, adipogenic marker fatty acid-binding protein 4 (FABP4) or B, osteogenic marker osteopontin (OPN) prior to differentiation or following differentiation. 4′,6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI) was used as a nuclear stain. Pictures are used to show presence of markers after differentiation and not as a quantitative measure for level of differentiation.
Alterations to Differentiation-Specific Gene Expressions
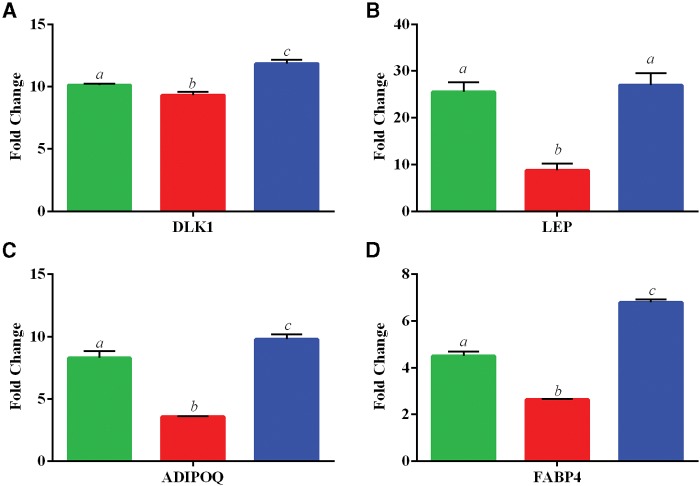
Expression levels of genes related to adipogenesis, osteogenesis, or chondrogenesis were assessed following the differentiation protocol of cultured ASCs by RT-qPCR. Adipogenic-related genes, including Pre Adipocyte Factor 1 (DLK1), Leptin (LEP), Adiponectin (ADIPOQ), and FABP4 increased by 2.6–27.0-fold depending on gene and iAs concentration when compared with the corresponding exposure-specific undifferentiated control (Figure 4). Three of the 4 genes assessed (LEP, ADIPOQ, and FABP4) had a biphasic curve in regards to mean fold change of differentiated over undifferentiated samples. This curve suggests an initial spike in adipogenic-related gene expression at 0 ppb iAs that was attenuated with 300 ppb and overall increased at 1000 ppb exposure when comparing differentiated samples over their undifferentiated controls.
FIG. 4.
Expression of adipogenic-related genes. Data is shown as the mean fold change ± SD (undifferentiated/differentiated) ASC for 3 in vivo exposed groups of 0 ppb (green/left), 300 ppb (red/middle), and 1000 ppb (blue/right). Genes investigated include A, Pre Adipocyte Factor 1 (DLK1), B, Leptin (LEP), C, Adiponectin (ADIPOQ), and D, Fatty Acid Binding Protein 4 (FABP4). Statistically significant groups (N = 3 per exposure group) as determined by One-Way ANOVA (P-value < 0.05) are depicted by the letters atop the bars for each gene set (ie a ≠ b ≠ c).
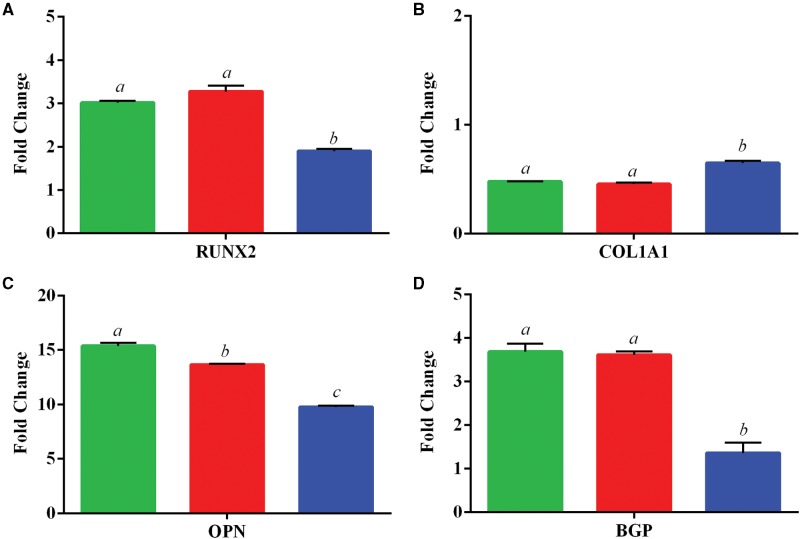
Following osteogenic differentiation, relative expression levels of osteogenic-related genes in cultured ASCs following in vivo exposure to iAs were assessed. Genes that were assayed include Runt-Related Transcription Factor 2 (RUNX2), Collagen, Type I, Alpha 1 (COL1A1), Osteopontin (OPN), and Osteocalcin (BGP). COL1A1 appeared not to be altered following the differentiation protocol, whereas the other genes examined showed an average increase in expression ranging from 1.4 to 15.4-fold change over undifferentiated controls (Figure 5). Multiple genes displayed a statistically significant (P < 0.05) decrease in expression including RUNX2 (3.0–1.9), OPN (15.4–9.7), and BGP (3.7–1.4) fold change, and the decreases were iAs concentration dependent.
FIG. 5.
Expression of osteogenic-related genes. Data is shown as the mean fold change ± SD (undifferentiated/differentiated) ASC for 3 in vivo exposed groups of 0 ppb (green/left), 300 ppb (red/middle), and 1000 ppb (blue/right). Genes investigated include A, Runt-Related Transcription Factor 2 (RUNX2), B, Collagen, Type I, Alpha 1 (COL1A1), C, Osteopontin (OPN), and D, Osteocalcin (BGP). Statistically significant groups (N = 3 per exposure group) as determined by One-Way ANOVA (P-value < 0.05) are depicted by the letters atop the bars for each gene set (ie a ≠ b ≠ c).
Expression of the chondrogenic-related genes, SRY (Sex Determining Region Y)-Box 9 (SOX9), Dermatan Sulfate Proteoglycan 3 (DSPG3), and Aggrecan (ACAN) were assayed following the chondrogenic differentiation protocol in cultured ASCs. Our results show changes in expression in these genes ranging from 1.2 to 5.0-fold when compared with their undifferentiated controls (Figure 6). Similar to osteogenesis, all 3 of these genes suggest an exposure-dependent significant decrease in differentiation-related gene expression, with SOX9 (2.5–1.4), DSPG3 (4.3–1.2), and ACAN (5.0–1.7) being progressively downregulated in response to increasing levels of iAs.
FIG. 6.
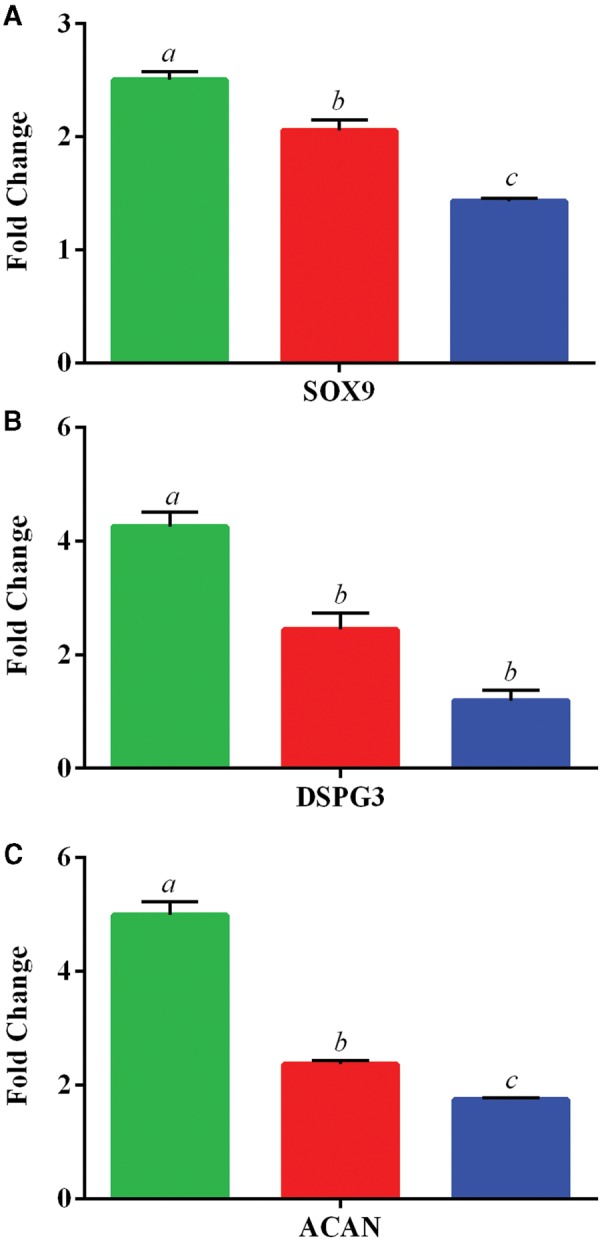
Expression of chondrogenic-related genes. Data is shown as the mean ± SD fold change (undifferentiated/differentiated) ASC for 3 in vivo exposed groups of 0 ppb (green/left), 300 ppb (red/middle), and 1000 ppb (blue/right). Genes investigated include A, SRY (Sex Determining Region Y)-Box 9 (SOX9), B, Dermatan Sulfate Proteoglycan 3 (DSPG3), and C, Aggrecan (ACAN). Statistically significant groups (N = 3 per exposure group) as determined by One-Way ANOVA (P-value < 0.05) are depicted by the letters atop the bars for each gene set (ie a ≠ b ≠ c).
Changes in Transforming Growth Factor Beta (TGFβ) Associated Gene Expression
Based on the literature implicating TGFβ as an important signaling pathway associated with MSC differentiation (Roelen and Duke, 2003) and on pathway analysis by PathGen (Clement et al., 2010), we hypothesized that TGFβ1 may follow the expression patterns of our significant differentiation gene sets. TGFβ1 expression was determined by RT-qPCR, and our results suggest that TGFβ1 expression (Figure 7A) mirrors differentiation-specific patterns for adipogenesis, osteogenesis, and chondrogenesis (Figs. 4–6). In particular, the biphasic curve seen with adipogenic-related genes, as well as the exposure-dependent decrease in osteogenic and chondrogenic-related genes appear to match the levels of TGFβ1 in cultured ASCs.
FIG. 7.
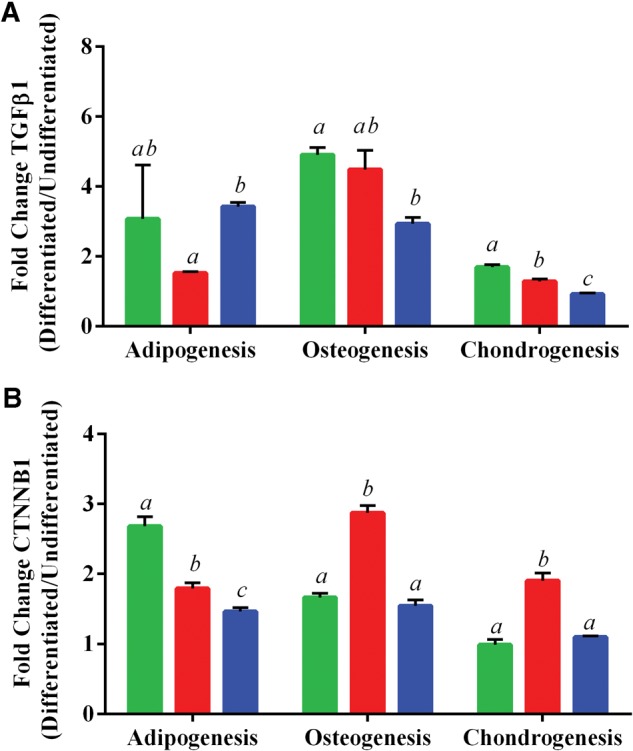
Expression of A, transforming growth factor beta 1 (TGFβ1) or B, β-catenin (CTNNB1) after differentiation of ASC. Data is shown as the mean ± SD fold change (undifferentiated/differentiated) ASC for 3 in vivo exposed groups of 0 ppb (green/left), 300 ppb (red/middle), and 1000 ppb (blue/right). Statistically significant groups (N = 3 per exposure group) as determined by One-Way ANOVA (P-value < 0.05) are depicted by the letters atop the bars (i.e. a ≠ b ≠ c).
Changes in Wnt/β-Catenin Associated Gene Expression
The Wnt/β-catenin also has been shown to be an important player in MSC differentiation (Kim et al., 2015), therefore, we investigated the effects iAs levels had on β-catenin (CTNNB1) expression during differentiation of isolated ASCs. Our results show a decrease in CTNNB1 expression in ASCs undergoing adipogenesis as levels of iAs increased from 0 to 1000 ppb, starting from a 2.7-fold increase in expression at 0 ppb and ending at half the level of expression (1.5-fold increase) at 1000 ppb when compared with their own undifferentiated controls (Figure 7B). In both osteogenesis and chondrogenesis, a biphasic change in CTNNB1 expression was detected that appeared to be exposure dependent, with an increase in expression at 300 ppb followed by a return to baseline level at 1000 ppb (Figure 7B) when comparing the fold change of differentiated cells over undifferentiated ASC.
Downstream Observations of TGFβ Signaling
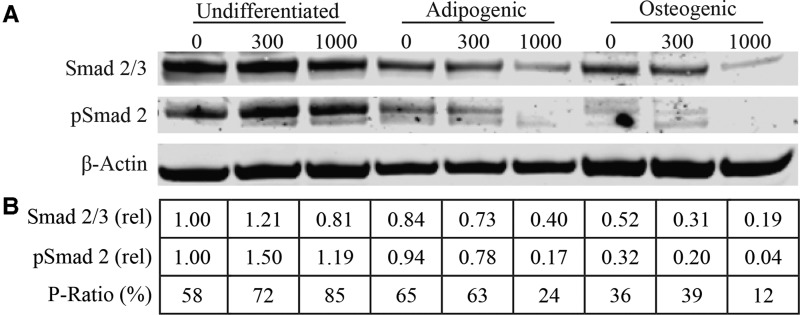
One of the major steps in TGFβ signaling transduction involves the phosphorylation of Smad proteins (Nakao et al., 1997). Because of aforementioned changes in gene expression of TGFβ1, we examined the relative levels of total and phosphorylated Smad protein in undifferentiated and differentiated ASC samples following in vivo exposures to iAs to determine the effects of iAs on downstream TGFβ signal transduction (Figure 8A). We observed that adipogenesis and osteogenesis may promote a reduction in the level of total Smad 2/3 and phospho-Smad 2 (pSmad 2) when compared with non-exposed and non-differentiated control (Figure 8B). Exposure to iAs may further reduce Smad2/3 expression and pSmad 2 levels in both adipogenic and osteogenic samples. Furthermore, when we compared the ratio of pSmad 2 to total Smad 2/3 for each sample (P-Ratio), we observed a concentration-dependent effect as iAs levels increased (from 0 to 1000 ppb) in the adipogenic (65%–24%) and osteogenic (36%–12%) samples following exposure.
FIG. 8.
Protein expression for TGFβ signaling. A, Western blot of Smad 2/3, phosphorylated Smad 2 (pSmad 2), and β-Actin protein levels. The blot shows ASC protein expression from undifferentiated or ASCs (N = 1) that have undergone adipogenic/osteogenic stimulation following 1 week of in vivo iAs exposure (0, 300, and 1000 ppb). B, Dosimetry of protein levels. All bands were normalized to their level of β-Actin expression. Values are shown relative to 0 ppb control samples for both Smad 2/3 (rel) and pSmad 2 (rel). P-Ratio was calculated by taking the β-Actin normalized values of pSmad 2 over total Smad 2/3 as an indicator of downstream activation of TGFβ signaling.
DISCUSSION
Mesenchymal stem cells have emerged over the last decade as a promising therapeutic strategy for a host of diseases. This is quite evident by the fact that as of September 2016 there were nearly 247 open clinical trials that were utilizing MSCs in various ways (clinicaltrials.gov, MSCs, 2016). Because, MSCs can now be effectively isolated and expanded from adipose tissue (ASCs), we would predict that the access to MSC-based therapies may grow even further in the near future. Compared with bone marrow-derived MSCs, much less is known about the biology of ASC-based therapies or what makes one donor more desirable over another. Whereas similar in many respects, including overall surface marker characteristics, and differentiation ability, subtle differences have been described in signaling and differentiation potential relating to the tissue of origin (Li et al., 2015). Even more striking is the heterogeneity of therapeutic outcomes associated with variables including age (Choudhery et al., 2014) and body mass index (Frazier et al., 2013) of ASC donors. As the utility of these cells for treating a wide range of disease conditions continues to grow, efforts need to be made to better understand what makes an optimal MSC donor cell population in order to maximize their therapeutic potential and avoid any undesirable effects such as a reduced capacity for tissue repair or dedifferentiation of tissues post-repair.
Environmental contaminants, such as iAs, are often overlooked as potential criteria when considering which factors might play a role in the ability of these cells to function and differentiate properly. Arsenical compounds have been implicated in altering the capacity of MSC or progenitor cells to differentiate following ex vivo exposure (Cheng et al., 2011; Hou, et al., 2013; Klei et al., 2013). These data suggested that these therapeutic stem cell populations might be sensitive to environmental exposures; however, there have been no reports yet on the effects of in vivo exposure to iAs on the ability of ASCs to differentiate.
We investigated the sensitivity of ASCs to iAs in a mouse exposure model. Mice are known to be much more efficient at iAs methylation compared with humans, which may be attributed to their enhanced ability to metabolize and excrete arsenic (Vahter, 1994). Due to this ability, mice generally require higher levels of arsenic in order to model human exposure. The differences in iAs metabolism are evident by the fact that mice receiving 100 times (1000 ppb) the safety limit suggested by the EPA show no apparent adverse phenotypic responses following exposure. Our in vivo results suggest that the majority of iAs appears to be readily excreted in the urine at concentrations nearly the same as the supplied water, suggesting iAs is not being stored in vivo. Although we did not measure iAs levels in different tissues types, previous reports have shown that adipose tissue stores iAs to a much lower degree than any other tissues (Paul et al., 2008). Our urinary excretion data suggests arsenic might not be stored in fat but might be dynamically (albeit transiently) interacting with ASCs in the adipose tissues where these stem cells reside. Our data show that the mice have reached a relative state of equilibrium following exposure, which is maintained throughout the duration of the study and that nearly matches the concentration of iAs provided in the water. This equilibrated state is more physiologically relevant to modeling human long-term low level exposures rather than acute, high-level toxic exposures.
A major strength of this study is that the ASCs were exposed in vivo, subsequently isolated from the mice, and then never were exposed to iAs again. This experimental design emulates how ASCs would be isolated from human subjects for passaging and expansion in culture prior to therapeutic use. Interestingly, our results suggest that sustained changes to differentiation-related gene expression, can occur already after only 1 week of exposure to iAs through drinking water. Although iAs does not abrogate the ability of ASCs to differentiate, which is evident by the fact that all 3 groups still maintain adipogenic, osteogenic, and chondrogenic capabilities, it shows how relatively short periods of time potentially can affect either temporal or spatial characteristics of induction of genes related to differentiation. This may have detrimental implications for therapeutic applications such as delayed or reduced ASC regenerative capacity. For example, ASCs from a donor that was exposed to environmental contaminants might not be able to restore cartilage in an osteoarthritic knee or might inadvertently differentiate towards an adipogenic phenotype upon use.
One gap in the current literature pertaining to the effects that iAs has on differentiation stems from the fact that different groups have examined the effect of iAs during cell culture (after cells have already been isolated), rather than examining the in vivo effects of exposure (prior to isolation). And although some reports have shown that exposure to iAs appears to inhibit adipogenesis (Hou et al., 2013), our results suggest this may be concentration dependent. We observed an initial decrease in adipogenic-related gene expression following 300 ppb, and an increase at higher levels (1000 ppb) compared with unexposed controls (0 ppb). We detected this trend in altered expression for 3 adipogenic-specific genes, including LEP, ADIPOQ, and FABP4. These findings suggest that different iAs-mediated mechanisms might be activated depending on the exposure levels.
Little evidence exists on how iAs exposure impacts osteogenesis, however, other arsenical compounds such as arsenic trioxide decrease osteogenesis in bone marrow stromal cells following exposure (Wu et al., 2014), which suggests these differentiation processes may be influenced by environmental contaminants such as iAs. Interestingly, unlike adipogenesis, osteogenesis, and chondrogenesis showed a strikingly similar response to increasing iAs concentrations, showing a concentration-dependent decrease in the expression of several differentiation markers. Although very little is known about the effects of iAs and chondrogenesis, one study has suggested that iAs inhibits chondrogenesis in chick limb bud mesenchymal cells (Lindgren et al., 1984). These findings emulate our chondrogenesis results following exposure to iAs, which showed concentration-dependent decreases in expression of chondrogenic-related genes including SOX9, DSPG3, and ACAN. These are interesting findings, however, additional work is needed to determine the potential impact of environmental contaminants on chondrogenesis, especially as the interest in ASC-based regenerative medicine grows.
Our last goal for this study was to identify potential molecular pathways that may explain how iAs is capable of mediating changes in 3 unique ASC differentiation programs. Two major pathways involving TGFβ and Wnt signaling have been implicated previously in MSC differentiation (Augello and De Bari, 2010). Therefore, using PathGen pathway analysis with our differentiation-specific gene expression results, we hypothesized that the TGFβ/Smad pathway may be a potential key mediator for the effects of iAs seen in all 3 differentiation lineages. Interestingly, the changes we identified in our differentiation-specific gene panels mirrored the changes detected in TGFβ expression as assessed by RT-qPCR. We have shown in previous studies that iAs is able to alter TGFβ signaling in human ASCs in vitro (Shearer et al, 2016), which might explain the concentration-dependent decrease in osteogenic/chondrogenic-related genes seen in the present study.
The biphasic response in adipogenesis gene expression following iAs exposure was an interesting finding, which might be attributed to several mechanisms. However, this type of differentiation response curve is not unique to MSC differentiation and has been reported for other stromal cells (osteoclasts) when fluctuations occur in the levels of TGFβ (Karst et al., 2004). This suggests that perturbations to the TGFβ signaling might promote differentiation changes that occur in a non-classic dose-response pattern. Our results of decreased osteogenic and increased adipogenic potential following increasing iAs exposure may not be mutually exclusive of one another because recent reports suggest that MSC adipogenic and osteogenic differentiation potential tend to oppose one another (James, 2013). Additionally, some reports have suggested that ASCs express adipogenic genes to a higher degree relative to bone marrow-derived MSC upon differentiation (Liu et al., 2007), thus they might display a predisposition towards adipogenesis that may supersede or dilute the effects of iAs. This might make ASCs more resistant to environmental or other insults, with regards to attenuation of adipogenesis, due to the fact they are already primed towards a pro-adipogenic state.
The Wnt/β-catenin has also been found to be an important pathway in differentiation of ASCs (Takada et al., 2009) and we sought to expand our examination of whether β-catenin expression was altered also by iAs in addition to TGFβ. Our results showed that β-catenin levels were altered with iAs exposure, however, it did not mirror either the TGFβ or differentiation-specific gene profiles assayed. This may suggest that β-catenin expression is sensitive to iAs, however, it might not be directly related to iAs-induced changes in ASC differentiation. Future work will help delineate the contributions and potential cooperation between these pathways with regards to iAs-induced changes in ASC differentiation.
With the gene expression of TGFβ1 emulating the changes in expression of differentiation genes, we examined whether this might have been mediated through downstream activation as indicated by the phosphorylation of Smad2 effector molecules. Interestingly, the observed changes in phosphorylated Smad2 appear not to directly coincide with changes in TGFβ1 gene expression. However, this observation has been previously described by Allison et al. (2013) showing that arsenic exposure increased relative expression of TGFβ1 but simultaneously attenuated phosphorylation of Smad proteins and loss of nuclear translocation. Because it appears that iAs exposure may affect the canonical TGFβ signaling pathway through a multifaceted mechanism that includes TGFβ, Smad proteins, and translocation, further work needs to be conducted to understand the complex interplay between in vivo arsenic exposure and the components of the TGFβ signaling cascade with regards to controlling the differentiation status of cells.
Although we have tried to capture to the best of our ability, the effects that in vivo exposure to an environmental contaminant may have on ASC biology we do recognize some limitations within our study. First, a full pathological assessment of mouse health was not performed upon sacrifice, which might have revealed health-related issues not obvious from phenotypic observations or from monitoring body weight. Body weight composition assessment might have provided a more sensitive marker of mouse health (Burkholder et al., 2012) as compared with body weight monitoring. Although we did not observe changes in body weight, a food intake measurement could have shown any potential body-composition changes in the mouse cohorts. The gene-expression profiles were measured purposely as differentiated/undifferentiated in order to reduce potential background effects associated with biological variance.
Another important consideration was the choice to pool ASC donors. Although this method has been used in the literature when investigating MSC biology (Goff et al., 2008), it is not optimal for identifying individual biological variance and remains a limitation of the study. However, using inbred C57/BL6 mice, whose genetic background should be identical between animals, removes much of the heterogeneity associated with human samples. For this reason we propose that pooling might influence the results to a much lesser degree than what would be seen if we were pooling MSCs isolated from genetically heterogeneous human samples. Additionally, our choice to pool samples may reflect a more clinically-relevant application of MSCs. For example, it has been proposed that pooled donor samples may be advantageous in overcoming the heterogeneity associated with the efficacy of MSC-based therapies (Kuçi et al., 2016). Thus, while we lose individual variance from pooling cells from different genetically identical donors, we gain an understanding of how an at-risk population may be influenced by environmental contaminants. Additionally, we can better understand how collecting ASCs from donors from such a population may influence fundamental ASC biology and potentially their utility as a therapeutic.
Cumulatively, our data suggests that exposure to environmental contaminants has the potential to influence stromal cell populations such as ASCs. We have shown that iAs levels in our mouse model do not appear to affect their overall health from our phenotypic observations but can subtly alter the expression of differentiation-specific genes in certain groups of stromal stem cells. Altering the ability of ASCs to properly express genes associated with differentiation might have important consequences that must be considered when employing them for therapeutic or regenerative medicine strategies. Exposure history may become an important criterion when considering potential donors for cell-based therapies.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr Karl Wood and Arlene Rothwell for their expertise and advice regarding ICP-MS analysis.
FUNDING
This work was supported by the National Institutes of Health (R21 CA153165) and Department of Basic Medical Sciences at the Purdue University College of Veterinary Medicine.
REFERENCES
- Allison P., Huang T., Broka D., Parker P., Barnett J. V., Camenisch T. D. (2013). Disruption of canonical TGFβ-signaling in murine coronary progenitor cells by low level arsenic. Toxicol. Appl. Pharmacol. 272, 147–153. doi:10.1016/j.taap.2013.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio F., Brown E., Stolz D., Ferrari R., Goodpaster B., Deasy B., Distefano G., Roperti A., Cheikhi A., Garciafigueroa Y., et al. (2014). Arsenic induces sustained impairment of skeletal muscle and muscle progenitor cell ultrastructure and bioenergetics. Free Radic. Biol. Med. 74, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augello A., De Bari C. (2010). The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 21, 1226–1238. [DOI] [PubMed] [Google Scholar]
- Buchet J., Lauwerys R., Roels H. (1981). Urinary excretion of inorganic arsenic and its metabolites after repeated ingestion of sodium metaarsenite by volunteers. Int. Arch. Occup. Environ. Health 48, 111–118. [DOI] [PubMed] [Google Scholar]
- Burkholder T., Foltz C., Karlsson E., Linton C., Smith J. (2012). Health evaluation of experimental laboratory mice. Curr. Protoc. Mouse Bio. 2, 145–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Qiu L., Zhang H., Cheng M., Li W., Zhao X., Liu K., Lei L., Ma J. (2011). Arsenic trioxide promotes senescence and regulates the balance of adipogenic and osteogenic differentiation in human mesenchymal stem cells. Acta Biochim. Biophys. Sin. (Shanghai) 43, 204–209. 10.1093/abbs/gmq130. [DOI] [PubMed] [Google Scholar]
- Choudhery M. S., Badowski M., Muise A., Pierce J., Harris D. T. (2014). Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 12, 8. 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement K., Gustafson N., Berbert A., Carroll H., Merris C., Olsen A., Clement M., Snell Q., Allen J., Roper R. J. (2010). PathGen: a transitive gene pathway generator. Bioinformatics 26, 423–425. 10.1093/bioinformatics/btp661. [DOI] [PubMed] [Google Scholar]
- Dai R., Wang Z., Samanipour R., Koo K. I., Kim K. (2016). Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016, 6737345. 10.1155/2016/6737345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA). (2001). National Primary Drinking Water Regulations; Arsenic and Clarifications to Compliance and New Source Contaminants Monitoring Available at: https://www.gpo.gov/fdsys/pkg/FR-2001-01-22/pdf/01-1668.pdf. Accessed August 12, 2016.
- Frazier T. P., Gimble J. M., Devay J. W., Tucker H. A., Chiu E. S., Rowan B. G. (2013). Body mass index affects proliferation and osteogenic differentiation of human subcutaneous adipose tissue-derived stem cells. BMC Cell. Biol. 14, 34. 10.1186/1471-2121-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J. M., Katz A. J., Bunnell B. A. (2007). Adipose-derived stem cells for regenerative medicine. Circ. Res. 100, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff L., Boucher S., Ricupero C., Fenstermacher S., Swerdel M., Chase L., Adams C., Chesnut J., Lakshmipathy U., Hart R. (2008). Differentiating human multipotent mesenchymal stromal cells regulat microRNAs: prediction of microRNA regulation by PDGF during Osteogenesis. Exp. Hematol. 36, 1354–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking A. M. (2015). The role of chemokines in mesenchymal stem cell homing to wounds. Adv. Wound Care (New Rochelle) 4, 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Xue P., Woods C. G., Wang X., Fu J., Yarborough K., Qu W., Zhang Q., Andersen M. E., Pi J. (2013). Association between arsenic suppression of adipogenesis and induction of CHOP10 via the endoplasmic reticulum stress response. Environ. Health Perspect. 121, 237–243. 10.1289/ehp.1205731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). (2012). Arsenic, Metals, Fibres And Dusts. IARC Monogr Eval Carcinog Risk Hum 100C:41–93 Available at: http://monographs.iarc.fr/ENG/Monographs/Vol15701C/mono100C.pdf. Accessed August 12, 2016.
- James A. W. (2013). Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica (Cairo) 2013, 684736.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst M., Gorny G., Galvin R. J., Oursler M. J. (2004). Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J. Cell. Physiol. 200, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Choi H., Kim T., Leem S., Oh I. (2015). Regulation of mesenchymal stromal cells through fine tuning of conocical Wnt signaling. Stem Cell Res. 14, 356–368. [DOI] [PubMed] [Google Scholar]
- Klei L. R., Garciafigueroa D. Y., Barchowsky A. (2013). Arsenic activates endothelin-1 Gi protein-coupled receptor signaling to inhibit stem cell differentiation in adipogenesis. Toxicol. Sci. 131, 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh Y. G., Choi Y. J., Kwon S. K., Kim Y. S., Yeo J. E. (2015). Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 23, 1308–1316. [DOI] [PubMed] [Google Scholar]
- Kozul C. D., Nomikos A. P., Hampton T. H., Warnke L. A., Gosse J. A., Davey J. C., Thorpe J. E., Jackson B. P., Ihnat M. A., Hamilton J. W. (2008). Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung. Chem. Biol. Interact. 173, 129–140. [DOI] [PubMed] [Google Scholar]
- Kuçi Z., Bönig H., Kreyenberg H., et al. (2016). Mesenchymal stromal cells from pooled mononuclear cells of multiple bone marrow donors as rescue therapy in pediatric severe steroid-refractory graft-versus-host disease: a multicenter survey. Haematologica 101, 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Wu X. Y., Tong J. B., Yang X. X., Zhao J. L., Zheng Q. F., Zhao G. B., Ma Z. J. (2015). Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 6, 55. 10.1186/s13287-015-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren A., Danielsson B. R., Dencker L., Vahter M. (1984). Embryotoxicity of arsenite and arsenate: distribution in pregnant mice and monkeys and effects on embryonic cells in vitro. Acta Pharmacol. Toxicol. (Copenh) 54, 311–320. [DOI] [PubMed] [Google Scholar]
- Liu T. M., Martina M., Hutmacher D. W., Hui J. H., Lee E. H., Lim B. (2007). Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 25, 750–760. [DOI] [PubMed] [Google Scholar]
- Mesenchymal Stem Cells (MSCs). (2016). ClinicalTrials.gov National Institutes of Health, Bethesda, MD. Available at: https://clinicaltrials.gov/ct2/results?term=mesenchymal+stem+cells&recr=Open&no_unk=Y. Accessed September 12, 2016.
- Nakao A., Imamura T., Souchelnytskyi S., Kawabata M., Ishisaki A., Oeda E., Tamaki K., Hanai J., Heldin C. H., Miyazono K., et al. (1997). TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 16, 5353–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D., Zavala A. H., Perez D. I., Thomas D. J., Styblo M. (2008) Tissue Distribution of Arsenic Species in Mice Chronically Exposed to Methylarsonous Acid [Abstract]. Presented at Society of Toxicology Annual Meeting, Seattle, WA, March 16–20, 2008. Available at: https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=185443&CFID=75210628&CFTOKEN=36240199. Accessed July 18, 2016.
- Roelen B., Duke P. (2003). Controlling mesenchymal stem cell differentiation by TGFβ family members. J. Orthop. Sci. 8, 740–748. [DOI] [PubMed] [Google Scholar]
- Shah K. (2012). Mesenchymal stem cells engineered for cancer therapy. Adv. Drug. Deliv. Rev. 64, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J. J., Wold E. A., Umbaugh C. S., Lichti C. F., Nilsson C. L., Figueiredo M. L. (2016). Inorganic arsenic-related changes in the stromal tumor microenvironment in a prostate cancer cell-conditioned media model. Environ. Health Perspect. 124, 1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel G., Kluba T., Hermanutz-Klein U., Bieback K., Northoff H., Schäfer R. (2013). Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 11, 146.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Lingas E., Rahman M. (2000). Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull. World Health Organ 78, 1093–1103. [PMC free article] [PubMed] [Google Scholar]
- Takada I., Kouzmenko A. P., Kato S. (2009). Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 5, 442–447. [DOI] [PubMed] [Google Scholar]
- Vahter M. (1994). Species differences in the metabolism of arsenic compounds. Appl.Organomet. Chem. 8, 175–182. [Google Scholar]
- Wu C. T., Lu T. Y., Chan D. C., Tsai K. S., Yang R. S., Liu S. H. (2014). Effects of arsenic on osteoblast differentiation in vitro and on bone mineral density and microstructure in rats. Environ. Health Perspect. 122, 559–565. 10.1289/ehp.1307832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Anbalagan M., Shi Y., Wang F., Wang H. (2013). Arsenic inhibits the adipogenic differentiation of mesenchymal stem cells by down-regulating peroxisome proliferator-activated receptor gamma and CCAAT enhancer-binding proteins. Toxicol. In Vitro 27, 211–219. 10.1016/j.tiv.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Yu G., Wu X., Kilroy G., Halvorsen Y. D., Gimble J. M., Floyd Z. E. (2011). Isolation of murine adipose-derived stem cells. Methods Mol. Biol. 702, 29–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.