Dual antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12 receptor antagonist is a fundamental component of acute coronary syndrome (ACS) management, with longstanding endorsements by both the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) and the European Society of Cardiology (ESC).1,2 Dual antiplatelet therapy reduces the risk of both stent thrombosis and spontaneous ischaemic events, at the cost of an increased bleeding risk.3,4 The management of antiplatelet therapy in ACS patients with thrombocytopenia poses a particular challenge for physicians, as they are at higher risk of both bleeding and, paradoxically, ischaemic events.5 Unfortunately, there are currently no guideline recommendations or consensus reports to guide clinicians on the management of this cohort. In this setting, we examine the evidence to date and provide our opinion on future directions and management strategies for this group (Figure 1).
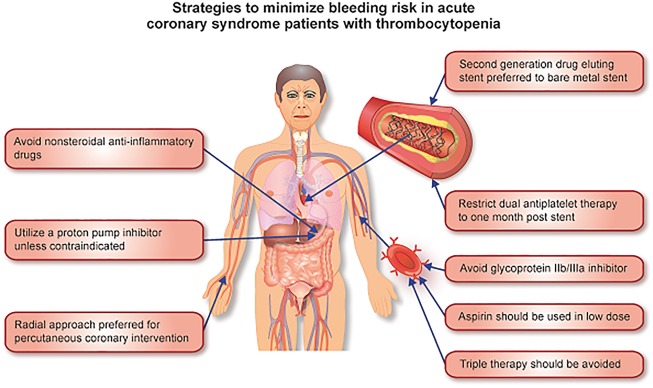
Figure 1.
Figure outlining strategies to minimize bleeding risk in acute coronary syndrome patients with thrombocytopenia.
Including the excluded
The reported prevalence and incidence of thrombocytopenia among patients with ACS has varied depending on the definition and the nature of the study. Observational evidence suggests that baseline thrombocytopenia, defined as a platelet count of <150 × 109/L, is present in approximately 5% of ACS patients,6,7 whereas incident thrombocytopenia can be expected in 13% of patients.6 Incident thrombocytopenia can be seen more frequently in patients who are older or have diabetes, renal insufficiency, heart failure, or prior cardiovascular disease.6
Despite making up a substantial proportion of ACS patients in clinical practice, these patients have been excluded or under-represented in important clinical trials evaluating antiplatelet therapies in ACS patients. Patients with thrombocytopenia were excluded from the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON–TIMI) 38,8 the PLATelet inhibition and patient Outcomes (PLATO) trial,9 the Cangrelor versus standard therapy to acHieve optimal Management of Platelet InhibitiON (CHAMPION) PHOENIX trial,10 and represented only 0.8% of the participants in the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial.11
Prognostic implications
The presence of thrombocytopenia in ACS patients predicts significantly worse outcomes.6,7 Yadav et al. examined the implication of baseline thrombocytopenia on clinical outcomes in 10 603 patients who underwent percutaneous coronary intervention (PCI) for non-ST-elevation ACS (NSTE-ACS) or ST-elevation myocardial infarction (STEMI) pooled from two large-scale randomized trials.7 In multivariable analysis, the presence of thrombocytopenia at baseline was an independent predictor of mortality at 1 year [hazard ratio (HR), 1.74; 95% confidence interval (CI), 1.12–2.69; P = 0.01], ischaemic target lesion revascularization (HR, 1.37; 95% CI: 1.04–1.81; P = 0.03), and major adverse cardiac events (HR, 1.39; 95% CI: 1.09–1.79; P = 0.009).7 The authors found no association between baseline thrombocytopenia and major or minor bleeding rates at 30 days, however, they noted that their cohort of patients only included patients with mild thrombocytopenia (platelet count of 100 × 109/L–150 × 109/L).
Wang et al.6 investigated the effect of incident thrombocytopenia on outcomes in 36 182 NSTE-ACS patients participating in the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines (CRUSADE) quality improvement initiative. Risks of inpatient mortality and bleeding correlated directly with severity of thrombocytopenia and even mild thrombocytopenia (platelet nadir 100 to 149 × 109/L) was associated with increased risks of mortality [adjusted odds ratio (OR), 2.01; 95% CI: 1.69 to 2.38] and bleeding (adjusted OR, 3.76; 95% CI: 3.43 to 4.12). Every 10% decrease in platelet count was associated with increased mortality (adjusted ORs: 1.39, 95% CI: 1.33 to 1.46) and bleeding risk (adjusted OR: 1.89, 95% CI: 1.83 to 1.95). Ominously, approximately one in four patients who developed moderate/severe thrombocytopenia did not survive the hospitalization.6 The influence of the aetiology of thrombocytopenia on prognosis and treatment has not been well investigated and warrants exploration. Liver disease in particular is associated with coagulation disorders as well and therefore may carry even greater bleeding risk in the setting of thrombocytopenia.
The association of thrombocytopenia and increased ischaemic events remains poorly understood. It may reflect the presence of a greater degree of comorbidities that are unmeasured. Some authors have suggested that thrombocytopenia in ACS may reflect a greater burden of atherosclerosis predisposing to heightened platelet consumption, or reflect clinically significant thrombosis, and consequently, its presence should be viewed as a marker of disease severity.5,12
Management strategies and future directions
Has this common and high-risk group of patients been neglected by the research community to date? This appears to be the case, with an absence of evidence to guide physicians on the management of this cohort leading to a sense of despair amongst clinicians, as summarized by Warkentin et al. in an editorial ‘We know that thrombocytopenia is bad, but we do not think we can do anything about it’.12 It is our belief that positive steps can be made and should begin with randomized clinical trials evaluating the safety and efficacy of various antiplatelet regimens in this cohort.
A comparative study of DAPT vs. aspirin or P2Y12 inhibitor monotherapy in ACS patients with thrombocytopenia would be valuable. Sub-analysis of patients with varying degrees of thrombocytopenia would help determine the platelet count at which these regimens reach a ‘tipping point’, where the bleeding risk outweighs any ischaemic benefit. Of the P2Y12 inhibitors, clopidogrel offers a lower bleeding risk compared with ticagrelor or prasugrel,3,8 and accordingly would be the agent of choice in patients receiving DAPT in such a trial. An interesting alternative approach would involve utilizing an intravenous antiplatelet agent such as cangrelor in this cohort. Cangrelor has a plasma half-life of approximately 3–6 min and platelet function is restored within 1 h after cessation of the infusion. Therefore, such an agent may be advantageous in patients at higher risk of bleeding events. Indeed, in the CHAMPION PHOENIX trial, cangrelor significantly reduced the rate of ischaemic events (including stent thrombosis) compared with clopidogrel, with no significant increase in severe bleeding or transfusions.10 However, patients with a platelet count of <100 × 109/L were excluded from this trial. As such, the bleeding risk associated with cangrelor might be significant if used in patients with platelets below this threshold. Nevertheless, an investigation of cangrelor in the inpatient setting as both monotherapy or as part of a combination regimen in this cohort might be worthwhile.
Thrombocytopenia can generally be classified as mild, moderate, or severe (Table 1). While a patient’s risk of bleeding can increase even with mild thrombocytopenia,6 we believe a platelet count of <100 × 109/L is clinically relevant, and warrants proactive measures to reduce the risk of bleeding (Table 2). What approach could clinicians take until further randomized trial evidence is available? We believe initial management should begin with identification and correction of any reversible causes of thrombocytopenia. If suspected as the instigating factor, medications associated with the development of thrombocytopenia such as unfractionated heparin, glycoprotein IIb/IIIa inhibitors, furosemide, NSAIDs, and penicillin-based antibiotics should be discontinued.
Table 1.
Classification of thrombocytopenia
| Classification | Platelet count |
|---|---|
| Mild | <150 × 109/L but ≥100 × 109/L |
| Moderate | <100 × 109/L but ≥50 × 109/L |
| Severe | <50 × 109/L |
Table 2.
Strategies to minimize bleeding risk in patients with significant thrombocytopenia
|
|
|
|
|
|
|
|
|
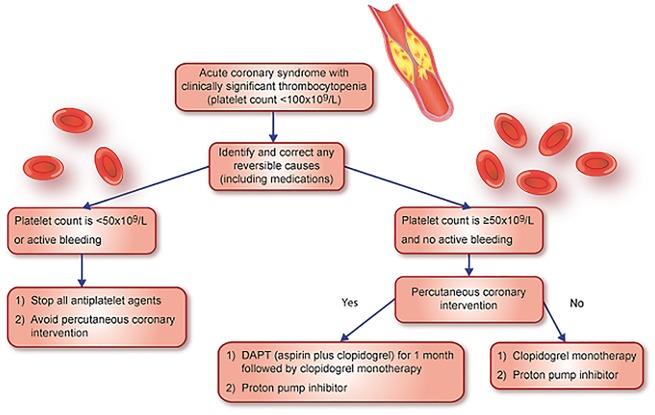
It is our opinion that clopidogrel monotherapy should be administered to ACS patients with thrombocytopenia who are not undergoing PCI if their platelet count is <100 × 109/L but ≥50 × 109/L and in the absence of bleeding (Figure 2). We suggest clopidogrel as opposed to aspirin monotherapy based on the results of the Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) randomized controlled trial. This trial demonstrated a statistically significant relative risk reduction in gastrointestinal bleeding with clopidogrel 75 mg as opposed to aspirin 325 mg (relative risk reduction of 31.8%, CI: 7.9–49.5, P = 0.012).13 Due to their association with higher bleeding rates3, we propose prasugrel and ticagrelor be avoided in this cohort, though their study as monotherapy, particularly of ticagrelor with its twice daily dosing and reversible binding, would be of great interest. In patients with a platelet count <50 × 109/L or in the setting of active bleeding, we advise stopping all antiplatelet therapy and avoidance of PCI. The addition of a proton pump inhibitor would likely reduce the rates of gastrointestinal bleeding and would be advised.14 In ACS patients with thrombocytopenia undergoing PCI, second generation drug-eluting stents are preferable to bare-metal stents. While traditional teaching would advise the utility of bare-metal stents in patients at higher risk of bleeding, two recent randomized trials—Prospective Randomized Comparison of the BioFreedom Biolimus A9 Drug-Coated Stent versus the Gazelle Bare-Metal Stent in Patients at High Bleeding Risk (LEADERS FREE) and Zotarolimus-eluting Endeavor Sprint Stent in Uncertain DES Candidates (ZEUS)—demonstrated superior outcomes with second generation drug-eluting stents compared with bare-metal stents in patients at high bleeding risk (including patients with thrombocytopenia) who may need a shorter duration of DAPT.15,16 Based on the limited data from these trials, we would recommend that stented patients with ACS who have a platelet count of <100 × 109/L but ≥50 × 109/L receive DAPT with aspirin and clopidogrel for 1 month followed by a single antiplatelet agent thereafter.
Figure 2.
Figure outlining approach to acute coronary syndrome patients with thrombocytopenia.
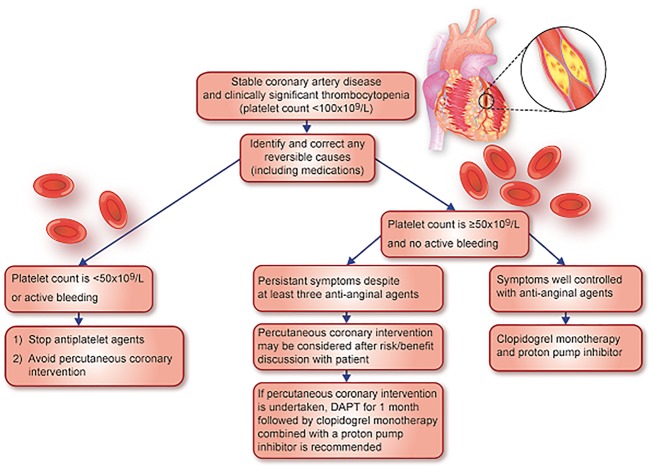
Finally in patients with stable coronary artery disease, in the absence of evidence, we suggest stopping antiplatelet therapy and avoiding PCI in patients with a platelet count <50 × 109/L. In thrombocytopenic patients with a platelet count ≥50 × 109/L and <100 × 109/L, we advise clopidogrel monotherapy and a proton pump inhibitor. If a patient’s symptoms persist despite three antianginal agents at maximally tolerated doses, then PCI is reasonable after a risk/benefit discussion with the patient (Figure 3). If PCI is undertaken, we recommend a second generation drug-eluting stent in combination with DAPT with aspirin and clopidogrel for 1 month followed by clopidogrel thereafter, along with a proton pump inhibitor.
Figure 3.
Figure outlining approach to stable coronary artery disease patients with thrombocytopenia.
Conflict of interest: Ph.G.S. discloses the following relationships—Research grants: Merck, Sanofi, and Servier; Speaking or consulting fees: Amarin, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, CSL-Behring, Daiichi-Sankyo, GlaxoSmithKline, Janssen, Lilly, Merck Novartis, Pfizer, Regeneron, Sanofi, Servier, and The Medicines Company. D.L.B. discloses the following relationships—Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee, including the ongoing RE-DUAL PCI), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor, Associate Editor), Population Health Research Institute (clinical trial steering committee, including the ongoing COMPASS), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. C.M.C has no conflict of interest to disclose.
References
- 1. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'Gara PT, Sabatine MS, Smith PK, Smith SC Jr. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines: An update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 2016;134:e123–e155. [DOI] [PubMed] [Google Scholar]
- 2. Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, Van't Hof A, Widimsky P, Zahger D, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Hasdai D, Astin F, Astrom-Olsson K, Budaj A, Clemmensen P, Collet J-P, Fox KA, Fuat A, Gustiene O, Hamm CW, Kala P, Lancellotti P, Maggioni AP, Merkely B, Neumann F-J, Piepoli MF, Van de Werf F, Verheugt F, Wallentin L.. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation, the task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 3. Bhatt DL, Hulot J-S, Moliterno DJ, Harrington RA.. Antiplatelet and anticoagulation therapy for acute coronary syndromes. Circ Res 2014;114:1929–1943. [DOI] [PubMed] [Google Scholar]
- 4. Udell JA, Bonaca MP, Collet JP, Lincoff AM, Kereiakes DJ, Costa F, Lee CW, Mauri L, Valgimigli M, Park SJ, Montalescot G, Sabatine MS, Braunwald E, Bhatt DL.. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: A collaborative meta-analysis of randomized trials. Eur Heart J 2016;37:390–399. [DOI] [PubMed] [Google Scholar]
- 5. McClure MW, Berkowitz SD, Sparapani R, Tuttle R, Kleiman NS, Berdan LG, Lincoff AM, Deckers J, Diaz R, Karsch KR, Gretler D, Kitt M, Simoons M, Topol EJ, Califf RM, Harrington RA.. Clinical significance of thrombocytopenia during a non-ST-elevation acute coronary syndrome. The platelet glycoprotein IIb/IIIa in unstable angina: Receptor suppression using integrilin therapy (PURSUIT) trial experience. Circulation 1999;99:2892–2900. [DOI] [PubMed] [Google Scholar]
- 6. Wang TY, Ou F-S, Roe MT, Harrington RA, Ohman EM, Gibler WB, Peterson ED.. Incidence and prognostic significance of thrombocytopenia developed during acute coronary syndrome in contemporary clinical practice. Circulation 2009;119:2454–2462. [DOI] [PubMed] [Google Scholar]
- 7. Yadav M, Généreux P, Giustino G, Madhavan MV, Brener SJ, Mintz G, Caixeta A, Xu K, Mehran R, Stone GW.. Effect of baseline thrombocytopenia on ischemic outcomes in patients with acute coronary syndromes who undergo percutaneous coronary intervention. Can J Cardiol 2016;32:226–233. [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM.. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 9. James S, Akerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, Skene A, Steg PG, Storey RF, Harrington R, Becker R, Wallentin L.. Comparison of ticagrelor, the first reversible oral P2Y (12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the platelet inhibition and patient outcomes (PLATO) trial. Am Heart J 2009;157:599–605. [DOI] [PubMed] [Google Scholar]
- 10. Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, Price MJ, Leonardi S, Gallup D, Bramucci E, Radke PW, Widimský P, Tousek F, Tauth J, Spriggs D, McLaurin BT, Angiolillo DJ, Généreux P, Liu T, Prats J, Todd M, Skerjanec S, White HD, Harrington RA.. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med 2013;368:1303–1313. [DOI] [PubMed] [Google Scholar]
- 11. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK.. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 12. Warkentin TE, Crowther MA.. Adverse prognostic significance of thrombocytopenia in acute coronary syndrome. Can anything be done about it? Circulation 2009;119:2420–2422. [DOI] [PubMed] [Google Scholar]
- 13. Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ.. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000;140:67–73. [DOI] [PubMed] [Google Scholar]
- 14. Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, Shook TL, Lapuerta P, Goldsmith MA, Laine L, Scirica BM, Murphy SA, Cannon CP.. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909–1917. [DOI] [PubMed] [Google Scholar]
- 15. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, Lipiecki J, Richardt G, Iñiguez A, Brunel P, Valdes-Chavarri M, Garot P, Talwar S, Berland J, Abdellaoui M, Eberli F, Oldroyd K, Zambahari R, Gregson J, Greene S, Stoll H-P, Morice M-C.. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015;373:2038–2047. [DOI] [PubMed] [Google Scholar]
- 16. Valgimigli M, Patialiakas A, Thury A, McFadden E, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, Menozzi A, de Cesare N, Garbo R, Meliga E, Testa L, Gabriel HM, Airoldi F, Ferlini M, Liistro F, Dellavalle A, Vranckx P, Briguori C.. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol 2015;65:805–815. [DOI] [PubMed] [Google Scholar]