Abstract
Background
Low birthweight (LBW) is a worldwide public health problem, demonstrating an increasing incidence in developed countries, including Japan. LBW is also a risk factor for later development of chronic kidney disease (CKD). To date, studies have not evaluated the population impacts of increasing LBW rates on renal function.
Methods
Estimated glomerular filtration rate (eGFR) was evaluated in 3737 Japanese adolescent males (15–16 years old) using annual cross-sectional data over an 18-year period (1998–2015).
Results
Between the initial (1998–2003) and final (2010–15) periods of the study, the mean birthweight decreased from 3213.4 ± 383.8 to 3116.2 ± 382.3 g and the LBW rate increased from 2.5 to 5.5% (both P ≤ 0.01). Additionally, the mean eGFR decreased from 105.1 ± 15.9 to 97.4 ± 13.8 mL/min/1.73 m2 and the prevalence of mildly reduced renal function (eGFR ≤ 60– <90 mL/min/1.73 m2) increased from 16.4 to 30.0% (both P ≤ 0.01), most evident in the LBW group (from 10.3 to 41.7%, P ≤0.01). The prevalence of proteinuria also increased significantly. Mildly reduced renal function was significantly associated with LBW [odds ratio (LBW 3000–3999 g) 1.51; 95% confidence interval 1.00–2.55; P = 0.047].
Conclusions
In this population of Japanese adolescents, the frequency of mildly reduced renal function increased as the LBW frequency increased. Our findings may have implications for the broader Japanese population as well as for other populations in which the prevalence of LBW is increasing.
Keywords: chronic kidney disease, epidemiology, low birth weight, renal function, trend
INTRODUCTION
Chronic kidney disease (CKD) is a strong risk factor for end-stage renal failure and cardiovascular disease [1, 2] and has become an important global public health problem due to its increasing prevalence and mortality [3–5]. Lifestyle-related diseases (e.g. obesity, diabetes and hypertension) are major contributors to the development of CKD, as is low birthweight (LBW) [6]. LBW is defined as a birthweight <2500 g (5.5 lb), irrespective of gestational age, and is associated with a lowered estimated glomerular filtration rate (eGFR), elevated proteinuria and an increased risk of later CKD development [7, 8]. Birthweight is linearly correlated with nephron number [9], and kidneys with reduced numbers of nephrons probably have reduced eGFR [10], as birthweight is a key determinant of eGFR in young individuals [11–13].
LBW is a global public health problem due to its association with both short- and long-term mortality [14], having a global prevalence of 15–20% and occurring in both developing and developed countries [6, 14, 15]. The proportion of LBW births has increased in most developed countries since 1990 [15]. Recently the rate of LBW births in Japan was the highest among the developed countries, showing an increase from ∼5.2% (1980) to 9.6% (2013); the mean rate of LBW births in developed countries was 6.6% (2013) [15]. LBW is associated with impaired renal function, therefore a concern regarding the global burden of LBW-related kidney disease exists [6, 16]. However, the actual impact of the serial increase in LBW rates on renal function is currently unknown.
We investigated birthweight and eGFR changes between 1998 and 2015 in healthy Japanese adolescents (15–16 years old). We also examined birthweight category trends to determine the contribution of birthweight changes on renal dysfunction rates.
MATERIALS AND METHODS
The study included Japanese male high school students attending an annual medical checkup performed by the Health Center, Keio University (Japan). The participants were enrolled in three private high schools in suburban Tokyo. Participants without birthweight data were excluded from this study, but we did not exclude participants known to have pre-existing CKD or hypertension. The study protocol was approved by the review committee of Keio University, the study was conducted in accordance with the Declaration of Helsinki and general informed consent was obtained.
During each medical checkup, each participant’s standing height, without shoes, was measured to the nearest centimetre. Body weights were measured to the nearest 100 g, with the participant wearing light clothing. Body mass indexes (BMIs) were calculated using the formula: weight (kg)/[height (m)]2. Blood pressures (BPs) were measured, after having the participant sit for at least 3 min, using an electronic sphygmomanometer. If the measured BP was >140/90 mmHg (pre-2011) or >140/85 mmHg (2011–15), the BP was remeasured. These cut-off values were based on the Japanese Society of Hypertension Guidelines for the Management of Hypertension. If more than one measurement was taken, the average BP was used. The first morning urine specimen was collected and protein levels were determined using a dipstick test. Serum levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), glucose and uric acid (UA) were determined, after overnight fasting, using standard methods. BP, HDL-C, TG and glucose levels were categorized according to the International Diabetes Federation consensus report [17]; TC and UA levels were categorized based on previous reports [18, 19]. Increased BMI was defined as a BMI ≥85th percentile; BMI cut-off values for Japanese adolescents [20] were used. Serum creatinine (SCr) levels were measured using the modified Jaffe assay until 2000; thereafter, SCr levels were measured using the enzymatic method. Jaffe assay values were converted to enzymatic method values using the following formula: SCr [enzymatic method (mg/dL)] = 0.977 × SCr [Jaffe assay (mg/dL)] − 0.199 [21]. eGFR was calculated per the following Cr-based equation for Japanese children and adolescents: eGFR = 110.2 × (reference SCr/patient SCr) + 2.93 [22]; reference SCr = −1.259 × [height (m)]5 + 7.815 × [height (m)]4 − 18.57 × [height (m)]3 + 21.39 × [height (m)]2 − 11.71 × height (m) + 2.628. Regardless of the presence or absence of proteinuria, mildly reduced renal function was defined as an eGFR ≤ 60– ≤ 90 mL/min/1.73 m2 [23].
Birthweight, height and gestational age, recorded at the time of delivery, were obtained from routine obstetric records. Gestational age was available for 2146 study participants.
Statistical analysis
Participant characteristics such as eGFR and serum parameters were summarized for three different time periods: 1998–2003, 2004–09 and 2010–15. Data were expressed as means ± SD or proportions; TG values were expressed as medians [interquartile range (IQR)]. The prevalence of LBW, high birthweight (HBW, >4000 g), preterm birth (<37 weeks), mildly reduced renal function and proteinuria were estimated for each time period. Continuous variable trends over each of the three time periods were assessed using trend tests with two contrasts (linear or plateau at the middle period) in a linear regression model; trends over the years were assessed using simple linear regression. Linear trends of proportions were assessed using the Cochran–Armitage test [24]. The odds ratio (OR) for each event was estimated using multivariate logistic regression analysis. To measure the impact of LBW, we calculated the number needed to be exposed (NNE) for one additional adolescent to demonstrate mildly reduced renal function and the population attributable risk (PAR, %) from the adjusted ORs obtained from the multivariate logistic regression analyses [25–27]. Associations between eGFR and potential confounders/mediators were examined using simple linear regression analysis or stepwise multiple linear regression analysis. Age; BP; levels of TG, glucose, HDL-C, UA and TC; BMI; birthweight; and year were taken into account in the multiple regression analysis. If an association was strong, such as between current body weight and BMI, we excluded one of them from the candidate confounders. Multicollinearity was assessed using the variance inflation factor (VIF), and all candidates had VIFs <1.5. We used a stepwise, bidirectional elimination algorithm, with inclusion and exclusion criteria of P < 0.20. Each statistical test was two-sided, with a significance level of 5%. All analyses were performed using JMP 12 (SAS Institute, Cary, NC, USA).
RESULTS
Characteristics
The study involved 3737 male Japanese high school students. Current body weight, birthweight, BP and serum metabolic parameter trends are shown in Table 1; demographics by birthweight category are shown in Supplementary Table S1. Current body weight and BMI trends were similar across the three time periods. However, for the participants surveyed in each time period, the mean birthweight decreased from 3213.4 ± 383.8 (1998–2003) to 3116.2 ± 382.3 g (2010–15). Although the proportions of preterm births were not different, the prevalence of LBW increased significantly from 2.5% (1998–2003) to 5.5% (2010–2015) (P ≤ 0.01 for the trend test) (Figure 1). In each survey period, only one participant was noted to have had a very LBW (VLBW < 1500 g). A significant decrease in mean eGFR was observed across the survey periods (P ≤ 0.01 for the trend test; Table 1): the mean eGFR was 105.1 ± 15.9 mL/min/1.73 m2 during the 1998–2003 period, 99.7 ± 14.1 during the 2004–09 period and 97.4 ± 13.8 during the 2010–15 period. The serum TG level was significantly higher during the 2010–15 period [56.0 (range 41.0–80.0) mg/dL] than during the 1998–2003 period [49.0 (range 36.0–66.0) mg/dL, P ≤ 0.01 for the trend test]. Although systolic BP values were similar, the diastolic and mean BPs increased significantly across the three time periods.
Table 1.
Demographic, anthropometric and laboratory characteristics of the study population
| 1998–2003 | 2004–09 | 2010–15 | ||||
|---|---|---|---|---|---|---|
| (n = 1164) | (n = 1261) | (n = 1312) | Trend test 1 | Trend test 2 | Trend test 3 | |
| P-value | P-value | P-value | ||||
| Height (cm) | 170.6 ± 5.6 | 170.9 ± 5.5 | 170.6 ± 5.7 | 0.99 | 0.52 | 0.99 |
| Weight (kg) | 60.3 ± 8.3 | 60.9 ± 8.7 | 60.5 ± 8.6 | 0.49 | 0.15 | 0.87 |
| BMI (kg/m2) | 20.7 ± 2.5 | 20.8 ± 2.6 | 20.8 ± 2.5 | 0.52 | 0.24 | 0.94 |
| SBP (mmHg) | 114.8 ± 12.6 | 116.9 ± 13.4 | 113.9 ± 12.9 | 0.07 | 0.20 | 0.07 |
| DBP (mmHg) | 61.5 ± 8.2 | 65.9 ± 9.1 | 63.1 ± 9.7 | <0.01 | <0.01 | <0.01 |
| Mean BP (mmHg) | 79.2 ± 8.2 | 82.8 ± 9.9 | 80.0 ± 9.8 | 0.047 | <0.01 | 0.04 |
| Cr (mg/dL) | 0.75 ± 0.10 | 0.79 ± 0.10 | 0.81 ± 0.10 | <0.01 | <0.01 | <0.01 |
| eGFR (mL/min) | 105.1 ± 15.9 | 99.7 ± 14.1 | 97.4 ± 13.8 | <0.01 | <0.01 | <0.01 |
| TC (mg/dL) | 169.7 ± 27.6 | 167.3 ± 27.1 | 167.2 ± 26.8 | 0.03 | 0.01 | 0.17 |
| TG (mg/dL)a | 49.0 (36.0–66.0) | 52.0 (39.0–75.0) | 56.0 (41.0–80.0) | <0.01 | <0.01 | <0.01 |
| High-density lipoprotein (mg/dL) | 63.3 ± 13.2 | 65.8 ± 13.4 | 62.6 ± 11.6 | 0.21 | 0.04 | <0.01 |
| UA (mg/dL) | 6.1 ± 1.2 | 5.9 ± 1.1 | 5.9 ± 1.1 | <0.01 | <0.01 | <0.01 |
| Glucose (mg/dL) | 88.7 ± 6.5 | 84.8 ± 9.4 | 86.0 ± 7.2 | <0.01 | <0.01 | <0.01 |
| Birth weight (g) | 3213.4 ± 383.8 | 3170.6 ± 382.7 | 3116.2 ± 382.3 | <0.01 | <0.01 | <0.01 |
| Gestational age (wk) | 39.1 ± 1.5 | 39.1 ± 1.6 | 39.0 ± 1.4 | 0.25 | 0.29 | 0.50 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate. Values are expressed as mean ± SD.
Trend test 1: a trend test with a linear contrast between three time periods; trend test 2: a trend test with a contrast showing a plateau at a middle category between three time periods; trend test 3: linear trend across years.
TG values are expressed as median (IQR).
FIGURE 1.
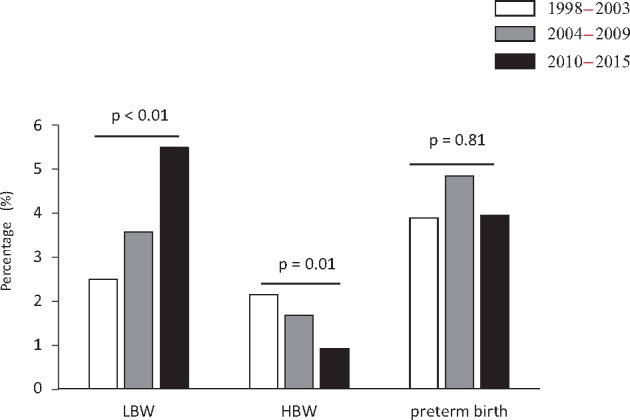
LBW, HBW and preterm birth prevalence rates during the 1998–2003, 2004–09 and 2010–15 survey periods. Categorical variables are presented as percentages. LBW, low birthweight (<2500 g); HBW, high birthweight (>4000 g); preterm birth, gestational age <37 weeks.
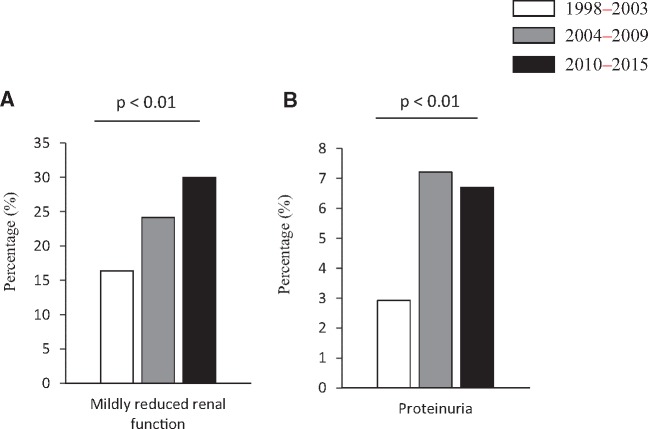
Only one participant had an eGFR <60 mL/min/1.73 m2, reflecting the overall health of the study population. The prevalence of mildly reduced renal function (eGFR ≤ 60– < 90 mL/min/1.73 m2) increased from 16.4% (1998–2003) to 30.0% (2010–15; P ≤ 0.01 for the trend test) (Figure 2A). A significant increase in the prevalence of proteinuria from 2.9% (1998–2003) to 6.7% (2010–15; P ≤ 0.01 for the trend test) was also observed (Figure 2B).
FIGURE 2.
Prevalence of (A) mildly reduced renal function (eGFR ≤ 60– < 90 mL/min/1.73 m2) and (B) proteinuria during the 1998–2003, 2004–09 and 2010–15 survey periods. Categorical variables are presented as percentages.
Mildly reduced renal function by birthweight
We examined the prevalence of mildly reduced renal function in each birthweight category to explore its contribution to mildly reduced renal function (Table 2). In the 1998–2003 period, the prevalence of mildly reduced renal function varied from 10.3 to 19.6% across the four birthweight categories. Within each birthweight category, except for HBW, the prevalence of mildly reduced renal function significantly increased across the three time periods and was most evident in the LBW group. The prevalence of mildly reduced renal function among adolescents with birthweights of 3000–3999 g increased in each subsequent time period (P for trend ≤ 0.01); the change from the earliest to latest time period was 12.2% [95% confidence interval (CI) 8.3–16.2]. Among adolescents with LBW, the prevalence increased from 10.3% in the 1998–2003 span to 41.7% in 2010–2015 span (P ≤ 0.01 for the trend), a 31.3% (95% CI 15.4–47.2) increase from the original frequency (Table 2). To measure the impact of LBW, we calculated the NNE and PAR. Among adolescents with LBW, the NNE needed to result in an additional case of mildly reduced renal function was low (12.2), confirming that LBW is an important risk factor for mildly reduced renal function in adolescents. Moreover, the PAR of LBW on mildly reduced renal function was 1.8%. These data were comparable with a previous report [26].
Table 2.
Prevalence of mildly reduced renal function based on birthweight category in 1998–2003, 2004–09 and 2010–15
| 1998–2003 | 2004–09 | 2010–15 | Total change (95% CI) | P-valuea | |
|---|---|---|---|---|---|
| Birthweight (g) | |||||
| <2500 | 10.3 (3/29) | 28.9 (13/45) | 41.7 (30/72) | 31.3 (15.4–47.2)b | <0.01 |
| 2500–2999 | 19.6 (58/296) | 32.9 (117/356) | 32.8 (136/415) | 13.2 (6.8–19.6)b | <0.01 |
| 3000–3999 | 15.6 (127/812) | 20.9 (175/839) | 27.9 (226/811) | 12.2 (8.3–16.2)b | <0.01 |
| ≥4000 | 11.1 (3/27) | 0 (0/21) | 14.3 (2/14) | 3.2 (−25.0 to 18.7) | 0.98 |
Cochran–Armitage trend test.
P < 0.05 for a comparison between 1998–2003 and 2010–15.
Risk for mildly reduced renal function and proteinuria
We tested the associations between mildly reduced renal function/proteinuria and various risk factors [7, 28] using multiple logistic regression analyses. Increased ORs for mildly reduced renal function were observed in the LBW participants [OR 1.51 (95% CI 1.00–2.55); P = 0.047], and for those with birthweights ranging from 2500 to 2999 g [OR 1.52 (95% CI 1.28–1.81); P ≤ 0.01] (Table 3, left panel). In addition to birthweight, increased BMIs and high UA levels were strongly associated with mildly reduced renal function (Table 3, left panel); high UA levels were also significantly related to proteinuria (Table 3, right panel). The continuous relationships between kidney function and birthweight, BP and metabolic parameters were also examined using stepwise multiple linear regression analyses (Supplementary Table S2). Each 1-kg decrease in birthweight was associated with a 3.45 mL/min/1.73 m2 decrease in eGFR (95% CI 2.16–4.74; P ≤ 0.01, model R2= 0.10), comparable to the association found in a previous study [26]. UA levels were negatively associated with eGFR [coefficient −2.13 (95% CI −2.57 to −1.70); P ≤ 0.01].
Table 3.
The OR for mildly reduced renal function and proteinuria
| Mildly reduced renal function |
Proteinuria |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Birthweight (g) | ||||||
| <2500 | 1.51 | 1.00–2.55 | 0.047 | 0.87 | 0.36–1.79 | 0.73 |
| 2500–2999 | 1.52 | 1.28–1.81 | <0.01 | 0.86 | 0.61–1.20 | 0.39 |
| 3000–3999 | 1.00 | 1.00 | ||||
| ≥4000 | 0.37 | 0.13–0.84 | 0.02 | 0.64 | 0.10–2.11 | 0.52 |
| Overweight | 1.60 | 1.32–1.95 | <0.01 | 0.82 | 0.54–1.19 | 0.30 |
| High BP | 0.80 | 0.62–1.03 | 0.09 | 1.02 | 0.64–1.56 | 0.94 |
| Hypertriglyceridemia | 0.84 | 0.51–1.33 | 0.47 | 1.42 | 0.63–2.86 | 0.37 |
| Low HDL-C | 0.65 | 0.25–1.44 | 0.30 | 0.87 | 0.14–3.05 | 0.85 |
| Hyperglycemia | 0.84 | 0.53–1.30 | 0.44 | 0.42 | 0.10–1.13 | 0.09 |
| Hypercholesterolemia | 1.08 | 0.82–1.40 | 0.59 | 1.01 | 0.60–1.61 | 0.97 |
| Hyperuricemia | 1.57 | 1.27–1.94 | <0.01 | 1.54 | 1.04–2.21 | 0.03 |
CI, confidence interval; HDL, high-density lipoprotein; overweight, BMI ≥85th percentile; high blood pressure, BP ≥ 130/85 mmHg; hypertriglyceridemia, TG ≥ 150 mg/dL; low HDL-C, HDL < 40 mg/dL; hyperglycemia, glucose≥110 mg/dL; hypercholesterolemia, TC ≥ 200 mg/dL; hyperuricemia, UA ≥ 7.0 mg/dL.
The OR and 95% CI were determined by logistic regression analysis. Adjusted for age, year, BMI, BP and metabolic parameters.
DISCUSSION
LBW is a global public health problem demonstrating an increasing prevalence in most developed countries [15, 29]. It is also a major contributor to non-communicable diseases and has been reported to be a risk factor for renal dysfunction, raising concern regarding the global burden of LBW-related kidney disease [6, 16]. However, the population impacts of progressive changes in birthweight over time on renal dysfunction are currently unknown. We found that the prevalence of mildly reduced renal function increased from 16.4% (1998–2003) to 30.0% (2010–15), with simultaneous decreases in mean birthweight in our study population. We also found that these outcome trends varied by birthweight category. The increased prevalence of mildly reduced renal function was particularly notable among the LBW participants. Multiple regression analyses demonstrated that LBW remained independently associated with mildly reduced renal function. Proteinuria also increased significantly over time, implying an increase in the renal dysfunction burden in the subject population. Thus this study demonstrates the impact of the serial increase in LBW on renal function.
In Japan, the mean birthweight has been decreasing since the 1980s [30] and the proportion of LBW births has been increasing, similar to the observations in many other developed countries [15, 29]. Several factors, such as shorter gestational duration, increased maternal age, parental socio-economic status and multiple fertilizations, have been reported to affect birthweight. Low pre-pregnancy BMI, due to dieting, and low maternal weight gain, due to strict weight management, also seem to be major contributors to the high incidence of LBW births in Japan [31]. In this study, the mean birthweight decreased from 3213.7 ± 383.8 g in the 1998–2003 survey (births in 1982–88) to 3116.2 ± 382.3 g in the 1998–2003 survey (births in 1994–2000). Thus a seemingly trivial (100 g) reduction in mean birthweight led to a near-doubling of the proportion of adolescents with mildly reduced renal function (from 16.4 to 30.0% between surveys). Therefore our study suggests that even minor population shifts in birthweight may have significant public health consequences. Overall, ∼6000 of the 1 200 000 Japanese adolescent males (15–16 years old) may be expected to have mildly reduced renal function due to LBW, based on the attributable population [26]. The impact of LBW in the Japanese population may be greater, as described in the study limitations below. The prevalence of mildly reduced renal function increased in the later surveys, particularly in adolescents with LBW. This is likely due to the tendency for a birthweight decrease in the LBW group to occur across the surveys and/or for LBW individuals to appear predisposed to renal dysfunction induced by a ‘year’ factor, as described below. Regarding the OR for mildly reduced dysfunction by birthweight category, we did not find the U-shaped trend shown in a previous study [8]. This discrepancy may be because the number of glomeruli increases and glomerular volume decreases until birthweight approaches 3000 g [9]; furthermore, HBW is associated with a genetic predisposition for future CKD [8].
LBW and the subsequent overnutrition synergistically augment the risk of CKD [6, 8]. In this study, LBW and increased BMI were significantly associated with renal dysfunction (Table 3). In Japan, the prevalence of obesity has increased, with ∼25% of the male population currently being overweight [30]. Therefore there is a concern that LBW and subsequent obesity will increase the prevalence of CKD, as is occurring in developing countries [32, 33]. Because the prevalence of CKD, in Japan, has increased significantly over the last three decades [34], we suggest that the incidence of CKD will increase further due to the increasing proportion of LBW babies and their increased risk of becoming obese.
The prevalence of mildly reduced renal function in the 2010–15 period was higher than in the 1998−2003 survey among those with birthweights in the 3000–3999 and 2500–2999 g ranges, suggesting that other changes besides birthweight affected these trends. Although multiple logistic regression analyses demonstrated that increased BMIs and UA levels were associated with an increased risk of renal dysfunction, we could not determine any factors associated with the increased prevalence of renal dysfunction. Risk factors for the decreasing eGFR in adolescents have not been fully elucidated [35]. Regardless, reductions in physical activity have been recorded in Japan [36] and diet composition has changed over time [37], potentially affecting the trends in mildly reduced renal function [38, 39]. Japan is a monoethnic society, and socio-economic differences occur to a lesser degree than in the USA or Europe [40]. However, socio-economic data, such as parental education and income, were not investigated, therefore we cannot exclude the potential effects of socio-economic status on the observed trends. These speculations need further investigation.
Several limitations impact the interpretation of this study. First, renal function was not measured directly by infusion of exogenous substances, such as inulin; instead, an equation for estimating GFR for Japanese adolescents with CKD [22] was used. Although this equation has not been validated in the general population [41], and may become less accurate at higher GFR levels, the equation was determined in individuals with GFR <150 mL/min/1.73 m2 [22]. Second, SCr conversions were performed to convert between Jaffe assay and enzymatic method values. The differences between these methods may have affected the SCr and eGFR trends. We converted Cr and used the single formula to compare eGFR, which was previously performed [34, 42], because switching from equations may affect the results [43, 44], and the Schwartz formulae are not suitable for Japanese adolescents due to muscle mass [45]. Indeed, we still observed a significant reduction in eGFR between 2004–09 and 2010–15, during which time SCr was measured using the enzymatic method. Third, the sensitivity of the serum sample measurement and urine dipstick methods may have changed throughout the surveys. Fourth, although we speculate that trends in our sample population are reflective of Japanese trends, the analysis of data from only three private high schools might limit this generalization. Specifically, the lower prevalence of VLBW observed in our study (0.08%) compared with a national survey (0.5–0.7%) and the lower eGFR associated with adolescents who were VLBW babies compared with those who were LBW babies [26] suggest that our results might represent an underestimation of the true effect of birthweight and the exposure impact of LBW on the Japanese population. Finally, this was a cross-sectional study, which limits conclusions on the causality of impaired renal function.
In conclusion, we found that the prevalence of mildly reduced renal function has increased in Japan concurrent with a decrease in the birthweights of healthy Japanese adolescent males. A low eGFR is a significant risk factor for a rapid decrease in eGFR [46, 47], and our findings suggest that adolescents with LBW might be more vulnerable to CKD later in life. Based on the increasing prevalence of LBW babies, we suspect the incidence of CKD will increase in the future.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Daiwa Securities Health Foundation. The Daiwa Securities Health Foundation had no role in the design of the study; the data collection, analysis and interpretation; or on the decision to approve publication of the finished manuscript. We would like to thank Midori Awazu, MD for helpful suggestions. We would like to thank Editage (www.editage.jp) for English language editing.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl 1): S1–S266 [PubMed] [Google Scholar]
- 2. Sarnak MJ. Cardiovascular complications in chronic kidney disease. Am J Kidney Dis 2003; 41(5 Suppl): 11–17 [DOI] [PubMed] [Google Scholar]
- 3. Lozano R, Naghavi M, Foreman K. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coresh J, Selvin E, Stevens LA. et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 5. Zhang L, Wang F, Wang L. et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379: 815–822 [DOI] [PubMed] [Google Scholar]
- 6. Luyckx VA, Brenner BM.. Birth weight, malnutrition and kidney-associated outcomes—a global concern. Nat Rev Nephrol 2015; 11: 135–149 [DOI] [PubMed] [Google Scholar]
- 7. White SL, Perkovic V, Cass A. et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 2009; 54: 248–261 [DOI] [PubMed] [Google Scholar]
- 8. Li S, Chen SC, Shlipak M. et al. Low birth weight is associated with chronic kidney disease only in men. Kidney Int 2008; 73: 637–642 [DOI] [PubMed] [Google Scholar]
- 9. Hughson M, Farris AB 3rd, Douglas-Denton R. et al. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 2003; 63: 2113–2122 [DOI] [PubMed] [Google Scholar]
- 10. Luyckx VA, Bertram JF, Brenner BM. et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013; 382: 273–283 [DOI] [PubMed] [Google Scholar]
- 11. Bakker H, Gaillard R, Franco OH. et al. Fetal and infant growth patterns and kidney function at school age. J Am Soc Nephrol 2014; 25: 2607–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keijzer-Veen MG, Kleinveld HA, Lequin MH. et al. Renal function and size at young adult age after intrauterine growth restriction and very premature birth. Am J Kidney Dis 2007; 50: 542–551 [DOI] [PubMed] [Google Scholar]
- 13. Hallan S, Euser AM, Irgens LM, et al. Effect of intrauterine growth restriction on kidney function at young adult age: the Nord Trondelag Health (HUNT 2) Study. Am J Kidney Dis 2008; 51: 10–20 [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization. Global Nutrition Targets2025: Low Birth Weight Policy Brief http://www.who.int/nutrition/publications/globaltargets2025_policybrief_lbw/en/ (29 November 2016, date last accessed)
- 15. Organization for Economic Coperation and Development. OECD Family Database https://www.oecd.org/els/family/CO_1_3_Low_birth_weight.pdf (29 November 2016, date last accessed)
- 16. Abitbol CL, Moxey-Mims M.. Chronic kidney disease: Low birth weight and the global burden of kidney disease. Nat Rev Nephrol 2016; 12: 199–200 [DOI] [PubMed] [Google Scholar]
- 17. Zimmet P, Alberti KG, Kaufman F. et al. The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatr Diabetes 2007; 8: 299–306 [DOI] [PubMed] [Google Scholar]
- 18. Hickman TB, Briefel RR, Carroll MD. et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4-19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med 1998; 27: 879–890 [DOI] [PubMed] [Google Scholar]
- 19. Oyama C, Takahashi T, Oyamada M. et al. Serum uric acid as an obesity-related indicator in early adolescence. Tohoku J Exp Med 2006; 209: 257–262 [DOI] [PubMed] [Google Scholar]
- 20. Inokuchi M, Hasegawa T, Anzo M. et al. Standardized centile curves of body mass index for Japanese children and adolescents based on the 1978–1981 national survey data. Ann Hum Biol 2006; 33: 444–453 [DOI] [PubMed] [Google Scholar]
- 21. Horio M, Orita Y.. Comparison of Jaffe rate assay and enzymatic method for the measurement of creatinine clearance. Nihon Jinzo Gakkai Shi 1996; 38: 296–299 [PubMed] [Google Scholar]
- 22. Uemura O, Nagai T, Ishikura K. et al. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol 2014; 18: 626–633 [DOI] [PubMed] [Google Scholar]
- 23. Kawamoto R, Kohara K, Tabara Y. et al. An association between body mass index and estimated glomerular filtration rate. Hypertens Res 2008; 31: 1559–1564 [DOI] [PubMed] [Google Scholar]
- 24. Asamoah-Odei E, Garcia Calleja JM, Boerma JT.. HIV prevalence and trends in sub-Saharan Africa: no decline and large subregional differences. Lancet 2004; 364: 35–40 [DOI] [PubMed] [Google Scholar]
- 25. Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 1974; 99: 325–332 [DOI] [PubMed] [Google Scholar]
- 26. Khalsa DD, Beydoun HA, Carmody JB.. Prevalence of chronic kidney disease risk factors among low birth weight adolescents. Pediatr Nephrol 2016; 31: 1509–1516 [DOI] [PubMed] [Google Scholar]
- 27. Bender R, Blettner M.. Calculating the “number needed to be exposed” with adjustment for confounding variables in epidemiological studies. J Clin Epidemiol 2002; 55: 525–530 [DOI] [PubMed] [Google Scholar]
- 28. Tomaszewski M, Charchar FJ, Maric C. et al. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int 2007; 71: 816–821 [DOI] [PubMed] [Google Scholar]
- 29. Oken E. Secular trends in birthweight. Nestle Nutr Inst Workshop Ser 2013; 71: 103–114 [DOI] [PubMed] [Google Scholar]
- 30. Ministry of Health, Labour and Welfare. Vital Statistics in Japan http://www.mhlw.go.jp/english/database/db-hw/vs01.html
- 31. Tsukamoto H, Fukuoka H, Koyasu M. et al. Risk factors for small for gestational age. Pediatr Int 2007; 49: 985–990 [DOI] [PubMed] [Google Scholar]
- 32. Gluckman PD, Seng CY, Fukuoka H. et al. Low birthweight and subsequent obesity in Japan. Lancet 2007; 369: 1081–1082 [DOI] [PubMed] [Google Scholar]
- 33. Gluckman PD, Hanson MA.. Living with the past: evolution, development, and patterns of disease. Science 2004; 305: 1733–1736 [DOI] [PubMed] [Google Scholar]
- 34. Nagata M, Ninomiya T, Doi Y. et al. Trends in the prevalence of chronic kidney disease and its risk factors in a general Japanese population: the Hisayama Study. Nephrol Dial Transplant 2010; 25: 2557–2564 [DOI] [PubMed] [Google Scholar]
- 35. Wong CJ, Moxey-Mims M, Jerry-Fluker J. et al. CKiD (CKD in children) prospective cohort study: a review of current findings. Am J Kidney Dis 2012; 60: 1002–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inoue S, Ohya Y, Tudor-Locke C. et al. Time trends for step-determined physical activity among Japanese adults. Med Sci Sport Exer 2011; 43: 1913–1919 [DOI] [PubMed] [Google Scholar]
- 37. National Institute of Health and Nutrition. The National Health and Nutrition Survey 1975–2002, 2005. http://www0.nih.go.jp/eiken/chosa/kokumin_eiyou/doc_theme/tbl_1100.xls (29 November 2016, date last accessed)
- 38. Hallan S, de Mutsert R, Carlsen S. et al. Obesity, smoking, and physical inactivity as risk factors for CKD: are men more vulnerable? Am J Kidney Dis 2006; 47: 396–405 [DOI] [PubMed] [Google Scholar]
- 39. Skov AR, Toubro S, Bulow J. et al. Changes in renal function during weight loss induced by high vs low-protein low-fat diets in overweight subjects. Int J Obes Relat Metab Disord 1999; 23: 1170–1177 [DOI] [PubMed] [Google Scholar]
- 40. Kagamimori S, Gaina A, Nasermoaddeli A.. Socioeconomic status and health in the Japanese population. Soc Sci Med 2009; 68: 2152–2160 [DOI] [PubMed] [Google Scholar]
- 41. Uemura O, Nagai T, Ishikura K. et al. Reference glomerular filtration rate levels in Japanese children: using the creatinine and cystatin C based estimated glomerular filtration rate. Clin Exp Nephrol 2015; 19: 683–687 [DOI] [PubMed] [Google Scholar]
- 42. Iseki K, Kohagura K, Sakima A. et al. Changes in the demographics and prevalence of chronic kidney disease in Okinawa, Japan (1993 to 2003). Hypertens Res 2007; 30: 55–62 [DOI] [PubMed] [Google Scholar]
- 43. Fadrowski JJ, Neu AM, Schwartz GJ. et al. Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol 2011; 6: 1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pottel H, Hoste L, Dubourg L. et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 2016; 31: 798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uemura O, Honda M, Matsuyama T. et al. Is the new Schwartz equation derived from serum creatinine and body length suitable for evaluation of renal function in Japanese children? Eur J Pediatr 2012; 171: 1401–1404 [DOI] [PubMed] [Google Scholar]
- 46. Imai E, Horio M, Yamagata K. et al. Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res 2008; 31: 433–441 [DOI] [PubMed] [Google Scholar]
- 47. Fox CS, Larson MG, Leip EP. et al. Predictors of new-onset kidney disease in a community-based population. JAMA 2004; 291: 844–850 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.