Abstract
Disruption of steroid hormone signaling has been implicated independently in the developmental abnormalities resulting from maternal phthalate plasticizer exposure and developmental zinc deficiency. This study investigated if secondary zinc deficiency may result from dietary exposure to a low level of di-2-ethylhexyl phthalate (DEHP) through gestation and if this could be associated with altered steroid metabolism. The interaction between marginal zinc nutrition and DEHP exposure to affect pregnancy outcome, zinc status, and steroid metabolism was also assessed. For this purpose, rats were fed a diet containing an adequate (25 mg/kg) or marginal (10 mg/kg) level of zinc without or with DEHP (300 mg/kg) from gestation day (GD) 0 until GD 19. Steroid profiles were measured in dam liver, plasma, adrenal glands, and in fetal liver by UPLC/MS-MS. In dams fed the adequate zinc diet, DEHP exposure decreased maternal weight gain and led to hepatic acute-phase response and zinc accumulation. The latter could compromise zinc availability to the fetus. DEHP and marginal zinc deficiency caused several adverse effects on the maternal and fetal steroid profiles. Interactions between DEHP exposure and marginal zinc deficient nutrition affected 17OH pregnenolone and corticosterone, while pregnenolone levels were specifically affected by DEHP exposure. Maternal marginal zinc deficiency specifically affected maternal progesterone and aldosterone, and presented evidence of increased androgen aromatization activity in maternal and fetal tissues. Results stress the potential major impact of mild DEHP exposure on maternal/fetal steroid metabolism that can be potentiated by nutritional and chronic disease states leading to zinc deficiency.
Keywords: zinc deficiency, DEHP, di-2-ethylhexyl phthalate (DEHP), steroid, inflammation, pregnancy.
Interaction between maternal phthalate plasticizer exposure and dietary zinc intake during pregnancy may contribute to reproductive toxicity and disruptions of fetal development. Recent studies found an increased risk of pregnancy complications, especially preterm birth, associated with maternal exposure to the phthalate plasticizer di-2-ethylhexyl phthalate (DEHP) (Cobellis et al., 2003; Ferguson et al., 2014a, b; Huang et al., 2014; Kim et al., 2011; Latini et al., 2003; Reddy et al., 2006). Whereas low maternal zinc intake was associated with similar pregnancy complications (Chaffee and King, 2012; Scholl et al., 1993), zinc supplementation reduced the risk of preterm birth in several randomized controlled trials (Ota et al., 2015).
Previous work demonstrated that secondary zinc deficiency may occur during pregnancy as a consequence of different conditions including infections, diabetes, alcohol consumption, and exposure to certain toxicants including DEHP (Bui et al., 1998; Keen et al., 1993). Coyle et al. found that the pro-inflammatory cytokine interleukin-6 (IL-6) interacts with glucocorticoid (GC) signaling to stimulate a hepatic acute-phase response involving increased expression of the zinc binding protein metallothionein (MT) (Coyle et al., 1993). In response to IL-6 and GC, the transcription factors STAT3 and the GC receptor (GR) bind to MT promoters leading to synergistic activation of MT expression (Kasutani et al., 1998). Increased MT expression in maternal liver was proposed as a mechanism leading to decreased zinc availability for the developing embryo, and this model was supported by the observation that MT knockout mice are protected from the hypozincemia and developmental defects resulting from maternal ethanol exposure (Carey et al., 2000). Similarly, hypozincemia resulting from turpentine exposure was absent in IL-6 knockout mice lending further support to the mechanism of secondary zinc deficiency resulting from an acute-phase response to toxicant exposure (Liuzzi et al., 2005). Peters et al. demonstrated that acute gestational exposure by intubation with 1000 mg DEHP/kg body weight resulted in secondary zinc deficiency associated with decreased maternal weight gain and increased incidence of neural tube defects (Peters et al., 1997). A subsequent study by Lee et al. demonstrated that lower levels of acute exposure (as low as 50 mg/kg) cause changes in the expression of MT consistent with secondary zinc deficiency (Lee et al., 2004). However, the lowest observed effect level for reproductive development in rats is approximately 23 mg DEHP/kg body weight/day resulting from the chronic consumption of a diet containing 300 mg DEHP/kg (Blystone et al., 2010).
Disruption of steroid hormone signaling during development has been implicated independently in the developmental abnormalities resulting from maternal phthalate plasticizer exposure (Chauvigne et al., 2009) and developmental zinc deficiency (Hamdi et al., 1997). We recently showed that although developmental marginal zinc deficiency does not affect placenta steroid concentrations (Huang et al., 2016), it causes a major increase in 11β-hydroxysteroid dehydrogenase type 2 expression. The latter could have a significant impact on fetal steroid homeostasis. In previous studies both phthalate exposure and zinc deficiency disrupted testosterone and estrogen signaling and disproportionately affected development in male rats compared with females (Andrade et al., 2006; Blystone et al., 2010; Om and Chung, 1996; Swenerton and Hurley, 1968). Increased GC signaling has also been implicated independently in the developmental defects resulting from phthalate exposure (Cooper et al., 2008; Xiao-feng et al., 2009) and zinc deficiency (DePasquale-Jardieu and Fraker, 1979; Takeda et al., 2007). Whereas altered glucocorticoid metabolism during early development can have a major impact on the offspring’s risk for disease in later life (Duthie and Reynolds, 2013), altered metabolism of mineralocorticoids leading to hypertension could directly affect maternal and fetal outcome (Escher and Mohaupt, 2007).
This study tested the hypothesis that secondary zinc deficiency results from dietary exposure to a low level of DEHP through gestation and is associated with altered steroid metabolism. The influence of marginally low zinc nutrition on DEHP-mediated changes in zinc status and steroid metabolism was also assessed. A 2 × 2 factorial design was used to compare the effects of maternal DEHP exposure with the effects of marginal zinc deficiency and investigate the potential interactions between maternal DEHP exposure and marginal zinc deficiency. Pregnant rats were fed diets containing an adequate (25 mg/kg) or marginal (10 mg/kg) level of zinc with or without DEHP at (300 mg/kg), the lowest observed adverse effect level for reproductive development (Blystone et al., 2010). The mechanism of secondary zinc deficiency resulting from maternal DEHP exposure was investigated in the maternal liver by measuring hepatic levels of zinc, IL-6, STAT3, and MT. To investigate mechanisms of endocrine disruption, steroid hormone profiles were measured in maternal liver, plasma, adrenal gland, and fetal liver.
MATERIALS AND METHODS
Animals and Animal Care
All procedures were in agreement with standards for the care of laboratory animals as outlined in the NIH Guide for the Care and Use of Laboratory Animals. All procedures were administered under the auspices of the Animal Resource Services of the University of California at Davis, which is accredited by the American Association for the Accreditation of Laboratory Animal Care. Experimental protocols were approved before implementation by the University of California at Davis Animal Use and Care Administrative Advisory Committee and were administered through the Office of the Campus Veterinarian.
Adult Sprague–Dawley female and male rats were purchased from Charles River (Wilmington, MA, USA). Female rats (200–225 g) were housed individually in stainless steel cages in a temperature (22–23 °C) and photoperiod (12 h light/dark) controlled room. Distilled water was provided through a daily flushed automatic watering system. An egg-white protein-based diet with adequate zinc (25 μg zinc/g) was the control diet (Keen et al., 1989). Animals were fed control diet for 5 days before breeding. Males and females were caged together overnight and the following morning, embryonic day (E)0, after the presence of a sperm plug confirmed successful breeding; female rats (6–9 animals/group) were randomly divided into four groups and fed ad libitum the control or one of three experimental diets. The control diet (C) contained 25 μg zinc/g diet. The marginally zinc deficient diet (MZ) contained 10 μg zinc/g diet. The remaining two diets were C and MZ with 300 mg/kg DEHP (C + D and MZ + D, respectively). Food intake was recorded daily, and body weight was measured at 5-day intervals. On GD19, the dams were anesthetized with isoflurane (2 mg/kg body wt), and laparotomies were performed. The gravid uterus was removed, and fetuses were weighed. Tissues (fetal and dam liver, dam adrenals) were weighed, frozen in liquid nitrogen, and stored at −80° C. Concentrations of zinc in the diets, maternal plasma and livers were measured by ICP-AES or ICP-MS as described by Clegg et al. (2005).
Immunoblots
Extraction of total cellular protein from tissue was done as previously described (Aimo et al., 2010). Protein concentration was measured by the Bradford assay (Ernst and Zor, 2010) and aliquots were alkylated with iodoacetamide to enable efficient transfer of small hydrophilic proteins such as MT as previously described (Xie et al., 2004). Aliquots containing 35–50 μg of protein were separated by reducing 10 or 13% (w/v) polyacrylamide gel electrophoresis and electroblotted to PVDF membranes from BioRad (Hercules, CA). Colored molecular weight standards from BioRad were run simultaneously. Membranes were blocked for 1 h in 5% (w/v) nonfat milk in tris-buffered saline containing 0.2% Tween 20 (TBST) and then incubated overnight in 1% w/v BSA in TBST containing the corresponding dilution (1:500 for MT; 1:1000 for STAT3, phosphor-STAT3 and α-tubulin; 1:5000 for hnRNP) of the primary antibodies. Primary antibodies for MT (sc-11377), hnRNP (sc-32301), STAT3 (sc-8019), and α-tubulin (sc-23948) were from Santa Cruz Biotech (Santa Cruz, CA). The antibody for phosphotyrosine-705 STAT3 (9145) was purchased from Cell Signaling Technologies (Danvers, MA). After incubation with a 1:10,000 dilution of horseradish peroxidase conjugated corresponding secondary antibody in 5% w/v BSA in TBST for 90 min at room temperature, the conjugates were visualized with chemifluorescent detection in a Storm 840 Phosphoimager from Amersham Pharmacia Biotech (Piscataway, NJ). Image Quant software from Molecular Dynamics (Sunnyvale, CA) was used to quantify band intensity.
Enzyme-Linked Immunosorbent Assay
IL-6 was measured in maternal liver according to the manufacturer’s procedures using the Rat Interleukin-6 ELISA Kit from Sigma–Aldrich Co (St. Louis, MO). Briefly, tissue was homogenized and protein concentration was measured. Aliquots containing 20 µg of protein in 100 µl of sample buffer were incubated overnight at 4 °C in a microplate coated with primary antibody against rat IL-6. The plate was developed with a biotin conjugated secondary antibody and avidin conjugated horseradish peroxidase with TMB substrate. Absorbance was measured at 450 nm and a standard curve run in parallel on the same microplate was used to calculate final IL-6 concentrations.
Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry (UPLC/MS-MS) Sample Preparation
Fetal and dam liver, dam adrenals and plasma were extracted using previous methods with minor modifications (Gaikwad, 2013; Huang et al., 2016; Jiang et al., 2014). Briefly, liver and adrenal samples were homogenized and extracted twice with 2:1 methanol to sample weight. The remaining pellets were extracted twice with 1 ml chloroform. In the case of plasma, samples were extracted twice with 1:1 chloroform to sample volume. The remaining pellet was lyophilized to dryness. Then the pellet was extracted twice with 1:1 methanol to sample volume. For both the plasma and tissue samples, the methanol and chloroform extracts were combined and dried. Then samples where re-suspended in 125 μl methanol, filtered through a 0.2 μm ultracentrifuge filter from Millipore Inc. (Billerica, MA), and injected into the UPLC/MS-MS.
Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry (UPLC/MS-MS) Detection of Steroid Hormones
Reference standards for pregnenolone, 17-OH-pregnenolone, progesterone, corticosterone, aldosterone, estradiol, and testosterone were purchased from Steraloids (Newport, RI). The steroid hormone detection method used was previously described (Gaikwad, 2013). Briefly, after sample extraction, UPLC analyses of extracts were carried out with a Waters Acquity UPLC system connected with the high performance Xevo-TQ mass spectrometer. The elutions from the UPLC column were introduced to the Xevo-TQ mass spectrometer. Analytical separations of the samples were conducted on the UPLC system using an Acquity Cortecs UPLC C18 1.6 um 2.1 × 50 mm analytical column at 50 °C and at a flow rate of 0.15 mL/min. The gradient started with 100% A (0.1% formic acid in H2O) and 0% B (0.1% formic acid in CH3CN), after 1 min changed to 80% A, then changed to 45% A over 4 min, followed by 20% A in 2 min. Finally, after 5.5 min, it was changed to the original 100% A over 1 min, resulting in a total separation time of 15 min. The elutions from the UPLC column were introduced to the mass spectrometer. All MS experiments were performed by using electrospray ionization (ESI) in positive ion (PI) and negative ion (NI) mode, with an ESI-MS capillary voltage of 3.0 kV, an extractor cone voltage of 2 V, and a detector voltage of 650 V. Following MS conditions were used: desolvation gas at 600 l/h, cone gas flow at 60 l/h, desolvation temperature at 350°C and source temperature 150 °C. Pure standards were used to optimize the UPLC/MS-MS conditions prior to analysis and make calibration curves. Elutions from the UPLC column were analyzed in the MRM mode, and resulting data were processed by TargetLynx 4.1 software from Waters (Milford, MA).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6 software (San Diego, CA). For steroid measurements, all zero values were deleted and then Grubbs’ test was run once to remove outliers. Tests for interaction were performed by 2-way analysis of variance (ANOVA) and post-tested using Fisher’s Least Significant Difference (LSD). Data were subsequently analyzed by one-way analysis of variance (ANOVA), and Fisher least significance difference test used to examine differences between group means. Differences were considered statistically significant at P < 0.05. All results are presented as mean ± SEM.
RESULTS
Maternal DEHP Exposure Combined with Marginal Zinc Deficiency Decreased Maternal Weight Gain During Pregnancy
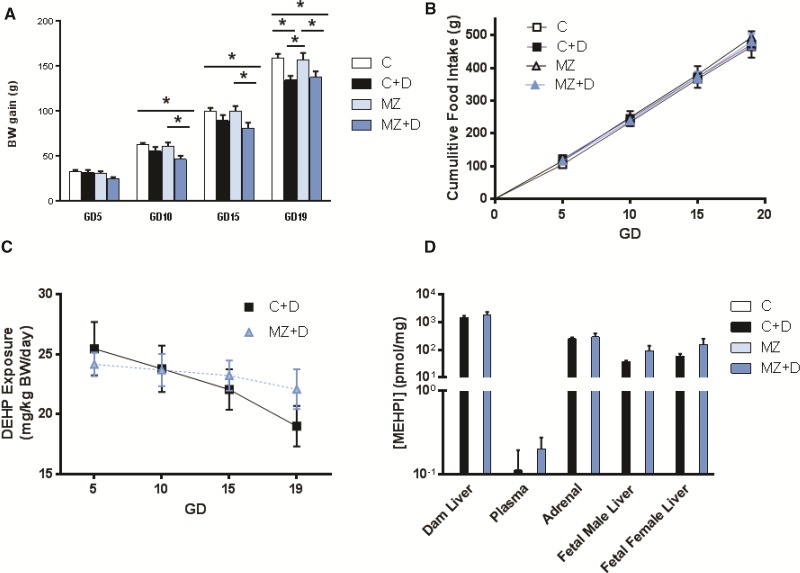
At GD10 and GD15, MZ + D dams had gained significantly lower body weight (∼20%, P < 0.05) compared with C and MZ (Figure 1A). At GD19, both MZ + D and C + D dams showed significantly lower maternal weight gain (∼14%, P < 0.05) compared with C and MZ dams. Decreased maternal weight gain was not related to food intake (Figure 1B). There were no effects related to the treatments on the number of pups per litter, fetal brain weight, fetal body weight, or fetal brain to body mass ratio (Table 1). The lower gravid uterine weight (P < 0.05) observed in C + D dams normalized when corrected for the number of pups per litter (Table 1).
FIG. 1.
Combined dietary DEHP exposure and MZ decreased maternal weight gain during pregnancy. Rat dams were fed control (25 µg zinc/g) (C) or marginal zinc (10 µg zinc/g) (MZ) diets without or with 300 mg/kg DEHP (C + D and MZ + D), from GD0 until GD19. (A) Body weight (BW) and (B) cumulative food intake were measured from GD0 until GD19 and used to estimate (C) DEHP exposure. D) The concentration of MEHP in dam liver, adrenals and plasma and fetal liver at GD19 was measured by UPLC-MS/MS. Results are shown as means ± S.E.M. *Significantly different (p < 0.05, n = 6-9).
TABLE 1.
Pregnancy Outcome
| Parameters | C |
C+D |
MZ |
MZ+D |
||||
|---|---|---|---|---|---|---|---|---|
| Sample size (n) | 9 |
6 |
7 |
8 |
||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Body wt gain (g) | 159 | 4 | 135** | 4 | 157 | 8 | 138** | 6 |
| Food intake (g) | 465 | 17 | 471 | 40 | 494 | 17 | 480 | 23 |
| Litter fetal wt (mg) | 2312 | 34 | 2294 | 55 | 2387 | 59 | 2301 | 63 |
| Litter placenta wt (mg) | 471 | 20 | 447.6 | 8.1 | 457.3 | 11.7 | 486.4 | 13.2 |
| Gravid uterine wt (g) | 69 | 2.5 | 57* | 3.7 | 69 | 3.0 | 65 | 2.4 |
| # of fetus/litter | 15.8 | 0.7 | 13.7 | 0.9 | 14.9 | 0.8 | 14.4 | 1 |
| Gravid uterine wt/# of fetus (g) | 4.3 | 0.1 | 4.4 | 0.1 | 4.7 | 0.1 | 4.7 | 0.4 |
| Fetal brain wt (mg) | 93.4 | 2.5 | 88.7 | 1.4 | 92.9 | 0.9 | 91.7 | 2.2 |
| Fetal brain wt/body wt | 0.04 | 0.001 | 0.039 | 0.001 | 0.04 | 0.001 | 0.04 | 0.001 |
| Plasma Zn (uM) | 14.17 | 0.79 | 14.59 | 1.02 | 6.22** | 0.47 | 6.32** | 0.51 |
Rat dams were fed control (25 µg zinc/g) (C) or marginal zinc (10 µg zinc/g) (MZ) diets without or with 300 mg/kg DEHP (C + D and MZ + D), from GD0 until GD19. Table shows data collected at GD19. Results are shown as means ± S.E.M analyzed by 2-way ANOVA with Fisher’s LSD (*p < 0.05 **p < 0.01 relative to control). Wt: weight, #: number.
Maternal Administration of DEHP Resulted in the Presence of Mono-2-Ethylhexyl Phthalate in Both Maternal and Fetal Tissues
DEHP is readily hydrolyzed to the major metabolite mono-2-ethylhexyl phthalate (MEHP), which served as our biomarker for DEHP distribution (Blount et al., 2000). Consumption of 300 mg DEHP/kg diet during pregnancy resulted not only in maternal but also fetal exposure to MEHP. Both C + D and MZ + D dams consumed approximately 20–25 mg DEHP/kg body weight; with a trend towards decreased exposure as maternal weight gain increased through gestation (Figure 1C). Gestational DEHP exposure resulted in an average MEHP concentration of 163± 55 nM in maternal plasma at GD19. The highest tissue MEHP levels were found in maternal liver with an average of 1630 ± 310 pmol/mg. Average MEHP levels in maternal adrenal gland, fetal male liver, and fetal female liver were 270 ± 60, 70 ± 30, and 110 ± 60 pmol/mg, respectively. No significant differences were observed in plasma or tissue MEHP concentrations between dams fed C + D and MZ + D diets (Figure 1D). As expected, MEHP was not detected in plasma or tissues from rats fed the C and MZ diets (Figure 1D).
IL-6 and MT Expression Were Associated With Accumulation of Zinc in Liver of Dams Exposed to DEHP
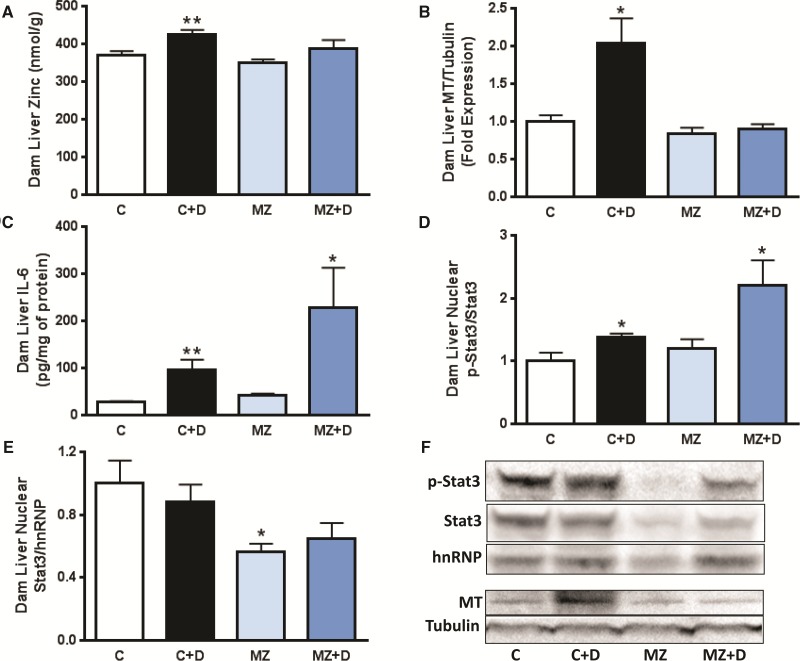
Both dam plasma and liver zinc concentrations followed similar trends, with C + D showing higher, and both MZ and MZ + D showing lower zinc concentrations compared with C dams. Marginal zinc deficiency (MZ and MZ + D) caused significantly lower (approximately 56%) maternal plasma zinc concentrations compared with C and C + D (P < 0.01) (Table 1). DEHP exposure caused a significant increase (P < 0.01) in hepatic zinc content when compared with C, whereas values were similar for C, MZ, and MZ + D (Figure 2A). A significant interaction between DEHP exposure and marginal zinc deficiency on hepatic MT expression was observed (interaction from 2-way ANOVA P < 0.05, Figure 2B). Maternal liver MT content was 2-fold higher in C + D (P < 0.01) compared with C, whereas no significant differences were observed among C, MZ, and MZ + D groups.
FIG. 2.
IL-6 and MT expression were associated with accumulation of zinc in the liver of dams exposed to DEHP. Rat dams were treated as described in legend to Fig. 1. A) Rat dam liver zinc content was measured at GD19 by ICP-AES and ICP-MS. (B) MT and (C) IL-6 levels in dam liver were measured at GD19 by immunoblot and ELISA, respectively. Western blots for B) MT and α-tubulin (loading control) in total liver homogenates, D,E) phospho (tyrosine-705)-STAT3, STAT3 and hnRNP A1 (loading control) in liver nuclear fractions, and (F) Western blot representative images. For Western blots, after quantification of the bands, results were expressed as the ratio MT/α-tubulin, phospho (p)-STAT3/STAT3 or STAT3/hnRNP and normalized to control (C) values. Results are shown as means ± S.E.M. *, ** Significantly different (*p < 0.05, **p < 0.01, n = 6-9).
To investigate if DEHP exposure-associated alterations in maternal zinc homeostasis were related to a hepatic acute-phase response, we measured IL-6 levels in maternal liver. DEHP exposure caused a significant increase in maternal liver IL-6 levels when comparing both C + D versus C (4-fold increase, P < 0.01) and MZ + D versus MZ (5-fold increase, P < 0.05) (Figure 2C). Zinc deficiency per se did not affect dam liver IL-6 concentrations (Figure 2C).
Total and Tyr705-phosphorylated STAT3 were measured to further investigate the mechanism underlying DEHP-mediated stimulation of MT expression in the maternal liver. DEHP exposure caused higher levels of STAT3 phosphorylation (activation) in nuclear fractions from maternal liver (P < 0.01, Figure 2D,F). Both C + D and MZ + D groups showed higher nuclear STAT3 phosphorylation levels compared with C and MZ (39 and 86%, respectively, P < 0.05). In contrast to DEHP exposure, marginal zinc deficiency led to a lower nuclear content of total STAT3, being differences significant between C and MZ (P < 0.05) (Figure 2E,F), whereas a trend for lower values (P = 0.075) was observed for MZ + D compared with C + D.
Both DEHP and Marginal Zinc Deficiency Disrupt Progestogen Levels in Maternal Adrenals, Plasma, and Liver
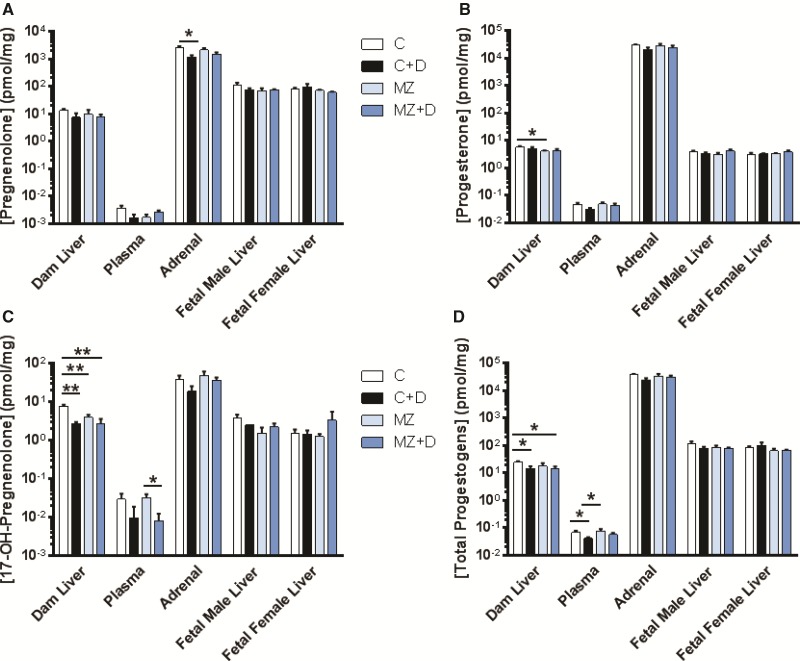
Diet-induced disruption of steroid hormone profiles varied across tissue type and was specific for the different steroids measured. Using UPLC/MS-MS analysis, we observed that only pregnenolone levels were affected in dam adrenals. In this regard, pregnenolone content was significantly lower in MZ (P < 0.05, Figure 3A) compared with C; with a trend (P = 0.06) towards lower pregnenolone levels in MZ +D versus C dams. Marginal zinc deficiency resulted in significantly lower progesterone levels in MZ dam liver (P < 0.05, Figure 3B) compared with C, with no other alterations observed for progesterone in other tissues or plasma.
FIG. 3.
DEHP exposure and marginal zinc deficiency interacted to disrupt maternal progestogen levels. Rat dams were treated as described in legend to Fig. 1. A) Pregnenolone, B) progesterone, C) 17-OH-pregnenolone, and D) total progestogen concentrations were measured in rat dam liver, adrenals and plasma; and fetal liver at GD19 by UPLC/MS-MS. Results are shown as means ± S.E.M. *, ** Significantly different (*p < 0.05, **p < 0.01, n = 6-9).
There was an interaction (P < 0.05) between DEHP exposure and marginal zinc deficiency causing lower maternal liver 17-hydroxypregnenolone (17HPG) levels (Figure 3C). 17HPG dam liver content in the C + D, MZ, and MZ + D groups were significantly lower (P < 0.01) than in C. 17HPG plasma concentration in the MZ + D dams was significantly lower (P < 0.05) than in MZ, with a trend for lower values (P = 0.057) compared with C (Figure 3C). Collectively, DEHP exposure significantly lowered dam liver total progestogen levels (C vs C + D and C vs MZ + D, P < 0.05, Figure 3D). In addition, plasma progestogen levels were significantly lower (P < 0.05) in C + D compared with C, and C + D compared with MZ. No significant effects on progestogen content were detected in dam adrenal or fetal liver.
DEHP Exposure and Marginal Zinc Deficiency Effects on Corticosteroids
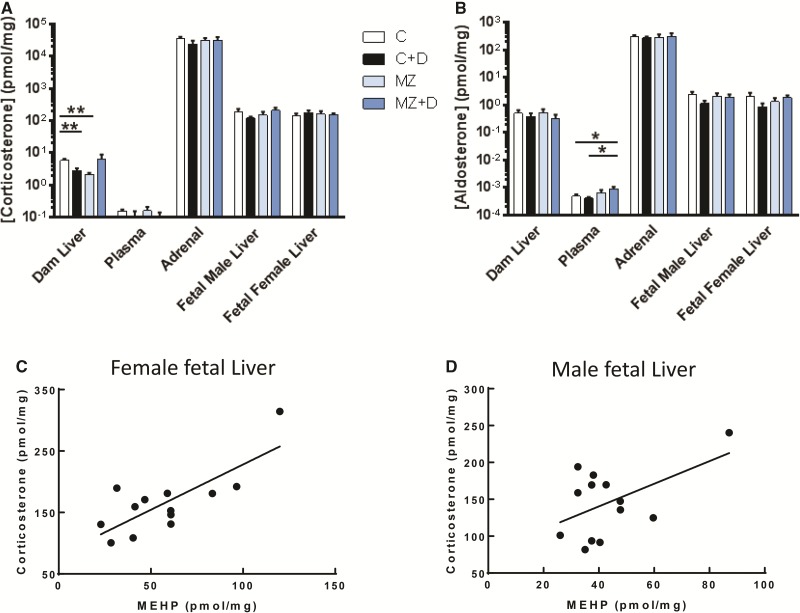
We observed that both maternal exposure to DEHP and/or in combination with marginal zinc deficiency during pregnancy altered dam corticosteroids concentrations. In the maternal liver, there was a significant interaction (P < 0.05) between DEHP exposure and marginal zinc deficiency to decrease corticosterone content. Both C + D and MZ dam livers had significantly lower corticosterone concentrations compared with C dams (P < 0.01, Figure 4A). No other direct maternal or fetal effects of DEHP exposure or marginal zinc deficiency on corticosterone levels were detected. However, we did observe positive correlations between corticosterone and MEHP concentrations in female fetal liver (r = 0.60, P < 0.01, Figure 4C), male fetal liver (r = 0.86, P < 0.01, Figure 4D), and in male and female liver combined (r = 0.79, P < 0.01). A significant correlation between maternal liver corticosterone and MEHP concentrations (R2 = 0.48, P = 0.01) was only observed when the value of one animal with very high MEHP concentration in all tissues was included.
FIG. 4.
DEHP exposure and marginal zinc deficiency interacted to disrupt maternal corticosteriod levels. Rat dams were treated as described in legend to Fig. 1. (A) Corticosterone and (B) aldosterone concentrations were measured in rat dam liver, adrenals and plasma; and fetal liver at GD19 by UPLC/MS-MS. Results are shown as means ± S.E.M. *, ** Significantly different (*p < 0.05, **p < 0.01, n = 6-9). C, D) Corticosterone and MEHP levels in female and male fetal livers were positively correlated (r = 0.60, p < 0.01 and r = 0.86, p < 0.01, respectively, from Pearson’s r, n = 6-9).
In the plasma MZ + D dams had significantly lower plasma aldosterone concentrations compared with C and C + D (P < 0.05, Figure 4B). All other maternal and fetal tissues aldosterone levels were unaffected by diet.
Marginal Zinc Deficiency and DEHP Exposure Disrupted Both Estrogen and Androgen Levels in Maternal and Fetal Liver
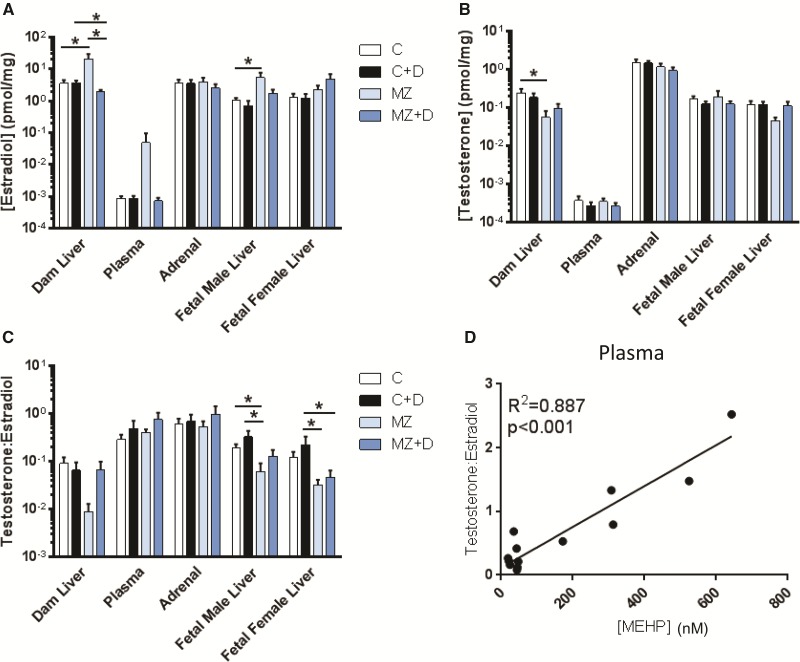
There were no significant interactions between treatments for testosterone, estradiol, nor testosterone/estradiol levels. We observed that the marginal zinc deficient diet disrupted estrogen levels in both maternal and male fetal liver. MZ dam livers had an approximate 4-fold higher estradiol concentration compared with the C group (P < 0.05, Figure 5A), but no significant differences were observed between C versus C + D, or C versus MZ + D. Conversely, MZ + D dam liver estradiol concentration was significantly lower compared with both C + D and MZ (P < 0.05). In the fetus, we observed a 3.5-fold, higher estradiol level in MZ male liver compared with C (P < 0.05). There were no significant effects related to diet in estradiol concentrations of maternal plasma, adrenal gland, or fetal female liver.
FIG. 5.
Marginal zinc deficiency increased maternal and fetal estradiol levels. Rat dams were treated as described in legend to Fig. 1. (A) Estradiol and (B) testosterone concentrations were measured in rat dam liver, adrenals and plasma; and fetal liver at GD19 by UPLC/MS-MS. C) Ratio of testosterone to estradiol concentration. Results are shown as means ± S.E.M. *Significantly different (*p < 0.05, n = 6-9). D) The testosterone/estradiol ratio was positively correlated with MEHP levels in maternal plasma. (r = 0.89, p < 0.001 from Pearson’s r, n = 6-9).
Tissue testosterone concentrations were only effected by the marginal zinc deficient diet. MZ dam liver testosterone levels were significantly lower compared with C (P < 0.05, Figure 5B), with no differences among C, C + D, or MZ + D. Testosterone levels in plasma, adrenal gland, or fetal liver were not affected by DEHP exposure or marginal zinc deficiency. However, there was a trend (P = 0.06) for low fetal female liver testosterone levels in MZ compared with C.
The effect of the different treatments on estrogen aromatization was evaluated through the ratio of testosterone to estradiol. The testosterone/estradiol ratio in MZ fetal male liver was significantly lower compared with C and C + D, and in the MZ and MZ + D fetal female liver compared with C + D (P < 0.05, Figure 5C). A trend (P = 0.09) for low testosterone/estradiol ratio was observed in MZD dam liver compared with C. Interestingly, there was a significant correlation between the testosterone/estradiol ratio and MEHP concentration in maternal plasma (r2 = 0.89, P < 0.001, Figure 5D).
DISCUSSION
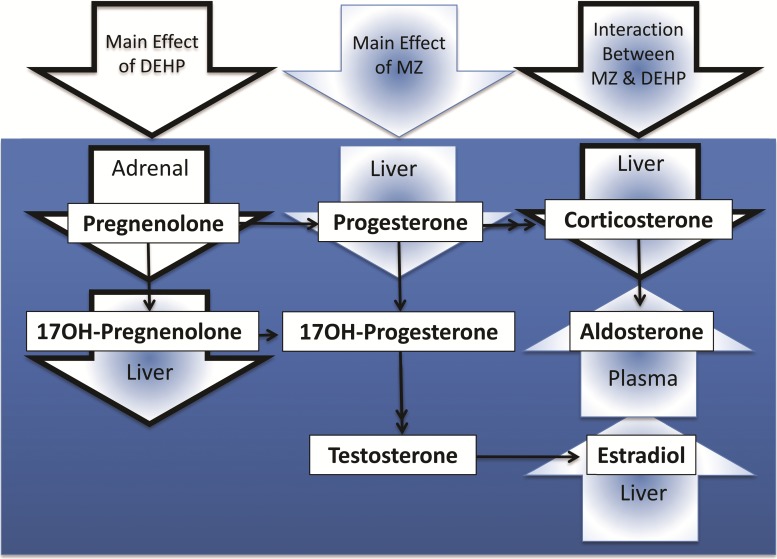
This study showed that DEHP exposure and dietary marginal zinc deficiency imposed throughout gestation in rats interacted to disrupt maternal weight gain and endocrine signaling. We present evidence supporting the concept that secondary zinc deficiency resulting from DEHP exposure may contribute to the observed disruptions in maternal endocrine signaling (summarized in Figure 6) and pregnancy complications. In this regard, DEHP exposure led to increased MT expression and the consequent accumulation of zinc in maternal liver which could cause decreased zinc availability to the mother and the conceptus.
FIG. 6.
Disruption of steroid levels in maternal tissues resulting from DEHP exposure and marginal zinc deficiency individually or combined during rat pregnancy. Arrows with black borders show the steroids mainly affected by DEHP exposure, arrows with blue border and blue fill show the steroids mainly affected by marginal zinc nutrition, and arrows with black border and blue fill show the steroids/pathway in which DEHP exposure/marginal zinc nutrition interacted to promote changes.
We observed a significant interaction between DEHP exposure and marginal zinc deficiency leading to a decrease in maternal weight gain during mid-gestation for the MZ + D group. Whereas the mechanism underlying this interaction remains unknown, secondary zinc deficiency may at least in part account for this effect. Both maternal DEHP exposure and low maternal weight gain during pregnancy are associated with increased risk of preterm birth (Carmichael and Abrams, 1997; Ferguson et al., 2014b). Importantly, randomized controlled trials of zinc supplementation during pregnancy have consistently found reduced risk of preterm birth (Ota et al., 2015). However, our study was completed before birth precluding evaluation of the length of gestation.
DEHP exposure stimulated IL-6/STAT3 signaling, increased MT expression, and led to the accumulation of zinc in maternal liver. These results demonstrate a redistribution of maternal zinc occurring at the lowest DEHP observed effect level to disrupt reproductive development in rats. However, it is important to note that recent estimates of DEHP exposure in humans have been considerably lower than the levels that produced reproductive toxicity in rats. For example, the highest estimates of human DEHP exposures were around 1–2 mg DEHP/kg/day (Koch et al., 2006). To some extent this discrepancy may be accounted for by allometric scaling to compensate for the increased rate of clearance in smaller animals (Sharma and McNeill, 2009). Differences in the rate of DEHP metabolism may also be related to the binding affinities and reaction rates of human and rat esterase and cytochrome P450 oxidase enzymes (Choi et al., 2013). Physiologically based pharmacokinetic modeling should enable a more accurate comparison of exposure levels between rat and human studies. Importantly, the exposure level used in this study may be relevant to human reproductive health because plasma concentrations of MEHP measured in this study are an order of magnitude lower than the levels of MEHP detected in fetal cord plasma associated with decreased gestational age at birth (Latini et al., 2003).
DEHP exposure and consumption of a marginal zinc diet interacted to affect maternal progestogen and corticosterone levels. The largest effects on steroid levels related to maternal diet were lower progestogen and corticosterone levels in maternal liver. MZ, C + D, and MZ + D treatments all led to an equivalent decrease in maternal liver 17HPG levels. In contrast to previous studies that reported increased corticosterone in response to severe zinc deficiency and to higher doses of DEHP exposure, we observed that both MZ and C + D treatments decreased corticosterone levels in maternal liver. Nevertheless, MEHP concentrations were positively correlated with corticosterone levels in maternal and fetal liver. It remains unclear if this association reflects an effect of DEHP exposure on maternal corticosterone or another relationship between DEHP exposure and corticosterone metabolism. However, it is important to consider the fact that chronic stress can result in decreased corticosterone levels (Rosmond and Bjorntorp, 2000). Collectively, these results suggest that disruption of maternal progestogen and glucocorticoid signaling may be involved in a mechanism leading to decreased maternal growth resulting from the interaction between DEHP exposure and consumption of a marginal zinc deficient diet.
Increased aldosterone signaling resulting from nutritional marginal zinc deficiency during pregnancy may contribute to gestational hypertension. By stimulating renal sodium reabsorption, aldosterone is a key regulator of blood pressure. Although we did not anticipate effects on aldosterone signaling, an increase in maternal plasma aldosterone resulted from marginal zinc deficiency. Increased plasma volume resulting from high aldosterone levels in zinc deficiency could provide a mechanism to account for the association between plasma zinc and gestational hypertension (Cherry et al., 1981). Indeed, a randomized controlled trial of zinc supplementation in a low income Mexican population found a decrease in the incidence of gestational hypertension (Hunt et al., 1985). However, another trial conducted in the United Kingdom found no effect of zinc supplementation on gestational hypertension (Mahomed et al., 1989). Preeclampsia is also associated with hypozincemia (Kim et al., 2012). However, plasma aldosterone levels are decreased in patients with preeclampsia (Siddiqui et al., 2013). Clinical trials of zinc supplementation during pregnancy found no effect on incidence of preeclampsia suggesting dietary zinc deficiency is unlikely to play a causal role (Jonsson et al., 1996; Mahomed et al., 1989). Whereas preeclampsia is associated with low aldosterone levels, high aldosterone during the third trimester is associated with gestational hypertension (Elsheikh et al., 2001). Therefore, future work should further investigate the relationship between dietary zinc intake, renin-angiotensin-aldosterone signaling, and gestational hypertension.
Whereas marginal zinc deficiency increased maternal and male fetal liver estradiol levels it caused significantly low testosterone concentration and a trend for low testosterone: estradiol ratio in dam liver. A trend for low fetal female liver testosterone levels was also observed. Estradiol levels were high in fetal male liver, and the testosterone:estradiol ratio was significantly lower in fetal male and female liver as a consequence of marginal zinc deficiency. These results suggest a higher androgen aromatization activity as a consequence of zinc deficiency. This is in agreement with previous observations of low testosterone and high estradiol concentrations in the liver of male adult rats fed a severe zinc deficient diet for 3 months (Om and Chung, 1996). Interestingly, MZ had an opposite effect on maternal liver estradiol levels in dams exposed to DEHP, and the testosterone to estradiol ratio was correlated with MEHP in maternal plasma. Inhibition of aromatase by hepatic zinc may provide a mechanism to account for both increased estradiol resulting from marginal zinc deficiency and the correlation between MEHP concentration and the testosterone to estradiol levels ratio in maternal plasma. Importantly, the observed decrease in MZ dam liver testosterone can have long term effects on the male offspring, which is in line with the significant alterations caused by zinc deficiency on male reproduction and fertility. Fetal exposure to a mild in utero androgen deficiency environment can cause male offspring reproductive alterations including cancer, cryptorchidism, hypospadias, and abnormal spermatogenesis (Skakkebaek et al., 2001, 2016). Whereas decreased fetal testicular testosterone production is associated with postnatal reproductive tract malformations, current efforts are directed to establish predictive values linking both alterations (Gray et al., 2016).
In conclusion, this study found a significant interaction between maternal DEHP exposure and dietary marginal zinc deficiency leading to decreased maternal weight gain and altered maternal and fetal tissues steroid hormone profiles. These results open the door for future research into the mechanisms of endocrine disruption and reproductive toxicity resulting from maternal DEHP exposure and marginal zinc deficiency. The observed interactions are particularly relevant given how frequently both DEHP exposure and primary/secondary marginal zinc deficiency occur in human populations.
ACKNOWLEDGMENTS
This project was funded by a West Coast Metabolomics Center pilot project grant from the National Institute of Health (NIH #DK097154) (P.I.O.) and NIFA-USDA (CA-D*-XXX-7244-H) (P.I.O.). J.R. Nuttall was supported by a National Institute of Environmental Health Sciences-funded training program in Environmental Health Sciences (T32 ES007058-33).
REFERENCES
- Aimo L., Mackenzie G. G., Keenan A. H., Oteiza P. I. (2010). Gestational zinc deficiency affects the regulation of transcription factors AP-1, NF-kappaB and NFAT in fetal brain. J. Nutr. Biochem. 21, 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade A. J., Grande S. W., Talsness C. E., Grote K., Chahoud I. (2006). A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology 227, 185–192. [DOI] [PubMed] [Google Scholar]
- Blount B. C., Silva M. J., Caudill S. P., Needham L. L., Pirkle J. L., Sampson E. J., Lucier G. W., Jackson R. J., Brock J. W. (2000). Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. 108, 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blystone C. R., Kissling G. E., Bishop J. B., Chapin R. E., Wolfe G. W., Foster P. M. (2010). Determination of the di-(2-ethylhexyl) phthalate NOAEL for reproductive development in the rat: importance of the retention of extra animals to adulthood. Toxicol. Sci. 116, 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui L. M., Taubeneck M. W., Commisso J. F., Uriu-Hare J. Y., Faber W. D., Keen C. L. (1998). Altered zinc metabolism contributes to the developmental toxicity of 2-ethylhexanoic acid, 2-ethylhexanol and valproic acid. Toxicology 126, 9–21. [DOI] [PubMed] [Google Scholar]
- Carey L. C., Coyle P., Philcox J. C., Rofe A. M. (2000). Maternal ethanol exposure is associated with decreased plasma zinc and increased fetal abnormalities in normal but not metallothionein-null mice. Alcohol. Clin. Exp. Res. 24, 213–219. [PubMed] [Google Scholar]
- Carmichael S. L., Abrams B. (1997). A critical review of the relationship between gestational weight gain and preterm delivery. Obstet. Gynecol. 89, 865–873. [DOI] [PubMed] [Google Scholar]
- Chaffee B. W., King J. C. (2012). Effect of zinc supplementation on pregnancy and infant outcomes: a systematic review. Paediatr. Perinat. Epidemiol. 26, 118–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvigne F., Menuet A., Lesne L., Chagnon M. C., Chevrier C., Regnier J. F., Angerer J., Jegou B. (2009). Time- and dose-related effects of di-(2-ethylhexyl) phthalate and its main metabolites on the function of the rat fetal testis in vitro. Environ. Health Perspect. 117, 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry F. F., Bennett E. A., Bazzano G. S., Johnson L. K., Fosmire G. J., Batson H. K. (1981). Plasma zinc in hypertension/toxemia and other reproductive variables in adolescent pregnancy. Am. J. Clin. Nutr. 34, 2367–2375. [DOI] [PubMed] [Google Scholar]
- Choi K., Joo H., Campbell J. L. Jr., Andersen M. E., Clewell H. J. 3rd (2013). In vitro intestinal and hepatic metabolism of Di(2-ethylhexyl) phthalate (DEHP) in human and rat. Toxicol. In Vitro 27, 1451–1457. [DOI] [PubMed] [Google Scholar]
- Clegg M. S., Hanna L. A., Niles B. J., Momma T. Y., Keen C. L. (2005). Zinc deficiency-induced cell death. IUBMB Life 57, 661–669. [DOI] [PubMed] [Google Scholar]
- Cobellis L., Latini G., De Felice C., Razzi S., Paris I., Ruggieri F., Mazzeo P., Petraglia F. (2003). High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum. Reprod. 18, 1512–1515. [DOI] [PubMed] [Google Scholar]
- Cooper B. W., Cho T. M., Thompson P. M., Wallace A. D. (2008). Phthalate induction of CYP3A4 is dependent on glucocorticoid regulation of PXR expression. Toxicol. Sci. 103, 268–277. [DOI] [PubMed] [Google Scholar]
- Coyle P., Philcox J. C., Rofe A. M. (1993). Corticosterone enhances the zinc and interleukin-6-mediated induction of metallothionein in cultured rat hepatocytes. J. Nutr. 123, 1464–1470. [DOI] [PubMed] [Google Scholar]
- DePasquale-Jardieu P., Fraker P. J. (1979). The role of corticosterone in the loss in immune function in the zinc-deficient A/J mouse. J. Nutr. 109, 1847–1855. [DOI] [PubMed] [Google Scholar]
- Duthie L., Reynolds R. M. (2013). Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology 98, 106–115. [DOI] [PubMed] [Google Scholar]
- Elsheikh A., Creatsas G., Mastorakos G., Milingos S., Loutradis D., Michalas S. (2001). The renin-aldosterone system during normal and hypertensive pregnancy. Arch. Gynecol. Obstet. 264, 182–185. [DOI] [PubMed] [Google Scholar]
- Ernst O., Zor T. (2010). Linearization of the bradford protein assay. J. Visual. Exp. JoVE doi: 10.3791/1918(38). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher G., Mohaupt M. (2007). Role of aldosterone availability in preeclampsia. Mol. Aspects Med. 28, 245–254. [DOI] [PubMed] [Google Scholar]
- Ferguson K. K., McElrath T. F., Ko Y. A., Mukherjee B., Meeker J. D. (2014a). Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ. Int. 70, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K. K., McElrath T. F., Meeker J. D. (2014b). Environmental phthalate exposure and preterm birth. JAMA Pediatrics 168, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaikwad N. W. (2013). Ultra performance liquid chromatography-tandem mass spectrometry method for profiling of steroid metabolome in human tissue. Anal. Chem. 85, 4951–4960. [DOI] [PubMed] [Google Scholar]
- Gray L. E. Jr., Furr J., Tatum-Gibbs K. R., Lambright C., Sampson H., Hannas B. R., Wilson V. S., Hotchkiss A., Foster P. M. (2016). Establishing the “Biological Relevance” of dipentyl phthalate reductions in fetal rat testosterone production and plasma and testis testosterone levels. Toxicol. Sci. 149, 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi S. A., Nassif O. I., Ardawi M. S. (1997). Effect of marginal or severe dietary zinc deficiency on testicular development and functions of the rat. Arch. Androl. 38, 243–253. [DOI] [PubMed] [Google Scholar]
- Huang Y., Li J., Garcia J. M., Lin H., Wang Y., Yan P., Wang L., Tan Y., Luo J., Qiu Z., et al. (2014). Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PloS One 9, e87430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. L., Supasai S., Kucera H., Gaikwad N. W., Adamo A. M., Mathieu P., Oteiza P. I. (2016). Nutritional marginal zinc deficiency disrupts placental 11beta-hydroxysteroid dehydrogenase type 2 modulation. Food Funct. 7, 84–92. [DOI] [PubMed] [Google Scholar]
- Hunt I. F., Murphy N. J., Cleaver A. E., Faraji B., Swendseid M. E., Browdy B. L., Coulson A. H., Clark V. A., Settlage R. H., Smith J. C. Jr. (1985). Zinc supplementation during pregnancy in low-income teenagers of Mexican descent: effects on selected blood constituents and on progress and outcome of pregnancy. Am. J. Clin. Nutr. 42, 815–828. [DOI] [PubMed] [Google Scholar]
- Jiang M., He J., Kucera H., Gaikwad N. W., Zhang B., Xu M., O'Doherty R. M., Selcer K. W., Xie W. (2014). Hepatic overexpression of steroid sulfatase ameliorates mouse models of obesity and type 2 diabetes through sex-specific mechanisms. J. Biol. Chem. 289, 8086–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson B., Hauge B., Larsen M. F., Hald F. (1996). Zinc supplementation during pregnancy: a double blind randomised controlled trial. Acta Obstet. Gynecol. Scand. 75, 725–729. [DOI] [PubMed] [Google Scholar]
- Kasutani K., Itoh N., Kanekiyo M., Muto N., Tanaka K. (1998). Requirement for cooperative interaction of interleukin-6 responsive element type 2 and glucocorticoid responsive element in the synergistic activation of mouse metallothionein-I gene by interleukin-6 and glucocorticoid. Toxicol. Appl. Pharmacol. 151, 143–151. [DOI] [PubMed] [Google Scholar]
- Keen C. L., Peters J. M., Hurley L. S. (1989). The effect of valproic acid on 65Zn distribution in the pregnant rat. J. Nutr. 119, 607–611. [DOI] [PubMed] [Google Scholar]
- Keen C. L., Taubeneck M. W., Daston G. P., Rogers J. M., Gershwin M. E. (1993). Primary and secondary zinc deficiency as factors underlying abnormal CNS development. Ann. N.Y. Acad. Sci. 678, 37–47. [DOI] [PubMed] [Google Scholar]
- Kim J., Kim Y. J., Lee R., Moon J. H., Jo I. (2012). Serum levels of zinc, calcium, and iron are associated with the risk of preeclampsia in pregnant women. Nutr. Res. 32, 764–769. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Chun S., Jang J. Y., Chae H. D., Kim C. H., Kang B. M. (2011). Increased plasma levels of phthalate esters in women with advanced-stage endometriosis: a prospective case-control study. Fertil. Steril. 95, 357–359. [DOI] [PubMed] [Google Scholar]
- Koch H. M., Preuss R., Angerer J. (2006). Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure– an update and latest results. Int. J. Androl. 29, 155–165. [DOI] [PubMed] [Google Scholar]
- Latini G., De Felice C., Presta G., Del Vecchio A., Paris I., Ruggieri F., Mazzeo P. (2003). In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ. Health Perspect. 111, 1783–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Park J., Jang B., Knudsen T. B. (2004). Altered expression of genes related to zinc homeostasis in early mouse embryos exposed to di-2-ethylhexyl phthalate. Toxicol. Lett. 152, 1–10. [DOI] [PubMed] [Google Scholar]
- Liuzzi J. P., Lichten L. A., Rivera S., Blanchard R. K., Aydemir T. B., Knutson M. D., Ganz T., Cousins R. J. (2005). Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. PNAS 102, 6843–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahomed K., James D. K., Golding J., McCabe R. (1989). Zinc supplementation during pregnancy: a double blind randomised controlled trial. BMJ 299, 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Om A. S., Chung K. W. (1996). Dietary zinc deficiency alters 5 alpha-reduction and aromatization of testosterone and androgen and estrogen receptors in rat liver. J. Nutr. 126, 842–848. [DOI] [PubMed] [Google Scholar]
- Ota E., Mori R., Middleton P., Tobe-Gai R., Mahomed K., Miyazaki C., Bhutta Z. A. (2015). Zinc supplementation for improving pregnancy and infant outcome. Cochrane Datab. Syst. Rev. 2, CD000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. M., Taubeneck M. W., Keen C. L., Gonzalez F. J. (1997). Di(2-ethylhexyl) phthalate induces a functional zinc deficiency during pregnancy and teratogenesis that is independent of peroxisome proliferator-activated receptor-alpha. Teratology 56, 311–316. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Rozati R., Reddy B. V., Raman N. V. (2006). Association of phthalate esters with endometriosis in Indian women. BJOG 113, 515–520. [DOI] [PubMed] [Google Scholar]
- Rosmond R., Bjorntorp P. (2000). [Low cortisol production in chronic stress. The connection stress-somatic disease is a challenge for future research]. Lakartidningen 97, 4120–4124. [PubMed] [Google Scholar]
- Scholl T. O., Hediger M. L., Schall J. I., Fischer R. L., Khoo C. S. (1993). Low zinc intake during pregnancy: its association with preterm and very preterm delivery. Am. J. Epidemiol. 137, 1115–1124. [DOI] [PubMed] [Google Scholar]
- Sharma V., McNeill J. H. (2009). To scale or not to scale: the principles of dose extrapolation. Br. J. Pharmacol. 157, 907–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A. H., Irani R. A., Zhang W., Wang W., Blackwell S. C., Kellems R. E., Xia Y. (2013). Angiotensin receptor agonistic autoantibody-mediated soluble fms-like tyrosine kinase-1 induction contributes to impaired adrenal vasculature and decreased aldosterone production in preeclampsia. Hypertension 61, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek N. E., Rajpert-De Meyts E., Buck Louis G. M., Toppari J., Andersson A. M., Eisenberg M. L., Jensen T. K., Jorgensen N., Swan S. H., Sapra K. J., et al. (2016). Male Reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol. Rev. 96, 55–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek N. E., Rajpert-De Meyts E., Main K. M. (2001). Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 16, 972–978. [DOI] [PubMed] [Google Scholar]
- Swenerton H., Hurley L. S. (1968). Severe zinc deficiency in male and female rats. J. Nutr. 95, 8–18. [DOI] [PubMed] [Google Scholar]
- Takeda A., Tamano H., Kan F., Itoh H., Oku N. (2007). Anxiety-like behavior of young rats after 2-week zinc deprivation. Behav. Brain Res. 177, 1–6. [DOI] [PubMed] [Google Scholar]
- Xiao-feng Z., Nai-qiang Q., Jing Z., Zi L., Yang Z. (2009). Di (n-butyl) phthalate inhibits testosterone synthesis through a glucocorticoid-mediated pathway in rats. Int. J. Toxicol. 28, 448–456. [DOI] [PubMed] [Google Scholar]
- Xie T., Tong L., McCann U. D., Yuan J., Becker K. G., Mechan A. O., Cheadle C., Donovan D. M., Ricaurte G. A. (2004). Identification and characterization of metallothionein-1 and -2 gene expression in the context of (+/-)3,4-methylenedioxymethamphetamine-induced toxicity to brain dopaminergic neurons. J. Neurosci. 24, 7043–7050. [DOI] [PMC free article] [PubMed] [Google Scholar]