Abstract
Aims
Although a true clinical challenge, high bleeding risk patients with an acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI) have never been specifically studied. Leaders Free ACS, a pre-specified Leaders Free sub-study, determined efficacy, and safety of a combination of 1-month dual anti-platelet therapy (DAPT) with implantation of either a polymer-free Biolimus-A9-coated stent (BA9-DCS) or a bare-metal stent (BMS) in these patients.
Methods and results
Leaders Free included 2466 patients undergoing PCI who had at least 1 of 13 pre-defined factors for an increased bleeding risk. Of these, 659 ACS patients were included in this analysis (BA9-DCS 330, BMS 329). At 12-month follow-up, treatment with the BA9-DCS was more effective (clinically driven target-lesion revascularization 3.9 vs. 9.0%, P = 0.009) and safer (cumulative incidence of cardiac death, myocardial infarction, or definite or probable stent thrombosis 9.3 vs. 18.5%, P = 0.001), driven by significantly lower rates of cardiac mortality (3.4 vs. 6.9%, P = 0.049) and myocardial infarction (6.9 vs. 13.8%, P = 0.005).
Conclusion
We believe that the results of this sub-analysis from the Leaders Free trial are likely to significantly impact clinical practice for high bleeding risk patients presenting with an ACS: the use of a BMS can, in our view, no longer be recommended, and, given the paucity of available data for second-generation DES with shortened DAPT in these patients, the BA9-DCS should currently be considered as the device with the strongest evidence to support its use for this indication.
Keywords: Acute coronary syndrome, High bleeding risk, Bare-metal stent, Drug-coated stent, Percutaneous coronary intervention
Introduction
Appropriate and timely antithrombotic therapy is essential for the outcome of patients presenting with acute coronary syndromes (ACS). The individual bleeding risk has to be balanced with the ischaemic threat. High bleeding risk patients presenting with an ACS, undergoing percutaneous coronary intervention (PCI), have never been specifically studied.
For these patients, current guidelines suggest the implantation of drug-eluting stents (DES) with 3–6 months dual anti-platelet therapy (DAPT) or bare-metal stents (BMS) with 1-month DAPT.1–3Leaders Free ACS is a pre-specified sub-study of Leaders Free4,5 which is a randomized double-blind trial, designed to assess the combination of 1 month of DAPT with either a polymer-free Biolimus-A9-coated stent (drug-coated stent; DCS) or a BMS in patients with at least one criterion for an increased bleeding risk such as advanced age, oral anticoagulant treatment, recent bleeding, anaemia, chronic renal failure, or cancer. In this patient group, DCS treatment displayed superiority not only with respect to clinically driven target-lesion revascularization at 390 days but also regarding the composite safety endpoint which was the incidence of cardiac death, myocardial infarction, or definite or probable stent thrombosis.
The aim of this analysis was to focus on the efficacy and safety of a polymer-free BA9-coated stent with 1-month DAPT in high bleeding risk patients presenting with non-ST-segment-elevation myocardial infarction (NSTEMI) or ST-segment-elevation myocardial infarction (STEMI).
Methods
Patients and methods
All patients from Leaders Free presenting with STEMI or NSTEMI, undergoing PCI were included. Methods and proceedings for Leaders Free have been described previously4,5 and are summarized here. Leaders Free is a randomized, double-blind, clinical trial, which enrolled 2466 patients at 68 sites in 20 countries. Patients were required to meet one or more of the criteria for an increased bleeding risk listed in Table 1. They were 1:1 randomly assigned to undergo PCI with a polymer-free BA9-DCS (BiofreedomTM DCS Biosensors Europe, Morges, Switzerland) or a similar bare-metal stent (GazelleTM, Biosensors Interventional Technologies, Singapore). Randomization was performed with the use of either a Web-based system or a telephone interactive voice-response system (Merge Healthcare, www.merge.com) in blocks of 16 with no further stratification. All patients received 1 month of DAPT followed by single anti-platelet therapy lifelong.
Table 1.
Baseline patient characteristics and inclusion criteriaa
| Drug-coated stent (N = 330) | Bare-metal stent (N = 329) | P-value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 76.9 ± 10.0 | 76.5 ± 9.9 | 0.17 |
| Female sex | 122 (37.0) | 110 (33.4) | 0.34 |
| Body mass index | 26.7 ± 4.8 | 26.7 ± 4.5 | 0.98 |
| Diabetes, n/total | 111/328 (33.8) | 108/329 (32.8) | 0.78 |
| Hypertension, n/total | 248/330 (75.2) | 249/327 (76.1) | 0.77 |
| Hypercholesterolaemia | 180/322 (55.9) | 156/321 (57.9) | 0.60 |
| STEMI | 57 (17.2) | 48 (14.5) | 0.40 |
| NSTEMI | 273 (82.8) | 281 (85.5) | 0.40 |
| Creatinine kinase (U/L) | 4.14 ± 7.16 | 3.12 ± 6.40 | 0.17 |
| Creatinine kinase MB (U/L) | 28.19 ± 73.06 | 16.38 ± 30.10 | 0.13 |
| High-sensitive troponin (ng/L) | 12.77 ± 18.94 | 13.80 ± 70.18 | 0.92 |
| Multi-vessel disease | 207/327 (64.1) | 220/323 (68.1) | 0.28 |
| Previous myocardial infarction | 63/329 (19.1) | 82/329 (24.9) | 0.07 |
| Previous PCI | 55/330 (16.7) | 67/328 (20.4) | 0.22 |
| Previous CABG | 24/330 (7.3) | 23/328 (7.0) | 0.90 |
| Congestive heart failure | 29/328 (8.8) | 43/329 (13.1) | 0.08 |
| Atrial fibrillation | 84/330 (25.5) | 109/329 (33.1) | 0.03 |
| Previous stroke | 46/326 (14.1) | 26/329 (7.9) | 0.01 |
| Peripheral vascular disease | 47/325 (14.5) | 49/328 (14.9) | 0.86 |
| Chronic obstructive lung disease | 39/330 (11.8) | 45/328 (13.7) | 0.47 |
| Crusade score | 36.4 ± 13.8 | 36.6 ± 14.1 | 0.87 |
| Criteria for high risk of bleeding | |||
| Oral anti-coagulation planned to continue after PCI | 79 (23.9) | 101 (30.7) | 0.05 |
| Age ≥75 years | 232 (70.3) | 229 (69.6) | 0.85 |
| Haemoglobin <11 g/L or transfusion within 4 weeks before randomization | 73 (22.1) | 79 (24.0) | 0.57 |
| Platelet count <100 000/mm3 | 4 (1.2) | 6 (1.8) | 0.57 |
| Hospital admission for bleeding in previous 12 months | 23 (7.0) | 15 (4.6) | 0.18 |
| Stroke in previous 12 months | 6 (1.8) | 5 (1.5) | 0.77 |
| Previous intracerebral bleed | 7 (2.1) | 7 (2.1) | 0.99 |
| Severe chronic liver disease | 4 (1.2) | 4 (1.2) | 0.99 |
| Creatinine clearance <40 mL/min | 62 (18.8) | 80 (24.3) | 0.08 |
| Cancer (non-skin) in the previous 3 years | 35 (10.6) | 37 (11.2) | 0.79 |
| Planned major surgery in next 12 months | 38 (11.5) | 36 (10.9) | 0.82 |
| Glucocorticoids or NSAID planned for >30 days after PCI | 14 (4.2) | 12 (3.6) | 0.70 |
| Expected non-adherence to >30 days of dual antiplatelet therapy | 18 (5.5) | 19 (5.8) | 0.86 |
STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST-segment myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
aEither presented as n (%) or mean ± SD.
Study proceedings
Percutaneous coronary intervention was performed according to standard techniques. Vascular access, peri-procedural antithrombotic regimen, and lesion preparation were left to the operator. All target lesions were treated with at least one study stent. Staged procedures were permitted within 1 week after the index procedure. The protocol mandated that all patients receive both aspirin and a P2Y12 inhibitor for 30 days, followed by a single anti-platelet agent.
Per protocol patients who were included in the trial because of planned oral anti-coagulation post-PCI should receive either the WOEST regimen or triple therapy.
A patient follow-up visit on site was performed at 30 days and 360 days. Further contacts were made at 60 and 120 days. Ischaemia testing and angiographic evaluation during follow-up were left to the discretion of the investigator.
Study endpoints
The primary safety endpoint was the cumulative incidence of a composite of cardiac death, myocardial infarction, or definite or probable stent thrombosis; the primary efficacy endpoint was the incidence of clinically driven target-lesion revascularization. Primary endpoint events and bleeding events were recorded for up to 390 days in order to capture events occurring soon after the 1-year visit. Myocardial infarction was defined according to the third universal definition of myocardial infarction,6 stent thrombosis according to the ARC definitions,7 and bleeding according to the BARC definitions.8
Clinically driven target-lesion revascularization was defined as PCI or surgery either for operator-defined restenosis in the treated lesion together with angina symptoms or documented ischaemia or, for a core-laboratory-defined restenosis of >70% of the artery diameter without symptoms or ischaemia.
Statistical analyses
Continuous variables are presented as mean ± SD, categorical data as counts and percentages. Categorical variables were compared using a χ2 test, continuous variables were compared using a two sample t-test. Whenever appropriate a Fisher exact test was used instead.
For time-to-event variables, hazard ratio or its 95% confidence interval was derived from an unadjusted Cox proportional hazard model. Cumulative incidence rates come from the Kaplan–Meier estimator with log-rank P-value to test if the plots differ over time. Proportional hazard assumptions were checked using Schoenfeld residuals. There was no adjustment for covariates or imputation for missing data. All available data were used in the analysis of all endpoints. We performed additional Cox proportional hazard models to analyze if the DAPT, P2Y12 inhibitor, or anticoagulant therapy prescription had an impact on the primary endpoints. The same method was used to analyze the potential impact of imbalances at baseline. All data were analyzed using SAS V.9.3 (SAS Institute, Cary, NC, USA).
Results
Patients and procedures
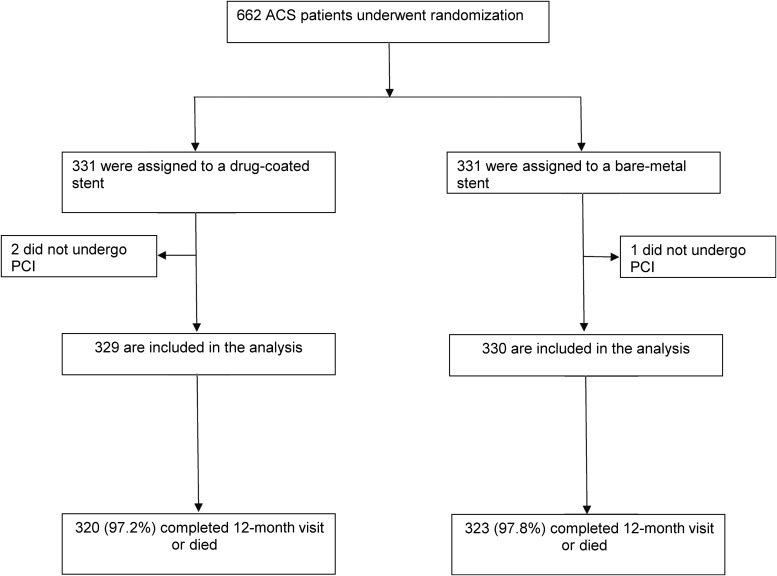
Six hundred and fifty-nine patients in Leaders Free presenting with an ACS, underwent PCI (Figure 1). Of these, 554 patients had an NSTEMI, 105 had an STEMI. 330 were assigned to the BA9-coated DCS and 329 were assigned to the BMS. Baseline biomarkers and other patient features are displayed in Table 1.
Figure 1.
Leaders Free ACS flow chart.
The patient population was of advanced age and displayed conditions indicative of an increased bleeding risk. Three or more of the criteria for high bleeding risk were met in 132 patients (20%), 2 criteria in 256 (39%), and only 1 criterion in 271 (41%). The criteria were well balanced between treatment groups.
Regarding baseline characteristics, there were no significant differences except previous stroke, being more frequent in the DCS group (14.1 vs. 7.9%; P = 0.01), while history of atrial fibrillation was more frequent in the BMS group (25.5 vs. 33.1%; P = 0.03). Given multiple testing across available baseline variables, these findings are compatible with the play of chance and had no significant impact on the primary endpoints.
Procedural data are displayed in Table 2. A total of 63.6% of the procedures in the DCS group and 64.0% in the BMS group were performed through radial access (P = 0.91). 3.8 and 8.9% of the procedures in the respective groups were staged (P = 0.01). The latter had no significant influence on the primary endpoints. 18.7% of the procedures in the DCS group and 23.0% in the BMS group involved multi-vessel revascularization (P = 0.16).
Table 2.
Procedure details and medicationa
| Drug-coated stent (N = 330) | Bare-metal stent (N = 329) | P-value | |
|---|---|---|---|
| Procedure details | |||
| Radial access | 218 (63.6) | 231 (64) | 0.91 |
| Staged procedure | 13 (3.8) | 32 (8.9) | 0.01 |
| Multi-lesion procedure | 113 (32.9) | 133 (36.8) | 0.28 |
| Multi-vessel procedure | 64 (18.7) | 83 (23.0) | 0.16 |
| LAD | 175 (51.0) | 193 (53.6) | 0.52 |
| LCX | 113 (32.9) | 114 (31.7) | 0.64 |
| LM | 8 (2.3) | 15 (4.2) | 0.16 |
| RCA | 110 (32.1) | 122 (33.9) | 0.17 |
| SVG | 5 (1.5) | 6 (1.7) | 0.93 |
| Bifurcation | 43 (12.5) | 59 (16.4) | 0.15 |
| ISR | 5 (1.5) | 5 (1.4) | 0.94 |
| CTO | 26 (7.6) | 24 (6.7) | 0.64 |
| Mean stent diameter | 2.90 ± 0.48 | 2.90 ± 0.50 | 0.93 |
| Total stent length | 32.20 ± 22.1 | 33.4 ± 23.3 | 0.50 |
| Number of stent | 1.70 ± 1.0 | 1.79 ± 1.1 | 0.27 |
| Lesion success | 468 (96.9) | 533 (97.4) | 0.60 |
| Device success | 560 (96.9) | 613 (96.7) | 0.97 |
| Procedural success | 326 (95.0) | 341 (94.7) | 0.85 |
| Medication | |||
| DAPT at Day 23 | 311 (95.6) | 317 (97.8) | 0.18 |
| DAPT at Day 37 | 28 (8.7) | 49 (15.2) | 0.01 |
| UFH | 282 (82.2) | 280 (77.6) | 0.12 |
| LMWH | 28 (8.2) | 34 (9.4) | 0.56 |
| Bivalirudin | 7 (2) | 16 (4.4) | 0.07 |
| GPIIbIIIa antagonist | 7 (2) | 5 (1.4)b | 0.50 |
LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCX, right coronary artery; SVG, saphenous vein graft; ISR, in-stent restenosis; CTO, chronic total occlusion; UFH, unfractionated heparin; LMWH, low-molecular-weight heparin.
aEither presented as n(%) or mean ± SD.
bFollowing the Leaders Free report, medication is reported at Day 23 post-PCI.
Among the 166 patients who were on oral anti-coagulation at Day 23 post-PCI, data were available for 164 patients. Most patients (n = 158; 96.3%) received triple therapy and only 6 (3.7%) followed the WOEST regimen. There was no difference between the treatment groups and no impact on outcomes.
At 23 days, DAPT was used in the DCS and BMS groups in 95.6 and 97.8%, respectively (P = 0.18). In detail, ASA was given in 97.3 and 99.1%, Clopidogrel in 85.0 and 85.2%, Ticagrelor in 11.7 and 11.1%, and Prasugrel in 1.8 and 2.5%. At 37 days, DAPT was continued in significantly more patients in the BMS arm (Table 2). This had no significant effect on the primary endpoints.
Primary endpoints
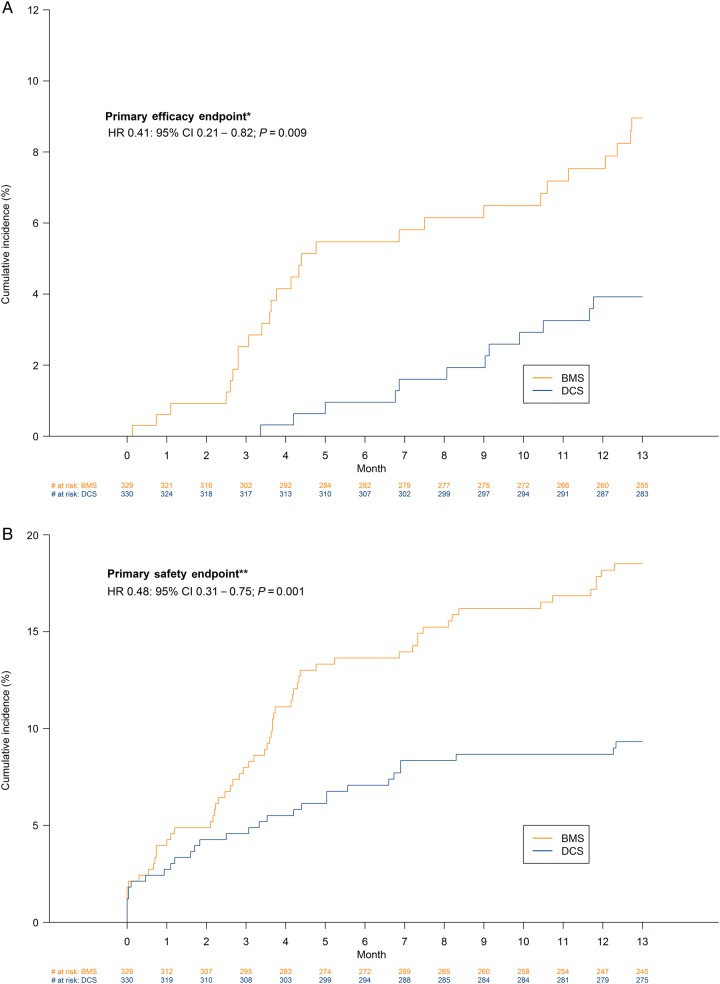
At 390 days, the primary efficacy endpoint (clinically driven target-lesion revascularization) had occurred in 12 patients (3.9%) in the DCS group and 27 patients (9.0%) in the BMS group (HR 0.41; 95% CI 0.21–0.82; P = 0.009) (Table 3).
Table 3.
Incidence of safety and efficacy endpoints at 390 days, presented as n (%) of patients affected
| Endpoint | Drug-coated stent (N = 330) | Bare-metal stent (N = 329) | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|
| Primary safety endpoint: cardiac death, myocardial infarction, or definite/probable stent thrombosis | 30 (9.3) | 59 (18.5) | 0.48 (0.31–0.75) | 0.001 |
| Cardiac death | 11 (3.4) | 22 (6.9) | 0.49 (0.23–1.01) | 0.049 |
| Myocardial infarction | 22 (6.9) | 43 (13.8) | 0.48 (0.29–0.81) | 0.005 |
| Definite or probable stent thrombosis | 4 (1.2) | 10 (3.1) | 0.39 (0.12–1.24) | 0.099 |
| Primary efficacy endpoint: clinically driven TLR | 12 (3.9) | 27 (9.0) | 0.41 (0.21–0.82) | 0.009 |
| Bleeding | ||||
| BARC 1–5 | 65 (20.2) | 67 (21.3) | 0.97 (0.69–1.36) | 0.86 |
| BARC 2–5 | 49 (15.2) | 54 (17.2) | 0.90 (0.61–1.32) | 0.60 |
| BARC 3–5 | 29 (9.0) | 29 (9.2) | 0.99 (0.59–1.66) | 0.99 |
TLR, target-lesion revascularization; BARC, bleeding according to Academic Research Consortium definition.
At 390 days, the primary safety endpoint (composite of cardiac death, myocardial infarction, or definite or probable stent thrombosis) had occurred in 30 patients (9.3%) in the DCS group and in 59 patients (18.5%) in the BMS group (HR 0.48; 95% CI 0.31–0.75; P = 0.001). The time-to-event curves for the primary efficacy and safety endpoints are shown in Figure 2.
Figure 2.
Time-to-event curves for the primary efficacy and safety endpoints *primary efficacy endpoint (clinically driven target-lesion revascularization) **primary safety endpoint (cardiac death, myocardial infarction, or definite and probable stent thrombosis).
Additional analyses
Significant differences between the treatment groups were also observed for cardiac death (DCS 3.4%; BMS 6.9%; HR 0.49; 95% CI 0.23–1.01; P = 0.049 and myocardial infarction (DCS 6.9%; BMS 13.8%; HR 0.48; 95% CI 0.29–0.81; P = 0.005). There were numerically fewer definite and probable stent thromboses in the DCS group (1.2 vs. 3.1%; HR 0.39, 95% CI 0.12–1.24; P = 0.099). Rates of bleeding according to BARC criteria were high and similar in the two groups (Table 3).
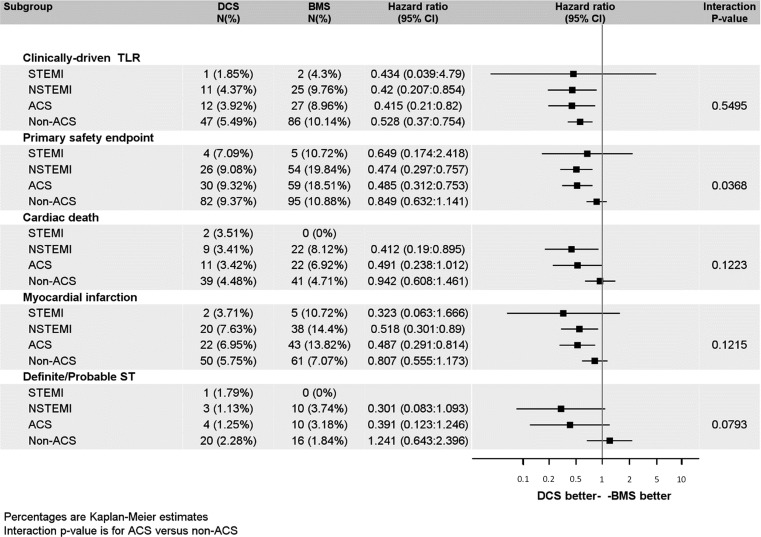
We analyzed if the treatment effects on the primary endpoints were comparable between patients with and without an ACS and with NSTEMI or STEMI, respectively. These analyses show that while the efficacy benefit of the treatment is consistent across all subgroups, the safety benefit in the Leaders Free population is mainly driven by the ACS subgroups (Figure 3).
Figure 3.
Forest plot of Leaders Free for the primary efficacy and safety endpoints and components by acute coronary syndrome status.
Discussion
At 390 days follow-up, Leaders Free ACS demonstrates that a polymer-free BA9-coated stent with 1-month DAPT is significantly more effective and safe than a BMS in high bleeding risk patients presenting with an ACS. The BA9-coated stent not only reduced the re-intervention rate and the incidence of the composite safety endpoint but also significantly reduced rates of cardiac mortality and myocardial infarction. These findings were not dependent on the post-procedural DAPT scheme, the oral anti-coagulation regimen, or imbalances at baseline.
There are two reasons why patients with an ACS receive prolonged DAPT: first for the prevention of secondary events,9–12 which is currently recommended for 12 months or beyond after the event,1–3 second for the prevention of stent thrombosis if they undergo PCI with stent implantation. With stable CAD, DAPT is currently recommended for at least 4 weeks after BMS—and 6 months after DES implantation.2,13
The bleeding risk induced by the prolonged DAPT in ACS patients is usually considered to be more than balanced by the benefits of a reduction of secondary thrombotic events,14 however, this has never been specifically studied in ACS patients with an increased risk of bleeding.15–18 Current guidelines suggest in this scenario the implantation of a DES with shortened DAPT or a BMS with 1-month DAPT.1–3 The use of BMS for ACS patients increases the rate of target-lesion revascularization and carries a significant risk for increasing major adverse cardiac events.19,20
The guideline's recommendations regarding shortening of DAPT are, besides the results of a network meta-analysis21 mainly based on the OPTIMIZE22 and RESET23 trials which assessed clinical non-inferiority of 3 months vs. 12 months of DAPT. The results of both trials suggest that it may be suitable to shorten DAPT after implantation of a fast-eluting Zotarolimus-eluting DES in selected patients at low-risk of bleeding, with no detectable benefit, but no significant trade off regarding clinical events. Observational data from new-generation Zotarolimus- and Everolimus-eluting stents have also raised the possibility that DAPT interruption may be safe in selected low-risk patients.24–26
A very recent sub-analysis of the ZEUS trial included patients with pre-defined risk factors for bleeding. Similar to Leaders Free, this analysis found a significant disadvantage regarding safety and efficacy in patients receiving a thin-strut BMS when compared with a fast-eluting Zotarolimus-eluting stent.27
Despite the fact that patients with a BMS in our analysis had a higher rate of DAPT use when compared with DCS patients at 37 days, definite and possible stent thrombosis tended to be lower in the DCS group, while both myocardial infarction and cardiovascular death were significantly less frequent in the DCS group at follow-up.
The fact that not only the primary efficacy endpoint but also stent thrombosis and ischaemic events are reduced in the DCS group is compelling. Spontaneous myocardial infarction (type 1), and myocardial infarction related to stent thrombosis (type 4b), as categorized according to the third Universal Definition of Myocardial Infarction, occurred significantly less frequent among patients with a drug-coated stent (see Appendix, Table A1). A comparable, however, non-significant trend was seen for myocardial infarction related to in-stent restenosis (type 4c). Because routine angiography was not systematically performed, it is likely that many of the spontaneous myocardial infarctions were also related to in-stent restenosis, although this uncertainty does not affect the comparison between treatment groups.
As demonstrated above, in Leaders Free this superior safety effect was mainly driven by the ACS subgroups. A similar effect has also been demonstrated in other studies of BA9 stents. Superior safety was also observed in the COMFORTABLE AMI study.19 The study, compared the same drug on the same stent platform as investigated in this trial, albeit with a biodegradable polymer, with the same BMS in the setting of acute myocardial infarction. Finally interaction testing in the LEADERS trial28 demonstrated heterogeneity of treatment effects in regards to the primary endpoint in the BA9 STEMI sub-group.
It is therefore tempting to speculate about a benefit for this type of therapy in the acute setting that is conferred by BA9. The high lipophilicity of this drug which may allow it to penetrate into lipid rich plaques more effectively than other drugs may explain part of the superior outcomes in the ACS population. This also means that our findings may not be extendable to polymer-coated DES or other polymer-free drug-coated stent designs.
In an ACS population at high risk of bleeding, our data show for the first time that it is possible to clinically reproduce an efficacy similar to that of current DES together with a significant safety benefit when compared with BMS implantation with only 1 month DAPT duration. Of note, despite a course of only 1 month DAPT, the incidence of any reported bleeding events was similar and >20% in both groups and the rate of severe bleeding was >9% during the 390 days follow-up. These figures are higher by one order of magnitude than those reported during the first year for several of the DAPT duration trials,22,23 underlining that a longer course of DAPT would be expected to be poorly tolerated in this high bleeding risk cohort.
Implications
Although the data presented herein are the result of a sub-analysis from the Leaders Free trial, and should be, thus, regarded as hypothesis generating, we believe that they are likely to significantly impact clinical practice for high bleeding risk patients presenting with an ACS: the use of a BMS can, in our view, no longer be recommended, and, given the paucity of available data for second-generation DES with shortened DAPT in these patients, the BA9-DCS should currently be considered as the device with the strongest evidence to support its use for this indication.
Limitations
Although pre-defined, Leaders Free ACS is a sub-study of Leaders Free and was, thus, not powered to detect clinical differences between the groups.
This pre-defined ACS sub-analysis was not intended to include unstable angina, since Troponin negative unstable angina was not thought to be precisely enough defined to serve as discriminator for an outcome analysis in a randomized trial. Therefore, these patients were not included in the analysis.
Despite the fact that observed effects tended to be similar in all groups, the number of STEMI patients in this analysis was limited and the ACS group consisted mainly of patients with NSTEMI.
In this analysis, we compared the combination of 1 month DAPT with implantation of a BA9-DCS or with implantation of a BMS only. Our results cannot be extended to other device or treatment regimens.
Authors’ contributions
S.C., S.J.P. performed statistical analysis. P.U., S.G., M.-C.M. handled funding and supervision. C.K.N., P.U., P.J.O., M.V.-C., F.F., C.D., M.-C.M., A.A.A. acquired the data. P.U., M.-C.M., C.K.N., A.A.A. conceived and designed the research. P.U., M.-C.M., S.P., S.C., C.K.N. drafted the manuscript. C.K.N., P.U., P.J.O., M.V.-C., A.A.A., S.J.P., F.F., C.D., S.C., S.G., M.-C.M. made critical revision of the manuscript for key intellectual content.
Funding
This work was funded by Biosensors Europe.
Conflict of interest: C.K.N. reports personal fees from Abbott, personal fees from Biosensors, grants and personal fees from Biotronik, personal fees from Medtronic, personal fees from Elixir, personal fees from REVA, personal fees from Microport, outside the submitted work; he is shareholder of CERC, the CRO responsible for running the LEADERS FREE study. P.U. reports personal fees from Biosensors Europe, during the conduct of the study; personal fees from Edwards Lifescience, personal fees from Terumo, personal fees from Abbott Vascular, personal fees from QUEST medical, outside the submitted work; he is Medical co-director and shareholder of CERC, the CRO responsible for running the LEADERS FREE study. A.A.A. reports grants from Abbott, grants from Medtronic, grants from Elixir, grants from Riva, during the conduct of the study. S.J.P. reports personal fees from Biosensors Europe SA, during the conduct of the study. C.D. reports personal fees from Boston Scientific, personal fees from Edwards Lifesciences, personal fees from Medtronic, grants from Abbott Vascular, grants from Biotronik, outside the submitted work. S.C. and S.G. are employees of Biosensors Internatonial. M.-C.M. is CEO and shareholder of CERC, the CRO responsible for running the LEADERS FREE study.
Acknowledgements
The authors would like to acknowledge the contribution of Ute Windhövel, PhD (CERC, Massy, France) to the data acquisition and management of the study.
Appendix
Table A1.
Myocardial infarction stratified by 3rd universial definition of myocardial infarction (6)
| Parameter | Statistics | Drug-coated stent (N = 330) | Bare-metal stent (N = 329) | Total (N = 659) | Hazard ratio | P-value |
|---|---|---|---|---|---|---|
| Myocardial infarction | n (%) | 22 (6.95%) | 43 (13.85%) | 65 (10.37%) | 0.487 (0.291:0.814) | 0.005 |
| Myocardial infarction—type 1 | n (%) | 14 (4.49%) | 26 (8.46%) | 40 (6.46%) | 0.515 (0.269:0.987) | 0.04 |
| Myocardial infarction—type 2 | n (%) | 4 (1.27%) | 5 (1.66%) | 9 (1.46%) | 0.784 (0.211:2.921) | 0.72 |
| Myocardial infarction—type 3 | n (%) | 0 (%) | 0 (%) | 0 (%) | N/A | – |
| Myocardial infarction—type 4a | n (%) | 5 (1.52%) | 6 (1.84%) | 11 (1.68%) | 0.83 (0.253:2.719) | 0.76 |
| Myocardial infarction—type 4b | n (%) | 0 (0%) | 8 (2.52%) | 8 (1.26%) | N/A | 0.004 |
| Myocardial infarction—type 4c | n (%) | 2 (0.65%) | 5 (1.66%) | 7 (1.15%) | 0.385 (0.075:1.983) | 0.24 |
| Myocardial infarction—type 5 | n (%) | 0 (%) | 0 (%) | 0 (%) | N/A | – |
Given that many patients did not undergo control angiography when readmitted during follow-up, a definite distinction between Types I, 4b and 4c was sometimes difficult to establish.
References
- 1. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D2, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 2. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'Gara PT, Sabatine MS, Smith PK, Smith SC Jr, Focused Update Writing Group. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease. J Am Coll Cardiol 2016. [Epub ahead of print]. [Google Scholar]
- 3. Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van't Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 4. Urban P, Abizaid A, Chevalier B, Greene S, Meredith I, Morice MC, Pocock S. Rationale and design of the LEADERS FREE trial: a randomized double-blind comparison of the BioFreedom drug-coated stent vs the Gazelle bare metal stent in patients at high bleeding risk using a short (1 month) course of dual antiplatelet therapy. Am Heart J 2013;165:704–709. [DOI] [PubMed] [Google Scholar]
- 5. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, Lipiecki J, Richardt G, Iñiguez A, Brunel P, Valdes-Chavarri M, Garot P, Talwar S, Berland J, Abdellaoui M, Eberli F, Oldroyd K, Zambahari R, Gregson J, Greene S, Stoll HP, Morice MC, LEADERS Free Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015;373:2038–2047. [DOI] [PubMed] [Google Scholar]
- 6. Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 7. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW, Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 8. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the BleedingAcademic Research Consortium. Circulation 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 9. Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001;358:527–533. [DOI] [PubMed] [Google Scholar]
- 10. Steinhubl SR, Berger PB, Mann JT III, Fry ET, DeLago A, Wilmer C, Topol EJ, CREDO Investigators.Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. J Am Med Assoc 2002;288:2411–2420. [DOI] [PubMed] [Google Scholar]
- 11. Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, Husted S, Katus H, Keltai M, Khurmi NS, Kontny F, Lewis BS, Steg PG, Storey RF, Wojdyla D, Wallentin L. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet 2010;375:283–293. [DOI] [PubMed] [Google Scholar]
- 12. Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITONTIMI 38): double-blind, randomised controlled trial. Lancet 2009;373:723–731. [DOI] [PubMed] [Google Scholar]
- 13. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 14. Wiviott SD, Steg PG. Clinical evidence for oral antiplatelet therapy in acute coronary syndromes. Lancet 2015;386:292–302. [DOI] [PubMed] [Google Scholar]
- 15. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 2010;55:2556–2566. [DOI] [PubMed] [Google Scholar]
- 16. Lopes RD, Alexander KP, Manoukian SV, Bertrand ME, Feit F, White HD, Pollack CV Jr, Hoekstra J, Gersh BJ, Stone GW, Ohman EM. Advanced age, antithrombotic strategy, and bleeding in non-ST-segment elevation acute coronary syndromes: results from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol 2009;53:1021–1030. [DOI] [PubMed] [Google Scholar]
- 17. Salisbury AC, Wang K, Cohen DJ, Li Y, Jones PG, Spertus JA. Selecting antiplatelet therapy at the time of percutaneous intervention for an acute coronary syndrome: weighing the benefits and risks of prasugrel versus clopidogrel. Circ Cardiovasc Qual Outcomes 2013;6:27–34. [DOI] [PubMed] [Google Scholar]
- 18. Ducrocq G, Schulte PJ, Becker RC, Cannon CP, Harrington RA, Held C, Himmelmann A, Lassila R, Storey RF, Sorbets E, Wallentin L, Steg PG. Association of spontaneous and procedure-related bleeds with short- and long-term mortality after acute coronary syndromes: an analysis from the PLATO trial. EuroIntervention 2015;11:737–745. [DOI] [PubMed] [Google Scholar]
- 19. Räber L, Kelbæk H, Ostojic M, Baumbach A, Heg D, Tüller D, von Birgelen C, Roffi M, Moschovitis A, Khattab AA, Wenaweser P, Bonvini R, Pedrazzini G, Kornowski R, Weber K, Trelle S, Lüscher TF, Taniwaki M, Matter CM, Meier B, Jüni P, Windecker S. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. J Am Med Assoc 2012;308:777–787. [DOI] [PubMed] [Google Scholar]
- 20. Taniwaki M, Stefanini GG, Räber L, Brugaletta S, Cequier A, Heg D, Iñiguez A, Kelbæk H, Serra A, Ostoijic M, Hernandez-Antolin R, Baumbach A, Blöchlinger S, Jüni P, Mainar V, Sabate M, Windecker S. Predictors of adverse events among patients undergoing primary percutaneous coronary intervention: insights from a pooled analysis of the COMFORTABLE AMI and EXAMINATION trials. EuroIntervention 2015;11:391–398. [DOI] [PubMed] [Google Scholar]
- 21. Palmerini T, Sangiorgi D, Valgimigli M, Biondi-Zoccai G, Feres F, Abizaid A, Costa RA, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Mariani A, Della Riva D, Genereux P, Leon MB, Bhatt DL, Bendetto U, Rapezzi C, Stone GW. Short- versus long-term dual antiplatelet therapy after drug-eluting stent implantation: an individual patient data pairwise and network meta-analysis. J Am Coll Cardiol 2015;65:1092–1102. [DOI] [PubMed] [Google Scholar]
- 22. Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, King SB III, Negoita M, Liu M, de Paula JE, Mangione JA, Meireles GX, Castello HJ Jr, Nicolela EL Jr, Perin MA, Devito FS, Labrunie A, Salvadori D Jr, Gusmão M, Staico R, Costa JR Jr, de Castro JP, Abizaid AS, Bhatt DL, OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. J Am Med Assoc 2013;310:2510–2522. [DOI] [PubMed] [Google Scholar]
- 23. Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Park BE, Kang WC, Lee SH, Yoon JH, Hong BK, Kwon HM, Jang Y, RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial. J Am Coll Cardiol 2012;60:1340–1348. [DOI] [PubMed] [Google Scholar]
- 24. Kedhi E, Stone GW, Kereiakes DJ, Serruys PW, Parise H, Fahy M, Simonton CA, Sudhir K, Sood P, Smits PC. Stent thrombosis: insights on outcomes, predictors and impact of dual antiplatelet therapy interruption from the SPIRIT II, SPIRIT III, SPIRIT IV and COMPARE trials. EuroIntervention 2012;8:599–606. [DOI] [PubMed] [Google Scholar]
- 25. Silber S, Kirtane AJ, Belardi JA, Liu M, Brar S, Rothman M, Windecker S. Lack of association between dual antiplatelet therapy use and stent thrombosis between 1 and 12 months following resolute zotarolimus-eluting stent implantation. Eur Heart J 2014;35:1949–1956. [DOI] [PubMed] [Google Scholar]
- 26. Généreux P, Rutledge DR, Palmerini T, Caixeta A, Kedhi E, Hermiller JB, Wang J, Krucoff MW, Jones-McMeans J, Sudhir K, Simonton CA, Serruys PW, Stone GW. Stent thrombosis and dual antiplatelet therapy interruption with everolimus-eluting stents: insights from the Xience V Coronary Stent System Trials. Circ Cardiovasc Interv 2015;8. [DOI] [PubMed] [Google Scholar]
- 27. Ariotti S, Adamo M, Costa F, Patialiakas A, Briguori C, Thury A, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, Menozzi A, de Cesare N, Garbo R, Meliga E, Testa L, Gabriel HM, Ferlini M, Vranckx P, Valgimigli M, ZEUS Investigators. Is bare-metal stent implantation still justifiable in high bleeding risk patients undergoing percutaneous coronary interventions? JACC Cardiovasc Interven 2015;9:426–436. [DOI] [PubMed] [Google Scholar]
- 28. Serruys PW, Farooq V, Kalesan B, de Vries T, Buszman P, Linke A, Ischinger T, Klauss V, Eberli F, Wijns W, Morice MC, Di Mario C, Corti R, Antoni D, Sohn HY, Eerdmans P, Rademaker-Havinga T, van Es GA, Meier B, Jüni P, Windecker S. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimuseluting stents in patients with coronary artery disease: final 5-year report of the LEADERS randomized, noninferiority trial. JACC Cardiovasc Interv 2013;6:777–789. [DOI] [PubMed] [Google Scholar]