Abstract
Skin tumorigenesis results from DNA damage, increased inflammation, and evasion of apoptosis. The peroxisome proliferator-activated receptors (PPARs) can modulate these mechanisms in non-melanoma skin cancer. However, limited data exists regarding the role of PPARs in melanoma. This study examined the effect of proliferator-activated receptor-β/δ (PPARβ/δ) and PPARγ on cell proliferation, anchorage-dependent clonogenicity, and ectopic xenografts in the UACC903 human melanoma cell line. Stable overexpression of either PPARβ/δ or PPARγ enhanced ligand-induced expression of a PPARβ/δ/PPARγ target gene in UACC903 cell lines as compared with controls. The induction of target gene expression by ligand activation of PPARγ was not altered by overexpression of PPARβ/δ, or vice versa. Stable overexpression of either PPARβ/δ or PPARγ reduced the percentage of cells in the G1 and S phase of the cell cycle, and increased the percentage of cells in the G2/M phase of the cell cycle in UACC903 cell lines as compared with controls. Ligand activation of PPARβ/δ did not further alter the distribution of cells within each phase of the cell cycle. By contrast, ligand activation of PPARγ enhanced these changes in stable UACC903 cells overexpressing PPARγ compared with controls. Stable overexpression of either PPARβ/δ or PPARγ and/or ligand activation of either PPARβ/δ or PPARγ inhibited cell proliferation, and anchorage-dependent clonogenicity of UACC903 cell lines as compared with controls. Further, overexpression of either PPARβ/δ or PPARγ and/or ligand activation of either PPARβ/δ or PPARγ inhibited ectopic xenograft tumorigenicity derived from UACC903 melanoma cells as compared with controls, and this was likely due in part to induction of apoptosis. Results from these studies demonstrate the antitumorigenic effects of both PPARβ/δ and PPARγ and suggest that targeting these receptors may be useful for primary or secondary melanoma chemoprevention.
Keywords: peroxisome proliferator-activated receptor-β/δ, (PPARβ/δ), peroxisome proliferator-activated receptor-γ, (PPARγ), melanoma, cell proliferation, xenografts, cancer
Melanoma is the sixth leading cause of cancer in the United States, and it is estimated that 87 000 people will be diagnosed with this disease in 2017 (Siegel etal. 2017). The prognosis and 5-year survival rate is very good for patients with localized melanomas (98.2%), but the 5-year survival rates precipitously decrease for regional (62.4%) and malignant (17.9%) melanoma (Howlader etal., 2016; Siegel etal., 2017). Based on the 5-year survival rate, malignant melanoma is more deadly than breast, ovarian, or prostate cancer (Siegel etal., 2017).
Melanoma originates from malignant proliferation of genetically altered melanocytes. Large-scale next-generation sequencing has identified several molecular signatures linked with the ontology of melanoma. For example, the mitogen-activated protein kinase and phosphatidylinositol 3 kinase/protein kinase B (AKT) pathways are frequently mutated, amplified, or deleted in malignant melanoma (reviewed in Kunz, 2014; Shtivelman etal., 2014; Zhang etal., 2016). Further, a number of related genes exhibit oncogenic mutations including BRAF (50% of tumors), NRAS (20%–25% of tumors), ERBB4 (15%–20% of tumors), AKT3 (25% of tumors), and PTEN (40%–60% of tumors) in melanomas (reviewed in Kunz, 2014; Shtivelman etal., 2014; Zhang etal., 2016). These genetic alterations modulate activity in these pathways that result in increased cell proliferation, increased cell survival, cell migration, and angiogenesis. Therapies targeting some of these pathways has led to significant improvement in the treatment of malignant melanoma, including the use of BRAF inhibitors and/or immunotherapies (reviewed in Franklin etal., 2016; Shtivelman etal., 2014). Despite the progress in therapeutically treating malignant melanoma, alternative strategies based on novel discoveries mediating the etiology and signaling pathways associated with this disease remains of high significance.
Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) and PPARγ are ligand-activated transcription factors that modulate numerous biological processes by several unique mechanisms. PPARβ/δ and PPARγ mediate dynamic changes in gene expression by association with specific chromatin binding sites, which is influenced by multiple factors including the presence of endogenous/exogenous ligands, expression and activity of chromatin remodeling proteins, co-repressors, co-activators, and other intracellular proteins (Biddie etal., 2010; Hager and Varticovski, 2012). The PPARs can also regulate gene expression through protein-protein interactions. The most classic example is the interaction between PPARs and the p65 subunit of NF-kB causing down-regulation of proinflammatory signaling (reviewed in Peters etal., 2012, 2015a). Combined, PPARs can modulate many biological effects by both direct transcription regulation of target genes and by indirect protein-protein interactions. Overall, the PPARs modulate many essential biological processes in the body, including lipid and glucose homeostasis, terminal differentiation, and inflammation (reviewed in Peters etal., 2012).
The role of PPARβ/δ in carcinogenesis remains controversial; while it is well accepted that activation of PPARγ inhibits or can be targeted for treating cancer (reviewed in Peters and Gonzalez, 2009; Peters etal., 2012, 2015a,b). Interestingly, PPARβ/δ is expressed at relatively high levels in melanocytes and melanoma cells (Eastham etal., 2008; Kang etal., 2004); suggesting that PPARβ/δ modulates melanocyte activity and function. Moreover, there is some evidence that relatively high expression of PPARβ/δ may interfere with ligand-dependent PPARγ activities (Shi etal., 2002; Wang etal., 2012). Thus, the present study examined the effect of PPARβ/δ or PPARγ in the human melanoma cancer cell line UACC903 using both invitro and invivo models.
MATERIALS AND METHODS
Materials and cell culture
[4-[[[2-[3-fluoro-4-(trifluoromethyl)phenyl]-4-methyl-5-thiazolyl]methyl]thio]-2-methylphenoxy acetic acid (GW0742) was synthesized by GlaxoSmithKline (Research Triangle Park, North Carolina). Rosiglitazone maleate was purchased from Santa Cruz Biotechnologies (Santa Cruz, Californina). Both PPAR ligands were dissolved in dimethylsulfoxide (DMSO). Primers for quantitative real-time polymerase chain reaction (qPCR) were purchased from Integrated DNA Technologies (Coralville, Iowa). The UACC903 melanoma cell line with the V600E-BRAF mutation was provided by Mark Nelson (University of Arizona, Tucson, Arizona). The UACC903 melanoma cell line was examined as a representative human melanoma cell line because it contains the BRAFV600E mutation and a deletion of PTEN, genotypes found in approximately 50% of all melanoma patients, which is considerably higher than other mutations in human melanoma (reviewed in Kunz, 2014; Shtivelman etal., 2014; Zhang etal., 2016). UACC903 cells were cultured in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin solution at 37 °C and 5% carbon dioxide, and used within a narrow passage range. The UACC903 cells were also annually monitored for genotypic characteristics, phenotypic behavior and tumorigenic potential to confirm identity. Athymic NCr-nu/nu mice were purchased from the National Cancer Institute (NCI, Frederick, Maryland).
Generation of stable UACC903 cell lines overexpressing PPARβ/δ or PPARγ
Stable human UACC903 malignant melanoma cell lines overexpressing PPARβ/δ or PPARγ were generated using the Migr1 bicistronic retrovirus vector (Pear etal., 1998). The Migr1 vector has a mouse stem cell promoter that drives expression of enhanced green fluorescent protein (eGFP), in addition to an internal ribosome entry site allowing for cloning different cDNAs of interest. This type of vector that yields relatively high expression of PPARs has been described previously in Borland etal., (2011), Foreman etal. (2011), Yao etal. (2014, 2015, 2017), and also causes marked expression of eGFP to facilitate identification and sorting of cells that have stably integrated the Migr1 vectors. The nomenclature for the melanoma cell lines used for these studies were: (1) UACC903 cells (control, parent cell line used for the infection with the Migr1 vectors); (2) the UACC903-Migr1 cells (control cell line with stable expression of only eGFP); (3) UACC903-hPPARβ/δ cells (the melanoma cell line with stable expression of human PPARβ/δ and eGFP); or (4) UACC903-hPPARγ cells (the melanoma cell line with stable expression of human PPARγ and eGFP).
Characterization of the Migr1-PPAR overexpression cell lines
Western blot analysis and qPCR were performed as described below to confirm that the PPARs were overexpressed at the mRNA and protein levels. The ability of the different cell lines to respond to ligand activation was examined by treating cells with either the high affinity agonists for PPARβ/δ (GW0742) or PPARγ (rosiglitazone). Ligand activation of PPARβ/δ was examined in the UACC903 cell lines cultured in medium with vehicle control (0.02% DMSO) or the PPARβ/δ ligand GW0742 (0.01–10.0 μM) for 8 h. Ligand activation of PPARγ was examined in the UACC903 cells cultured in medium with vehicle control (0.02% DMSO) or the PPARγ ligand rosiglitazone (0.01–10.0 μM) for 24 h. Analysis of gene expression was performed by qPCR as described below
Target gene analyses of PPAR activation
qPCR was used to measure the mRNA expression of PPARβ/δ, PPARγ, the PPAR target genes angiopoietin-like protein 4 (ANGPTL4) (Heinaniemi etal., 2007) or adipocyte differentiation-related protein (ADRP), and the putative PPARβ/δ target gene 3-phosphoinositide-dependent protein kinase 1 (PDPK1) (Di-Poi etal., 2002) as previously described (Borland etal., 2011). Each assay included a standard curve with greater than 85% efficiency and a no-template control. The relative mRNA expression of PPARβ/δ, PPARγ, ADRP, ANGPTL4, and PDPK1 was normalized to the relative mRNA value for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Western blot analysis
Soluble protein lysates were isolated from 90% to 95% confluent 100 mm culture dishes as previously described in Borland etal. (2011). Fifty micrograms of protein per sample was separated using SDS-polyacrylamide gels and transferred to a PVDF membrane using an electroblotting method. The membranes were blocked with 5% dried milk in Tris buffered saline/Tween-20 (TBST) and incubated overnight at 4 °C with primary antibodies for anti-human PPARβ/δ (ab21209, Abcam, Cambridge, Massachusetts), anti-human PPARγ (2430, Cell Signaling Technology, Danvers, Massachusetts) or anti-ACTIN (Rockland, Gilbertsville, Pennsylvania). The membranes were washed 3 times with TBST prior to the incubation with a biotinylated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania) for 1 h at room temperature. Membranes were then washed 3 more times with TBST before incubation in 125I-streptavidin, and then washed 3 more times with TBST after this final incubation. Membranes were exposed to phosphoimager plates and the level of radioactivity was quantified with filmless autoradiographic analysis (Packard Phosphoimager, PerkinElmer, Waltham, Massachusetts). Hybridization signals for specific proteins were normalized to the hybridization signal for ACTIN.
Flow cytometric analysis of cell cycle progression
The UACC903 cells were seeded onto 6-well tissue culture dishes at a concentration of 250 000 cells per well and cultured in DMEM with 10% FBS and 1% penicillin/streptomycin for 24 h. After this initial 24 h culture period without additional treatment, flow cytometric analysis was performed as described below. Additionally, in separate cohorts of cells, 24 h postplating, cells were cultured in medium with or without GW0742 (0.0, 0.01, 0.1, 1.0, or 10.0 µM) or rosiglitazone (0.0, 0.01, 0.1, 1.0, or 10.0 µM) for 24 h. After these treatments, culture medium was removed and the cells were trypsinized, pelleted and fixed in ice cold 70% ethanol. Prior to analysis, cells were stained with propidium iodide (PI) solution containing 1 µg PI/µl and 0.125% RNase A (Sigma Aldrich, St. Louis, Missouri). Approximately 10 000 cells/sample were analyzed using an EPICS-XL-MCL flow cytometer (Beckman Coulter, Miami Lakes, Florida) fitted with a single 15-mW argon ion laser providing excitation at 488 nm. The percentage of cells at each phase of the cell cycle was determined with MultiCycle analysis software. Values were calculated from a minimum of 3 independent samples per treatment.
Effect of ligand activation and overexpression of PPARβ/δ or PPARγ on cell proliferation
UACC903 cells were plated on a 12-well plate at a density of 25 000 cells/well 24 h before cell counting at time 0. Cell proliferation was determined using a Z1 Coulter particle counter (Beckman Coulter, Hialeah, Florida). After the first 24 h, cells were cultured in DMEM containing the vehicle control (0.02%DMSO), GW0742 (0.01–10.0 μM), or rosiglitazone (0.01–10.0 μM). Cells were counted every 24–72 h postligand treatment. Triplicate samples for each treatment were used for each time point, and each replicate was counted 3 times. Cell population doubling time was calculated from the 24- to 72-h time point to prevent log phase growth bias. Doubling time for each replicate was calculated as follows:
Effect of ligand activation and overexpression of PPARβ/δ and PPARγ on anchorage-dependent clonogenicity
UACC903 cells were plated onto 60-mm dishes at 200 cells per dish. After allowing the cells to adhere for 6 h, cell culture medium was replaced with medium containing either, vehicle control (0.02% DMSO), GW0742 (0.01–10.0 μM), or rosiglitazone (0.01–10.0 μM). After 14 days in culture, cell colonies were fixed and stained with a 6% (v/v) glutaraldehyde/0.5% (w/v) crystal violet solution. Colonies were counted with a stereomicroscope. Plating efficiency and surviving fractions were calculated as previously described in Franken etal. (2006) from 3 independent samples per treatment group.
Ectopic xenografts
Ectopic xenografts were induced in athymic nude mice as previously described in Yao etal. (2014). Briefly, 6-week-old female immune-deficient athymic nude (nu/nu) mice were injected subcutaneously with 1 × 106 cells per hind flank. The UACC903-Migr1 cells were injected in the left rear flank and the UACC903-hPPARβ/δ cells were injected in the right rear flank. Alternatively, the UACC903-Migr1 cells were injected in the left rear flank and the UACC903-hPPARγ cells were injected in the right rear flank. Groups of mice were then treated with or without GW0742 (2.5 mg/kg/d) or rosiglitazone (10 mg/kg/d) for up to 36 days. The PPAR ligands were provided by daily dosing with a pellet made with Bacon-flavored Transgenic Dough Diet (Bioserv, Inc., Flemington, New Jersey) mixed with either vehicle control (0.02% DMSO), GW0742 or rosiglitazone. Body weight and tumor volumes were measured 3 times a week. Mice were euthanized by overexposure to carbon dioxide, and tumors were carefully dissected. Half of each tumor was fixed in 10% phosphate buffered formalin, and the other half was snap frozen in liquid nitrogen for subsequent analysis of proteins by western blot analysis or mRNA expression by qPCR as described above. Fixed tumor sections were processed for staining as previously described (Yao etal., 2015). Hematoxylin-eosin (H&E) stained tumor sections were examined by a pathologist. A terminal deoxy-nucleotidyl transferase-mediated digoxigenin-dUTP nick end labeling (TUNEL) assay was performed to detect apoptotic fragmentation of DNA in paraffin-embedded tumor sections using the ApopTag kit (Chemicon, Temecula, California) following the manufacturer’s instructions. Twenty fields per section and 2 sections per tumor sample were analyzed. The relative level of apoptosis was determined by normalizing the intensity of 3,3′-diaminobenzidine to hematoxylin signals using ImageJ software (Version 1.47c).
Data analysis
Data were analyzed for statistical significance using one-way analysis of variance (ANOVA) and the Bonferroni’s multiple comparison tests, or Student’s t-test using Prism 5.0 (GraphPad Software Inc., La Jolla, California). All data are presented as the mean ± SEM.
RESULTS
Enhanced PPAR Activity in UACC903 Human Malignant Melanoma Cells Overexpressing PPARβ/δ or PPARγ
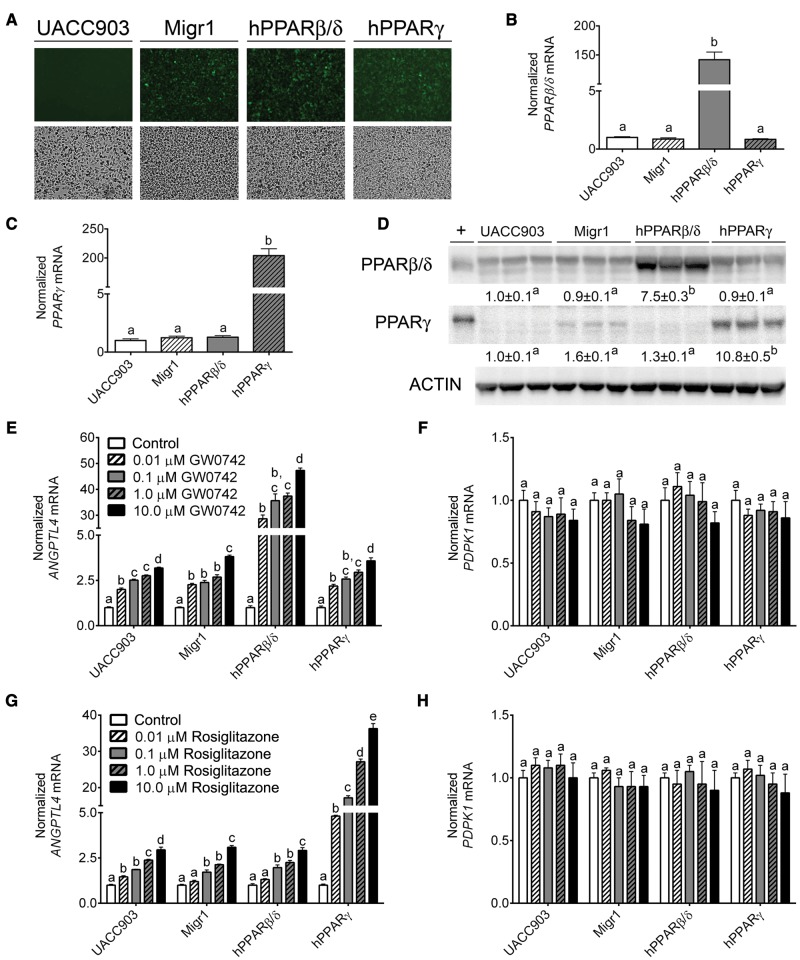
UACC903 stable cell lines with the control Migr1 vector, Migr1 vector encoding hPPARβ/δ and eGFP, or Migr1 vector encoding PPARγ and eGFP were compared with control UACC903 cells by fluorescent microscopy to validate stable integration of the vector. The control UACC903 cell line did not express eGFP whereas the 3 stable UACC903 cell lines exhibited marked expression of eGFP (Figure 1A). Increased expression of both PPARβ/δ and PPARγ mRNA and protein was confirmed by qPCR and western blot analysis in the stable cell lines as compared with controls (Figs. 1B–D). In particular, the mRNA expression of PPARβ/δ and PPARγ was increased 150- and 200-fold in the respective cell lines compared with control UACC903 cells (Figs. 1B and C). Similarly, a 7.5- and 10.8-fold increase in protein expression for PPARβ/δ and PPARγ, respectively, compared with control UACC903 cells, was also observed (Figure 1D). Although ligand activation of PPARβ/δ by GW0742 increased the expression of the target gene ANGPTL4 in all 4 cell lines, the induction was markedly higher in UACC903 cells overexpressing PPARβ/δ (Figure 1E). Ligand activation of PPARβ/δ did not influence expression of the putative PPARβ/δ target PDPK1 in any of the cell lines as compared with controls (Figure 1F). Similarly, ligand activation of PPARγ caused markedly enhanced expression of ANGPTL4 mRNA in UACC903 cells overexpressing PPARγ as compared with controls (Figure 1G) while expression of PDPK1 mRNA was unchanged following ligand activation of PPARγ by rosiglitazone (Figure 1H).
Figure 1.
Characterization of a human malignant melanoma cell line (UACC903) overexpressing PPARβ/δ or PPARγ. A, Representative photographs of control UACC903 cells, UACC903-Migr1 control cells (Migr1), UACC903-hPPARβ/δ cells (hPPARβ/δ), and UACC903-hPPARγ cells (hPPARγ) examined by fluorescent microscopy (upper panels) or light microscopy (lower panels). qPCR analysis for mRNA expression of PPARβ/δ (B) and PPARγ (C) in the UACC903 cell lines, normalized to the GAPDH mRNA. D, Western blot analysis of PPARβ/δ and PPARγ in the UACC903 cell lines, normalized to ACTIN expression. + indicates positive control: cell lysate from COS-1 cells transfected with a hPPARβ/δ or a hPPARγ expression vector. qPCR analysis of ANGPTL4 (E,G) and PDPK1 (F,H) mRNA expression in response to 0.0 – 10.0 µM of the PPARβ/δ ligand GW0742 (E,F) for 8 h or the PPARγ ligand rosiglitazone (G,H) for 24 h, normalized to the GAPDH mRNA. Data represents triplicate independent sample means ± SEM. Values with different letters are significantly different (p ≤ .05) using ANOVA with Bonferroni’s multiple comparison. The color image is available in the online version of the article.
Overexpression of PPARs and Ligand Activation of PPARs Modulates Cell Cycle Kinetics and Proliferation of UACC903 Human Melanoma Cells
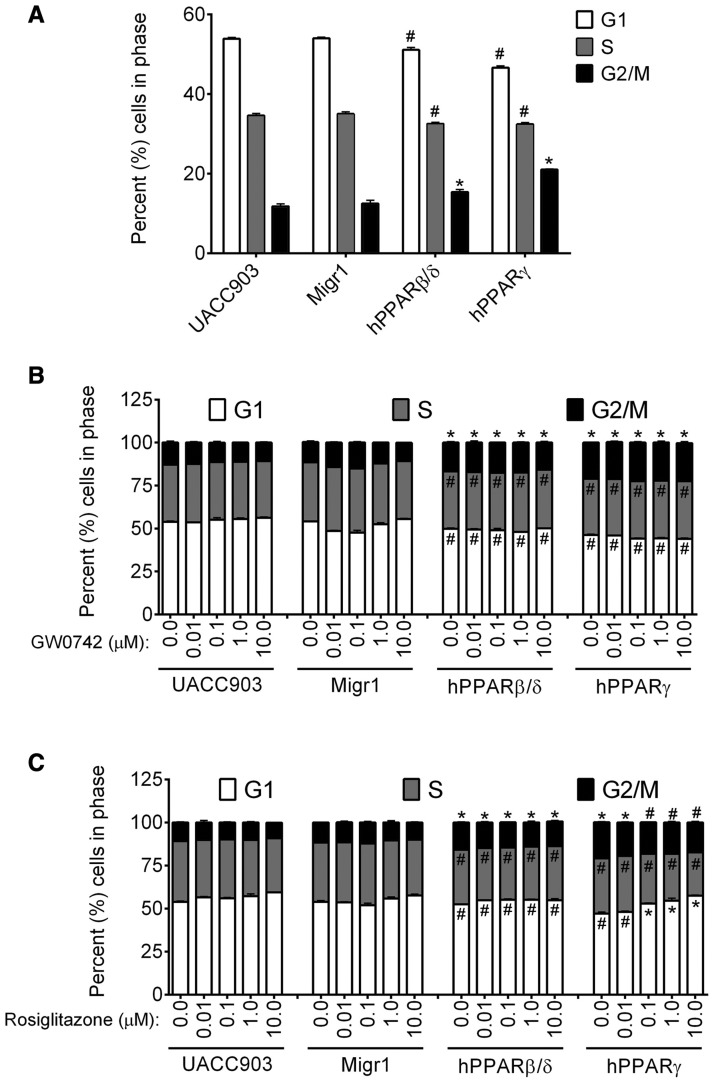
Twenty-four hours after plating the 4 different cell lines, overexpression of either PPARβ/δ or PPARγ resulted in a decrease in the percentage of cells in both the G1 and S phase, and an increase in the percentage of cells in the G2/M phase as compared with control UACC903 or UACC903-Migr1 cells (Figure 2A). It is also worth noting that the percentage of cells within each phase of the cell cycle was not different between the control UACC903 and control UACC903-Migr1 cell lines (Figure 2A). Although changes in G1, S and G2/M phases as described above were noted in the UACC903-hPPARβ/δ cells as compared with controls, ligand activation of PPARβ/δ did not further alter cell cycle kinetics in any of the 4 UACC903 cell lines (Figure 2B). By contrast, although the changes in G1, S, and G2/M phases as described above were observed in the UACC903-hPPARγ cells, ligand activation of PPARγ dose-dependently increased the percentage of cells in the G1 phase and decreased the percentage of cells within both the S and G2/M phase in UACC903-hPPARγ cells, and this effect was not found in control UACC903, UACC903-Migr1, or UACC903-hPPARβ/δ cells (Figure 2C).
Figure 2.
Effect of ligand activation and/or overexpression of PPARβ/δ or PPARγ on cell cycle progression. Cell cycle progression was examined in control UACC903, UACC903-Migr1 cells (Migr1), UACC903-hPPARβ/δ cells (hPPARβ/δ), and UACC903-hPPARγ cells (hPPARγ) by flow cytometry. A, Effect of PPARβ/δ or PPARγ overexpression on cell cycle progression. B, Effect of PPARβ/δ ligand activation on cell cycle progression. Cells were treated for 24 h with 0.0 – 10.0 µM GW0742. C, Effect of PPARγ ligand activation on cell cycle progression. Cells were treated for 24 h with 0.0–10.0 µM rosiglitazone. Data represents triplicate independent sample means ± SEM. *Significantly higher compared with UACC903 control (p ≤ .05) by ANOVA with Bonferroni’s multiple comparison. #Significantly lower compared with UACC903 control (p ≤ .05) by ANOVA with Bonferroni’s multiple comparison.
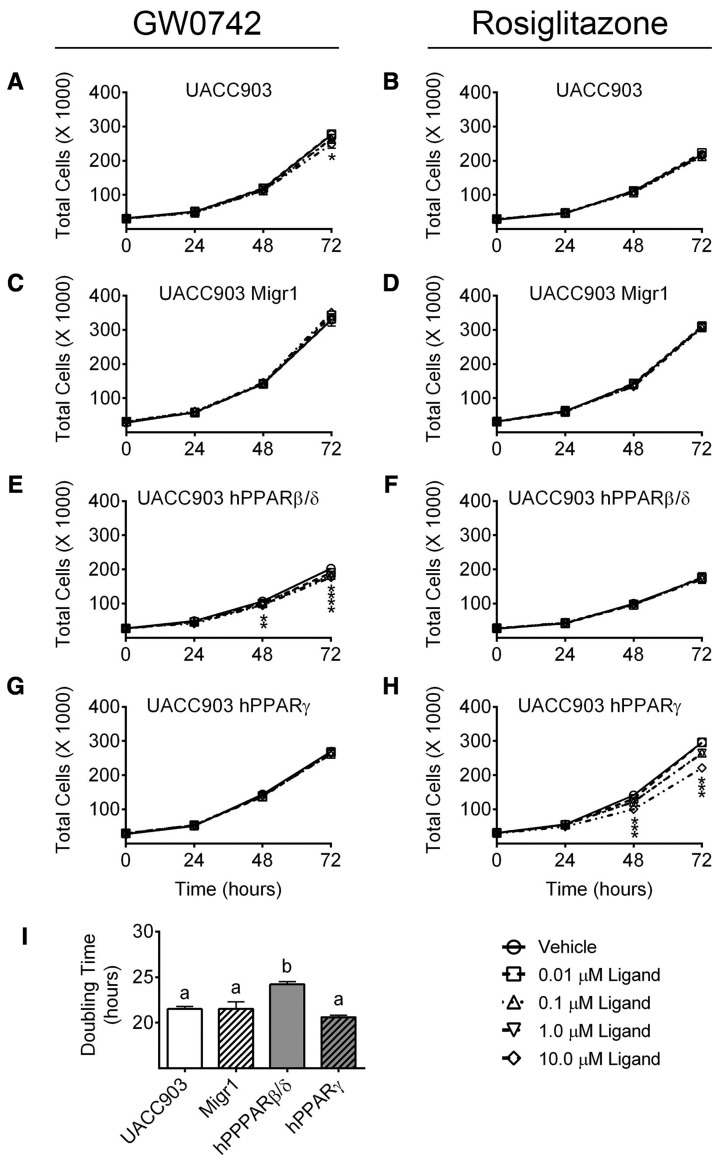
Given that both PPARβ/δ and PPARγ modulated cell cycle kinetics, the ability of both receptors to modulate cell proliferation in the presence or absence of ligand activation was examined using cell counting over a 72-h period. Ligand activation of PPARβ/δ with GW0742 reduced cell proliferation in cells overexpressing PPARβ/δ; these effects were observed as early as 48 h posttreatment (Figure 3E). Although ligand activation of PPARβ/δ had no effect on cell proliferation in UACC903-Migr1, or UACC903-hPPARγ cells (Figs. 3A, C, and G), proliferation of control UACC903 cells was inhibited by ligand activation of PPARβ/δ by GW0742 (10.0 µM) after 72 h (Figure 3A). Similarly, ligand activation of PPARγ by rosiglitazone markedly reduced cell proliferation in cells overexpressing PPARγ within 48 h (Figure 3H). Ligand activation of PPARγ by rosiglitazone did not alter proliferation in control UACC903 cells, UACC903-Migr1 cells, or UACC903-hPPARβ/δ cells (Figs. 3B, D, and F). Interestingly, human melanoma cancer cells overexpressing PPARβ/δ never achieved cell confluency equivalent to the other cell lines. The doubling time for the control UACC903, UACC903-Migr1, and UACC903-hPPARγ cell lines were all similar at approximately 21 h; however, cells overexpressing PPARβ/δ required more time, approximately 24 h, to double in population (Figure 3I).
Figure 3.
Effect of ligand activation and/or overexpression of PPARβ/δ or PPARγ on cell proliferation. The growth of UACC903, UACC903-Migr1 vector control cells (Migr1), UACC903-hPPARβ/δ cells (hPPARβ/δ), or UACC903-hPPARγ cells (hPPARγ) was examined over a 72-h period by Coulter Counting as described in “Materials and Methods” section. Cells were treated with indicated concentration of ligand, GW0742 (A,C,E,G) or rosiglitazone (B,D,F,H), at Time 0. Data represent triplicate independent sample means ± SEM. *Significantly different value (p ≤ .05) from cell line-specific vehicle control at the particular time point, as determined by ANOVA with Bonferroni’s multiple comparison. (I) Calculated doubling time from the 24- to 72-h growth period of the vehicle control in each cell line, as described in the “Materials and Methods” section. Values with different letters are significantly different (p ≤ 05) using ANOVA with Bonferroni’s multiple comparison.
PPARβ/δ and PPARγ Overexpression Inhibits Anchorage-Dependent Clonogenicity
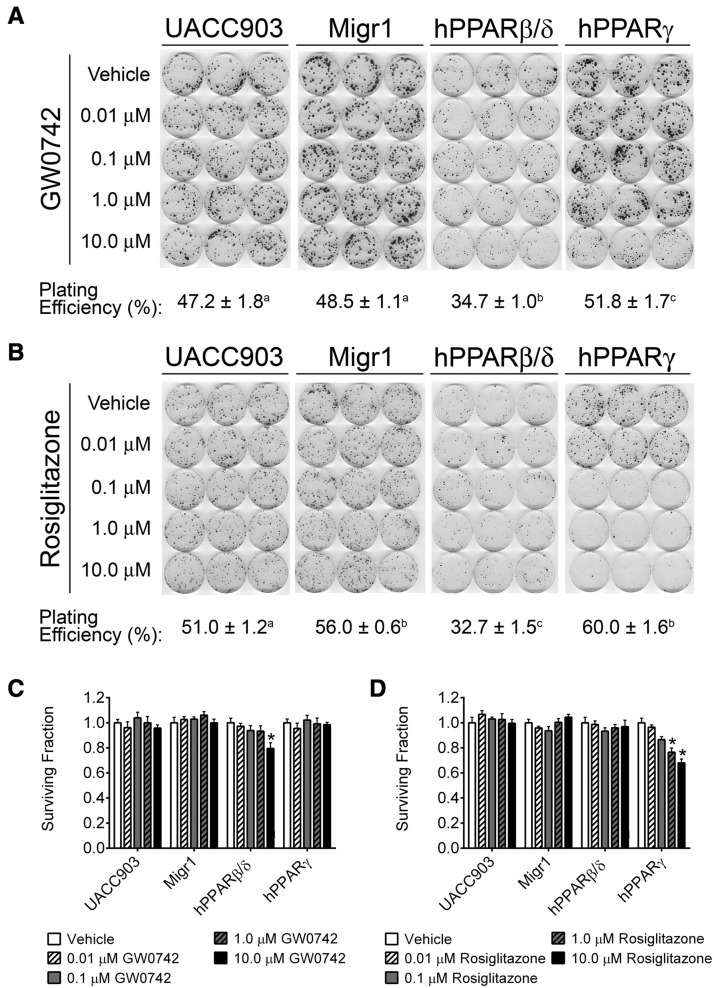
The ability of PPAR overexpression and/or ligand activation to modulate tumorigenicity was next examined by assessing anchorage-dependent clonogenicity. The plating efficiency of cells overexpressing PPARβ/δ was markedly reduced compared with the other 3 UACC903 cell lines (UACC903, UACC903-Migr1, or UACC903-hPPARγ; Figs. 4A and B). The foci formed from cells in the UACC903-hPPARβ/δ cells also appeared smaller than those formed from the other cell lines. Ligand activation of PPARβ/δ by GW0742 caused a 20% reduction in surviving fraction by administration of 10.0 μM GW0742 in UACC903-hPPARβ/δ cells (Figure 4C).
Figure 4.
Effect of ligand activation and/or overexpression of PPARβ/δ or PPARγ on anchorage-dependent clonogenicity. Anchorage-dependent clonogenicity was examined in control UACC903, UACC903-Migr1 vector control cells (Migr1), UACC903-hPPARβ/δ cells (hPPARβ/δ), or UACC903-hPPARγ cells (hPPARγ). Effect of GW0742 (A) or rosiglitazone (B) on clonal expansion. Cells were plated and treated as described in “Materials and Methods” section, and the dishes were left undisturbed for 14 days. The plating efficiency for each cell line is presented. Values with different letters are significantly different (p ≤ .05) using ANOVA with Bonferroni’s multiple comparison. Surviving fraction quantification from administration of GW0742 (C) or rosiglitazone (D). *Significantly different compared with cell line-specific vehicle control (p ≤ .05) by ANOVA with Bonferroni’s multiple comparison.
Ligand activation of PPARγ by rosiglitazone caused a 33% reduction in the colony surviving fraction (Figure 4D). Although the reduction was only observed in cells overexpressing PPARγ, a strong dose-dependent effect was observed in this cell line (Figure 4D).
Ligand Activation and/or Overexpression of PPARβ/δ and PPARγ Inhibit Ectopic Xenografts
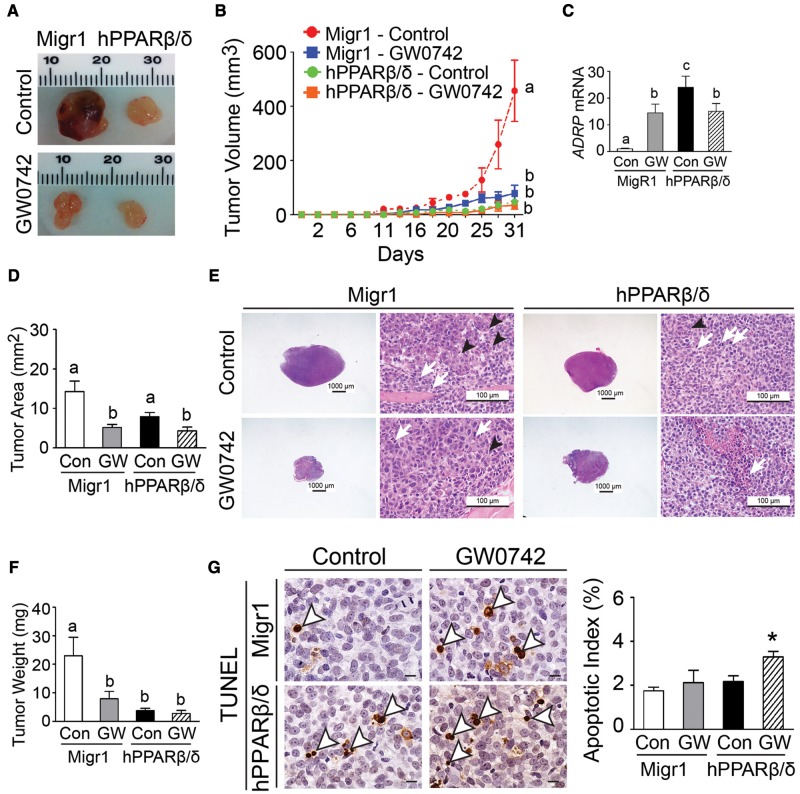
Ligand activation of PPARβ/δ by GW0742 markedly inhibited tumor volume, tumor weight, and tumor area in ectopic xenografts derived from UACC903-Migr1 cells as compared with controls (Figs. 5A–F). A similar phenotype was also observed in ectopic xenografts derived from UACC903-hPPARβ/δ cells as that observed with ligand activation of PPARβ/δ in ectopic xenografts derived from UACC903-Migr1 cells (Figs. 5A–F). The effects observed in ectopic xenografts derived from UACC903-hPPARβ/δ cells, with or without ligand activation of PPARβ/δ were similar to tumors derived from UACC903-Migr1 cells in response to ligand activation of PPARβ/δ (Figs. 5A–F). The latter suggests the presence of an endogenous ligand and is consistent with the observed increase in relative expression of the PPARβ/δ target gene ADRP in ectopic xenografts derived from UACC903-hPPARβ/δ cells as compared with controls (Figure 5C). Histological examination of the tumor tissues revealed infiltrating neutrophils within the tumor area and the infiltration was notably reduced in tumors in response to ligand activation of PPARβ/δ (Figure 5E). Although overexpression PPARβ/δ alone did not alter apoptotic index compared with tumors derived from UACC903-Migr1 cells, ligand activation of PPARβ/δ increased apoptosis in tumors derived from UACC903-hPPARβ/δ cells as compared with controls (Figure 5G).
Figure 5.
Ligand activation and/or overexpression of PPARβ/δ suppresses ectopic xenografts derived from UACC903 melanoma cancer cell lines. A, Representative photomicrographs of xenografts derived from UACC903-Migr1 (Migr1) or UACC903-hPPARβ/δ (hPPARβ/δ) cells. B, Average tumor volumes over time. C, Relative expression of the PPARβ/δ target gene ADRP in tumors. D, Average tumor area in paraffin-embedded tumor sections. E, Representative photomicrographs of H&E-stained xenografts. Tumor cells frequently exhibited mitotic figures (white arrows) and rosette structure (black arrowheads). Magnification = 12.5 × (left) and 400 × (right). F, Average tumor weight at the end of the study. G, Apoptosis in xenograft tumors was determined by TUNEL assay. Left panel, apoptotic tumor cells are indicated by white arrowheads. Magnification = 1000×. Bar = 10 μm. Right panel, quantification of apoptotic index in tumor sections. Control = (Con); GW0742-treated (2.5 mg/kg/d) = (GW). Values represent the mean ± SEM. Values with different superscript letters are significantly different at p ≤ .05. *Significantly different than control, p ≤ .05. The color image is available in the online version of the article.
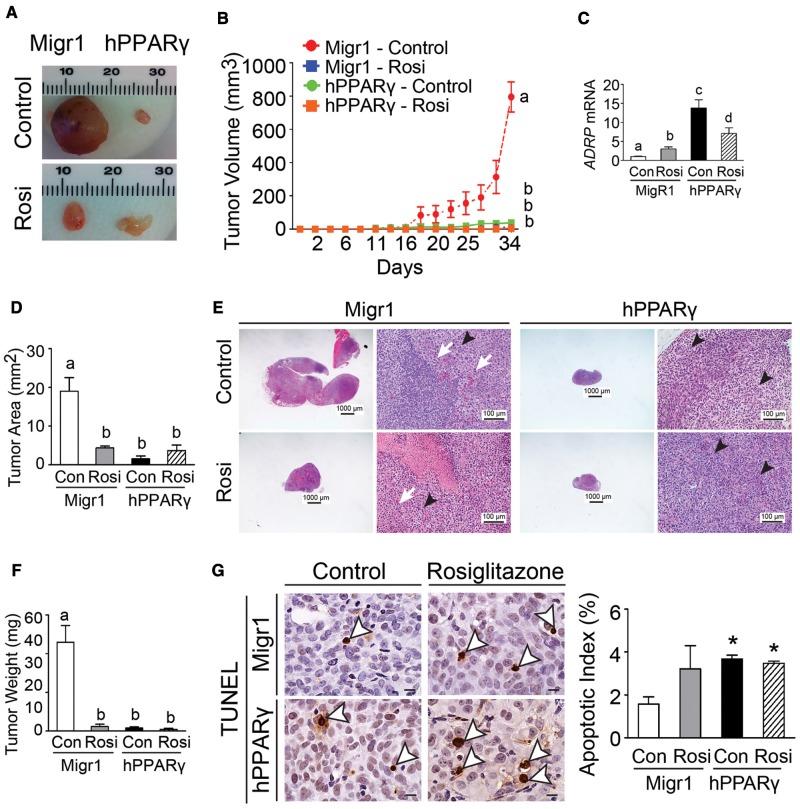
Ligand activation of PPARγ by rosiglitazone markedly inhibited tumor volume, tumor weight, and tumor area in ectopic xenografts derived from UACC903-Migr1 cells as compared with controls (Figs. 6A–F). A similar phenotype was also observed in ectopic xenografts derived from UACC903-hPPARγ cells as that observed with ligand activation of PPARγ in ectopic xenografts derived from UACC903-Migr1 cells (Figs. 6A–F). The effects observed in ectopic xenografts derived from UACC903-hPPARγ cells, with or without ligand activation of PPARγ were similar to tumors derived from UACC903-Migr1 cells in response to ligand activation of PPARγ (Figs. 6A–F). The latter suggests the presence of an endogenous ligand and is consistent with the observed increase in relative expression of the PPARγ target gene ADRP in ectopic xenografts derived from UACC903-hPPARγ cells as compared with controls (Figure 6C). These effects were comparable to tumors derived from UACC903-Migr1 cells in response to ligand activation of PPARγ (Figs. 5A–E). Histological examination of the tumor tissues revealed infiltrating neutrophils within the tumor area and the infiltration was inhibited in tumors in response to ligand activation of PPARγ (Figure 6E). Overexpression of PPARγ alone increased the apoptotic index compared with tumors derived from UACC903-Migr1 cells, whereas ligand activation of PPARγ by rosiglitazone did not further increase the apoptotic index in tumors derived from UACC903-hPPARγ cells as compared with controls (Figure 6G).
Figure 6.
Ligand activation and/or overexpression of PPARγ suppresses ectopic xenografts derived from UACC903 melanoma cancer cell lines. A, Representative photomicrographs of xenografts derived from UACC903-Migr1 (Migr1) or UACC903-hPPARγ (hPPARγ) cells. B, Average tumor volumes over time. C, Relative expression of the PPARβ/δ target gene ADRP in tumors. D, Average tumor area in paraffin-embedded tumor sections. E, Representative photomicrographs of H&E-stained xenografts. Tumor cells frequently exhibited mitotic figures (white arrows) and rosette structure (black arrowheads). Magnification = 12.5 × (left) and 400 × (right). F, Average tumor weight at the end of the study. G, Apoptosis in xenograft tumors was determined by TUNEL assay. Left panel, apoptotic tumor cells were indicated by white arrowheads. Magnification = 1000×. Bar = 10 μm. Right panel, quantification of apoptotic index in tumor sections. Control = (Con); Rosiglitazone-treated (10 mg/kg/d) = (Rosi). Values represent the mean ± SEM. Values with different superscript letters are significantly different at p ≤ .05. *Significantly different than control, p ≤ .05. The color image is available in the online version of the article.
DISCUSSION
Results from the present studies are substantive for many reasons. The role of PPARβ/δ remains controversial because there are conflicting studies, with some showing that activation of PPARβ/δ promotes tumorigenesis and others showing that ligand activation of PPARβ/δ inhibits tumorigenesis (Peters etal., 2012, 2015a,b). Thus, more rigorous studies are needed to more precisely determine the role of PPARβ/δ in carcinogenesis so that this nodal transcription factor can be targeted for cancer chemoprevention and/or chemotherapy. To date, there are limited studies that have examined the role of PPARβ/δ in melanoma.
The first study that reported a role for PPARβ/δ in melanoma demonstrated that ligand activation PPARβ/δ with either GW501516 or GW0742 inhibited proliferation of human UACC903 melanoma cells as compared with controls (Girroir etal., 2008). A second study examined the role of PPARβ/δ in a human (A375) or a mouse (B16F0) melanoma cell line and also observed that ligand activation PPARβ/δ with either GW501516 or GW0742 inhibited proliferation, but this change was not due to increased apoptosis (Michiels etal., 2010). Results from the present studies are consistent with these 2 studies as they demonstrate that ligand activation and/or overexpression of PPARβ/δ inhibits tumorigenesis in a human melanoma cancer cell line. Moreover, these studies extend the former studies with complementary invitro and invivo analysis, and most importantly, demonstrate that ligand activation PPARβ/δ inhibits ectopic xenografts derived from a human melanoma cancer cell line with one of the most common mutations found in this disease. The mechanism underlying this preventive effect is likely mediated in part by the induction of apoptosis and a block in the G2/M phase of the cell cycle, consistent with previous studies (Borland etal., 2008, 2011; Zhu etal., 2012). It is unclear why the studies by Michiels and colleagues did not detect changes in apoptosis following ligand activation PPARβ/δ in human A375 melanoma cells compared with controls. It remains possible that this could be due to differences present in the invitro analysis versus the invivo analysis, or to differences in the sensitivities between A375 cells versus UACC903 melanoma cell lines. Further studies are needed to distinguish between these possibilities. It is also worth noting that the present studies also provide additional data showing that ligand activation and/or overexpression of PPARβ/δ does not increase expression of PDPK1, which is consistent with other studies (Ahmed etal., 2008; Borland etal., 2008, 2011; Zhu etal., 2014), but in contrast to another report (Di-Poi etal., 2002). The reason for this difference cannot be determined from these studies but the weight of evidence that PDPK1 is not a target gene of PPARβ/δ is becoming stronger.
Results from these studies also demonstrated that overexpression and/or ligand activation of PPARγ inhibits a human melanoma cancer cell line proliferation and this effect is reflected by the observed inhibition of ectopic xenograft tumorigenicity and enhanced apoptosis as compared with controls. In contrast to the effect of ligand activation of PPARβ/δ in carcinogenesis, the effect of the role of PPARγ in cancer is relatively less contentious. Results from these studies show that ligand activation and/or overexpression of PPARγ inhibits UACC903 cell cycle progression, proliferation and ectopic xenografts as compared with controls. These results are consistent with numerous studies showing similar effects in melanoma cancer cell lines (Botton etal., 2009; Chen etal., 2014; Eastham etal., 2008; Freudlsperger etal., 2006, 2007, 2008; Klopper etal., 2009, 2010; Meyer etal., 2010; Mossner etal., 2002; Nunez etal., 2006; Papi etal., 2009; Paulitschke etal., 2012; Placha etal., 2003; Sertznig etal., 2008, 2009). Of particular interest is the study showing that increased expression of ANGPTL4 prevents migration and invasion of melanoma cells (Galaup etal., 2006), as this target gene is increased by both ligand activation and/or overexpression of either PPARβ/δ or PPARγ.
The relative expression of PPARβ/δ in cancer cells as compared with control untransformed tissue is another area of contention. It has become increasingly clear that relative expression of PPARβ/δ is lower in most, but not all, tumor cells as compared with untransformed tissue (reviewed in (Peters etal., 2012, 2015a,b). In contrast to the original suggestion that PPARβ/δ mRNA expression is higher in colon tumors as compared with control tissue (He etal., 1999), it has been shown that expression of PPARβ/δ protein is markedly lower in most human and mouse colon tumors as compared with controls (Foreman etal., 2011; reviewed in Peters etal., 2012, 2015a,b). The hypothesis that relative expression of PPARβ/δ is higher in most tumors as compared with control tissue has been disputed for years and led to an alternative hypothesis that relatively high expression of PPARβ/δ in cancer cells could interfere with PPARγ signaling (Wang etal., 2012). Since it is known that ligand activation of PPARγ inhibits carcinogenesis by inducing differentiation and apoptosis, this was of interest because relatively high expression of PPARβ/δ in cancer cells as compared with untransformed tissue, this could interfere with the chemopreventive effects mediated by ligand activation of PPARγ. In addition to the fact that there are large databases showing that relative expression of PPARβ/δ protein is markedly lower in most human and mouse colon tumors as compared with controls (reviewed in Peters etal., 2012, 2015a,b), results from the present studies provide new evidence to address the hypothesis that relatively high expression of PPARβ/δ in cancer cells could prevent the chemopreventive activities associated with ligand activation of PPARγ. For example, relatively high expression of PPARβ/δ did not influence the relative efficacy of rosiglitazone to activate PPARγ in UACC903 cells as compared with controls, as shown by the relative increase ANGPTL4 expression. Further, relatively high expression of PPARβ/δ did not influence the relative ability of the PPARγ ligand rosiglitazone to modulate cell cycle or cell proliferation as compared with controls in UACC903 cells. Combined, these additional new data provide striking new evidence that increased expression of PPARβ/δ in a human melanoma cancer cell line can not only be beneficial because it can inhibit tumorigenesis as assessed both invitro and invivo, but they also strongly argue against the hypothesis that relatively high expression of PPARβ/δ in cancer cells could interfere with PPARγ signaling as suggested by others (Wang etal., 2012).
The results from the present studies and those by Girroir and Michiels examining melanoma cancer cell lines are in contrast to the original observation reported by others suggesting that overexpression of PPARβ/δ promotes tumorigenesis in human colon cancer (He etal., 1999). Interestingly, at least 5 published studies describing the effects of overexpression of PPARβ/δ in human cancer cell lines indicate that relatively higher expression of PPARβ/δ inhibits, but does not promote, proliferation of human cancer cell lines (Borland etal., 2011; Foreman etal., 2011; Yao etal., 2014, 2015, 2017). This is of interest to note because recent studies attempting to replicate results from studies examining druggable cancer targets demonstrated that the reported results are not consistently reproducible (Errington etal., 2014). This is important to note because the effect of ligand activation of PPARβ/δ in 2 A375 variant human melanoma cell lines (A375P and A375SM; the former with lower metastatic potential, the latter with greater metastatic potential) suggested that ligand activation PPARβ/δ promoted migration and invasion of human A375SM melanoma cells but did not influence migration or invasion in human A375P melanoma cells as compared with controls (Ham etal. 2014). The reason(s) for the differences between the present studies and others (Girroir etal., 2008; Michiels etal., 2010), and the study suggesting that ligand activation PPARβ/δ promotes melanoma migration and invasion cannot be determined from the present studies. However, these disparate results illustrate the need for future, more rigorous studies. This will provide for stronger rationales for the targeting of either PPARβ/δ or PPARγ for melanoma chemoprevention and/or chemotherapy.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr Gary H. Perdew at The Pennsylvania State University for the use of a fluorescent microscope, and the Penn State Microscopy and Cytometry Facility for their technical support in sorting the fluorescent cells.
FUNDING
This work supported in part by the National Institutes of Health (CA124533, CA140369, J.M.P.), Bloomsburg University (BU) Academic Enhancement Funds (M.G.B. and E.M.K.), a BU Research & Scholarship Grant (M.G.B.), and a BU Margin of Excellence Grant (M.G.B.).
REFERENCES
- Ahmed N., Riley C., Quinn M. A. (2008). An immunohistochemical perspective of PPARβ and one of its putative targets PDK1 in normal ovaries, benign and malignant ovarian tumours. Br. J. Cancer 98, 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddie S. C., John S., Hager G. L. (2010). Genome-wide mechanisms of nuclear receptor action. Trends Endocrinol. Metab. 21, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland M. G., Foreman J. E., Girroir E. E., Zolfaghari R., Sharma A. K., Amin S., Gonzalez F. J., Ross A. C., Peters J. M. (2008). Ligand activation of peroxisome proliferator-activated receptor-β/δ inhibits cell proliferation in human HaCaT keratinocytes. Mol. Pharmacol. 74, 1429–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland M. G., Khozoie C., Albrecht P. P., Zhu B., Lee C., Lahoti T. S., Gonzalez F. J., Peters J. M. (2011). Stable over-expression of PPARβ/δ and PPARγ to examine receptor signaling in human HaCaT keratinocytes. Cell. Signal. 23, 2039–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botton T., Puissant A., Bahadoran P., Annicotte J. S., Fajas L., Ortonne J. P., Gozzerino G., Zamoum T., Tartare-Deckert S., Bertolotto C., et al. (2009). In vitro and invivo anti-melanoma effects of ciglitazone. J. Invest. Dermatol. 129, 1208–1218. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Chang J. L., Chen P. R., Chuang Y. J., Tang S. T., Pan S. F., Lin T. B., Chen K. H., Chen M. J. (2014). Inhibition of peroxisome proliferator-activated receptor γ prevents the melanogenesis in murine B16/F10 melanoma cells. Biomed. Res. Int. 2014, 695797.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di-Poi N., Tan N. S., Michalik L., Wahli W., Desvergne B. (2002). Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol. Cell 10, 721–733. [DOI] [PubMed] [Google Scholar]
- Eastham L. L., Mills C. N., Niles R. M. (2008). PPARα/γ expression and activity in mouse and human melanocytes and melanoma cells. Pharm. Res. 25, 1327–1333. [DOI] [PubMed] [Google Scholar]
- Errington T. M., Iorns E., Gunn W., Tan F. E., Lomax J., Nosek B. A. (2014). An open investigation of the reproducibility of cancer biology research. Elife 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. E., Chang W. C., Palkar P. S., Zhu B., Borland M. G., Williams J. L., Kramer L. R., Clapper M. L., Gonzalez F. J., Peters J. M. (2011). Functional characterization of peroxisome proliferator-activated receptor-β/δ expression in colon cancer. Mol. Carcinog. 50, 884–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken N. A., Rodermond H. M., Stap J., Haveman J., van Bree C. (2006). Clonogenic assay of cells invitro. Nat. Protoc. 1, 2315–2319. [DOI] [PubMed] [Google Scholar]
- Franklin C., Livingstone E., Roesch A., Schilling B., Schadendorf D. (2016). Immunotherapy in melanoma: Recent advances and future directions. Eur. J. Surg. Oncol. 43, 604–611. [DOI] [PubMed] [Google Scholar]
- Freudlsperger C., Moll I., Schumacher U., Thies A. (2006). Anti-proliferative effect of peroxisome proliferator-activated receptor γ agonists on human malignant melanoma cells invitro. Anticancer Drugs 17, 325–332. [DOI] [PubMed] [Google Scholar]
- Freudlsperger C., Schumacher U., Reinert S., Hoffmann J. (2008). The critical role of PPARγ in human malignant melanoma. PPAR Res. 2008, 503797.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudlsperger C., Thies A., Pfuller U., Schumacher U. (2007). The proteasome inhibitor bortezomib augments anti-proliferative effects of mistletoe lectin-I and the PPAR-γ agonist rosiglitazone in human melanoma cells. Anticancer Res. 27, 207–213. [PubMed] [Google Scholar]
- Galaup A., Cazes A., Le Jan S., Philippe J., Connault E., Le Coz E., Mekid H., Mir L. M., Opolon P., Corvol P., et al. (2006). Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc. Natl. Acad. Sci. U. S. A. 103, 18721–18726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girroir E. E., Hollingshead H. E., Billin A. N., Willson T. M., Robertson G. P., Sharma A. K., Amin S., Gonzalez F. J., Peters J. M. (2008). Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) ligands inhibit growth of UACC903 and MCF7 human cancer cell lines. Toxicology 243, 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager G. L., Varticovski L. (2012). Chromatin in time and space. Biochim. Biophys. Acta 1819, 631.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham S. A., Yoo T., Hwang J. S., Kang E. S., Lee W. J., Paek K. S., Park C., Kim J. H., Do J. T., Lim D. S., et al. (2014). Ligand-activated PPARδ modulates the migration and invasion of melanoma cells by regulating Snail expression. Am. J. Cancer Res. 4, 674–682. [PMC free article] [PubMed] [Google Scholar]
- He T. C., Chan T. A., Vogelstein B., Kinzler K. W. (1999). PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinaniemi M., Uski J. O., Degenhardt T., Carlberg C. (2007). Meta-analysis of primary target genes of peroxisome proliferator-activated receptors. Genome Biol. 8, R147.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N., Noone A., Krapcho M., Miller D., Bishop K., Altekruse S. F., Kosary C. L., Yu M., Ruhl J., et al. (eds). (2016). SEER Cancer Statistics Review, 1975-2013. National Cancer Institute. [Google Scholar]
- Kang H. Y., Chung E., Lee M., Cho Y., Kang W. H. (2004). Expression and function of peroxisome proliferator-activated receptors in human melanocytes. Br. J. Dermatol. 150, 462–468. [DOI] [PubMed] [Google Scholar]
- Klopper J. P., Sharma V., Berenz A., Hays W. R., Loi M., Pugazhenthi U., Said S., Haugen B. R. (2009). Retinoid and thiazolidinedione therapies in melanoma: An analysis of differential response based on nuclear hormone receptor expression. Mol. Cancer 8, 16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopper J. P., Sharma V., Bissonnette R., Haugen B. R. (2010). Combination PPARγ and RXR agonist treatment in melanoma cells: functional importance of S100A2. PPAR Res. 2010, 729876.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz M. (2014). Oncogenes in melanoma: An update. Eur. J. Cell Biol. 93, 1–10. [DOI] [PubMed] [Google Scholar]
- Meyer S., Vogt T., Landthaler M., Berand A., Reichle A., Bataille F., Marx A. H., Menz A., Hartmann A., Kunz-Schughart L. A., et al. (2010). Cyclooxygenase 2 (COX2) and peroxisome proliferator-activated receptor gamma (PPARG) are stage-dependent prognostic markers of malignant melanoma. PPAR Res. 2009, 848645.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels J. F., Perrin C., Leccia N., Massi D., Grimaldi P., Wagner N. (2010). PPARβ activation inhibits melanoma cell proliferation involving repression of the Wilms' tumour suppressor WT1. Pflugers Arch. 459, 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossner R., Schulz U., Kruger U., Middel P., Schinner S., Fuzesi L., Neumann C., Reich K. (2002). Agonists of peroxisome proliferator-activated receptor γ inhibit cell growth in malignant melanoma. J. Invest. Dermatol. 119, 576–582. [DOI] [PubMed] [Google Scholar]
- Nunez N. P., Liu H., Meadows G. G. (2006). PPAR-γ ligands and amino acid deprivation promote apoptosis of melanoma, prostate, and breast cancer cells. Cancer Lett. 236, 133–141. [DOI] [PubMed] [Google Scholar]
- Papi A., Rocchi P., Ferreri A. M., Guerra F., Orlandi M. (2009). Enhanced effects of PPARγ ligands and RXR selective retinoids in combination to inhibit migration and invasiveness in cancer cells. Oncol. Rep. 21, 1083–1089. [DOI] [PubMed] [Google Scholar]
- Paulitschke V., Gruber S., Hofstatter E., Haudek-Prinz V., Klepeisz P., Schicher N., Jonak C., Petzelbauer P., Pehamberger H., Gerner C., et al. (2012). Proteome analysis identified the PPARγ ligand 15d-PGJ2 as a novel drug inhibiting melanoma progression and interfering with tumor-stroma interaction. PLoS One 7, e46103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear W. S., Miller J. P., Xu L., Pui J. C., Soffer B., Quackenbush R. C., Pendergast A. M., Bronson R., Aster J. C., Scott M. L., et al. (1998). Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92, 3780–3792. [PubMed] [Google Scholar]
- Peters J. M., Gonzalez F. J. (2009). Sorting out the functional role(s) of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in cell proliferation and cancer. Biochim. Biophys. Acta 1796, 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. M., Gonzalez F. J., Muller R. (2015a). Establishing the Role of PPARβ/δ in Carcinogenesis. Trends Endocrinol. Metab. 26, 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. M., Shah Y. M., Gonzalez F. J. (2012). The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer 12, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. M., Yao P. L., Gonzalez F. J. (2015b). Targeting peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) for cancer chemoprevention. Curr. Pharmacol. Rep. 1, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placha W., Gil D., Dembinska-Kiec A., Laidler P. (2003). The effect of PPARγ ligands on the proliferation and apoptosis of human melanoma cells. Melanoma Res. 13, 447–456. [DOI] [PubMed] [Google Scholar]
- Sertznig P., Dunlop T., Seifert M., Tilgen W., Reichrath J. (2009). Cross-talk between vitamin D receptor (VDR)- and peroxisome proliferator-activated receptor (PPAR)-signaling in melanoma cells. Anticancer Res. 29, 3647–3658. [PubMed] [Google Scholar]
- Sertznig P., Seifert M., Tilgen W., Reichrath J. (2008). Peroxisome proliferator-activated receptors (PPARs) and the human skin: Importance of PPARs in skin physiology and dermatologic diseases. Am. J. Clin. Dermatol. 9, 15–31. [DOI] [PubMed] [Google Scholar]
- Shi Y., Hon M., Evans R. M. (2002). The peroxisome proliferator-activated receptor δ, an integrator of transcriptional repression and nuclear receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 99, 2613–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Davies M. Q., Hwu P., Yang J., Lotem M., Oren M., Flaherty K. T., Fisher D. E. (2014). Pathways and therapeutic targets in melanoma. Oncotarget 5, 1701–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2017). Cancer Statistics, 2017. CA Cancer J. Clin. 67, 7–30. [DOI] [PubMed] [Google Scholar]
- Wang D., Ning W., Xie D., Guo L., DuBois R. N. (2012). Peroxisome proliferator-activated receptor δ confers resistance to peroxisome proliferator-activated receptor γ-induced apoptosis in colorectal cancer cells. Oncogene 31, 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P. L., Chen L., Dobrzanski T. P., Zhu B., Kang B. H., Muller R., Gonzalez F. J., Peters J. M. (2017). Peroxisome proliferator-activated receptor-β/δ inhibits human neuroblastoma cell tumorigenesis by inducing p53- and SOX2-mediated cell differentiation. Mol. Carcinog. 56, 1472–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P. L., Chen L. P., Dobrzanski T. P., Phillips D. A., Zhu B., Kang B. H., Gonzalez F. J., Peters J. M. (2015). Inhibition of testicular embryonal carcinoma cell tumorigenicity by peroxisome proliferator-activated receptor-β/δ- and retinoic acid receptor-dependent mechanisms. Oncotarget 6, 36319–36337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P. L., Morales J. L., Zhu B., Kang B. H., Gonzalez F. J., Peters J. M. (2014). Activation of peroxisome proliferator-activated receptor-β/δ (PPAR-β/δ) inhibits human breast cancer cell line tumorigenicity. Mol. Cancer Ther. 13, 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Dutton-Regester K., Brown K. M., Hayward N. K. (2016). The genomic landscape of cutaneous melanoma. Pigment. Cell Melanoma Res. 29, 266–283. [DOI] [PubMed] [Google Scholar]
- Zhu B., Ferry C. H., Blazanin N., Bility M. T., Khozoie C., Kang B. H., Glick A. B., Gonzalez F. J., Peters J. M. (2014). PPARβ/δ promotes HRAS-induced senescence and tumor suppression by potentiating p-ERK and repressing p-AKT signaling. Oncogene 33, 5348–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Khozoie C., Bility M. T., Ferry C. H., Blazanin N., Glick A. B., Gonzalez F. J., Peters J. M. (2012). Peroxisome proliferator-activated receptor β/δ cross talks with E2F and attenuates mitosis in HRAS-expressing cells. Mol. Cell. Biol. 32, 2065–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]