Abstract
Aims
Diabetes mellitus (DM) is associated with the development of cardiovascular disease (CVD). Morphological changes in the left atrium (LA) may appear before symptoms. We aimed to investigate the association between cardiac magnetic resonance imaging (CMR) measured LA structure and function and incident CVD in asymptomatic individuals with DM.
Methods and results
Tissue tracking CMR was used to measure LA size and phasic function (emptying fractions and strain) on all 536 Multi-Ethnic Study of Atherosclerosis (MESA) participants with DM and available CMR at baseline in 2000–2002. At the time of enrolment, all participants were free of clinically recognized CVD, which was defined as MI, resuscitated cardiac arrest, angina, stroke, heart failure, and atrial fibrillation. Cox regression was used to assess the association of LA parameters with incident CVD adjusted for traditional cardiovascular risk factors, LV mass, NT Pro-BNP and maximum LA volume. Kaplan–Meier curves, adjusted for traditional risk factors, were generated for each LA measurement for the 25% of participants with the most abnormal values versus the remaining 75%. After a mean follow up of 11.4 ± 3.4 years, 141 individuals developed CVD. Individuals with incident CVD (mean age 66 years, 66% male vs. mean age 64 years, 50% male) had larger maximum and minimum LA volume index (LAVI) (32.1 vs. 26.8 mm3/m2; 19.4 vs. 14.2 mm3/m2 respectively, P < 0.001 for both), and lower total, passive, and active EF than those without CVD (P < 0.01 for all). In the fully adjusted model, there was a significant association of minimum LAVI, LA total EF, LA passive EF and LA active EF with incident CVD (HR 1.12 per mm3/m2, P < 0.001; HR 0.95 per %, P < 0.001; HR 0.97 per %, P = 0.021; HR 0.98 per %, P < 0.027, respectively).
Conclusions
CMR measured LA minimum volume and LA function as measured by emptying fraction are predictive of CVD in a diabetic multi-ethnic population free of any clinically recognized CVD at baseline.
Keywords: atrium , diabetes mellitus , magnetic resonance imaging , left atrial function
Introduction
Diabetes mellitus is increasingly prevalent in the United States with current estimates suggesting that greater than 11% of adults meet diagnostic criteria. Projected trends are even more concerning; the Center for Disease Control estimates that 1 in 3 American adults will have diabetes in 2050 if the current incidence continues.1 Cardiovascular disease is the most important cause of mortality in diabetics2,3 and adults with diabetes have age-specific mortality rates that are four-fold greater than the general population.1 It is important to examine this population for identifiable predictors of cardiovascular disease.
Early cardiac remodelling such as LA enlargement has been shown to occur in patients with diabetes.4,5 LA dilation is thought to reflect either coexisting volume overload or LV pressure abnormalities in the absence of valvular or rhythm abnormalities.6 Likely due to these associations, LA enlargement is a predictor of outcomes including MI and heart failure, and additionally provides prognostic information for asymptomatic patients.7–10 Not surprisingly, LV diastolic dysfunction and, recently, LA volume have been shown to independently predict all-cause mortality in patients with diabetes.5,11 Little is known, however, about the association of function with incident cardiovascular disease (CVD) in this population.11
Cardiac MRI (CMR) is the most accurate non-invasive method to assess LA size and function, though it has lower temporal resolution compared to echocardiogram.12 It offers the ability to both confirm earlier findings and identify additional LA features that are associated with adverse cardiovascular outcomes of DM.12 Studies in the Multi-Ethnic Study of Atherosclerosis (MESA) investigating the association of CMR measured LA function and cardiovascular outcomes have been promising, with key parameters explored including LA volumes, function, and strain.13,14
In this study, we aimed to evaluate the association of LA structure and function measured with CMR and cardiovascular outcomes in diabetic participants of MESA. We hypothesized that increased LA size and impaired LA function are associated with the subsequent development of cardiovascular disease in this population.
Methods
Data
Subjects
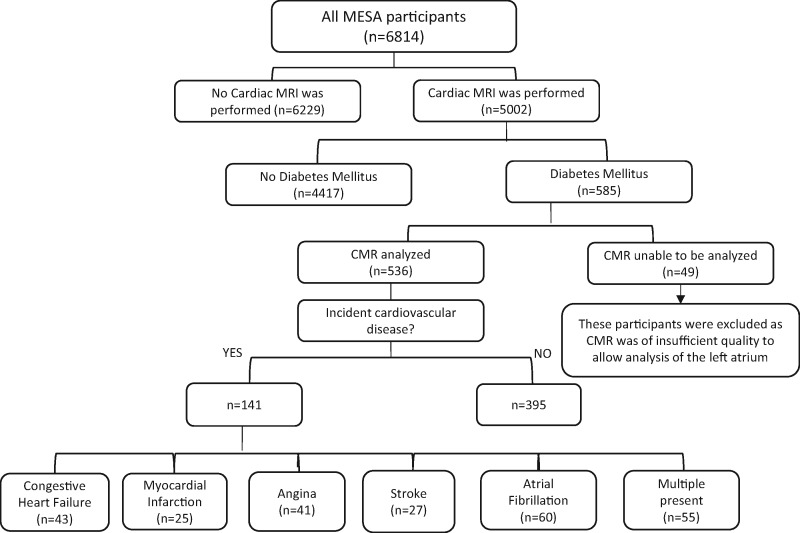
MESA was initiated to investigate prevalence, correlates, and progression of subclinical cardiovascular disease. Between July 2000 and August 2002, 6814 men and women who were 45 to 84 years old and free of clinically apparent cardiovascular disease were recruited from 6 US communities: Baltimore City and Baltimore County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan and the Bronx, NY; and St. Paul, MN.15 Approval was received from Institutional Review Boards at each participating university before the start of the study and protocol modifications were reviewed and approved each year. All participants of MESA with a diagnosis of type 2 DM, defined by a fasting glucose ≥126 mg/dL or current treatment with insulin or oral agents, who also had a baseline CMR, were included in this analysis (n = 585, Figure 1).
Figure 1.
Flow chart of MESA participants who were included in this study.
Data collection
Covariates
The follow covariates were analysed: age, gender, race, cigarette smoking status, systolic and diastolic blood pressure, heart rate, HDL, total cholesterol, hypertension medication use, body mass index (BMI), left ventricular mass by CMR, and N-terminal pro-brain natriuretic peptide (NT-Pro BNP).16 Demographic information was collected by standardized questionnaire. Laboratory studies were drawn at the time of baseline imaging and blood pressure was measured using an automated oscillometric cuff on three consecutive readings with the final two being averaged for analysis.
MRI protocol
The details of CMR protocol has been described previously.17 Images were acquired by 1.5-T MR scanners (SIGNA LX and CVi, GE Electric Medical Systems, and Somatom Vision and Sonata, Siemens Medical Solutions). Dedicated phase-array coils were used for signal reception. After the standard protocol was completed, 3 tagged short-axis slices were obtained. All images were obtained by ECG-triggered segmented k-space fast gradient-echo (SPGR or FLASH) pulse sequence during breath holds (12 to 18 s). All CMR studies were submitted to the core MESA MRI Reading Center at Johns Hopkins Hospital where all the analyses were performed.
Assessment of LA structure and function
The method of LA functional and structural analysis has been described previously with excellent reproducibility.18 Multimodality Tissue Tracking software (MTT; version 6.0, Toshiba, Japan) was used to obtain LA volume and global longitudinal strain from baseline 4-chamber and 2-chamber cine CMR images.
LA volume measurement
An experienced operator, who was blinded to the CVD outcomes of the participants, marked points along the endocardial and epicardial borders in the LA. Using the marked points, the software searched for the most closely matching borders in the subsequent frames. The operator then followed endocardial and epicardial contours generated by the software during the cardiac cycle for quality control. Based on the biplane area–length method from apical 4-and 2-chamber views, the software generated a volume/time curve during the cardiac cycle. The maximum, minimum and pre-atrial contraction LA volumes were extracted from the volume/time curves.18 We have previously demonstrated excellent inter-observer and intra-observer reliability.19
Maximum LA volume (LAVmax) is defined as the volume at end-systole, before the mitral valve opens. Minimum LA volume (LAVmin) is defined as volume at end-diastole, after the mitral value closes. Pre-atrial contraction LA volume (LAVPreA) is defined as volume before the initiation of atrial contraction. LA volume index (LAVI) is indexed by body surface area.
Total LA emptying fraction (LAEF) was calculated as 100 × (LAVmax–LAVmin)/LAVmax. Passive LAEF as 100 × (LAVmax–LAVpre-a)/LAVmax, and the active LAEF as 100 × (LAVpre-a–LAVmin)/LAVpre-a.
Strain measurement
By averaging longitudinal strain in all LA segments in 4 and 2 chamber cine images, the software generated global longitudinal strain curves during the cardiac cycle. Global peak longitudinal atrial strain (PLAS) and longitudinal strain before atrial contraction (PreA-S) were measured from the global longitudinal strain curve.20
Clinical follow-up
Study participants were contacted every 9–12 months during follow-up to identify clinically detected CVD events. Medical records, including discharge diagnoses, were obtained for each hospitalization. The participants were followed for a mean of 11.4 ± 3.4 years and the development of the primary endpoint, incident cardiovascular disease, was identified. Incident cardiovascular disease was defined as the MESA CVD outcome with heart failure and atrial fibrillation added.15,16 Medical records were reviewed and the occurrence of CVD events, except for atrial fibrillation, were adjudicated by the MESA Morbidity and Mortality Committee.
Myocardial infarction was classified based on a combination of symptoms, ECG changes, and cardiac biomarker levels (≥2 times upper limits of normal). Definite angina required objective evidence of reversible myocardial ischemia or obstructive coronary artery disease. Stroke was classified as present with documented rapid onset focal neurologic deficit lasting >24 h, or, if <24 h, when there was a clinically relevant lesion on brain imaging. Deficits secondary to brain trauma, tumour, infection, or other non-vascular cause were excluded. Atrial fibrillation was identified based on ICD-9 hospital discharge diagnosis codes for atrial fibrillation and atrial flutter (427.31 and 427.32, respectively) ascertained by MESA events detection protocol or from Medicare inpatient claims data, and from a study ECG conducted about 10 years after baseline. ‘Probable’ heart failure was defined by symptoms, such as shortness of breath or oedema plus a physician diagnosis of heart failure. ‘Definite’ heart failure also included objective evidence such as pulmonary oedema, dilated ventricles, or reduced ejection fraction on imaging.
Statistical analysis
Continuous variables are presented as mean ± standard deviation. Categorical variables are presented as numbers and percentages. The Student t-test was used to test for between-group differences in independent continuous variables. The chi2 test was used to test for differences between categorical variables.
The association between LA parameters and incidence of cardiovascular events was assessed using Cox proportional hazards. Failure time in the individuals with incident CVD was the time between the baseline CMR and the time of the diagnosis. For participants without cardiovascular events, the failure time was the time between baseline examination and the latest follow-up, death, or loss to follow-up.
The association of each LA parameter with combined cardiovascular events was assessed in separate Cox models. Spline curve analysis was performed for each parameter to justify the use of linear Cox models.20 In the first model (model 1), the analysis was adjusted for traditional cardiovascular risk factors including age (coefficient ± standard error; 0.01 ± 0.005), gender (0.25 ± 0.10), race (0.02 ± 0.04), cigarette smoking status(0.16 ± 0.06), BMI (0.004 ± 0.009), resting heart rate(0.002 ± 0.004), diastolic blood pressure (0.007 ± 0.005), hypertension medication use (0.12 ± 0.09), total cholesterol(0.001 ± 0.001), and HDL cholesterol (0.007 ± 0.003).21 In model 2, we additionally adjusted for LV mass and NT-proBNP. Logarithmic transformation was applied to NT-proBNP before entry into the models because of its skewed distribution. In the third model, we also adjusted for maximum LAV. Hazard Ratios were calculated with associated 95% confidence intervals (CI) and reported for one-unit increase in continuous variables.
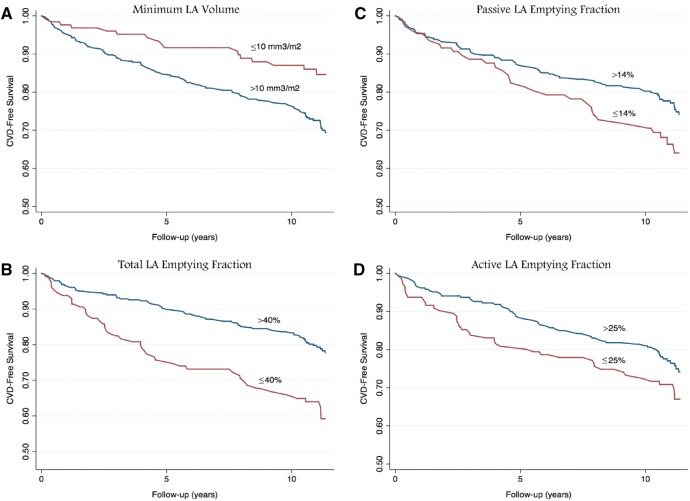
Overall event rates were presented in adjusted Kaplan–Meier curves based on 25th percentile cut offs (Figure 2). Receiver-operating characteristic curves were generated to assess the overall performance of these 3 models and LA parameters in predicting CVD events.
Figure 2.
Traditional risk factor adjusted Kaplan–Meier survival functions of incident cardiovascular disease for (A) minimum LAVI, (B) total emptying fraction, (C) passive emptying fraction, (D) active emptying fraction.
Results
Participants
Of the 585 participants with DM and a baseline CMR, it was feasible to measure LA parameters in a total of 536 (91.6%) subjects. After a mean follow up of 11.4 ± 3.4 years, 141 of the 536 enrolled participants (26%) experienced incident CVD. Baseline demographics of participants are summarized in Table 1. Though not meeting significance, diastolic blood pressure was lower and HDL was higher among those without incident CVD (71.6 10.0 vs 73.3 11.6 mmHg, P = 0.056; 46.7 13.0 vs. 44.7 12.8 pmol/L, P = 0.077). Of the 141 individuals who developed CVD, 43 developed congestive heart failure, 25 had a myocardial infarction, 41 had definite or probable angina, 27 had a stroke, and 60 developed atrial fibrillation. Some participants developed more than one event with 12 of 25 with myocardial infarction also having angina. A total of 32 participants died from a CVD related cause.
Table 1.
Participant demographic information
| Total (n = 536) | No cardiovascular disease (n = 395) | With cardiovascular disease (n = 141) | P-value | |
|---|---|---|---|---|
| Age, years | 64.19.2 | 63.69.5 | 66.47.5 | 0.004 |
| Male | 290 (54) | 197 (50) | 93 (66) | <0.001 |
| Caucasian | 107 (20) | 75 (19) | 32(22) | 0.330 |
| BMI (kg/m2) | 29.65.2 | 29.65.2 | 29.75.3 | 0.847 |
| Systolic BP, mm Hg | 131.922.0 | 130.621.2 | 137.424.6 | 0.004 |
| Diastolic BP, mm Hg | 71.910.2 | 71.610.0 | 73.311.6 | 0.056 |
| Heart Rate, beats per min | 66.910.3 | 66.910.1 | 67.41.3 | 0.666 |
| Current Cigarette Smoking | 70 (13) | 43 (11) | 27 (19) | 0.332 |
| Pack years | 15.012.9 | 14.612.3 | 16.415.3 | 0.184 |
| Total cholesterol, pmol/L | 188.437.9 | 188.937.0 | 186.441.7 | 0.271 |
| HDL cholesterol, pmol/L | 46.313.0 | 46.713.0 | 44.712.8 | 0.077 |
| Antihypertensive use (%) | 338 (63) | 237 (60) | 101 (72) | 0.014 |
| Statin use (%) | 158 (29) | 102 (26) | 56 (40) | 0.003 |
| Left ventricular mass, g | 157.342.7 | 152.339.6 | 179.348.6 | <0.001 |
| Fasting glucose (mg/dL) | 159.9 | 159.6 | 162.0 | 0.724 |
| Creatinine (mg/dL) | 0.980.63 | 0.950.12 | 1.19 | 0.001 |
The Student t-test was used to test for between-group differences in independent continuous variables. The chi2 test was used to test for differences between categorical variables.
Individuals who developed cardiovascular disease were older (66 ± 9 vs. 64 ± 9 years, P = 0.004), had higher systolic blood pressure (137 ± 25 vs. 131 ± 21 mmHg, P = 0.004), were more likely to be male (66 vs. 50%, P < 0.001), and had larger LV mass (179.3 ± 48.6 vs. 152.3 ± 39.6 g, P < 0.001).
Baseline LA parameters in participants with and without incident CVD are presented in Table 2. Incident cases had a larger LA compared with those who did not develop CVD (maximum LAVI: 32.1 ± 12.5 mm3/m2 vs. 26.8 ± 9.6 mm3/m2, P < 0.001 and minimum LAVI: 19.4 ± 9.7 mm3/m2 vs. 14.2 ±6.0 mm3/m2, P < 0.001). Lower total, passive, and active LAEF were seen in those with incident CVD (41.5 ± 11.4% vs. 47.6 ± 9.2%, P < 0.001; 17.9 ± 8.3 vs. 21.4 ± 9.9, P = 0.002; 28.8 ± 11.3% vs. 32.3 ± 10.6%, P = 0.003, respectively). In unadjusted analysis, peak global longitudinal strain was not significantly different between individuals with and without incident CVD.
Table 2.
Comparing LA parameters between individuals with and without cardiovascular disease
| LA parameter | Total (n = 536) | No cardiovascular disease (n = 395) | With cardiovascular disease (n = 141) | P-value |
|---|---|---|---|---|
| LA maximum volume index, mm3/m2 | 27.810.4 | 26.89.6 | 32.112.5 | <0.001 |
| LA minimum volume index, mm3/m2 | 15.17.1 | 14.26.0 | 19.49.7 | <0.001 |
| Total LAEF, % | 46.59.9 | 47.6 | 41.511.4 | <0.001 |
| Passive LAEF, % | 20.79.7 | 21.49.9 | 17.98.3 | 0.002 |
| Active LAEF, % | 31.610.9 | 32.310.6 | 28.811.3 | 0.003 |
| LA maximum strain | 28.511.7 | 28.811.6 | 27.012.3 | 0.086 |
The association between LA parameters and incidence of cardiovascular events was assessed using Cox proportional hazards. Failure time in the individuals with incident CVD was the time between the baseline CMR and the time of the diagnosis. For participants without cardiovascular events, the failure time was the time between baseline examination and the latest follow-up, death, or loss to follow-up. LA, Left Atrium; LAEF, Left Atrium Emptying Fraction.
Association of LA volume and function with incident CVD
In the initial model, higher maximum and minimum LAVI, and a lower total EF, passive EF, and active EF were all significantly associated with incident CVD when adjusted for traditional risk factors. After additionally adjusting for LV mass and NT-pro BNP (model 2), minimum LA volume, total, passive, and active LAEF were significantly associated with incident CVD. In the final model (model 3), additionally adjusted for maximum LA volume, total, passive, and active LAEF (HR for total LAEF = 0.95 per unit, P <0.001; HR for passive LAEF = 0.97, P = 0.021; HR for active LAEF = 0.98, P = 0.027) were all associated with incident CVD. Global longitudinal LA strain was not significantly associated with incident CVD in any of the three models. Details of all three models are shown in Table 3.
Table 3.
Association of left atrial parameters and incident cardiovascular disease
| LA parameter | Model 1 |
Model 2 |
Model 3 |
|||
|---|---|---|---|---|---|---|
| HR*(95% CI) | P-value | HR*(95% CI) | P-value | HR*(95% CI) | P-value | |
| LA maximum volume index, mm3/m2 | 1.03 (1.04, 1.14) | <0.001 | 1.01 (1.00, 1.03) | 0.083 | – | – |
| LA minimum volume index, mm3/m2 | 1.07 (1.05, 1.10) | <0.001 | 1.06 (1.03, 1.08) | <0.001 | 1.12 (1.06, 1.19) | <0.001 |
| LA passive EF, %LA total EF, % | 0.95 (0.93, 0.97) | <0.001 | 0.95 (0.93, 0.97) | <0.001 | 0.95 (0.93, 0.97) | <0.001 |
| LA passive EF, % | 0.97 (0.95, 1.00) | 0.022 | 0.97 (0.94, 1.00) | 0.020 | 0.97 (0.95, 1.00) | 0.021 |
| LA active EF, % | 0.98 (0.96, 0.99) | 0.007 | 0.97 (0.95, 1.00) | 0.009 | 0.98 (0.96, 1.00) | 0.027 |
| LA maximum strain | 0.99 (0.97, 1.00) | 0.159 | 0.99 (0.97, 1.00) | 0.162 | 0.99 (0.97, 1.00) | 0.300 |
Model 1: adjusted for traditional risk factors: age, race, gender, cigarette smoking status, diastolic blood pressure, heart rate, HDL, total cholesterol, hypertension medication use, BMI.
Model 2: adjusted for traditional risk factors, LV mass, and NT-pro BNP level.
Model 3: adjusted for traditional risk factors, LV mass, NT-pro BNP level, and LA maximum volume index.
Hazard Ratio (HR) calculated per unit change in LA parameter.
LA, Left Atrium; EF, Emptying Fraction.
In the area under the curve (AUC) analysis, maximum LA volume, minimum LA volume, total, passive, and active LA EF (P < 0.001 for each) each improved the ROC curve compared with traditional risk factors and NT-pro BNP (Table 4). AUC was derived from receiver operating characteristic curve analysis (ROC), which were obtained by cox regression and compared using a previously described method by DeLong et al.22
Table 4.
Area under the curve
| Parameter analysed | AUC (SE) | P-value |
|---|---|---|
| TRF | 0.67 (0.03) | — |
| TRF + NT-ProBNP | 0.73 (0.03) | P < 0.01 |
| TRF + NT-ProBNP + Vmaxi | 0.74 (0.03) | P < 0.001 |
| TRF + NT-ProBNP + Vmini | 0.77 (0.03) | P < 0.001 |
| TRF + NT-ProBNP + LA Total EF | 0.77 (0.03) | P < 0.001 |
| TRF + NT-ProBNP + LA Passive EF | 0.75 (0.03) | P < 0.001 |
| TRF + NT-ProBNP + LA Active EF | 0.75 (0.03) | P < 0.001 |
The first row represents traditional risk factors with each subsequent row demonstrating the improved predictive value of models that consider the listed parameters relative to the model with only traditional risk factors. AUC, Area under the curve; SE, Standard Error; TRF, Traditional Risk Factors; LA, Left Atrium; EF, Emptying Fraction.
Discussion
Our study cohort demonstrates that in diabetics free of symptomatic cardiovascular disease, increased LA size, especially LA minimum volume, and decreased LA function, as assessed by CMR, are associated with increased incident CVD.
Left atrium in patients with diabetes mellitus–what is known?
Both LA volume and LA function reflect LV diastolic and systolic function and have been implicated as predictive of cardiovascular disease in a range of patient populations.23
LA volume is increased in diabetic patients, although this finding depends upon the imaging modality; echocardiography studies have often failed to identify such an link while those using CT or CMR more consistently demonstrate significant associations.24–27 The prognostic value of increased LA volume was examined in a small echocardiography based study of 305 individuals. In this study, Poulsen et al. demonstrated increased maximum LAV to be a predictor of cardiovascular morbidity and mortality over approximately 5 years of clinical follow-up.11 Our study expands on this with CMR by additionally examining LA function parameters and showing decreased active, passive, and total emptying fraction to be associated with incident CVD.
Cardiac magnetic resonance imaging
In one study to date that has used CMR, Graca et al. demonstrated that CMR is able to detect LA dysfunction in asymptomatic patients with DM. They additionally identified an association between the diagnosis of DM and decreased LA total emptying fraction and passive emptying fraction, but not active emptying fraction.26 This cross sectional study was limited by a small sample size of only 45 patients with diabetes and lack of follow up for events.
CMR has important differences from echocardiography for the assessment of the LA. CMR has higher image resolution given the anatomic location of the LA, with associated limited availability, increased cost, and lower temporal resolution. Echocardiography relies on the quality of acoustic windows obtained, provides challenges in identification of the endocardial border, and less reliably images all components of the chamber, especially the appendage, although it is far more accessible than CMR.28
The results of our study add to knowledge about LA structure and function in patients with DM. Studies to date have suggested that changes in LA size and function might be associated with DM and, furthermore, with incident CVD. We therefore examined these parameters using the most precise imaging techniques available in a study with long-term follow-up. We determined that LA size and function are both associated with the development of CVD. The association of LA function with CVD is independent of LA volume, and minimum LA volume was more strongly related to incident CVD than maximum LA volume.
Minimum left atrium volume
A link between DM and diastolic dysfunction causing increased LV filling pressure with subsequent LA dilation has previously been hypothesized to explain the association between maximum LA volume and increased cardiovascular risk.11 The present study suggests, however, that even adjusting for measurable ventricular changes, minimum LA volume and LA function may be important predictors of incident CVD in people with DM.
There are several possible explanations of why these LA parameters are associated with long-term development of CVD. The first and most prominent theory assumes that LA volume reflects diastolic dysfunction. Our results suggest that there is a stronger association between minimum LA volume with CVD compared with maximum LAV. Other studies have shown that minimum LA volume has a stronger association with diastolic dysfunction than maximal LA volume and may be present in earlier stages of progressive dysfunction.14,19 This is consistent with our results, suggesting that minimal LA volume may be a more sensitive predictor of significant subsequent cardiovascular dysfunction than maximum LA volume by reflecting subtle diastolic dysfunction.
Why the left atrium matters
The limitations of cardiac MRI, including accessibility and cost, are important and highlighted in our paper. Though further research is necessary before any direct clinical applications, CMR analysis of the left atrium may ultimately prove valuable for patients with diabetes mellitus. As reflected in the Kaplan–Meier curves. These left atrial parameters provide additional prognostic information for the development of CVD over the next 10 years. In addition to reflecting diastolic dysfunction, other possible explanations for the strong association between LA parameters, especially LA function, and the development of CVD involve systemic effects on the heart. These effects are likely modulated through inflammation and microvascular changes (both frequently associated with diabetes) that may be detected early in the LA as subclinical markers of damage to the vascular system. Previous studies have shown an association between markers of inflammation and LA size and function, suggesting the inflammation may cause atrial remodeling.29–31 Subendocardial fibrosis, which occurs in diabetes, likely contributes to the increased blood pressure common in this population and may be reflected in these measured parameters.32 Further investigation is necessary into the aetiology of these changes.
Conclusion
Our study shows that LA volume and function measured with CMR are associated with the development of CVD in asymptomatic individuals with DM. Assessment of LA function may have additional value in risk stratification for cardiovascular events in this population.
Study limitations
We examine a population consisting exclusively of diabetics, given their increased rate of CVD, and therefore are unable to draw any conclusions about the uniqueness of our findings to this population. Follow-up data for such a prolonged study was inherently limited and was collected from a variety of sources including public files, medical records from hospitalizations, interviews from participants and their physicians, and Centers for Medicare and Medicaid services (CMS) claims data, which relies on billing paperwork submitted to CMS. Four different 1.5 T MR scanners were used in the acquisition of CMR images, though all images were reviewed at a single institution by a single operator who was blinded to cardiovascular outcomes.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Conflict of interest: None declared.
Funding
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2. Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, Holman RR.. UKPDS 59: hyperglycemia and other potentially modifiable risk facts for peripheral vascular disease in type 2 diabetes. Diabetes Care 2002;25:894–9. [DOI] [PubMed] [Google Scholar]
- 3. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M.. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–34. [DOI] [PubMed] [Google Scholar]
- 4. Boyer JK, Thanigaraj S, Schechtman KB, Perez JE.. Prevelence of ventricular diastoloic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 2004;93:870–5. [DOI] [PubMed] [Google Scholar]
- 5. From AM, Scott CG, Chem HH.. Changes in diastolic dysfunction in diabetes mellitus over time. Am J Cardiol 2009;103:1463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roelandt J. The increasing importance of left atrial size assessment. J Cardiovasc Med (Hagerstown) 2011;12:147. [DOI] [PubMed] [Google Scholar]
- 7. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ. et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006;47:2357–63. [DOI] [PubMed] [Google Scholar]
- 8. Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS. et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation 2003;107:2207–12. [DOI] [PubMed] [Google Scholar]
- 9. Dahl JS, Videbaek L, Poulsen MK, Pellikka PA, Veien K, Andersen LI. et al. Noninvasive assessment of filling pressure and left atrial pressure overload in severe aortic valve stenosis: relation to ventricular remodeling and clinical outcomes after aortic valve replacement. J Thorac Cardiovasc Surg 2011:142:e77–83. [DOI] [PubMed] [Google Scholar]
- 10. Meris A, Amigoni M, Uno H, Thune JJ, Verma A, Køber L. et al. Left atrial remodelling in patients with myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the VALIANT echo study. Eur Heart J 2009:30:56–65. [DOI] [PubMed] [Google Scholar]
- 11. Poulsen MK, Dahl JS, Henriksen JE, Hey TM, Hoilund-Carlsen PF, Beck-Nielsen H. et al. Left atrial volume index; relation to long term clinical outcomes in type 2 diabetes. J Am Coll Cardio 2013:62:2416–21. [DOI] [PubMed] [Google Scholar]
- 12. Hof IE, Velthuis BK, Van Driel VJ, Wittkampf FH, Hauer RN, Loh P.. Left atrial volume and function assessment by magnetic resonance imaging. J Cardiovasc Electrophysiol 2010;21:1247–50. [DOI] [PubMed] [Google Scholar]
- 13. Habibi M, Ambale Venkatesh B, Lima J.. Feature tracking cardiac magnetic resonance imaging in the assessment of left atrial function. J Am Coll Cardiol 2014;63:2434–5. [DOI] [PubMed] [Google Scholar]
- 14. Imai M, Ambale Venkatesh B, Samiei S, Donekal S, Habibi M, Armstrong AC. et al. Multi-ethnic study of atherosclerosis: association between left atrial function using tissue tracking from cine MR imaging and myocardial fibrosis. Radiology 2014;273:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR. et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 16. Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL. et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52:2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M. et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. Am J Roentgenol 2006;186:S357–65. [DOI] [PubMed] [Google Scholar]
- 18. Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E.. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson 2012;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle-Valle TM, Heckbert SR. et al. Association of CMR-measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging 2014;7:570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marrie RA, Dawson NV, Garland A.. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol, 2009; 62:511–17. [DOI] [PubMed] [Google Scholar]
- 21. Nieto FJ, Coresh J.. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol 1996;143:1059–68. [DOI] [PubMed] [Google Scholar]
- 22. DeLong ER, DeLong DM, Clarke-Pearson DL.. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 23. Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 24. Huang G, Zhang L, Xie M, Fu M, Huang J, Lv Q.. Assessment of left atrial function in diabetes mellitus by left atrial volume tracking method. J Huazhong Univ Sci Technolog Med Sci 2010;30:819–23. [DOI] [PubMed] [Google Scholar]
- 25. Muranaka A, Yuda S, Tsuchihashi K, Hashimoto A, Nakata T, Miura T. et al. Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography 2009;26:262–71. [DOI] [PubMed] [Google Scholar]
- 26. Graca B, Ferreira MJ, Donato P, Gomes L, Castelo-Branco M, Caseiro-Alves F.. Left atrial dysfunction in type 2 diabetes mellitus: insights from cardiac MRI. Eur Radiol 2014;24:2669–76. [DOI] [PubMed] [Google Scholar]
- 27. Gweon HM, Kim SJ, Kim TH, Lee SM, Hong YJ, Rim SJ.. Evaluation of left atrial volumes using multidetector computed tomography: comparison with echocardiography. Korean J Radiol 2010;11:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. To AC, Flamm SD, Marwick TH, Klein AL.. Clinical utility of multimodality LA imaging: assessment of size, function, and structure. JACC Cardiovasc Imaging 2011;4:788–98. [DOI] [PubMed] [Google Scholar]
- 29. Rao AK, Djamali A, Korcarz CE, Aeschlimann SE, Wolff MR, Stein JH.. Left atrial volume is associated with inflammation and atherosclerosis in patients with kidney disease. Echocardiography 2008;25:264–9. [DOI] [PubMed] [Google Scholar]
- 30. Barberato SH, Elias Bucharles SG, Moraes de Souza A, Costantini CO, Frack Costantini CR, Pecoits-Filho R.. Association between inflammatory markers and left atrial enlargement in patients on hemodialysis. Arq Bras Cardiol 2013;100:141–146. [DOI] [PubMed] [Google Scholar]
- 31. Habibi M, Lima JA, Khurram IM, Zimmerman SL, Zipunnikov V, Fukumoto K. et al. Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study. Circ Cardiovasc Imaging 2015;8:e002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lamberts RR, Lingam SJ, Wang H-Y, Bollen IA, Hughes G, Galvin IF. et al. Impaired relaxation despite upregulated calcium-handling protein atrial myocardium from type 2 diabetic patients with preserved ejection fraction. Cardiovasc Diabetol 2014;13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]