Abstract
Background
Physical inactivity is a highly modifiable risk factor for the development of osteoporosis but, due to a lack of research that has precisely and objectively meaured physical activity (PA) relevant to bone, the specific contribution that PA can make to bone health is poorly understood. This study examined whether a more precise measure of PA relelvant to bone was associated with meaures of bone health in pre- and post-menopausal women in UK Biobank.
Methods
Time spent at intensities specific to bone health [≥750 milli-gravitational units (mg) and ≥1000 mg] were analysed from raw tri-axial acceleration data averaged over 1-second epochs from 7-day monitoring of habitual PA using accelerometry-based activity monitors (100 Hz; AX3, Axivity, UK) of 1218 pre- and 1316 post-menopausal healthy women. In a cross-sectional analysis, associations between categories of time (<1, 1–2 and ≥2 minutes) spent above the intensity thresholds and calcaneal quantitative ultrasound measures of bone health (bone mineral density T-score, BMDT-score; speed of sound, SOS; and broadband ultrasound attenuation, BUA) were examined.
Results
Compared with <1 minute, spending 1–2 or ≥2 minutes/day at intensities ≥1000 mg in pre-menopausal and ≥750 mg in post-menopausal women was positively associated with BMDT-score, SOS and BUA.
Conclusion
Brief bursts of high-intensity PA relevant to bone health can be captured by applying bone-specific thresholds of intensity to raw tri-axial accelerations averaged over 1-second epochs. Accumulating 1–2 minutes/day of high-intensity PA, equivalent to running in pre-menopausal women and slow jogging in post-menopausal women, is associated with better bone health.
Keywords: osteoporosis, accelerometer, raw acceleration, quantitative ultrasound
Key Messages
Brief bursts of high-intensity habitual physical activity (PA) beneficial to bone health can be quantified from accelerations measured at the wrist with accelerometry-based activity monitors.
This method provides a step-change in the ability to precisely and objectively measure PA relevant to bone from commercially available tri-axial wrist-worn monitors typically employed in large population studies.
Accumulating 1–2 minutes or ≥2 minutes per day of high-intensity PA, equivalent to running in pre-menopausal women and slow jogging in post-menopausal women, is associated with better bone health.
Future research should further exploit high-resolution accelerometry-based activity monitor data to determine the optimal temporal characteristics of PA for bone health to inform the development of manageable and effective PA interventions.
Introduction
Osteoporosis is a brittle bone disease that affects women (one in three) more than men (one in five) especially over the age of 50.1,2 It causes over 300 000 people a year in the UK3 and over 2 million in the USA4 to suffer a fragility fracture resulting in significant pain, disability, loss of independence and increased risk of morbidity, especially in the first 6 months after fracture.2,3 In women, the incidence of osteoporosis increases dramatically post menopause1–3; therefore, identification of strategies that may optimize bone health in both pre- and post-menopausal women is a priority.
Physical inactivity is a highly modifiable risk factor for the development of osteoporosis5–7 but the specific contribution that physical activity (PA) can make to accruing, maintaining or minimizing the loss of bone mass is poorly understood compared with other modifiable lifestyle risk factors such as diet, smoking and alcohol.2,7–9 Whereas PA guidelines recommending the accumulation of at least 150 minutes/week of moderate PA, in bouts of 10 minutes or more, exist for cardivascular and metabolic health,10,11 there are no specific PA recommendations for reducing the risk of poor bone health that likely benefits from a different dose of activity characterized by short, dynamic, sporadic bursts.12,13 The development of bone-specific PA guidelines is limited by a lack of research that has precisely and objectively assessed the influence of exercise interventions2,14 or habitual PA on bone-health outcomes. Consequently, there is a lack of evidence for positive associations between bone mineral density (BMD) and moderate or vigorous intensities of PA in women.15,16
Until recently, the outcome for objectively measured PA in large cross-sectional bone-health studies has been time-accumulated in sedentary-, light-, moderate- or vigorous-intensity categories determined from proprietary counts (device-specific) from hip-worn monitors summed over user-defined 15- or 60-second epochs.15,16 The classification of the intensities corresponds to energy expenditure during steady-state exercise, making them most relevant to cardiovascular and metabolic health.17–19 Chastin and colleagues16 suggest that their counterintuitive finding for the absence of an association between BMD and PA at moderate and vigorous intensities may be due to summarising proprietary counts from hip-worn accelerometry-based activity monitors over 60-second epochs. For short dynamic episodes of activity, averaging has the effect of over-smoothing, misclassifying and underestimating time spent in moderate or vigorous intensities, thus failing to capture the very activities that are likely to benefit bone.16 Classification of activity into intensity categories calibrated with energy expenditure from steady-state activity relevant to cardiovascular and metabolic health outcomes may also contribute to the failure to detect an association between more dynamic intensities of PA and bone health.20–22
The commercial availability of high-resolution tri-axial accelerometry-based activity monitors that collect and store raw acceleration data at up to 100 Hz for 7 days provides the opportunity to more precisely measure intensities of PA beneficial to bone. We calibrated raw peak acceleration from these monitors worn on the hip and wrist with external ground reaction force in adults21 and determined the magnitude of acceleration associated with ground reaction forces that are beneficial to bone in pre-menopausal women.23 Providing a valid measure of activity relevant to bone from wrist-worn monitors is particularly important because, compared with hip-worn monitors, they result in higher levels of participant compliance, greater wear-time and therefore more accurate measures of habitual PA.24 The use of wrist-worn monitors to objectively measure PA is becoming more common in large population surveys and national health databases including UK Biobank.
UK Biobank is a new open-access large-scale prospective epidemiological resource that holds baseline measures on 500 000 adults including quantitative ultrasound scanning (QUS) of the heel and, in a sub-sample of approximately 100 000 participants, objective measurement of habitual (free-living) PA from 7-day monitoring using a commercial wrist-worn tri-axial accelerometer that sampled and stored raw accelerations at 100 Hz. These high-resolution files present a unique opportunity to derive a more precise measure of PA relevant to bone from raw acceleration data in a large cross-sectional study. Brief bursts of high-intensity activity can be quantified using intensity thresholds specific to bone health. We hypothesize that precise bone-specific measures of PA will predict measures of bone health in both pre- and post-menopausal women independently of PA accrued at all other intensities and other factors thought to influence bone.
Methods
Questionnaire and baseline physical measures including QUS of the heel were collected from 500 000 adults aged 40–69 years attending one of 21 assessment centres across Britain between 2006 and 2010. Objective measurements of PA were collected in a sub-sample (approximately 100 000) of the same cohort between 2013 and 2015. Details of recruitment and measurements used to obtain data for this resource can be found on the UK Biobank website: https://www.ukbiobank.ac.uk.
Study sample
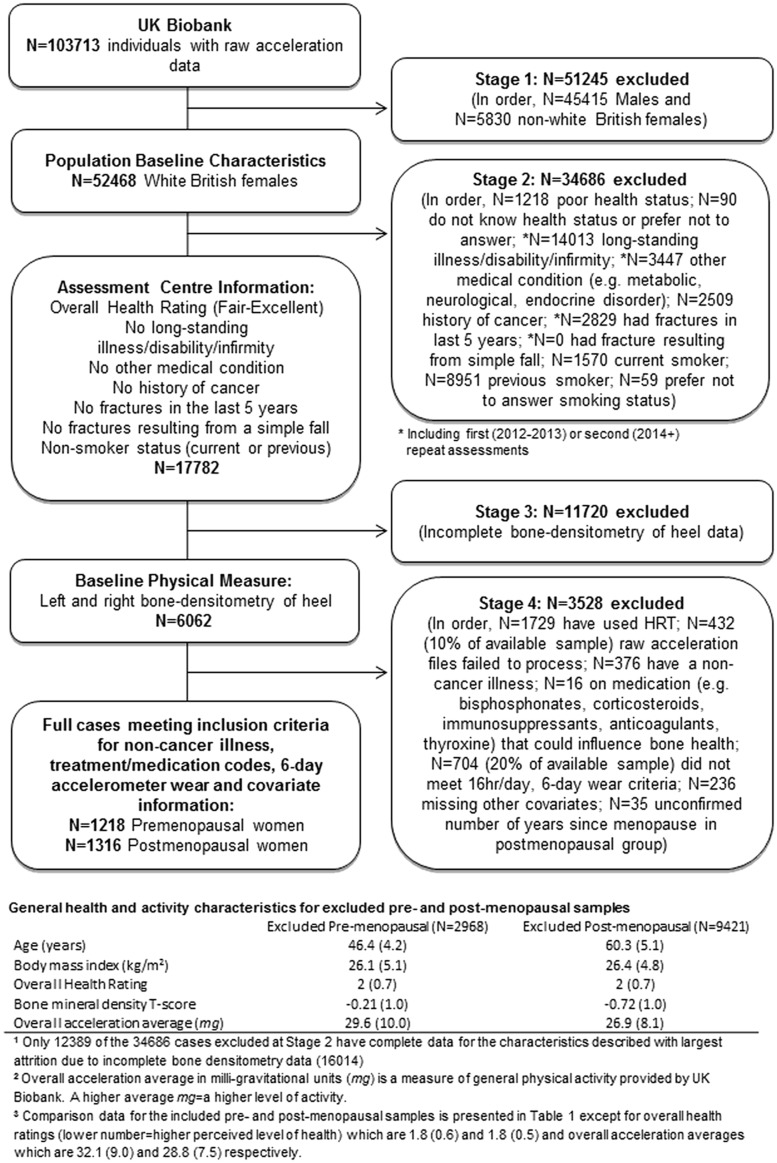
To reduce the influence of conditions or treatments affecting either bone health or PA, only ‘healthy’ individuals, in the order outlined in Figure 1, were selected. For comparison, where complete sets of data were available, general health and activity characteristics for excluded pre- and post-menopausal samples are presented (Figure 1). Pre-menopausal (n = 1218) and post-menopausal (n = 1316) women forming the included sample were analysed separately due to the potential for different PA intensity thresholds to predict bone health in each group.
Figure 1.
Study-inclusion flow chart.
Bone-health outcome measures
Participants had calcaneal QUS measurements of their left and right calcaneus performed using the Sahara Clinical Bone Sonometer (Hologic, Bedford, MA). BMDT-scores (number of standard deviations above or below peak BMD from a young sex-matched average) were derived from estimated BMD, calculated using the following formula:
where SOS is the speed of sound (m/s) and BUA is the broadband ultrasound attenuation (dH/MHz).25 The QUS measurements were averaged between the left and right calcaneus (one measurement from each) for each participant. In accordance with good practice, daily quality-control and cleaning procedures were conducted in line with the manufacturer’s recommendations across all assessment centres. Further details of the QUS testing protocol are available on the UK Biobank website.
PA monitoring
Raw acceleration files (.cwa) containing 7-day, 100-Hz data from tri-axial AX3 (Axivity, Newcastle, UK) accelerometers worn on the dominant wrist were downloaded from UK Biobank and auto-calibrated, re-sampled (100 Hz) and converted to .wav format using open-source software (Omgui Version 1.0.0.28; Axivity). An open-access package (GGIR Version 1.3–2) in R (http://cran.r-project.org/) was used to convert raw accelerations (x-, y- and z-axes) in .wav files to magnitudes of dynamic acceleration [resultant vector magnitude, corrected for gravity, expressed as Euclidean Norm Minus One (ENMO) in milli-gravitational units, mg26,27] averaged over 1-second epochs from which time accumulated at different intensities from 6 valid days (16 hours/day), including one weekend day, of wear was used to calculate an average day of activity. Month of PA measurement was extracted to allow for any adjustments in PA due to seasonal variation to be made.
Using wrist-worn monitors that produce acceleration magnitudes equivalent to the AX3,28 Hildebrand and colleagues19 found thresholds of approximately 100 mg and 400 mg represented moderate and vigorous intensities of activity based on energy expenditure for 3 and 6 METs, respectively, in adults (aged 34 ± 10 years). The moderate intensity approximated brisk walking, with the vigorous threshold just over half the 750 mg output elicited during running (8 km/h)—an activity that has been found to exceed impact magnitudes and loading rates beneficial to bone.23,29,30 When calibrating acceleration magnitudes with ground reaction force beneficial to bone,23 the thresholds we identified corresponded to the acceleration magnitudes found during running at 8 km/h (slow jogging) and 10 km/h, equivalent to 750 mg and 1000 mg when averaging over 1-second epochs.19,31 Time spent at intensities ≥750 mg (PA ≥ 750 mg) and ≥1000 mg (PA ≥ 1000 mg) were therefore used in the present study to examine thresholds of activity specific to bone.
Covariates
Variables collected by UK Biobank that were believed to be, or have previously been shown to be, associated with bone health and/or PA were treated as potential covariates. Baseline measures for age, height, fat mass and fat-free mass (bioelectrical impedance; Tanita BC418MA), self-reported alcohol, nutritional intake and current medications were extracted from UK Biobank. Whereas estimated calcium intake (mg) could intuitively be an important determinant of bone health, it was not included as a covariate in this report due to only half the sample providing data for it and the absence of any correlation (r = 0.001) between calcium intake and BMDT-score in the half that did provide a measure. Estimated alcohol consumption (units/week) was calculated from self-reported volumes of intake multiplied by units for each alcohol type.32 Continuous variables for age at menarche, the number of years taking contraceptive and years since the menopause (where applicable) were extracted or calculated from female-specific factors from the touchscreen questionnaire. The number of years between baseline and PA measures was also calculated to allow any influence in time between measures of bone health and PA to be examined. Covariates for PA (50–99 mg and 100–749 or 100–999 mg) were created to allow associations between time spent at higher intensities and measures of bone health to be analysed independently of time spent being in activities at all other intensities. To reduce the amount of dilution that light-intensity activity (which may also be beneficial to bone)16 has on measures of moderate activity and above, time spent in 50–99 mg was used as a separate PA covariate to time spent between 100–749 mg and 100–999 mg for respective analyses.
Statistical data analysis
For the first stage of the model-building process, all of the covariates were entered simultaneously into the regression model (Model 1) without removal (e.g. all entered covariates remained in the model irrespective of their p-value). Plotting the residuals of this covariate model against PA ≥ 750 mg or PA ≥ 1000 mg indicated that the relationship was curvilinear, requiring a second-order polynomial to model it. For ease of interpretation, we decided to address the curvilinear relationship by converting the continuous PA ≥ 750 mg/PA ≥ 1000 mg variables into categorical variables (<1, 1–2, ≥2 minutes/day). The parameters of these categories were chosen after examining the distribution of time spent at intensities ≥1000 mg and ≥750 mg for pre- and post-menopausal women, respectively, and consideration of the lowest accumulated dose of PA that would lend itself to a plausable public health message. Consequently, for the second stage of the model-building process (Model 2), we entered the categorical variables for PA ≥ 750 mg/PA ≥ 1000 mg (<1 minute/day being the reference category) into the model that contained all of the covariates with BMDT-score as the outcome measure. The models were repeated with BUA and SOS as the outcome measures. A sample size of n ∼ 1200 and n ∼ 1300 in each group provides ∼90% power (at p = 0.05) to detect very small (∼1%, partial R2 change = 0.011) increases in the explained variance of bone health by adding PA ≥ 750 mg/PA ≥ 1000 mg to a covariate model that already explains ∼10% of the variance. All analyses were carried out in IBM SPSS Version 23 (IBM, Chicago, IL).
Results
Descriptive statistics for measures of bone health, covariates and PA-by-intensity variables are reported in Table 1 for pre-menopausal and post-menopausal women separately. Means and standard deviations are reported for normally distributed variables and medians and interquartile ranges for variables that are positively skewed. There was no need to adjust PA data for the potential effects of seasonality, as there was no evidence in this sample that PA differed by season (e.g. summer vs autumn, vs winter and vs spring were all p ≥ 0.20 for PA ≥ 1000 mg in the pre-menopausal group and PA ≥ 750 mg in the post-menopausal group). Tables 2 and 3 report the beta-coefficients [with 95% confidence intervals (CIs) and p-values] for all the PA-by-intensity variables obtained from the full model (Model 2) that best predicted bone-health measures for pre-menopausal and post-menopausal women. In addition, the R2 increase for the PA ≥ 750 mg or PA ≥ 1000 mg variable was reported.
Table 1.
Summary characteristics of pre-menopausal and post-menopausal women
| Measures | Pre-menopausal (n = 1218) | Post-menopausal (n = 1316) |
|---|---|---|
| Age and time | ||
| Age at baseline (years)a | 46.2 (3.9) | 58.9 (5.0) |
| Years since menopause (years)* | – | 7 (3–11) |
| Age at menarche (years) | 13.1 (1.5) | 12.9 (1.5) |
| Contraceptive pill (years from first to last)* | 10 (4–18) | 6 (0–13) |
| Years between baseline and PA (years) | 4.8 (0.7) | 4.8 (0.7) |
| Body size and composition | ||
| Weight (kg)* | 65.4 (59.5–74.0) | 66.4 (60.3–74.0) |
| Height (m) | 1.65 (6.0) | 1.63 (6.1) |
| Body mass index (BMI, kg/m2)* | 24.0 (22.0–27.0) | 24.9 (22.6–27.6) |
| Fat mass (kg)* | 21.3 (17.1–27.4) | 23.1 (18.7–28.9) |
| Fat-free mass (kg)* | 44.5 (41.8–47.5) | 43.3 (40.8–46.1) |
| Dietary information | ||
| Consumption of alcohol (units/week)* | 6.4 (1.8–12.8) | 6.4 (1.4–11.7) |
| Physical activity (by intensity) | ||
| PA = 50–99 mg (min/day) | 131 (26) | 130 (26) |
| PA = 100–999b/749cmg (min/day) | 142 (44) | 125 (42) |
| PA ≥ 1000b/750cmg: | ||
| (<1 min/day)# | 73% (887) | 62% (816) |
| (1–2 min/day)# | 12% (151) | 21% (276) |
| (≥2 min/day)# | 15% (180) | 17% (224) |
| Bone health | ||
| Bone mineral density (BMDT-score) | –0.11 (0.95) | –0.63 (0.96) |
| Speed of sound (SOS, m/s) | 1563 (28) | 1548 (28) |
| Broadband ultrasound attenuation (BUA, dH/MHz) | 78.4 (14.3) | 71.2 (15.1) |
All values are means (standard deviations) unless indicated otherwise. *Median (inter quartile range). #Percentage (n). PA, physical activity; mg, milli-gravitational units; min/day = minutes per day. aNo participant was less than 40 years old at their baseline measure; bthreshold for pre-menopausal women; cthreshold for post-menopausal women.
Table 2.
Relationship between PA (by intensity) and measures of bone health in pre-menopausal women (n = 1218)
| Bone health | PA intensity | Beta (unstd) | 95%CI for Beta (unstd) | Beta (std) | p-value |
|---|---|---|---|---|---|
| BMD T-score | PA = 50–99 mg (per 30 min/day) | 0.003 | (–0.087 to 0.093) | 0.003 | 0.943 |
| PA = 100–999 mg (per 30 min/day) | –0.0004 | (–0.060 to 0.060) | –0.001 | 0.987 | |
| PA ≥ 1000 mg: | |||||
| (<1 min/day) | – | – | – | – | |
| (1–2 min/day) | 0.196 | (0.026 to 0.366) | 0.068 | 0.024 | |
| (≥2 min/day) | 0.291 | (0.130 to 0.452) | 0.109 | <0.001 | |
| R2 change for PA ≥ 1000 mg = 0.012 (p = 0.001) | |||||
| SOS (m/s) | PA = 50–99 mg (per 30 min/day) | 0.390 | (–2.000 to 2.770) | 0.011 | 0.754 |
| PA = 100–999 mg (per 30 min/day) | 0.060 | (–1.440 to 1.560) | 0.003 | 0.943 | |
| PA ≥ 1000 mg: | |||||
| (<1 min/day) | – | – | – | – | |
| (1–2 min/day) | 6.083 | (1.021 to 11.145) | 0.071 | 0.019 | |
| (≥2 min/day) | 9.817 | (5.014 to 14.620) | 0.123 | <0.001 | |
| R2 change for PA ≥ 1000 mg = 0.015 (p < 0.001) | |||||
| BUA (dH/MHz) | PA = 50–99 mg (per 30 min/day) | –0.240 | (–1.455 to 0.975) | –0.015 | 0.683 |
| PA = 100–999 mg (per 30 min/day) | –0.060 | (–0.825 to 0.705) | –0.008 | 0.849 | |
| PA ≥ 1000 mg: | |||||
| (<1 min/day) | – | – | – | – | |
| (1–2 min/day) | 2.379 | (–0.192 to 4.950) | 0.055 | 0.070 | |
| (≥2 min/day) | 2.771 | (0.332 to 5.210) | 0.069 | 0.026 | |
| R2 change for PA ≥ 1000 mg = 0.005 (p = 0.034) | |||||
PA, physical activity; mg, milli-gravitational units; min/day, minutes per day; unstd, unstandardized; std, standardized; BMD T-score, age-adjusted bone mineral density; SOS, speed of sound; BUA, broadband ultrasound attenuation; Beta, beta-coefficient from multiple regression analysis; CI, confidence interval.
Table 3.
Relationship between PA (by intensity) and measures of bone health in post-menopausal women (n = 1316)
| Bone health | PA intensity | Beta (unstd) | 95%CI for Beta (unstd) | Beta (std) | p-value |
|---|---|---|---|---|---|
| BMD T-score | PA = 50–99 mg (per 30 min/day) | –0.008 | (–0.085 to 0.065) | –0.008 | 0.823 |
| PA = 100–749 mg (per 30 min/day) | –0.032 | (–0.092 to 0.028) | –0.047 | 0.226 | |
| PA ≥ 750 mg: | |||||
| (<1 min/day) | – | – | – | – | |
| (1–2 min/day) | 0.156 | (0.021 to 0.292) | 0.066 | 0.024 | |
| (≥2 min/day) | 0.272 | (0.114 to 0.431) | 0.107 | 0.001 | |
| R2 change for PA ≥ 750 mg = 0.009 (p = 0.002) | |||||
| SOS (m/s) | PA = 50–99 mg (per 30 min/day) | –0.360 | (–2.475 to 1.755) | –0.012 | 0.731 |
| PA = 100–749 mg (per 30 min/day) | –0.840 | (–2.340 to 0.660) | –0.042 | 0.277 | |
| PA ≥ 750 mg: | |||||
| (<1 min/day) | – | – | – | – | |
| (1–2 min/day) | 4.660 | (0.693 to 8.628) | 0.068 | 0.021 | |
| (≥2 min/day) | 8.031 | (3.386 to 12.677) | 0.108 | <0.001 | |
| R2 change for PA ≥ 750 mg = 0.009 (p = 0.002) | |||||
| BUA (dH/MHz) | PA = 50–99 mg (per 30 min/day) | 0.016 | (–1.109 to 1.141) | 0.001 | 0.977 |
| PA = 100–749 mg (per 30 min/day) | –0.538 | (–1.348 to 0.272) | –0.050 | 0.187 | |
| PA ≥ 750 mg: | |||||
| (<1 min/day) | – | – | – | – | |
| (1–2 min/day) | 2.098 | (–0.004 to 4.200) | 0.057 | 0.050 | |
| (≥2 min/day) | 3.734 | (1.273 to 6.196) | 0.093 | 0.003 | |
| R2 change for PA ≥ 750 mg = 0.007 (p = 0.008) | |||||
PA, physical activity; mg, milli-gravitational units; min/day, minutes per day; unstd, unstandardized; std, standardized; BMD T-score, age-adjusted bone mineral density; SOS, speed of sound; BUA, broadband ultrasound attenuation; Beta, beta-coefficient from multiple regression analysis; CI, confidence interval.
Pre-menopausal women
Whereas there was some evidence that the time spent in PA ≥ 750 mg was positively associated with BMDT-score (p = 0.04), the evidence for PA ≥ 1000 mg was much stronger (p = 0.001). Additional analysis implied that time spent in PA at 750–999 mg (p = 0.16) did not contribute at all to the association of PA ≥ 750 mg; it was due almost completely to time spent at PA ≥ 1000 mg. For this reason, we are not reporting the PA ≥ 750 mg variable for pre-menopausal women, as this would lead to inappropriate recommendations; we are only reporting the results of the model that examined PA ≥ 1000 mg. In this final model, PA ≥ 1000 mg was the only PA-by-intensity variable that was associated with BMD [e.g. BMD was 0.20 (p = 0.024) and 0.29 (p < 0.001) T-scores higher in pre-menopausal women who spent 1–2 minutes/day and ≥ 2 minutes/day, respectively, in PA ≥ 1000 mg than in pre-menopausal women who spent<1 minute/day at that intensity; R2 increased by 1.2% (p = 0.001) from the 1.4% covariate model]. There was no evidence that time spent in PA at 50–99 mg or PA at 100–999 mg were related to BMD with or without PA ≥ 1000 mg in the model (with: p = 0.943 and p = 0.987, respectively; without: p = 0.674 and p = 0.211, respectively). The pattern of results was very similar when SOS and BUA were used as the markers of bone health.
Post-menopausal women
In post-menopausal women, the association was much stronger between BMD and PA ≥ 750 mg than between BMD and PA ≥ 1000 mg (unlike in pre-menopausal women). Additional analysis showed that the association with PA ≥ 750 mg was due almost completely to time spent in PA at 750–999 mg (p < 0.001), and not at all to time spent in PA ≥ 1000 mg (p = 0.79). For this reason, we are not reporting the PA ≥ 1000 mg variable for post-menopausal women, as this would lead to inappropriate recommendations; we are only reporting the results of the model that examined the PA ≥ 750 mg variable (which clearly includes time in PA ≥ 1000 mg). In this final model, PA ≥ 750 mg was the only PA-by-intensity variable that was associated with BMD [e.g. BMD was 0.16 (p = 0.024) and 0.27 (p = 0.001) T-scores higher in post-menopausal women who spent 1–2 minutes/day and≥2 minutes/day, respectively, in PA ≥ 750 mg than in post-menopausal women who spent <1 minute/day at that intensity; R2 increased by 0.9% (p = 0.002) from the 7.2% covariate model]. There was no evidence that time spent in PA at 50–99 mg or PA at 100–749 mg were related to BMD with or without PA ≥ 750 mg in the model (with: p = 0.823 and p = 0.226, respectively; without: p = 0.408 and p = 0.808, respectively). The pattern of results was very similar when SOS and BUA were used as the markers of bone health.
Discussion
Using a bone-health-specific, precise and objective measures of time spent in high-intensity dynamic activity, we have demonstrated a step-change in the ability to measure PA relevant to bone and revealed a positive association between habitual physical activity and bone health in both pre- and post-menopausal women. In contrast to previous research, which summed proprietary counts from commercially available accelerometers over 15- or 60-second epochs,15,16 the averaging of raw accelerations over 1-second epochs ensured that brief bursts of high-intensity habitual PA more relevant to bone were captured, enabling bone-specific intensity thresholds to be applied. With a view to developing realistic and achievable bone- and population-specific public health messages, it is promising to find that relatively small amounts (1–2 minutes) of habitual PA at ≥1000 mg in pre-menopausal and ≥750 mg in post-menopausal women are positively associated with measures of bone health. High-impact activity is generally considered necessary to stimulate bone cells to benefit BMD,13 but this osteogenic effect has not always been found in post-menopausal women.29
To explain why bone-health measures are associated with a different threshold of intensity in pre- and post-menopausal women, it is possible as a result of bone strength declining with age that a lower-intensity activity in post-menopausal women produces a local bone strain equivalent to a higher-intensity activity in pre-menopausal women.33 This is further supported by higher loading rates in mature women (55 BW/s, ±9) compared with younger women (37 BW/s, ±8) when running at the same speed.34 Therefore, a lower threshold of high-intensity activity (750 mg equivalent to a slow jog) in post-menopausal women may provide the same mechanical stimulation as a higher threshold of high-intensity activity (e.g. 1000 mg equivalent running at 10 km/h) in pre-menopausal women. By extension, it may also be interesting to consider the potential for a lower intensity of activity to create sufficient local strain to stimulate bone formation in a less healthy population with lower levels of bone health. However, the close proximity of BMDT-scores of the excluded and included participants observed in the current study (–0.21 and –0.11, respectively, for pre-menopausal women and –0.72 and –0.63, respectively, for post-menopausal women) suggests that the activity intensities associated with bone health in each excluded menopausal group may not be that dissimilar to respective intensities of the included samples. Nonetheless, it would be interesting to further explore these intensities in a wider, potentially less healthy population with full consideration of a comprehensive list of covariates relevant to the sample.
To our knowledge, no other research producing dynamic measures of acceleration (ENMO) from raw accelerations (100 Hz) averaged over 1-second epochs to quantify PA relevant to bone is available for comparison. However, methods using a non-commercial uniaxial waist-worn accelerometer with an on-board processor to count the number of impact peaks in vertical acceleration during an activity intervention found that positive changes in BMD and calcaneal BUA were evident from fewer than 100 daily impacts over 3.9 g (standard acceleration due to gravity)—a threshold that is indicative of running and jumping.30 This supports the positive associations found for time spent above magnitudes equivalent to running in the present study.
Our results are counter to reports of osteogenic benefits35 and changes in bone structural properties8 from walking, which yield average (1-second epochs) accelerations of 170 mg during steady-state activity.19 A high number (approximately 8500) of peak accelerations at low intensity (0.3–1 g represents walking30) have been found to significantly predict changes in bone structure, e.g. circumference and cortical thickness at the proximal tibia.8 Given that low-level stimulations normally ‘ignored’ by bone may become highly anabolic if performed at higher frequencies,36,37 it may be that osteogenic benefits from lower-intensity accelerations averaged over 1-second epochs can only be recognized if wider characteristics of PA frequency, bout length and intermittence are also described.8,38,39 Therefore, further research should also consider the temporal characteristics of PA such as the distribution of activity bouts and rest periods over discrete periods of time.8,13,22,40,41
The development of a primary population-based strategy to increase PA at all ages in order to prevent osteoporosis and reduce the risk of fragility fractures has been limited by a scarcity of research that has accurately determined the influence of exercise intervention type, uptake and compliance on bone-health outcomes using precise, objective measurements of PA.2,14 This study demonstrates that the method used to analyse raw accelerations from commercially available tri-axial wrist-worn monitors, typically employed in large population studies, can be used to precisely and objectively capture high-intensity PA relevant to bone. This could be used to evaluate the influence of PA interventions on bone health and to inform the development of manageable PA guidelines specific to bone.
A number of limitations of the present study are acknowledged. Averaging accelerations over 1-second epochs captured high-intensity activity relevant to bone more accurately than previous studies summing counts over 15- or 60-second epochs; however, it was not possible to count the magnitude of individual peaks in raw acceleration using this method. The thresholds used in this study, however, were specific to the intensities of activity beneficial to bone and are meaningful in that they can be described in relation to running speed and duration. In UK Biobank, accelerometer data were collected from monitors worn on the dominant wrist, whereas our thresholds and those of Hildebrand et al.19 were developed using the non-dominant wrist. Evidence suggests, however, that differences in accelerometer output between the dominant and non-dominant wrist are minimal at higher intensities.31 Therefore, unless an activity that dominates on one side is taking place, e.g. racket sports, these high-intensity thresholds are likely appropriate for either wrist. It should also be acknowledged that accelerometers only measure acceleration and are not able to capture loading, e.g. from resistance-type training, which can also benefit bone health.
QUS measurements were used in UK Biobank rather than the current gold standard of DXA for measuring bone, as it provides a radiation-free and inexpensive method for measuring the density and micro-architectural properties of bone. The ultrasound-derived modulus of elasticity, as measured by the SOS, correlates strongly with values of bone-breaking strength derived from static loading, whereas BUA values are reported to be dependent upon trabecular orientation in vitro and to be significantly associated with bone structure independently of BMD. These results can be combined to provide a single estimate, which is an analogue of BMD.42 Whereas QUS is not used clinically in the UK, it provides a useful research tool to measure calcaneal estimated BMD and is affected by weight-bearing activity, with the calcaneus having a trabecular content similar to that of the spine and representing more metabolically active bone, which is likely to respond to mechanical and hormonal stimuli more rapidly than cortical bone sites.42 Finally, as this is a cross-sectional study, it may be susceptible to reverse causality whereby time spent being physically active at a high intensity could be influenced by bone health.
In conclusion, using precise, objective measures of high-intensity dynamic activity, we found that 1–2 minutes per day of high-intensity dynamic PA, equivalent to running in pre-menopausal women and slow jogging in post-menopausal women, is associated with better bone health.
Funding
This work was supported by an internal grant from the University of Exeter (UK) Project Development Fund (Science). No external sources of funding were accessed.
Acknowledgements
This research has been conducted using the UK Biobank Resource (Reference 10995). A.R. is with the National Institute for Health Research (NIHR) Biomedical Research Centre based at University Hospitals of Leicester and Loughborough University, the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care—East Midlands (NIHR CLAHRC—EM) and the Leicester Clinical Trials Unit. The views expressed here are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1. Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. The Lancet 2002;359:1929–36. [DOI] [PubMed] [Google Scholar]
- 2. National Osteoporosis Guideline Group. Osteoporosis—clinical guideline for prevention and treatment: Executive Summary, updated January 2016. https://www.shef.ac.uk/NOGG/NOGG_Executive_Summary.pdf (25 April 2017, date last accessed). [Google Scholar]
- 3. Mitchell P, Dolan L, Sahota O. et al. Osteoporosis in the UK at breaking point. UK: 2010. http://www.ilcuk.org.uk/images/uploads/publication-pdfs/pdf_pdf_143.pdf (25 April 2017, date last accessed). [Google Scholar]
- 4. Burge R, Dawson-Hughes B, Solomon DH. et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007;22:465–75. [DOI] [PubMed] [Google Scholar]
- 5. Bass S, Pearce G, Bradney M. et al. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. J Bone Miner Res 1998;13:500–7. [DOI] [PubMed] [Google Scholar]
- 6. Carter MI, Hinton PS. Physical activity and bone health. Missouri Med 2014;111:59–64. [PMC free article] [PubMed] [Google Scholar]
- 7. Weaver CM, Gordon CM, Janz KF. et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporosis Int 2016;27:1281–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vainionpää A, Korpelainen R, Vihriälä E. et al. Effect of impact exercise and its intensity on bone geometry in weight-bearing tibia and femur. Bone 2007;40:604–11. [DOI] [PubMed] [Google Scholar]
- 9. Jämsä T, Ahola R, Korpelainen R. Measurement of osteogenic exercise—how to interpret accelerometric data? Frontiers in Physiol 2011;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Donovan G, Blazevich AJ, Boreham C. et al. The ABC of Physical Activity for Health: a consensus statement from the British Association of Sport and Exercise Sciences. J Sports Sci 2010;28:573–91. [DOI] [PubMed] [Google Scholar]
- 11. Garber CE, Blissmer B, Deschenes MR. et al. American College of Sports Medicine position stand: quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–59. [DOI] [PubMed] [Google Scholar]
- 12. Robling AG, Hinant FM, Burr DB. et al. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res 2002;17:1545–54. [DOI] [PubMed] [Google Scholar]
- 13. Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev 2003;31:45–50. [DOI] [PubMed] [Google Scholar]
- 14. Peto L, Allaby M. January Screening for Osteoporosis in Postmenopausal Women: A Report for the UK National Screening Committee. Oxford, UK: Solutions for Public Health; 2013. http://legacy.screening.nhs.uk/osteoporosis (25 April 2017, date last accessed). [Google Scholar]
- 15. Gába A, Kapus O, Pelclová J. et al. The relationship between accelerometer-determined physical activity (PA) and body composition and bone mineral density (BMD) in postmenopausal women. Arch Gerontology and Geriatrics;54:e315–21. [DOI] [PubMed] [Google Scholar]
- 16. Chastin SF, Mandrichenko O, Helbostadt JL. et al. Associations between objectively-measured sedentary behaviour and physical activity with bone mineral density in adults and older adults, the NHANES study. Bone 2014;64:254–62. [DOI] [PubMed] [Google Scholar]
- 17. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777–81. [DOI] [PubMed] [Google Scholar]
- 18. Matthews CE, Chen KY, Freedson PS. et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol 2008;167:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hildebrand M, van Hees VT, Hansen BH. et al. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 2014;46:1816–24. [DOI] [PubMed] [Google Scholar]
- 20. Langsetmo L, Hitchcock CL, Kingwell EJ. et al. Physical activity, body mass index and bone mineral density-associations in a prospective population-based cohort of women and men: the Canadian Multicentre Osteoporosis Study (CaMos). Bone 2012;50:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rowlands AV, Stiles VH. Accelerometer counts and raw acceleration output in relation to mechanical loading. Journal of Biomechanics 2012;45:448–54. [DOI] [PubMed] [Google Scholar]
- 22. Hannam K, Deere KC, Hartley A. et al. A novel accelerometer-based method to describe day-to-day exposure to potentially osteogenic vertical impacts in older adults: findings from a multi-cohort study. Osteoporosis Int 2016;10.1007/s00198–016–3810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stiles VH, Griew PJ, Rowlands AV. Use of accelerometry to classify activity beneficial to bone in premenopausal women. Med Sci Sports Exerc 2013;45:2353–61. [DOI] [PubMed] [Google Scholar]
- 24. van Hees VT, Renström F, Wright A. et al. Estimation of daily energy expenditure in pregnant and non-pregnant women using a wrist-worn tri-axial accelerometer. PLoS One 2011;6:e22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frost ML, Blake GM, Fogelman I. Contact quantitative ultrasound: an evaluation of precision, fracture discrimination, age-related bone loss and applicability of the WHO criteria. Osteoporos Int 1999;10:441–9. [DOI] [PubMed] [Google Scholar]
- 26. van Hees VT, Gorzelniak L, Dean Leon EC. et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS ONE 2013;8:e61691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Hees VT, Fang Z, Langford J. et al. Auto-calibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol 2014;117:738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ladha C, Ladha K, Jackson D. et al. Shaker Table Validation of Openmovement AX3 Accelerometer. In: et al. (P Freedson, P Freeman, A Libertine, S Long, C Oliver, J Sasaki, R Spencer, J Staudenmayer, D John, S Crouter, D Bassett, eds). 3rd Int Conference on Ambulatory Monitoring of Physical Activity and Movement 2013;17–19 June 2013; Amherst, MA, USA; pp. 69–70. [Google Scholar]
- 29. Bassey EJ, Rothwell MC, Littlewood JJ. et al. Pre- and post menopausal women have different bone mineral responses to the same high-impact exercise . J Bone Miner Res 1998;13:1805–13. [DOI] [PubMed] [Google Scholar]
- 30. Vainionpää A, Korpelainen R, Vihriälä E. et al. Intensity of exercise is associated with bone density change in premenopausal women. Osteoporosis Int 2006;17:455–63. [DOI] [PubMed] [Google Scholar]
- 31. Esliger DW, Rowlands AV, Hurst TL. et al. Validation of the GENEA Accelerometer. Med Sci Sports Exerc 2011;43:1085–93. [DOI] [PubMed] [Google Scholar]
- 32. Goddard E. Estimating alcohol consumption from survey data: improved method of converting volume to units. ONS, 2007. http://webarchive.nationalarchives.gov.uk/20160105160709/http://www.ons.gov.uk/ons/guide-method/method-quality/specific/gss-methodology-series/index.html (31 May 2017, date last accessed). [Google Scholar]
- 33. Deere KC, Hannam K, Coulson J. et al. Quantifying habitual levels of physical activity according to impact in older people: accelerometry protocol for the VIBE study. J Aging Phys Act 2016;24:290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lilley K, Stiles VH, Dixon S. The influence of motion control shoes on the running gait of mature and young females. Gait and Posture 2013;37:331–5. [DOI] [PubMed] [Google Scholar]
- 35. Bonaiuti D, Shea B, Iovine R. et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev 2002;3:CD000333. [DOI] [PubMed] [Google Scholar]
- 36. Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 1995;57:344–58. [DOI] [PubMed] [Google Scholar]
- 37. Judex S, Carlson KJ. Is bone’s response to mechanical signal dominated by gravitational loading. Med Sci Sports Exerc 2009;41:2037–43. See comment in PubMed Commons below. [DOI] [PubMed] [Google Scholar]
- 38. Gross TS, Srinivasan S. Building bone mass through exercise: could less be more? Br J Sports Med 2006;40:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chastin SFM, Mandrichenko O, Skelton DA. The frequency of osteogenic activities and the pattern of intermittence between periods of physical activity and sedentary behaviour affects bone mineral content: the cross-sectional NHANES study. BMC Pub Health 2014;14:14. doi: 10.1186/1471-2458-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nikander R, Sievänen H, Heinonen A. et al. Femoral neck structure in adult female athletes subjected to different loading modalities. J Bone Miner Res 2005;20:520–8. [DOI] [PubMed] [Google Scholar]
- 41. Jämsä T, Vainionpää A, Korpelainen R. et al. Effect of daily physical activity on proximal femur. Clin Biomech 2006;21:1–7. [DOI] [PubMed] [Google Scholar]
- 42. Knapp KM. Quantitative ultrasound and bone health. Salud Publica Mex 2009;51(Suppl 1):S18–24. [DOI] [PubMed] [Google Scholar]