Abstract
The diverse community of microbes present in the human gut has emerged as an important factor for cancer risk, potentially by altering exposure to chemical carcinogens. In the present study, human gut bacteria were tested for their capacity to transform the carcinogenic heterocyclic amine 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MelQx). Eubacterium hallii, Lactobacillus reuteri, and Lactobacillus rossiae were able to convert MelQx to a new microbial metabolite characterized on the basis of high-resolution mass spectrometry and NMR as 9-hydroxyl-2,7-dimethyl-7,9,10,11-tetrahydropyrimido[2′,1′:2,3]imidazo[4,5-f]quinoxaline (MelQx-M1), resulting from conjugation with activated glycerol. Acrolein derived from the decomposition of 3-hydroxypropionaldehyde, which is the product of bacterial glycerol/diol dehydratase activity, was identified as the active compound responsible for the formation of MelQx-M1. A complex human gut microbial community obtained from invitro continuous intestinal fermentation was found to also transform MelQx to MelQx-M1. MelQx-M1 had slightly reduced cytotoxic potency toward human colon epithelial cells invitro, and diminished mutagenic potential toward bacteria after metabolic activation. As bacterially derived acrolein also transformed 2 other HCAs, namely 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 2-amino-3-methylimidazo[4,5-f]quinoline, these results generalize the capacity of gut microbiota to detoxify HCAs in the gut, potentially modulating cancer risk.
Keywords: gut microbiota, dietary carcinogen, acrolein, metabolism, heterocyclic amines
The human diet can influence the risk of developing cancer. Amongst putative contributing factors are regular exposure to genotoxic chemicals present in food and gut microbial dysbiosis driven by diet. For example, consuming well-cooked meat is associated with increased prevalence of colorectal cancer in humans (Sinha etal., 2001), and red and processed meat are probable and known human carcinogens, respectively (Bouvard etal., 2015). High-temperature or prolonged cooking of meat results in the formation of mutagenic and carcinogenic heterocyclic amines (HCAs) such as 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) (Felton etal., 1986), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MelQx) (Jägerstad etal., 1984), and 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) (Kasai etal., 1980). PhIP, MelQx, and IQ are among the most potent mutagens toward bacterial and mammalian cells (Sugimura, 1988; Thompson etal., 1987) and are carcinogens in multiple organs in rodent models (Chen etal., 2016; Kato etal., 1988; Ohgaki etal., 1987; Nishikawa etal., 2005). Overall, these data suggest that HCA exposure from consuming meat may contribute to an increased colorectal cancer prevalence.
The basis of HCA mutagenicity is well characterized. Cytochrome P450 enzymes convert HCAs to hydroxylamines, which are converted to corresponding esters by N-acetyltransferases or sulfotransferases (Hein etal., 1994; Kim and Guengerich, 2005). These esters are not stable and hydrolyze spontaneously, yielding reactive aryl nitrenium ions that covalently bind to DNA (Kim and Guengerich, 2005), thus producing HCA-DNA adducts, which are prone to induce frameshift or point mutations (Turesky and Le Marchand, 2011). Consequently, HCA-induced mutagenesis is critically modulated by its metabolism.
Gut microbes may alter HCAs in the human colon, for instance by hydrolyzing their glucuronide conjugates (Humblot etal., 2007), and catalyzing their oxidation (Humblot etal., 2005). More recently, a novel transformation involving the conjugation of PhIP to a glycerol-derived conjugate by complex human fecal microbiota invitro (Fekry etal., 2016; Vanhaecke etal., 2006) as well as by Enterococcus spp., Lactobacillus reuteri (Vanhaecke etal., 2008a), Lactobacillus rossiae, and Eubacterium hallii (Fekry etal., 2016), has gained attention (Engels etal., 2016a; Nicken etal., 2010, 2015; Vanhaecke etal., 2008b,c). This transformation requires the conversion of glycerol to 3-hydroxypropionaldehyde (3-HPA), and is catalyzed by bacterial glycerol/diol dehydratases encoded by the pduCDE gene (Fekry etal., 2016), which is present in E. hallii, L. reuteri, as well as L. rossiae (De Angelis etal. 2014; Engels etal., 2016a; Fekry etal., 2016). Finally, 3-HPA is excreted, chemically decomposes to produce acrolein, which then reacts with PhIP yielding PhIP-M1 (Engels etal., 2016a).
The microbial transformation of HCAs to their glycerol-derived conjugates may have toxicological relevance as a detoxification process. First, chemical inference on the basis of the involvement of the primary amine moiety in HCA activation, suggests this metabolic step will be blocked. Additionally, the mutagenic activity of PhIP-M1 in the Ames test is only 2%–4% of that of PhIP (Vanhaecke etal., 2008b) and PhIP-M1 did not induce malignant transformation of BALB/c 3T3 cells (Nicken etal., 2015) as well as not being cytotoxic to Caco-2 cells (Vanhaecke etal., 2008c) or rat intestinal tissues (Nicken etal., 2015). The substrate generality of this process is not known, eg, whether other HCAs, such as MelQx are conjugated.
MelQx is present in the diet at similar concentrations as PhIP, but is more mutagenic (about 10 times higher in the Ames test; Pfau etal., 1999) and has a higher capacity to induce tumors in rodents (Ohgaki etal., 1987). It is present in well-cooked meats at levels up to 1.1 µg/g (Oz and Kaya, 2011) and daily intake was estimated to be 0.2–2.6 µg for a “normal diet” (Ushiyama etal., 1991). Based on detection frequency in food, daily intake, and tumourigenic potency, the theoretical excess lifetime cancer risk from MelQx can be estimated to be higher than the cancer risk emanating from PhIP (Zimmerli etal., 2001). In addition, MelQx-DNA adducts have been observed in human hepatocytes (Nauwelaers etal., 2011), human colon and kidney from surgical and autopsy specimens (Totsuka etal., 1996). Furthermore, the profile of MelQx and its metabolites in feces was different than in bile (Turesky etal., 1988), implying that gut microbes may influence the fate of MelQx present in the colon.
On the basis of the chemical mechanism of the glycerol conjugation process (Engels etal., 2016a), we hypothesized that microbes with glycerol/diol dehydratase activity might transform MelQx, thereby altering its cytotoxicity and mutagenicity. Therefore, we examined the capacity of selected gut microbes as well as complex human gut microbiota to transform MelQx under strict anaerobic conditions. Furthermore, we evaluated the toxicological relevance of the transformation by characterizing the cytotoxicity, mutagenicity, and cell transforming potential of MelQx and its metabolite invitro. The relevance of the findings is discussed in the context of how individual risk of HCA carcinogenesis may be modulated by biotransformation catalyzed by human gut microbiota.
MATERIALS AND METHODS
Hazard information
Acrolein is toxic (oral, dermal, inhalation), forms flammable gases, is corrosive and hazardous to the environment. MelQx is a probable human carcinogen (IARC group 2B) (IARC Working Group on the Evaluation of Carcinogenic risk to Humans, 1993).
Bacterial strains and medium
Bacterial strains E. hallii DSM 3353, L. rossiae DSM 15814, L. reuteri DSM 20016 and ATCC 55730, Eubacterium rectale DSM 17629 and microbiota effluent derived from the invitro continuous fermentation model PolyFermS were cultured in a modified yeast extract-casitone-fatty acid medium (mYCFA; Duncan etal., 2002)) containing 10 g/l amicase, 2.5 g/l yeast extract, 4 g/l NaHCO3, 10 g/l glucose, 2 g/l maltose, 2 g/l cellobiose, 15% mineral solution I (3 g/l K2HPO4), 15% mineral solution II (3 g/l K2HPO4, 6 g/l (NH4)2SO4, 6 g/l NaCl, 0.6 g/l MgSO4, 0.6 g/l CaCl2), 0.1% vitamin solution (10 mg/l biotin, 10 mg/l cobalamin, 30 mg/l p-aminobenzoic acid, 50 mg/l folic acid, 75 mg/l pyridoxamine), 0.31% volatile fatty acid mix (59% acetate, 14% butyrate, 21% propionate, 3% n-valeric acid, 3% isobutyric acid), 0.02% hemin (0.5 g/l) as an iron source and 0.1% resazurin (1 g/l) as a redox indicator. The mYCFA medium was supplemented with 110 mM glycerol and 2 g/l glucose. All components except L-cysteine-HCL were dissolved in deionized water, and adjusted to pH 6.8 by adding 1.0 N NaOH. The medium was flushed with CO2 and boiled until the color changed from blue to pink/light yellow, at which point L-cysteine-HCl (0.1%, w/v) was added. The medium was transferred to Hungate tubes flushed with CO2, and tubes were sealed and autoclaved. Cultures of E. hallii and L. reuteri that were frozen at −20 °C in mYCFA agar (1.5% (w/v) agar) were used as stock cultures. For each experiment, a fresh agar stock was thawed and 1 ml of liquid YCFA medium added and thoroughly mixed before being transferred to 9 ml mYCFA medium. In the case of L. rossiae, the glycerol stock was inoculated on an mYCFA agar plate. After incubation at 37 °C overnight, single colonies were inoculated into 9 ml mYCFA medium. After incubation at 37 °C for 24 h, the culture was transferred at least once to fresh mYCFA medium before being used for an experiment.
Chemicals
MelQx was purchased from Chemie Brunschwig AG (Basel, Switzerland). Acrolein and the constituents of the culture media were all obtained from Sigma-Aldrich (Buchs, Switzerland). HPLC-grade acetonitrile and methanol were from Merck (Darmstadt, Germany) and Sigma-Aldrich (Steinheim, Germany), respectively. Formic acid for LC-MS was purchased from Fisher Scientific (Geel, Belgium) and water was purified with a Milli-Q Integral Water Purification System (Millipore Corporation, Billerica, Massachusetts). NMR solvents were purchased from ARMAR Chemicals (Döttingen, Switzerland).
Instrumentation
High-resolution MS analysis was carried out on an Exactive Orbitrap (Thermo Fisher) mass spectrometer set as follows: scan range: 100–600 m/z; nominal resolution: 100 000 at m/z 400; sheath and aux gas: helium; sheath gas flow rate: 4 U; aux gas flow rate: 2 U; spray voltage: 3.5 kV; capillary temperature: 250 °C; capillary voltage: 30 V; tube lens voltage: 100 V; skimmer voltage: 15 V; polarity: positive. NMR spectra were recorded on a 400 MHz NMR Spectrometer (1H, 400 MHz; 13C, 100 MHz). Chemical shifts are expressed in ppm relative to the solvent peak of D2O. Ultraviolet (UV) absorbance was recorded using 1 mM MelQx or MelQx-M1 in water (10% acetonitrile, v/v) at 25 °C with a Cary 100 UV-vis spectrophotometer, monitoring from 500 to 200 nm.
Microbial MelQx transformation
The bacteria L. reuteri DSM 20016 and ATCC 55730, L. rossiae DSM 15184, and E. hallii DSM 3353 possess the pdu gene (Supplementary Table 1), harboring the potential of glycerol/diol dehydratase activity. To characterize the capacity of these strains to transform MelQx, fresh mYCFA with and without glycerol was inoculated with overnight cultures at 10% (v/v) and the resulting mixture was allowed to incubate at 37 °C for 3 days in the dark. MelQx stock solution (10 mM in DMSO, 50 µl) was added to the mixture, yielding a final concentration of 100 µM MelQx (1% DMSO). DMSO used in this study did not influence bacterial growth, as measured by optical density (OD) (600 nm) during the 72-h incubation. Each experiment was carried out 3 times starting from a different bacterial sample.
The rate of transformation of MelQx was studied for incubations carried out in mYCFA supplemented with glycerol and 100 µM MelQx. Overnight cultures (0.5 ml) of L. reuteri DSM 20016 and E. hallii DSM 3353 were inoculated in 4.5 ml mYCFA with 110 mM glycerol and were incubated at 37 °C under anaerobic conditions for 72 h. The mixture was sampled at different time points up to 72 h. Bacterial growth was measured on the basis of OD measurements at 600 nm. MelQx degradation and MelQx-M1 formation were determined by LC-electrospray ionization-mass spectrometry (LC-ESI-MS2) as described below. All the experiments were performed in triplicate.
MelQx transformation by complex human colon microbiota
The transformation of MelQx by complex human colon microbiota was performed according to Fehlbaum etal. (2015) and Fekry etal. (2016). In brief, the fecal microbiota from a 78-year-old healthy woman was immobilized in gellan-xanthan gel beads under strict anaerobic conditions and the fecal beads were inoculated (30% v/v) in an inoculum reactor to mimic the conditions of the proximal colon (PC, 37 °C, pH 5.7, strictly anaerobic atmosphere). The inoculum PC reactor was connected to a reactor mounted in parallel mimicking the conditions of the distal colon (DC, 37 °C, pH 6.8, strictly anaerobic atmosphere). Effluent collected from PC and DC reactors were used to inoculate batch fermentations in mYCFA containing glycerol to investigate MelQx transformation in triplicate. Supernatants were collected from batch fermentations after incubation for 3 d at 37 °C and were analyzed for MelQx and MelQx-M1 using LC-ESI-MS2 as described below. Total bacterial DNA was extracted from bacterial effluents from the continuous intestinal fermentation with a FastDNA SPIN Kit for Soil according to the manufacturer’s instructions. Quantification of total bacterial and E. hallii DNA was performed by following the procedure reported by Fekry etal. (2016).
LC-ESI-MS2 analysis
Following microbial transformation reactions as described earlier, samples were transferred into 1.5 ml centrifuge tubes and centrifuged at 16 900 rcf (14 000 rpm) for 10 min with an Eppendorf R centrifuge. A 500 µl portion of the supernatants was removed and rendered alkaline by addition of 150 µl 1 M Na2CO3. This solution was extracted 3 times with 0.5 ml ethyl acetate. Phase separation was achieved by centrifugation at 14 000 rpm and 4 °C for 5 min. Ethyl acetate phases were combined and concentrated in a centrifugal evaporator (MiVac, GeneVac, Suffolk, England). Resulting residues were reconstituted in 300 µl acetonitrile. Samples were diluted by a factor of 50 before LC-ESI-MS2 analysis. The LC system, equipped with a Waters BEH130 C18 column (1.7 µm, 300 µm × 150 mm), was connected to a linear trap quadrupole ion trap Velos mass spectrometer (Thermo Fisher, San Jose, California, USA) equipped with an ESI interface. The injection volume was 1 µl. Solvent A was water containing 10% acetonitrile (v/v) and 0.1% of formic acid (v/v) and solvent B was acetonitrile with 0.1% formic acid (v/v). The linear gradient was as follows: 0–3 min: 0% B; 3–13 min: 0%–95% B; 13–15 min 95% B; 15–16 min: 95%–0% B; 16–22 min: 0% B. The flow rate was 5 µl/min. The MS ionization parameters were optimized by tuning with a 1 µM MelQx solution in solvent A in ESI positive mode. The spray voltage and capillary temperature were 3.5 kV and 250 °C, respectively. Fragmentation was carried out in collision-induced dissociation mode with the collision energy set at 35 eV. System control and data acquisition were performed using Xcalibur (version 2.2 SP1.48, Thermo Fisher).
9-hydroxyl-2,7-dimethyl-7,9,10,11-tetrahydropyrimido[2′,1′:2,3]imidazo[4,5-f]quinoxaline
MelQx (20 mg, 94 µmol) was mixed with acrolein (200 µl, 2.98 mmol) in 10 ml acetonitrile in an aluminum-foil-covered glass vial. The solution was allowed to react at 37°C for 2 h. Acetonitrile was then removed by vacuum evaporation, and the resulting residue was dissolved in 1 ml water with 10% acetonitrile. The sample was purified by HPLC using an Agilent 1100 series system comprised of a binary pump, a fraction collector and a UV/vis detector set to 265 nm. A Luna C18 (2) column (5 µm, 250 × 10 mm, Phenomenex, Schlieren, Switzerland) was used. The flow rate was 3.5 ml/min and the injection volume was 100 µl. Distilled water with 0.1% (v/v) of formic acid, and acetonitrile were used as mobile phases with a linear gradient from 3% to 30% acetonitrile from 0–35 min, followed by 5 min 100% acetonitrile, and finally re-equilibration to 3% acetonitrile. Fractions corresponding to MelQx-M1 were combined and freeze dried to yield the title compound as a reddish powder (yield: 53%). 1H NMR (400 MHz, D2O) δ 8.67 (s, 1H), 7.70 (d, J = 3.6 Hz, 2H), 5.57 (t, J = 2.8 Hz, 1H), 5.21 (ddd, J = 13.5, 5.8, 2.3 Hz, 1H), 4.33 (td, J = 13.2, 4.3 Hz, 1H), 3.71 (s, 3H), 2.73 (s, 3H), 2.51-2.36 (m, 1H), 2.20 (tdd, J = 13.9, 5.9, 2.8 Hz, 1H)., 13C NMR (100 MHz, D2O) 155.22, 145.97, 144.68, 136.09, 130.57, 129.12, 123.72, 121.27, 112.31, 72.03, 39.41, 29.12, 25.93, 21.91. HRMS (ESI) calculated for C14H16N5O: [M + H]+m/z 270.1349, found: 270.1352.
Cytotoxicity
The HCEC1CT clone was obtained in August 2011 from the laboratory of Professor Jerry Shay (University of Texas Southwestern Medical Center, Dallas, TX), grown under previously reported conditions (Roig etal., 2010) and regularly confirmed to be mycoplasma-free using the MycoAlert mycoplasma detection kit (Lonza Group Ltd, Basel, Switzerland). Cells were seeded in 96 well plates (3000 cells/well) and allowed to attach. After 24 h, medium was replaced by fresh medium containing 0, 10, 50, 100, 150, 200, 350, 500, 1000, and 2500 µM of MelQx or MelQx-M1 as well as 1% DMSO (v/v). 24 h after addition of the compounds, the medium was again replaced by fresh medium, before the CellTiter-Glo (Promega, Dübendorf, Switzerland) luminescent cell viability assay was performed according to manufacturer’s protocol (Promega, 2015). As a positive control, 2% (v/v) Triton X-100 was used and the experiment was performed 3 times. The resulting data were normally distributed based on the D’Agostino & Pearson omnibus normality test (p = .5643 and .1494 for MelQx and MelQx-M1, respectively). The 5% and 10%-response benchmark dose (BMD5 and BMD10) was calculated with PROAST version 38.9 in R by following the manual provided by European Food Safety Authority (EFSA) (EFSA Scientific Committee, 2009).
Bacterial reverse mutation test
The bacterial reverse mutation test (Ames test) was performed using the preincubation method as described by Mortelmans and Zeiger (2000), with slight alterations. Briefly, Salmonella Typhimurium TA98 (Trinova, Gießen, Germany) was cultivated in nutrient broth (nutrient broth no. 2; Oxoid, Wesel, Germany) overnight and then used at an OD of 0.9–1.0 in the test. Bacteria were thereby incubated with top agar (Kobe I agar; Carl Roth), S9 originating from rat liver (Trinova) and sodium phosphate buffer (2.8 g/l NaH2PO4·H2O, 12.8 g/l Na2HPO4·2H2O, 2.7 g/l KCl, 1.8 g/l MgCl, 3.5 g/l NADP, 1.6 g/l glucose-6-phosphate, pH = 7.4) for 20 min at 37 °C in 15 ml reaction tubes (CELLSTAR polypropylene tubes; Greiner Bio-One; Frickenhausen, Germany). This mixture was then poured into petri dishes (Sarstedt, Nümbrecht, Germany) containing base agar (Kobe I agar, Carl Roth). Finally, the plates were incubated for 48 h at 37 °C and the colonies on each plate were counted. To test the stability of MelQx-M1 under the assay conditions, buffer containing 10% of rat liver S9 and 1 µM MelQx-M1 were incubated for 30 min at 37 °C, before the mixture was centrifuged and the supernatant analyzed in the same manner as the samples described in LC-ESI-MS2 section.
BALB/c 3T3 cell transformation assay
The cell transformation assay (CTA) was performed exactly according to the ECVAM-prevalidated and recommended protocol (Sasaki etal., 2012a). All cell culture reagents for the CTA were supplied by Biochrom (Berlin, Germany) unless otherwise stated. Briefly, 20,000 Balb/c 3T3 clone A31-1-1 cells (a kind gift from Dr. A. Poth, Harlan Cytotest Cell Research, Roßdorf, Germany) were seeded on 10 cm culture dishes (TPP, Trasadingen, Switzerland) and incubated for 24 h in minimum essential medium supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (100 IU/ml and 100 mg/ml, respectively). Then, the cells were exposed to the different concentrations of MelQx and MelQx-M1 as well as 0.1% DMSO (solvent control) and 3-methylcholanthrene (4 µg/ml; positive control) for 72 h. After that period, the culture medium was changed twice weekly (from day 7 onwards, the medium described earlier was exchanged for DMEM/F12 supplemented with 2% FBS, 100 mg/ml streptomycin/100 IU/ml penicillin and 2 µg/ml bovine insulin) until day 31, when the cells were fixed and stained using methanol and Giemsa solution (both purchased from Carl Roth, Karlsruhe, Germany). Finally, the foci were scored using an Olympus SZX2-ILLT stereo microscope (Olympus, Hamburg, Germany) and the recommended photo catalogue (Sasaki etal., 2012b) as reference of focus appearance. A dose-range-finding study (colony forming efficiency method) was performed prior to the actual transformation assay exactly as described in the recommended protocol (Sasaki etal., 2012a).
Statistical analyses
All statistical analyses were performed using GraphPad Prism (version 6; GraphPad, La Jolla, California). A sigmoidal (4 parameter logistic) model was used to fit the changes in relative abundance of MelQx and MelQx-M1 in the presence of E. hallii, L. reuteri, acrolein, as well as 3-HPA and 1-way ANOVA multiple comparisons were performed to compare the cytotoxicity of MelQx and MelQx-M1.
RESULTS
Transformation of MelQx by Strains of Intestinal Bacteria
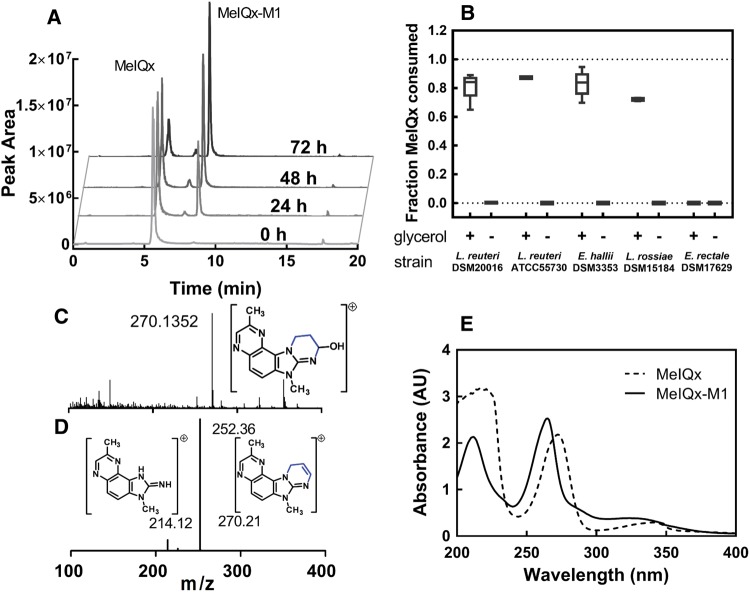
To determine whether selected gut microbes with confirmed glycerol/diol dehydratase activity (Supplemetary Table 1; Engels etal., 2016a; Fekry etal., 2016) have the capacity to transform MelQx, L. reuteri DSM 20016 and ATCC 55730, L. rossiae DSM 15184, and E. hallii DSM 3353 were tested for their ability to transform MelQx in presence or absence of glycerol (Figure 1). E. rectale DSM 17629, which lacks a glycerol/diol dehydratase-encoding gene (gene bank accession number: FP929042), served as negative control. All tested strains except E. rectale degraded MelQx when incubated under anaerobic conditions in mYCFA in the presence of glycerol. Analysis of the reaction mixture by LC-MS indicated the formation of a single new product with a mass to charge ratio (m/z) of 270.1352 (Figure 1A). In the absence of glycerol, none of the new product was formed even after 3 days of incubation (Figure 1B). The transformation efficiency on the basis of peak area determined by LC-ESI-MS2 was similar for all tested strains, ranging from 72% ± 1.5% for L. rossiae, to 87% ± 0.9% for L. reuteri ATCC 55730 (Figure 1B).
Figure 1.
Detection and characterization of MelQx metabolite (MelQx-M1) in bacterial culture. A, Presence of MelQx and MelQx-M1 during growth of E. hallii DSM 3353 in mYCFA containing 0.8% (v/v) glycerol (approximately 100 mM) and 100 µM MelQx, at 37 °C. B, Degradation of MelQx by L. reuteri DSM 20016 and ATCC 55730, E. hallii DSM 3353, L. rossaie DSM 15184 and E. rectale DSM 17629 in the presence and absence of glycerol in mYCFA medium. Data are displayed as mean values ± SD from 3 independent experiments. C, high resolution mass spectra of peak MelQx-M1 showed a m/z 270.1352. D, fragmentation of m/z 270.1352 at 8.5 min of the sample 72-h MelQx-incubation. E, UV absorbance of MelQx (dashed line) and MelQx-M1 (solid line).
Analysis of the product by high-resolution mass spectrometry (HRMS), LC-MS2 and NMR (Supplementary Table 2) was consistent with the structure being 9-hydroxyl-2,7-dimethyl-7,9,10,11-tetrahydropyrimido[2',1':2,3]imidazo[4,5-f]quinoxaline (MelQx-M1), analogous to the structure of PhIP-M1, and thus likely resulting from the reaction of MelQx with acrolein (Figure 2). The high-resolution m/z (Figure 1C and Supplementary Figure 1) is consistent with the formula C14H15N5O, and the main fragments were m/z 252, 226, and 214, corresponding to a loss of H2O, C2H4O, and C3H4O, respectively (Figure 1D and Supplementary Figure 2). This fragmentation pattern was similar to that of PhIP-M1 described by Vanhaecke etal. (2006). The UV absorption spectra of MelQx and MelQx-M1 had 2 absorbance peaks at 272 and 217 nm for MelQx and a hypochromic shift to 265 and 212 nm for MelQx-M1 (Figure 1E).
Figure 2.
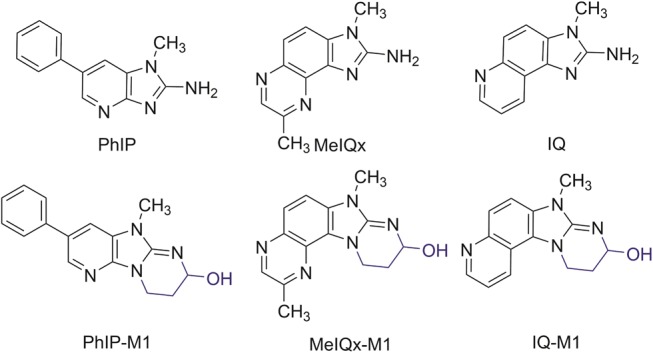
Structures of PhIP, PhIP-M1, MelQx, MelQx-M1, IQ, and IQ-M1.
An authentic standard of MelQx-M1 was synthesized by reacting MelQx with acrolein in acetonitrile. It gave rise to the same high resolution mass spectrum and co-eluted with the microbial transformation product (Supplementary Figure 2). NMR data, ie, H-NMR, 13C-NMR, distortionless enhancement by polarization transfer, homonuclear correlation spectroscopy, heteronuclear multiple-quantum correlation spectroscopy NMR further confirmed the identity of the new metabolite as MelQx-M1 (Supplementary Table 2 and Supplementary Figs. 3–7).
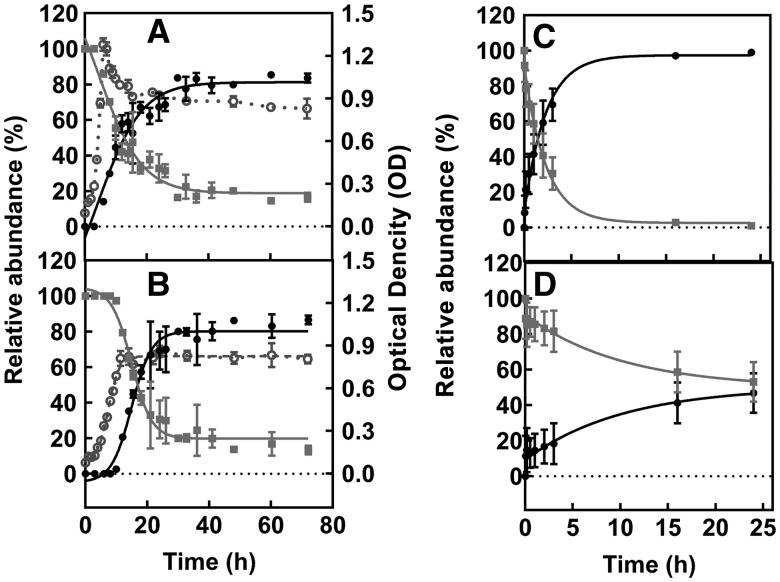
The rate of conversion of MelQx to MelQx-M1 was determined during growth of E. hallii DSM 3353 and L. reuteri DSM 20016 in mYCFA supplied with glycerol, as well as during incubation with 3-HPA or acrolein. The disappearance of MelQx and appearance of MelQx-M1 were monitored using LC-ESI-MS2. The transformation of MelQx by E. hallii and L. reuteri displayed similar kinetic profiles. MelQx-M1 was first observed when maximal growth was reached, i.e. after 6 h of incubation for E. hallii and 12 h for L. reuteri (Figs. 3A and B). Over the course of 20 h, MeIQ-M1 levels increased exponentially, and plateaued at around 80% conversion. The yields of MelQx-M1 were 86% ± 3% for E. hallii and 87% ± 2% for L. reuteri. Reaction of MelQx with 3-HPA or with acrolein resulted in the formation of 55 % ± 18% and 94% ± 6% MelQx-M1, respectively. Moreover, the reaction of MelQx with acrolein was found to be about 5 times faster than with 3-HPA (Figs. 3C and D). These data are consistent with the mechanism by which 3-HPA spontaneously decomposes to acrolein, with which MelQx reacts to give rise to MelQx-M1.
Figure 3.
MelQx to MelQx-M1 transformation in the presence of E. hallii and L. reuteri, acrolein, or 3-HPA. Disappearance of MelQx and formation of MelQx-M1 mediated by representative bacterial strains. Changes in relative abundance of MelQx (filled square) and MelQx-M1 (filled circle) and OD (open circle) of (A) E. hallii DSM 3353, and (B) L. reuteri DSM 20016 cultures at 37 °C in mYCFA-glyc medium in the presence of glycerol. MelQx to MelQx-M1 tranformation in the presence of (C) 10 mM acrolein, and (D) 10 mM 3-HPA at 37 °C and pH 7. Data are displayed as mean values ± SD from 3 independent experiments.
Transformation of MelQx by Complex Human Gut Microbiota
To check the capacity of complex human gut microbiota to perform the MelQx-to-MelQx-M1 transformation, we utilized effluent microbiota derived from a continuous intestinal fermentation PolyFermS model mimicking healthy elderly PC and DC microbiota (Fehlbaum etal., 2015). E. hallii was present at 0.02%–0.06% relative abundance in fermenter effluents. Effluent was inoculated (10% v/v) into mYCFA containing 100 μM MelQx in the presence and absence of glycerol, and incubations were carried out at 37 °C for 72 h under strict anaerobic conditions. When glycerol was added, MelQx was converted to MelQx-M1 by proximal and distal colon effluent microbiota at similar yields, ie, 29% ± 1% and 30% ± 2%, respectively. In contrast, no transformation of MelQx to MelQx-M1 was detected in the absence of glycerol.
Cytotoxicity of MelQx and MelQx-M1 to Human Colon Epithelial Cells
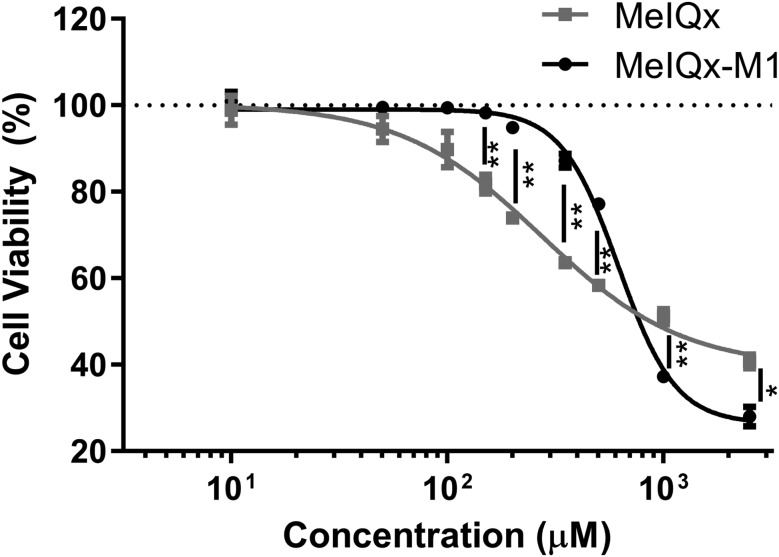
To compare the relative cytotoxic potencies of MelQx-M1 and MelQx, we exposed human colon epithelial cells (HCECs) to increasing concentrations of MelQx-M1 and MelQx and evaluated the impact on cell viability. HCECs are derived from immortalization of cells from a human colon biopsy of noncancerous tissue (Roig etal., 2010). The cells actively proliferate but retain multilineage epithelial differentiation capability and express stem cell makers, suggesting their utility as an invitro model for healthy colon epithelium (Erzinger etal., 2016; Roig etal., 2010; von Moos etal., 2017). After 24 h exposure to MelQx or MelQx-M1, changes in cell viability were determined with a luminescent cell viability assay to measure released ATP. MelQx-M1 did not reduce cell viability at concentrations below or equal to 150 µM, whereas MelQx reduced cell viability at concentrations ≥ 50 µM (Figure 4). Using these data, we calculated BMD levels (EFSA Scientific Committee, 2009) and determined the No Observed Adverse Effect Level (NOAEL) and Lowest Observed Adverse Effect Level (LOAEL) (Table 1). All indicators were higher when cells were treated with the microbial metabolite MelQx-M1 than with MelQx. For example, the BMD5 lower bound (BMDL5) for MelQx-M1 was 204 µM, or 50 times higher than that of MelQx, and the BMD5 upper bound (BMDU5) for MelQx-M1 was 334 µM, which is 6 times higher than that of MelQx (Table 1). Similarly, the BMD10, BMDL10 BMDU10 as well as the LOAEL and NOAEL for MelQx-M1 were 4–31 and 7–20 times higher than that of MelQx.
Figure 4.
Cytotoxicity of MelQx (filled square) and MelQx-M1 (filled cirlce) to HCEC. Data are displayed as mean values ± SD from 3 biological replicates. ** indicates a p-value < .01, * indicates a p-value < .05.
Table 1.
IC10, IC50, 5% Benchmark Dose (BMD5), 10% Benchmark Dose (BMD10), NOAEL, and LOAEL (µM) of MelQx and MelQx-M1 Observed in the Human Colonic Epithelial Cell Line (HCEC)
| Metabolite |
|||
|---|---|---|---|
| MelQx | MelQx-M1 | Fold change | |
| IC10 (95% CI) | 52 (31–87) | 316 (285–351) | 6 |
| IC50 (95% CI) | 272 (212–349) | 617 (575–663) | 2 |
| BMDL5a | 4.1 | 204.1 | 50 |
| BMDU5a | 55.8 | 333.7 | 6 |
| BMD5a | 26.5 | 262.2 | 10 |
| BMDL10a | 8.8 | 272.8 | 31 |
| BMDU10a | 87.6 | 366.1 | 4 |
| BMD10a | 55.2 | 334.6 | 6 |
| NOAELa | 10 | 200 | 20 |
| LOAELa | 50 | 350 | 7 |
Values of IC10 and IC50 are means of 3 independent experiments, the values in brackets are corresponding 95% confidence intervals.
BMDL, lowest benchmark dose lower bound from exponential models; BMDU, highest benchmark dose upper bound Hill models; BMD, bench mark dose, the subscripted number means the benchmark response used when calculating BMD, ie, 5% or 10%; NOAEL, no-observed-adverse-effect level; LOAEL, lowest-observed-adverse-effect level.
Mutagenicity of MelQx and MelQx-M1
On the basis of the established mechanism of MelQx mutagenicity, we expected MelQx-M1 to have lower mutagenic activity than MelQx, and the well-established bacterial reverse mutation test (Ames test) with and without a metabolic activation system (S9) was used to compare mutagenicity of both compounds. In the absence of metabolic activation, treatment of the bacteria with MelQx or MelQx-M1 did not induce a significantly higher number of revertant colonies when compared with the vehicle control (7.7% DMSO). 2-Nitrofluorene (2-NF) and 4-nitro-o-phenylenediamine (4-NPD), 2 positive controls, gave rise to 608 ± 16 (2-NF) and 680 ± 19 (4-NPD) revertants at the dose of 5 µg/plate (Table 2). In the presence of S9 however, both MelQx and MelQx-M1 induced a significant and concentration-dependent increase of the number of revertants (Table 2). Treatment with 0.23 µM MelQx (0.03 µg/plate equivalent) led to the formation of 980 ± 20 revertants/plate, whereas the microbial transformation product MelQx-M1 led to the formation of 66 ± 5 revertants/plate, suggesting that the mutagenicity of MelQx-M1 was diminished compared with its precursor MelQx.
Table 2.
Mutagenic Activities of MelQx and MelQx-M1 in the Presence and Absence of Metabolic Activation Determined Using Ames Test
| Compound | Dose (µg/plate)a | Dose (µM) | Mutagenic activity (revertants/plate) |
|
|---|---|---|---|---|
| −S9 | +S9 | |||
| MelQx | 0.01 | 0.08 | 23 ± 1 | 352 ± 4 |
| 0.03 | 0.2 | 21 ± 2 | 980 ± 20 | |
| 0.11 | 0.8 | 20 ± 1 | 1264 ± 38 | |
| 0.32 | 2.3 | 21 ± 1 | 1449 ± 132 | |
| 1.1 | 7.7 | 20 ± 1 | >1500 | |
| 3.2–107 | 23–769 | 21–22 | >1500 | |
| MelQx-M1 | 0.01 | 0.08 | 21 ± 1 | 44 ± 1 |
| 0.04 | 0.2 | 21 ± 1 | 66 ± 5 | |
| 0.13 | 0.8 | 21 ± 1 | 182 ± 13 | |
| 0.40 | 2.3 | 22 ± 2 | 358 ± 4 | |
| 1.3 | 7.7 | 31 ± 2 | 770 ± 7 | |
| 4.0 | 23 | 32 ± 3 | 1000 ± 20 | |
| 14–135 | 77–769 | 41–54 | >1500 | |
| Solvent control | NA | NA | 20 ± 1 | 31 ± 1 |
| 2-nitrofluorene | 5 | NA | 616 ± 18 | NA |
| 4-NPD | 5 | NA | 695 ± 5 | NA |
| Benzen[α]pyrene | 5 | NA | NA | 302 ± 3 |
The differences on the doses(µg/plate) of MelQx and MelQx-M1 were due to their different molecular weight. NA, not applicable. Data are displayed as mean ± SD (n = 2).
Malignant Transformation of MelQx and MelQx-M1
To test the potential of MelQx and MelQx-M1 to induce malignant transformation in mammalian cells, we used the Balb/c 3T3 CTA. The CTA is a well-established invitro assay for predicting carcinogenic risk of chemicals in humans (Lilienblum etal., 2008). Both compounds were tested at concentrations of up to 50 µM and neither MelQx nor MelQx-M1 induced a significant amount of foci in Balb/c 3T3 cells (Table 3). Higher concentrations were not tested due to their significant cytotoxicity.
Table 3.
Transformation of BALB/c 3T3 Cells by MelQx and MelQx-M1
| Doses (µM) | Number of foci per plate |
|
|---|---|---|
| MelQx | MelQx-M1 | |
| 0.1 | 0.1 ± 0.3 | 1.1 ± 1.2 |
| 0.5 | 0.5 ± 0.7 | 0.1 ± 0.3 |
| 1 | NT | 0.5 ± 0.7 |
| 2 | NT | 0.1 ± 0.3 |
| 3 | NT | 0.1 ± 0.3 |
| 4 | NT | 0.2 ± 0.4 |
| 5 | 0.1 ± 0.3 | 0 ± 0 |
| 6 | NT | 0.6 ± 0.8 |
| 7 | NT | 0.7 ± 1.5 |
| 10 | 0.6 ± 0.7 | 1.4 ± 2.2 |
| 50 | 0.6 ± 1.2 | 0 ± 0 |
Medium (control), 0.1% DMSO, 15 µM 3-methylcholanthrene (MCA, positive control) induced 0.2 ± 0.4, 0 ± 0, and 14.1 ± 4.5 foci per plate, respectively. NT, not tested in this study. Data are displayed as mean ± SD (n = 9–10).
DISCUSSION
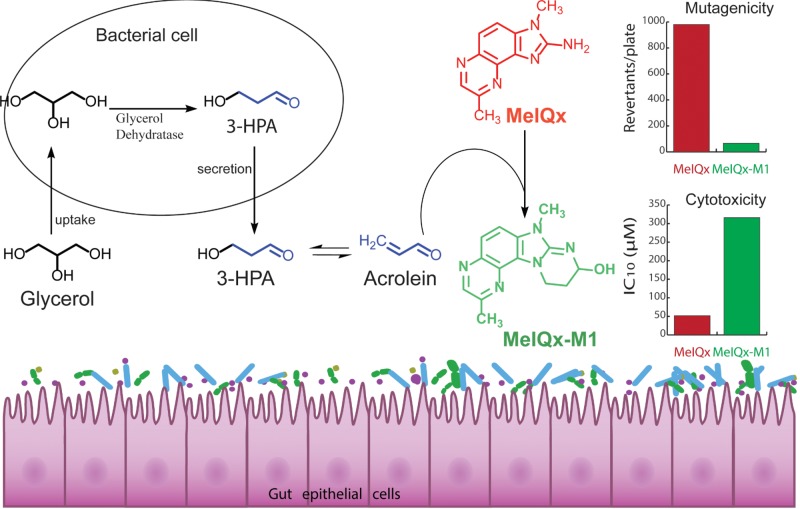
MelQx is one of the most abundant and mutagenic dietary HCAs that people ingest by eating high-temperature cooked red meat. In this study, we identified gut microbes that efficiently transform MelQx to its acrolein conjugate MelQx-M1 and observed that this transformation was also mediated by complex human gut microbiota. On the basis of chemical structure elucidation, direct reaction of MelQx with acrolein derived from bacterial glycerol metabolism appeared to be the chemical basis of the transformation of MelQx. The microbial metabolite MelQx-M1 was less cytotoxic towards human colon epithelial cells and showed lower mutagenicity in the bacterial reverse mutation assay than MelQx after metabolic activation, and no cell transformation potential was observed.
Bacterial Glycerol Metabolism and the Transformation of MelQx
Bacterial glycerol metabolism by glycerol/diol dehydratases was critical for the formation of MelQx-M1, similar to what was previously observed for the transformation of PhIP to PhIP-M1 (Fekry etal., 2016; Vanhaecke etal., 2008a). Glycerol is a nutritional component of dietary fat, a backbone for glycerides, and can be liberated after hydrolysis by digestive lipases (Mattson and Volpenhein, 1964). Furthermore, glycerol is a common additive in formulated foods, as a sweetener, humectant, moisturing as well as thickening agent (U.S. Food and Drug Administration, 2016). Glycerol has been observed in human feces (Marchesi etal., 2007) and may accumulate in the colon due to saturated glycerol absorption in small intestine and colon cells (Fujimoto etal., 2006; Yuasa etal., 2003). On the other hand, commensal bacteria harboring a glycerol dehydratase gene are a regular component of the human fecal microbiota (Engels etal., 2016b). For example, E. hallii occurs early in life and reaches adult abundance levels at approximately 2 years of age in populations from Venezuela, Malawi, the United States and Switzerland, which is indicative of global presence (Schwab etal., 2017). E. hallii glycerol/diol dehydratase-encoding genes were detected in up to 81% of adult human fecal metagenomes (Engels etal., 2016b). In contrast, L. reuteri and L. rossiae are rarely occurring in the adult intestine (Engels etal., 2016b; Fekry etal., 2016). The ubiquitous presence of glycerol, together with steady occurrence of gut microbes with potential for glycerol/diol dehydratase activity (Engels etal., 2016b), suggests that bacterially trigged formation of HCA-M1-type products occurs in the human intestine (Figure 5). Indeed, PhIP-M1 could be recovered from feces of consumers that obtained a single portion of cooked chicken meat (Vanhaecke etal., 2008b).
Figure 5.
Proposed transformation pathway of MelQx by gut microbes.
The observations that a substantial amount of acrolein could be derived from 3-HPA, and that the reaction of MelQx with acrolein is 5 times faster than with a 3-HPA solution, suggest that MelQx conjugation occurs by direct reaction with acrolein via 1,4-addition and 1,2-addition at the 2-amino group (Engels etal., 2016a). Our results for MelQx, in conjunction with those for PhIP (Engels etal., 2016a; Fekry etal., 2016; Vanhaecke etal., 2008a), suggest a general potential for microbes that secrete 3-HPA, which then dehydrates to acrolein, to assist in the formation of M1-type metabolites for other dietary HCAs bearing 2-aminoimidazole moiety. In agreement with this assertion, we could confirm that IQ was transformed to IQ-M1 by E. hallii with 31% efficiency (Figure 2 and Supplementary Figure 8) during growth in mYCFA in the presence of glycerol. This observation is in accordance with the well-established reactivity of acrolein with primary and secondary amines such as lysine, histidine and imidazole (Arai etal., 2014; Uchida, 2015; Yoshida etal., 2009). Furthermore, adducts similar to PhIP-M1, which contains an extra tetrahydropyrimidine ring, were identified as products of the reaction of acrolein with deoxyguanosine (Chung etal., 1984), adenine (Sodum and Shapiro, 1988), cytosine (Sodum and Shapiro, 1988) and arginine (Lambert etal., 2007).
Impact on Toxicity
We found that microbial transformation of MelQx to MelQx-M1 reduced the mutagenic activity of MelQx to 3%–26% in terms of revertants/plate. Similarly, the mutagenicity of PhIP-M1 was only 5%–10% of that described for PhIP (Vanhaecke etal., 2008b). Together, these results suggest that blocking the amino group of HCAs likely reduces toxic potency. This result is consistent with the observation that the 2-aminoimidazole group, and not the quinoxaline group, contributes to biological activities of HCAs (Conti and Crebelli, 2016; Grivas and Jagerstad, 1984), and that the chemical basis of HCA activation involves activation of the primary amine by oxidation and resulting formation of DNA adducts (Turesky etal., 2002). The presence of the 2-amino-substituted imidazole ring has been shown to be essential for activation by rat liver homogenate (S9) and mutagenicity to bacteria (Conti and Crebelli, 2016; Grivas and Jagerstad, 1984) and N-Methylation of the imidazole increases mutagenicity around 500- to 3000-fold (Nagao etal., 1981; Vikse etal., 1993). Indeed, we were somewhat surprised that mutagenicity was not completely devoid for MelQx-M1, and were able to confirm that the observed mutagenicity arose from the generation of small amounts of MelQx as a decomposition product of MelQx-M1 in the presence of S9 at 37 °C. Interestingly, 1 µg/plate PhIP produced 1700 revertants/µg under similar experimental conditions, while 1 µg/plate PhIP-M1 could be expected to give 50 revertants/µg, which is consistent with the observed value of 46 ± 2 revertants/µg (Vanhaecke etal., 2008b). These data suggest that mutagenic activity previously attributed to PhIP-M1 may have also been a result of residual PhIP formed under assay conditions.
Although use of the Ames test provides an indicator of possible genotoxicity/mutagenicity of chemicals, the CTA is an invitro assay well established for predicting carcinogenic risk of chemicals in humans (Lilienblum etal., 2008). However, using BALB/c 3T3 cells to test the transformation potential of MelQx and MelQx-M1 up to a concentration of 50 µM resulted in no detectable cell transformation for either compound. This result is in agreement with the observation that PhIP-M1 does not induce cell transformation in BALB/c 3T3 cells at non-cytotoxic concentrations (Nicken etal., 2015). Similarly, Pfau et al (1999) used mouse fibroblast C3H/M2 cells to test cell transformation by several HCAs, including MelQx, PhIP, and IQ. They found none of the HCAs induced cell transformation without metabolic activation, whereas all induced malignant transformation in the presence of rat-liver S9 (Pfau etal., 1999). Similarly, using human colon epithelial cells from a different origin, Herbst etal. (2006) showed that the active metabolite of PhIP, ie, N-OH-PhIP, induced malignant transformation in presence of sulfotransferases, and injection of N-OH-PhIP-treated human colon epithelial cells into mice led to the development of tumors at the injection sites. This result points out a limitation of the CTA, which is its current incompatibility with an S9-based metabolizing system, consistent withthe lack of cell transforming potential observed for MelQx in the present study. Moreover, these findings are consistent with mutagenicity data observed in the Ames test without S9. In human colon epithelial cells grown invitro, MelQx-M1-related cytotoxicity was reduced relative to MelQx. There is no cytotoxicity data for MelQx-M1 to compare, but the cytotoxicity of MelQx is in agreement with data observed in different cell lines including human liver HepG2 (Pezdirc etal., 2013) and HepaRG cells (Dumont etal., 2010).
CONCLUSION
We have demonstrated that in the presence of glycerol, gut microbes with glycerol/diol dehydratase activity (ie, E. hallii, L. reuteri, and L. rossiae) and complex human gut microbiota efficiently transform MelQx to its conjugate MelQx-M1. The chemical basis of MelQx conjugation was found to involve the bacterial metabolism of glycerol to 3-HPA, subsequent decomposition to acrolein and direct conjugation with MelQx. As IQ and PhIP were also transformed to M1-like transformation products, our data suggest that this is a general reaction of acrolein and 2-amino-imidazoquinoxaline HCAs. Results from multiple invitro toxicological assays indicate that MelQx-M1 is less cytotoxic and mutagenic than MelQx. Thus, the toxic potency of dietary HCAs may be reduced invivo by the activity of gut microbes. Moreover, amine conjugation with glycerol may constitute a general detoxification process emanating from gut microbiota, which diminishes the exposure to and toxic potency of HCAs and which should be considered in the evaluation of a potential health risk in the future.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Julia Hausmann and Nicole Brauer, Institute for Food Toxicology, University of Veterinary Medicine Hannover, for performing the CTA and Ames test experiments, respectively, and Nathalie Ziegler at ETH Zürich for assistance in instrument analysis. Thanks also to Dr Todor Angelov, Dr Marc Stevens, and Dr Simon Sieber at ETH Zürich for helpful discussion.
FUNDING
This work is supported by Swiss National Science Foundation (grant number CRSII3_136247) and the China Scholarship Council (J.Z.).
REFERENCES
- Arai T., Koyama R., Yuasa M., Kitamura D., Mizuta R. (2014). Acrolein, a highly toxic aldehyde generated under oxidative stress invivo, aggravates the mouse liver damage after acetaminophen overdose. Biomed. Res. 35, 389–395. [DOI] [PubMed] [Google Scholar]
- Bouvard V., Loomis D., Guyton K. Z., Grosse Y., Ghissassi F., El Benbrahim-Tallaa L., Guha N., Mattock H., Straif K. (2015). Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 16, 1599–1600. [DOI] [PubMed] [Google Scholar]
- Chen J. X., Wang H., Liu A., Zhang L., Reuhl K., Yang C. S. (2016). PhIP/DSS-Induced colon carcinogenesis in CYP1A-humanized mice and the possible role of Lgr5+ stem cells. Toxicol. Sci. 155, 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F., Young R., Hecht S. S. (1984). Formation of cyclic 1, N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 44, 990–995. [PubMed] [Google Scholar]
- Conti L., Crebelli R. (2016). Evaluation of the mutagenicity of simple substituted quinoxalines in Salmonella typhimurium. Drug Chem. Toxicol. 39, 213–216. [DOI] [PubMed] [Google Scholar]
- De Angelis M., Bottacini F., Fosso B., Kelleher P., Calasso M., Di Cagno R., Ventura M., Picardi E., van Sinderen D., Gobbetti M. (2014). Lactobacillus rossiae, a vitamin B12 producer, represents a metabolically versatile species within the genus Lactobacillus. PloS One 9, e107232.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J., Jossé R., Lambert C., Anthérieu S., Le Hegarat L., Aninat C., Robin M., Guguen-Guillouzo C., Guillouzo A. (2010). Differential toxicity of heterocyclic aromatic amines and their mixture in metabolically competent HepaRG cells. Toxicol. Appl. Pharmacol. 245, 256–263. [DOI] [PubMed] [Google Scholar]
- Duncan S. H., Hold G. L., Harmsen H. J. M., Stewart C. S., Flint H. J. (2002). Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 52, 2141–2146. [DOI] [PubMed] [Google Scholar]
- EFSA Scientific Committee. (2009). Guidance of the scientific committee on use of the benchmark dose approach in risk assessment. EFSA J 1150, 1–72. [Google Scholar]
- Engels C., Ruscheweyh H. J., Beerenwinkel N., Lacroix C., Schwab C. (2016b). The common gut microbe Eubacterium hallii also contributes to intestinal propionate formation. Front. Microbiol. 7, 713.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels C., Schwab C., Zhang J., Stevens M., Bieri C., Ebert M.-O., McNeill K., Sturla S., (2016a). Acrolein contributes strongly to antimicrobial and heterocyclic amine transformation activities of reuterin. Sci. Rep. 6, 36246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzinger M. M., Bovet C., Hecht K. M., Senger S., Winiker P., Sobotzki N., Cristea S., Beerenwinkel N., Shay J. W., Marra G., et al. (2016). Sulforaphane preconditioning sensitizes human colon cancer cells towards the bioreductive anticancer prodrug PR-104A. PLoS One 11, e0150219.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlbaum S., Chassard C., Haug M. C., Fourmestraux C., Derrien M., Lacroix C. (2015). Design and investigation of PolyFermS invitro continuous fermentation models inoculated with immobilized fecal microbiota mimicking the elderly colon. PLoS One 10, e0142793.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekry M. I., Engels C., Zhang J., Schwab C., Lacroix C., Sturla S. J., Chassard C. (2016). The strict anaerobic gut microbe Eubacterium hallii transforms the carcinogenic dietary heterocyclic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Environ. Microbiol. Rep. 8, 201–209. [DOI] [PubMed] [Google Scholar]
- Felton J. S., Knize M. G., Shen N. H., Lewis P. R., Andresen B. D., Happe J., Hatch F. T. (1986). The isolation and identification of a new mutagen from fried ground beef: 2-amino-l-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP). Carcinogenesis 7, 1081–1086. [DOI] [PubMed] [Google Scholar]
- Fujimoto N., Inoue K., Hayashi Y., Yuasa H. (2006). Glycerol uptake in HCT-15 human colon cancer cell line by Na(+)-dependent carrier-mediated transport. Biol. Pharm. Bull. 29, 150–154. [DOI] [PubMed] [Google Scholar]
- Grivas S., Jagerstad M. (1984). Mutagenicity of some synthetic quinolines and quinoxalines related to IQ, MeIQ or MelQx in Ames test. Mutat. Res. 137, 29–32. [DOI] [PubMed] [Google Scholar]
- Hein D. W., Rustan T. D., Ferguson R. J., Doll M. A., Gray K. (1994). Metabolic activation of aromatic and heterocyclicN-hydroxyarylamines by wild-type and mutant recombinant human NAT1 and NAT2 acetyltransferases. Arch. Toxicol. 68, 129–133. [DOI] [PubMed] [Google Scholar]
- Herbst U., Fuchs J. I., Teubner W., Steinberg P. (2006). Malignant transformation of human colon epithelial cells by benzo[c]phenanthrene dihydrodiolepoxides as well as 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Toxicol. Appl. Pharmacol. 212, 136–145. [DOI] [PubMed] [Google Scholar]
- Humblot C., Combourieu B., Väisänen M.-L., Furet J.-P., Delort A.-M., Rabot S. (2005). 1H nuclear magnetic resonance spectroscopy-based studies of the metabolism of food-borne carcinogen 2-amino-3-methylimidazo [4, 5-f] quinoline by human intestinal microbiota. Appl. Environ. Microbiol. 71, 5116–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humblot C., Murkovic M., Rigottier-Gois L., Bensaada M., Bouclet A., Andrieux C., Anba J., Rabot S. (2007). Beta-glucuronidase in human intestinal microbiota is necessary for the colonic genotoxicity of the food-borne carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline in rats. Carcinogenesis 28, 2419–2425. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic risk to Humans. (1993). MelQx 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline. 56, 211–228. [PMC free article] [PubMed]
- Jägerstad M., Olsson K., Grivas S., Negishi C., Wakabayashi K., Tsuda M., Shigeaki S., Takashi S. (1984). Formation of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in a model system by heating creatinine, glycine and glucose. Mutat. Res. Fundam. Mol. Mech. Mutagen. 126, 239–244. [DOI] [PubMed] [Google Scholar]
- Kasai H., Yamaizumi Z., Wakabayashi K., Nagao M., Sugimura T., Yokoyama S., Miyazawa T., Spingarn N. E., Weisburger J. H., Nishimura S. (1980). Potent novel mutagens produced by broiling fish under normal conditions. Proc. Japan Acad. Ser. B. 56, 278–283. [Google Scholar]
- Kato T., Ohgaki H., Hasegawa H., Sato S., Takayama S., Sugimura T. (1988). Carcinogenicity in rats of a mutagenic compound, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline. Carcinogenesis 9, 71–73. [DOI] [PubMed] [Google Scholar]
- Kim D., Guengerich F. P. (2005). Cytochrome P450 activation of arylamines and heterocyclic amines. Annu. Rev. Pharmacol. Toxicol. 45, 27–49. [DOI] [PubMed] [Google Scholar]
- Lambert C., Li J., Jonscher K., Yang T.-C., Reigan P., Quintana M., Harvey J., Freed B. M. (2007). Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J. Biol. Chem. 282, 19666–19675. [DOI] [PubMed] [Google Scholar]
- Lilienblum W., Dekant W., Foth H., Gebel T., Hengstler J. G., Kahl R., Kramer P. J., Schweinfurth H., Wollin K. M. (2008). Alternative methods to safety studies in experimental animals: Role in the risk assessment of chemicals under the new European Chemicals Legislation (REACH). Arch. Toxicol. 82, 211–236. [DOI] [PubMed] [Google Scholar]
- Marchesi J. R., Holmes E., Khan F., Kochhar S., Scanlan P., Shanahan F., Wilson I. D., Wang Y. (2007). Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J. Proteome Res. 6, 546–551. [DOI] [PubMed] [Google Scholar]
- Mattson F. H., Volpenhein R. A. (1964). The digestion and absorption of triglycerides. J. Biol. Chem. 239, 2772–2777. [PubMed] [Google Scholar]
- Mortelmans K., Zeiger E. (2000). The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. Fundam. Mol. Mech. Mutagen. 455, 29–60. [DOI] [PubMed] [Google Scholar]
- Nagao M., Wakabayashi K., Kasai H., Nishlmura S., Sugimura T. (1981). Effect of methyl substitution on mutagenicity of 2-amino-3-methylimidazo [4, 5-f] quinoline isolated from broiled sardine. Carcinogenesis 2, 1147–1149. [DOI] [PubMed] [Google Scholar]
- Nauwelaers G., Bessette E. E., Gu D., Tang Y., Rageul J., Fessard V., Yuan J.-M., Yu M. C., Langouët S., Turesky R. J. (2011). DNA adduct formation of 4-aminobiphenyl and heterocyclic aromatic amines in human hepatocytes. Chem. Res. Toxicol. 24, 913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicken P., Hamscher G., Breves G., Steinberg P. (2010). Uptake of the colon carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by different segments of the rat gastrointestinal tract: Its implication in colorectal carcinogenesis. Toxicol. Lett. 196, 60–66. [DOI] [PubMed] [Google Scholar]
- Nicken P., Willenberg I., Keutz A., von, Elsner L. von, Hamscher G., Vanhaecke L., Schröder B., Breves G., Schebb N. H., Steinberg P. (2015). Intestinal absorption and cell transforming potential of PhIP-M1, a bacterial metabolite of the heterocyclic aromatic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Toxicol. Lett. 234, 92–98. [DOI] [PubMed] [Google Scholar]
- Nishikawa A., Imazawa T., Kuroiwa Y., Kitamura Y., Kanki K., Ishii Y., Umemura T., Hirose M. (2005). Induction of colon tumors in C57BL/6J mice fed MelQx, IQ, or PhIP followed by dextran sulfate sodium treatment. Toxicol. Sci. 84, 243–248. [DOI] [PubMed] [Google Scholar]
- Ohgaki H., Hasegawa H., Suenaga M., Sato S., Takayama S., Sugimura T. (1987). Carcinogenicity in mice of a mutagenic compound, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MelQx) from cooked foods. Carcinogenesis 8, 665–668. [DOI] [PubMed] [Google Scholar]
- Oz F., Kaya M. (2011). Heterocyclic aromatic amines in meat. J. Food Process. Preserv. 35, 739–753. [Google Scholar]
- Pezdirc M., Žegura B., Filipič M. (2013). Genotoxicity and induction of DNA damage responsive genes by food-borne heterocyclic aromatic amines in human hepatoma HepG2 cells. Food Chem. Toxicol. 59, 386–394. [DOI] [PubMed] [Google Scholar]
- Pfau W., Martin F. L., Cole K. J., Venitt S., Phillips D. H., Grover P. L., Marquardt H. (1999). Heterocyclic aromatic amines induce DNA strand breaks and cell transformation. Carcinogenesis 20, 545–551. [DOI] [PubMed] [Google Scholar]
- Promega. (2015). CellTiter-Glo luminescent cell viability assay. Retrieved from https://ch.promega.com/-/media/files/resources/protocols/technical-bulletins/0/celltiter-glo-luminescent-cell-viability-assay-protocol.pdf. Accessed May 30, 2017.
- Roig A. I., Eskiocak U., Hight S. K., Kim S. B., Delgado O., Souza R. F., Spechler S. J., Wright W. E., Shay J. W. (2010). Immortalized epithelial cells derived from human colon biopsies express stem cell markers and differentiate invitro. Gastroenterology 138, 1012–1021. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Bohnenberger S., Hayashi K., Kunkelmann T., Muramatsu D., Phrakonkham P., Poth A., Sakai A., Salovaara S., Tanaka N., et al. (2012b). Recommended protocol for the BALB/c 3T3 cell transformation assay. Mutat. Res. - Genet. Toxicol. Environ. Mutagen. 744: 30–35. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Bohnenberger S., Hayashi K., Kunkelmann T., Muramatsu D., Poth A., Sakai A., Salovaara S., Tanaka N., Thomas B. C. (2012a). Photo catalogue for the classification of foci in the BALB/c 3T3 cell transformation assay. Mutat. Res. - Genet. Toxicol. Environ. Mutagen. 744, 42–53. [DOI] [PubMed] [Google Scholar]
- Schwab C., Ruscheweyh H.-J., Bunesova V., Pham V., Beerenwinkel N., Lacroix C. (2017). Trophic interactions of infant Bifidobacteria and Eubacterium hallii during L-fucose and fucosyllactose degradation. Front. Microbiol. 8, 95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Kulldorff M., Chow W. H., Denobile J., Rothman N. (2001). Dietary intake of heterocyclic amines, meat-derived mutagenic activity, and risk of colorectal adenomas. Cancer Epidemiol. Biomarkers Prev. 10, 559–562. [PubMed] [Google Scholar]
- Sodum R. S., Shapiro R. (1988). Reaction of acrolein with cytosine and adenine derivatives. Bioorg. Chem. 16, 272–282. [Google Scholar]
- Sugimura T. (1988). Successful use of short-term tests for academic purposes: Their use in identification of new environmental carcinogens with possible risk for humans. Mutat. Res. 205, 33–39. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Tucker J. D., Stewart S. A., Christensen M. L., Salazar E. P., Carrano A. V., Felton J. S. (1987). Genotoxicity of compounds from cooked beef in repair-deficient CHO cells versus salmonella mutagenicity. Mutagenesis 2, 483–487. [DOI] [PubMed] [Google Scholar]
- Totsuka Y., Fukutome K., Takahashi M., Takahashi S., Tada A., Sugimura T., Wakabayashi K. (1996). Presence of N2-(deoxyguanosin-8-yl)-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (dG-C8-MelQx) in human tissues. Carcinogenesis 17, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Turesky R. J., Aeschbacher H. U., Würzner H. P., Skipper P. L., Tannenbaum S. R. (1988). Major routes of metabolism of the food-borne carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in the rat. Carcinogenesis 9, 1043–1048. [DOI] [PubMed] [Google Scholar]
- Turesky R. J., Guengerich F. P., Guillouzo A., Langouët S. (2002). Metabolism of heterocyclic aromatic amines by human hepatocytes and cytochrome P4501A2. Mutat. Res. Fundam. Mol. Mech. Mutagen. 506–507, 187–195. [DOI] [PubMed] [Google Scholar]
- Turesky R. J., Le Marchand L. (2011). Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: Lessons learned from aromatic amines. Chem. Res. Toxicol. 24, 1169–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. (2016). Food additive status list. Available at: www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm091048.htm
- Uchida K. (2015). Aldehyde adducts generated during lipid peroxidation modification of proteins. Free Radic. Res. 49, 896–904. [DOI] [PubMed] [Google Scholar]
- Ushiyama H., Wakabayashi K., Hirose M., Itoh H., Sugimura T., Nagao M. (1991). Presence of carcinogenic heterocyclic amines in urine of healthy volunteers eating normal diet, but not of inpatients receiving parenteral alimentation. Carcinogenesis 12, 1417–1422. [DOI] [PubMed] [Google Scholar]
- Vanhaecke L., Derycke L., Le Curieux F., Lust S., Marzin D., Verstraete W., Bracke M. (2008c). The microbial PhIP metabolite 7-hydroxy-5-methyl-3-phenyl-6,7,8,9-tetrahydropyrido[3’,2’:4,5]imidazo[1,2-a]pyrimidin-5-ium chloride (PhIP-M1) induces DNA damage, apoptosis and cell cycle arrest towards Caco-2 cells. Appl. Environ. Microbiol. 178, 61–69. [DOI] [PubMed] [Google Scholar]
- Vanhaecke L., Knize M. G., Noppe H., Brabander H. De, Verstraete W., Van de W. T. (2008b). Intestinal bacteria metabolize the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine following consumption of a single cooked chicken meal in humans. Food Chem. Toxicol. 46, 140–148. [DOI] [PubMed] [Google Scholar]
- Vanhaecke L., Van Hoof N., Van Brabandt W., Soenen B., Heyerick A., De Kimpe N., De Keukeleire D., Verstraete W., Van de Wiele T. (2006). Metabolism of the food-associated carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human intestinal microbiota. J. Agric. Food Chem. 54, 3454–3461. [DOI] [PubMed] [Google Scholar]
- Vanhaecke L., Vercruysse F., Boon N., Verstraete W., Cleenwerck I., De Wachter M., De Vos P., van de Wiele T. (2008a). Isolation and characterization of human intestinal bacteria capable of transforming the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Appl. Environ. Microbiol. 74, 1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikse R., Knapstad A., Klungsøyr L., Grivas S. (1993). Mutagenic activity of the methyl and phenyl derivatives of the food mutagen 2-amino-3-methylimidazo [4, 5-f] quinoxaline (IQx) in the Ames test. Mutat. Res. Toxicol. 298, 207–214. [DOI] [PubMed] [Google Scholar]
- von Moos L. M., Schneider M., Hilty F. M., Hilbe M., Arnold M., Ziegler N., Sales Mato D., Winkler H., Tarik M., Ludwig C., et al. (2017). Iron phosphate nanoparticles for food fortification: Biological effects in rats and human cell lines. Nanotoxicology 11, 496–506. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Tomitori H., Machi Y., Hagihara M., Higashi K., Goda H., Ohya T., Niitsu M., Kashiwagi K., Igarashi K. (2009). Acrolein toxicity: Comparison with reactive oxygen species. Biochem. Biophys. Res. Commun. 378, 313–318. [DOI] [PubMed] [Google Scholar]
- Yuasa H., Hamamoto K., Dogu S., Marutani T., Nakajima A., Kato T., Hayashi Y., Inoue K., Watanabe J. (2003). Saturable absorption of glycerol in the rat intestine. Biol. Pharm. Bull. 26, 1633–1636. [DOI] [PubMed] [Google Scholar]
- Zimmerli B., Rhyn P., Zoller O., Schlatter J. (2001). Occurrence of heterocyclic aromatic amines in the swiss diet: Analytical method, exposure estimation and risk assessment. Food Addit. Contam. 18, 533–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.