Abstract
Aims
Cardiac resynchronization therapy (CRT) device implantation has been shown to reduce morbidity and mortality in selected patients with heart failure. We sought to investigate the utilization and in-hospital complications of cardiac resynchronization therapy defibrillator (CRT-D) and pacemaker (CRT-P) implantations in the United States from 2003 to 2013.
Methods and results
Patients receiving CRT-D or CRT-P were identified in the National Inpatient Sample database (NIS), using the International Classification of Diseases-Ninth Revision-Clinical Modification procedure codes. Annual implantation rates, patient demographics, co-morbidities, in-hospital complications, and length of stay were analysed. From 2003 to 2013, an estimated total of 439 010 (95% CI: 406 723–471 296) inpatient CRT implantations were performed in the U.S. The median age of patients was 72 and 71% were male. Overall, 6.1% had at least one complication. During the study period, comorbidity index and overall complication rate increased (P = 0.002 and P = 0.01, respectively). Mortality and length of stay showed no significant trend. Predictors of complications included: age 65 and older, female sex (OR: 1.19; 95% CI: 1.12–1.27), Deyo–Charlson Comorbidity Index, and elective admission (OR: 0.61; 95% CI: 0.57–0.66).
Conclusion
From 2003 to 2013, the severity of comorbid conditions increased and a rising trend was observed in the rate of periprocedural complications among patients undergoing CRT in the United States. In-hospital mortality and length of stay showed no uniform trend.
Keywords: Cardiac resynchronization therapy , Complication , Defibrillator , Pacemaker
Introduction
Cardiac resynchronization therapy (CRT) has been shown to reduce morbidity and mortality in selected patients with heart failure.1–3 The long-term clinical benefits of both CRT with (CRT-D) and without (CRT-P) a defibrillator have been demonstrated in several randomized clinical trials.4,5 These positive findings have resulted in more widespread use of CRT over the past decade.6
While the benefits of CRT have been confirmed by large clinical trials and population-based studies, data regarding periprocedural complication rates of CRT are scarce.1–5 Moreover, most of the existing evidence of procedure-related adverse events and the safety and effectiveness of CRT stems from clinical trials. While these studies provide robust data on efficacy, their selected patient populations and clinical settings limit their generalizability to real-world practice. It has been shown that, in terms of the clinical characteristics and associated comorbidities of patients with heart failure, significant differences exist between clinical practice and published trials.7 As a result, real-world data regarding complications of CRT are missing.
We therefore sought to collect data on the baseline characteristics, comorbidity index, in-hospital complications, mortality, and length of stay for patients undergoing CRT device implantation during the period from 2003 to 2013 on a nationwide scale.
Methods
Data source
The data were obtained from the National Inpatient Sample and the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP), and Agency for Healthcare Research and Quality (AHRQ) from 2003 to 2013.8,9 The NIS is the largest all-payer inpatient database of hospital discharge records in the United States. It represents approximately 20% of all discharges from a broad spectrum of U.S. hospitals. National estimates can be calculated using sampling weights provided by the NIS. The provided discharge sample weights were calculated within each sampling stratum as the ratio of discharges in the universe to discharges in the sample. Detailed information on the implementation of sampling weights are provided in the Supplementary material online.
Study design
The International Classification of Diseases- 9th Revision-Clinical Modification (ICD-9-CM) was used to identify patients age 18 years or older with a primary procedure code of CRT-P (code 00.50) or CRT-D (code 00.51) device implantation. Data regarding age, sex, race, primary and secondary procedures, and length of stay were collected using discharge records.
Associated comorbidities were identified by measures from the Agency for Healthcare Research and Quality (AHRQ). The severity of comorbidities was calculated using the Deyo modification of the Charlson Comorbidity Index (CCI), which includes 17 comorbid conditions with differential weights and a total score ranging from 0 to 33. Higher scores correspond to greater severity of comorbid diseases.10
Rates of acute in-hospital periprocedural complications were determined using the corresponding ICD-9-CM diagnosis. These included cardiac complications; pericardial complications; post-operative haemorrhage/haematoma; vascular injury; vascular injury requiring surgical repair; pulmonary complications (pneumothorax, haemothorax, chest tube placement, and other iatrogenic complications); neurological complications (stroke and transient ischaemic attack); post-operative infectious complications; and in-hospital death. ICD-9-CM codes used in this study are provided in the Supplementary material online.
Statistical analysis
For all analyses, we used survey estimation (svyset and svy) and followed the recommendations from AHRQ for analysis of survey data to account for the complex survey design of the NIS database. For calculation of national estimates and correct variances, trend weight files (called ‘TRENDWT’11) provided by AHRQ were used as sampling weights (pweight). For categorical variables, the χ2 test was used for comparisons between patients receiving CRT-D and CRT-P. For continuous variables, the Wilcoxon signed rank test was used when appropriate. Trends for continuous variables were tested using the non-parametric test for trend by Cuzick.12 Two level mixed-effects multivariable logistic regression was used to identify independent predictors of post-procedural complications. Candidate variables included those on a patient and hospital level, with patient level factors clustered within hospital level factors, as well as the comorbidity index (Deyo-CCI) and year, with a term to adjust for the interaction effect between weekend admission and elective admission. All analyses were performed using Stata/IC 12.1 (College Station, TX: StataCorp LP.). P-value <0.05 was considered statistically significant.
Results
Baseline clinical characteristics
Baseline clinical characteristics and associated comorbidities of patients who underwent CRT-D or CRT-P implantation are listed in Table 1. A total of 92 480 unweighted observations were analysed from 2003 to 2013. After weighting the observations, it represents an estimated total of 376 045 (95% CI: 348 190–403 890) CRT-D and 62 965 (57 012–68 917) CRT-P implantations on an inpatient basis in the United States. No consistent trend was observed for the annual volume of CRT-D or CRT-P device implantations.
Table 1.
Comparison of baseline characteristics of CRT-D and CRT-P implantations 2003–2013
| Demographic variable | All CRT | CRT-D | CRT-P | P-value |
|---|---|---|---|---|
| Total numbera | 92 480 | 13 293 | 79 187 | |
| Weighted total numberb | 439 010 | 376 045 | 62 965 | |
| Age (%) | <0.001 | |||
| 18–49 | 5.51 | 5.90 | 3.23 | |
| 50–64 | 22.44 | 23.98 | 13.26 | |
| 65–79 | 49.57 | 50.75 | 42.51 | |
| >80 | 22.48 | 19.37 | 41.00 | |
| Mean ± SE | 70.15 ± 0.11 | 69.34 ± 0.11 | 75.03 ± 0.16 | |
| Sex (%) | <0.001 | |||
| Male | 70.98 | 73.06 | 58.52 | |
| Female | 29.02 | 26.94 | 41.48 | |
| Comorbidities (%) | ||||
| Ischaemic heart disease | 66.54 | 68.83 | 52.87 | <0.001 |
| History of hypertension | 54.10 | 54.00 | 54.71 | 0.2 |
| History of diabetes mellitus | 27.95 | 28.62 | 23.86 | <0.001 |
| History of chronic pulmonary disease | 20.79 | 20.82 | 20.58 | 0.5 |
| History of chronic kidney disease | 17.04 | 17.01 | 17.22 | 0.6 |
| History of peripheral vascular disease | 8.24 | 8.40 | 7.30 | <0.001 |
| Obesity | 7.59 | 7.79 | 6.34 | <0.001 |
The percentage of patients over 80 years of age who underwent CRT-P implantation was twice that of those who received CRT-D devices.
Represents the number of observations in the NIS dataset.
Represents total national estimates after applying sampling weights.
The median age of CRT recipients was 72 years (IQR: 63–79) and the majority of patients (71%) were male (Table 1). The proportion of females receiving CRT devices increased from 26.1% in 2003 to 32% in 2013 (P = 0.002).
Patients receiving CRT-D were younger than those receiving CRT-P (69.34 ± 0.11 vs. 75.03 ± 0.16, respectively). The proportion of patients older than 80 who received CRT-P was more than twice that of recipients of CRT-D (Table 1). While the mean age of patients remained unchanged for recipients of CRT-D (68.8 ± 0.3 years in 2003 vs. 69.7 ± 0.2 years in 2013, P = 0.54), patients receiving CRT-P showed an increase in mean age (from 72.4 ± 0.4 years in 2003 to 76.26 ± 0.2 years in 2013, P = 0.004).
Overall, the most common comorbidity among all CRT recipients was a history of ischaemic heart disease (66.5%), followed by hypertension (54.1%), diabetes mellitus (27.9%), chronic pulmonary disease (20.8%), chronic kidney disease (17%), and peripheral vascular disease (8.2%) (Table 1). The severity of comorbid diseases, as indicated by Deyo-CCI, showed a steady increase in both groups over the study period (Table 2). The percentage of patients receiving CRT with a Deyo-CCI of two or more increased from 55.9% in 2003 to 70.2% in 2013 (P = 0.002). Patients with CRT-D had higher rates of ischaemic heart disease, diabetes mellitus, peripheral vascular diseases, and obesity compared with CRT-P recipients (Table 1).
Table 2.
Temporal trends in complication rates, mortality, and length of stay for all CRT implantations 2003–2013
| Overall | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total CRT (n) | 92 480 | 5550 | 8699 | 9820 | 11 512 | 9931 | 9610 | 9869 | 8201 | 7893 | 6222 | 5173 | 0.4 |
| Weighted total CRT (n) | 439 010 | 25 767 | 40 966 | 46 956 | 53 766 | 47 151 | 44 900 | 47 303 | 39 311 | 35 915 | 31 110 | 25 865 | 0.4 |
| ≥1 complication | 6.11 | 5.76 | 5.58 | 4.93 | 4.49 | 5.54 | 6.34 | 6.75 | 6.75 | 7.45 | 7.34 | 6.98 | 0.01 |
| Haemorrhage or haematoma | 1.41 | 1.43 | 1.15 | 0.94 | 0.78 | 1.02 | 1.38 | 1.53 | 1.70 | 2.21 | 2.03 | 2.22 | 0.01 |
| Vascular injury | 0.32 | 0.50 | 0.43 | 0.28 | 0.34 | 0.32 | 0.28 | 0.35 | 0.33 | 0.23 | 0.10 | 0.33 | 0.07 |
| Vascular injury requiring surgery | 0.12 | 0.17 | 0.15 | 0.07 | 0.09 | 0.10 | 0.18 | 0.08 | 0.17 | 0.16 | 0.14 | 0.12 | 0.8 |
| Cardiac | 0.87 | 1.04 | 0.96 | 0.96 | 0.91 | 0.96 | 0.89 | 0.74 | 0.79 | 0.67 | 0.72 | 0.85 | 0.007 |
| Pericardial | 0.65 | 0.40 | 0.41 | 0.37 | 0.39 | 0.54 | 0.75 | 0.88 | 0.93 | 0.75 | 1.00 | 1.01 | 0.005 |
| Pulmonary | 1.48 | 1.42 | 1.45 | 1.52 | 1.34 | 1.31 | 1.66 | 1.47 | 1.61 | 1.54 | 1.54 | 1.49 | 0.1 |
| Neurological | 0.24 | 0.16 | 0.32 | 0.14 | 0.25 | 0.22 | 0.26 | 0.32 | 0.19 | 0.24 | 0.34 | 0.25 | 0.2 |
| Infection | 1.17 | 0.69 | 0.67 | 0.52 | 0.90 | 1.08 | 1.18 | 1.67 | 1.59 | 1.94 | 1.85 | 0.93 | 0.01 |
| Mortality | 0.76 | 0.98 | 1.00 | 0.73 | 0.81 | 0.70 | 0.61 | 0.77 | 0.63 | 0.84 | 0.53 | 0.77 | 0.1 |
| Length of stay(days) | 2 (1–7)±0.06 | 3 (1–7)±.18 | 3 (1–7) | 2 (1–7) | 2 (1–6) | 2 (1–6) | 2 (1–6) | 3 (1–7) | 3 (1–7) | 3 (1–7) | 3 (1–7) | 3 (1–7) | 0.9 |
| Deyo-CCI | 2.27 ±0.01 | 1.85 ±0.03 | 1.92 ±0.02 | 1.96 ±0.02 | 2.07 ±0.03 | 2.26 ±0.03 | 2.27 ±0.03 | 2.42 ±0.03 | 2.49 ±0.04 | 2.61 ±0.04 | 2.66 ±0.03 | 2.70 ±0.03 | 0.002 |
| (0.01) | (0.03) | (0.02) | (0.02) | (0.03) | (0.03) | (0.03) | (0.03) | (0.04) | (0.04) | (0.03) | (0.03) |
Values are n, %, mean (SEM), or median (25th, 75th percentile) (due to the skewed distribution).
P-value for trend using the non-parametric test for trend by Cuzick. P < 0.05 considered significant.
In-hospital complications, length of stay, and mortality
As shown in Figure 1, of all CRT procedures completed from 2003 to 2013, 6.1% had at least one complication (6.04% and 6.54% for CRT-D and CRT-P implants, respectively). Patients in older age groups had significantly higher rates of complications than those in younger groups (Figure 2). Overall, in patients over 80 years of age, 7.1% had at least one complication. The rate of at least one complication increased in the overall population during the study (P = 0.01). While cardiac complications decreased among all CRT recipients (P = 0.007), they were offset by increasing trends in the rates of haemorrhage/haematoma (P = 0.02), pericardial complications (P = 0.005), and post-operative infection (P = 0.01).
Figure 1.
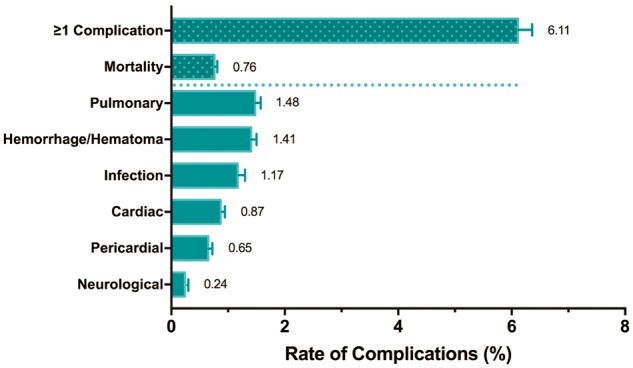
Types and frequencies of complications following CRT implantation. Pulmonary complications were the most common adverse events in CRT recipients.
Figure 2.
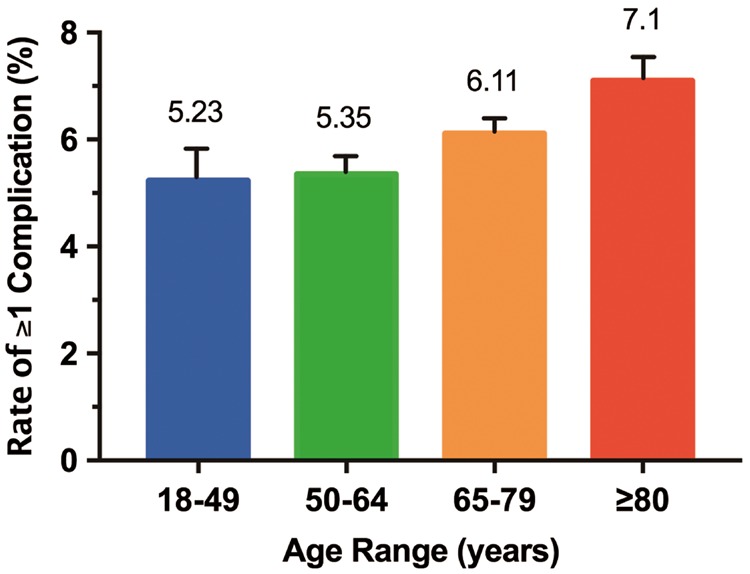
Overall in-hospital complications by age group. The highest rate of complications occurred in patients ≥80 years of age.
Trends in the rates of complication for CRT-D and CRT-P groups are reported in Tables 2 and 3 of the Supplementary material online, respectively. Over the study period, the overall rate of at least one complication in recipients of CRT-D devices increased from 5.86 to 6.95% (P = 0.01), and from 5.46 to 7.11% (P = 0.01) in CRT-P recipients. In the CRT-D group, this trend was driven by increasing trends in pericardial complications (P = 0.008), vascular complications (P = 0.02), and post-operative infections (P = 0.01). In contrast, the increase in the rate of complications for CRT-P recipients appeared to be driven only by an increase in vascular complications (P = 0.01), since no uniform trend was observed for any other complication. The most common adverse outcomes for CRT-P implants were pulmonary complications, while in the CRT-D group, vascular complications were most common. Among CRT-D recipients, the rate of cardiac complications decreased over time (P = 0.02).
Table 3.
Independent predictors of CRT implantation complications
| Predictor | P-value | Odds ratio* (95% CI) |
|---|---|---|
| Age | ||
| 18–54 | Ref | Ref |
| 55–64 | 0.952 | 1.01 (0.89–1.14) |
| 65–74 | 0.024 | 1.16 (1.02–1.32) |
| 75–84 | <0.001 | 1.29 (1.13–1.47) |
| ≥85 | <0.001 | 1.40 (1.19–1.64) |
| Female | <0.001 | 1.19 (1.12–1.27) |
| Elective admission | <0.001 | 0.61 (0.57–0.66) |
| Deyo-CCI | ||
| 0–1 | Ref | Ref |
| 2–4 | 0.017 | 1.08 (1.02–1.16) |
| ≥5 | <0.001 | 1.22 (1.09–1.37) |
Model c-statistics: 0.67 (0.66–0.68).
Adjusted for insurance status, weekend admission, hospital bedsize, hospital region, hospital teaching status, and calendar year.
The median (IQR) length of stay (LOS) for all CRT recipients was 2 (1–7), similar to the CRT-D group’s LOS. In the CRT-P group, median (IQR) LOS was 3 (1–7). The total in-hospital mortality rate was 0.76% (0.7 and 1.08% for CRT-D and CRT-P implants, respectively). No significant trend was observed in mortality or length of stay for either group (see Supplementary material online).
Predictors of in-hospital complications
Results of the multivariable logistic regression model are shown in Table 3. Age was a significant predictor of complications. There was a stepwise increase in the odds of in-hospital complication with increasing age and increasing Deyo-CCI. Female sex (OR: 1.19; 95% CI: 1.12–1.27, P < 0.001) was also an independent predictor of complications.
In univariate analysis, weekend admissions had a higher rate of complications. Upon further investigation, we found that this ‘weekend effect’ was due to a higher proportion of non-elective admissions on weekends (89% on weekends vs. 45% on weekdays). After adjusting for the observed interaction, only elective admission remained a significant independent predictor of complications (OR: 0.61; 95% CI: 0.57–0.66, P < 0.001).
Discussion
This study reports trends of in-hospital CRT device implantations from 2003 to 2013 in the U.S. The results demonstrate an increase in the comorbidity index and frequency of in-hospital complications. In-hospital mortality and length of stay remained unchanged over the study period.
The mean (SEM) age of patients receiving CRT implants was 70.15 (0.11) and women comprised 29% of the total population. These demographic characteristics are in line with those published for clinical trials.1–3 The mean age increased over the study period (P = 0.02), driven mainly by an increase in the mean age of patients receiving CRT-P. A recent study reported advanced age as an independent predictor for CRT-P device selection.13 Patients receiving CRT-D devices comprised approximately 86% of the total CRT implantations in the present study, which is in the higher range of the proportions published using European registries.14
Statistical analysis revealed an overall in-hospital complication rate of 6.11%, similar to those reported in randomized clinical trials.3,15,16 In the CARE-HF trial, adverse events occurred in 10% of patients within the first 24 h of implantation.17 In a study of Medicare beneficiaries from 2006 to 2010 using NCDR registry data, 6.5% of CRT-D recipients had at least one device-related complication.18 While direct comparisons are not possible for every complication, similarities do exist between the present findings and previously reported clinical trials. Jamerson et al.15 investigated major procedure-related adverse events within the first 30 days of CRT implantation in women vs. men in the MADIT-CRT trial. They reported adverse events in 6.3% of women, comparable to 6.8% of women in our population. Importantly, the adverse event rates reported in this study reflect only inpatient procedural complications. Results of 30-day post-procedural complications could not be estimated, given the nature of the database.
Cardiac perforation is one of the most serious complications related to CRT implantation. It is also associated with an increase in the rate of other major complications, length of stay, and in-hospital mortality.19 In this study, the rate of pericardial complications (i.e. cardiac perforation) increased significantly. This increase was mainly driven by the CRT-D population, which is concordant with other studies that have reported an increase in the risk of perforation with an increase in the number of leads.20,21 Female gender and older age are also associated with an increased risk of cardiac perforation due to thinner chamber wall thickness in those populations.19 Correspondingly, in our study, women had higher rates of pericardial complications (1.09% of females vs. 0.47% of males, P < 0.001). Body mass index (BMI) has also been reported to be a significant predictor for early procedure-related complications in females.15 Consistent with this and other studies,15,22 both female sex and older age were associated with higher odds of developing any complication. This fact, along with a significant rising trend in the age of patients and the proportion of females undergoing CRT implantation over the study period, may account for the overall rise we observed in complication rates.
Device pocket haematoma and haemorrhage are among the most common complications of CRT implantation. This is in part due to the high proportion of CRT candidates who are under anticoagulant therapy for cardiac comorbidities, in particular atrial fibrillation and atrial flutter. In our study, an increasing trend was observed in haematoma/haemorrhage, alongside an 11% increase in the proportion of CRT candidates who had a history of atrial fibrillation or atrial flutter (from 34.99% in 2003 to 46.26% in 2013, P < 0.001). Management of haematoma/haemorrhage may necessitate temporary interruption of anticoagulation therapy, which could have significant ramifications for a population already at risk for thrombo-embolic events.23,24 Haematoma has also been associated with an increased risk of infection in recipients of cardiac devices.25,26 This could serve as a partial explanation for the observed increase in the rate of post-operative infections during our study period.
Pneumothorax complications following cardiac device implantation have been reported to occur in 0.6 to 1.9% of procedures.27–29 In this study, pulmonary complications (i.e. pneumothorax) occurred in 1.48% of all CRT procedures. CRT-P recipients had higher pulmonary complications compared with CRT-D recipients. This could be because the CRT-P population consisted of more females and older patients, characteristics which have been reported as predictors of pneumothorax complications in cardiac device recipients.28
The increase in some complications is also potentially attributable to a higher rate of comorbid conditions over time. From 2003 to 2013, a steady increase was observed in the mean CCI of both groups, as well as the number of patients with two or more comorbidities (Deyo-CCI ≥ 2). Deyo-CCI was a significant independent predictor of in-hospital complications. Previous studies have documented the association between preexisting comorbidities as important risk factors for post-implantation complications.30 In addition, increases in comorbidities have been linked with an increased rate of infection in CRT recipients.31 These findings indicate that, while the use of CRT has expanded, strategies to reduce associated complications are needed.
Previous studies have reported weekend admission (aka the ‘weekend effect’) and non-elective admission as predictors of mortality and morbidity among hospitalized cardiovascular patients.21,32,33 While weekend admissions had a higher rate of complication in this study, this effect was in fact due to the high proportion (89%) of non-elective admissions on weekends. Ultimately, elective admission was the only type of admission that was found to be an independent predictor of complications. This is likely because elective patients are more stable and there is more time to prepare the procedure.
The overall in-hospital mortality rate during the study period was 0.76%. There was no uniform trend in the rate of mortality, even though Deyo-CCI increased significantly. Recipients of CRT-P devices had a higher overall mortality rate in comparison to CRT-D recipients (1.08% vs. 0.7%, P < 0.001).
The present study has several limitations. First, data were collected from an administrative database, which may have errors associated with coding inaccuracies. Second, there is the potential for selection bias, given that this study is retrospective and limited to inpatient procedures, and there has been an increase in outpatient CRT implantations in recent years. Third, the NIS does not provide a way to trace the progression of events during a given hospital stay. Therefore, in contrast to outpatient care, it is possible that some patients may have received CRT therapies at the end of treatment for another reason of admission (e.g. decompensated CHF) than solely a stay for CRT implantation. This could partly explain the lengthier stays observed. Fourth, the present study was unable to capture complications that occurred after hospital discharge. Nevertheless, results from recent studies have shown that most complications of CRT implantation occur within 24 h, therefore increasing the applicability of our findings.17 Fifth, several factors that could affect complication rate are not provided by the NIS: duration of procedure, re-intervention, device explants, and medical management (e.g. anticoagulants). In addition, we could not include operator volume in our analyses because operator ID was not provided for over half the discharges in this dataset. Sixth, the New York Heart Association (NYHA) classification could not be studied, since it is not provided by the NIS. Recent changes in heart failure guidelines regarding preventive CRT therapy in NYHA class II patients could have affected the trends in CRT implantation in the final two years of this study.34 Even so, these changes might have affected outpatient procedures more than inpatient ones, given that NYHA II patients are more likely to be implanted on an outpatient basis.
Conclusion
In summary, this study represents real-world experience in a large population of patients undergoing inpatient CRT implantation in the U.S. from 2003 to 2013. While year-to-year fluctuations in procedure volumes were observed, the average number of implants changed very little. However, the number and severity of comorbidities, particularly in older age groups, increased significantly. These changes, accompanied by an increase in the rate of some procedural complications, underscore the need for strategies to reduce periprocedural risks in this patient population.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centres. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centres, or the National Institutes of Health.
Funding
This work was supported by the Al Sagri Research Fund and the Deane Institute for Integrative Research in Atrial Fibrillation and Stroke at Massachusetts General Hospital.
Conflict of interest: Dr Ruskin (all significant in amount): Gilead Sciences (Data Monitoring Committees), Medtronic (Scientific Advisory Board), Pfizer (Steering Committee), NewPace Ltd. (Scientific Advisory Board, equity), Element Science (Scientific Advisory Board, equity), InfoBionic (Scientific Advisory Board, equity). Dr Heist (all modest in amount): Biotronik (consultant, research grant), Biosense-Webster (consultant), Boston Scientific (consultant), Janssen (consultant), Medtronic (consultant), St Jude Medical (consultant, research grant). All other authors declare no conflict of interest.
References
- 1. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM.. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 2. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J.. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–1853. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NAMIII, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W.. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 4. Lam SKH, Owen A.. Combined resynchronisation and implantable defibrillator therapy in left ventricular dysfunction: Bayesian network meta-analysis of randomised controlled trials. BMJ 2007;335:925–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhan C, Baine WB, Sedrakyan A, Steiner C.. Cardiac device implantation in the United States from 1997 through 2004: a population-based analysis. J Gen Intern Med 2008;23 Suppl 1:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, van Veldhuisen DJ.. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 7. Masoudi FA, Havranek EP, Wolfe P, Gross CP, Rathore SS, Steiner JF, Ordin DL, Krumholz HM.. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am Heart J 2003;146:250–257. [DOI] [PubMed] [Google Scholar]
- 8. Healthcare Cost and Utilization Project (HCUP). 2000-2011. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nisoverview.jsp (15 February 2016). [PubMed]
- 9. HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012-2013. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nisoverview.jsp (15 February 2016).
- 10. Deyo RA, Cherkin DC, Ciol MA.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol Pergamon 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 11. Trend Weights for 1993-2011 HCUP NIS Data. Healthcare Cost and Utilization Project (HCUP). 2015. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. (27 April 2015).
- 12. Cuzick J. A Wilcoxon‐type test for trend. Stat Med 1985;4:543–547. [DOI] [PubMed] [Google Scholar]
- 13. Lindvall C, Chatterjee NA, Chang Y, Chernack B, Jackson VA, Singh JP, Metlay JP.. National trends in the use of cardiac resynchronization therapy with or without implantable cardioverter-defibrillator. Circulation 2016;133:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Veldhuisen DJ, Maass AH, Priori SG, Stolt P, Van Gelder IC, Dickstein K, Swedberg K.. Implementation of device therapy (cardiac resynchronization therapy and implantable cardioverter defibrillator) for patients with heart failure in Europe: changes from 2004 to 2008. Eur J Heart Fail 2009;11:1143–1151. [DOI] [PubMed] [Google Scholar]
- 15. Jamerson D, Mcnitt S, Polonsky S, Zareba W, Moss A, Tompkins C.. Early procedure-related adverse events by gender in MADIT-CRT. J Cardiovasc Electrophysiol 2014;25:985–989. [DOI] [PubMed] [Google Scholar]
- 16. Niu H-X, Hua W, Wang F-Z, Zhang S, Chen K-P, Chen X.. Complications of cardiac resynchronization therapy in patients with congestive heart failure. Chin Med J 2006;119:449–453. [PubMed] [Google Scholar]
- 17. Gras D, Böcker D, Lunati M, Wellens HJJ, Calvert M, Freemantle N, Gervais R, Kappenberger L, Tavazzi L, Erdmann E, Cleland JGF, Daubert JC.. Implantation of cardiac resynchronization therapy systems in the CARE-HF trial: procedural success rate and safety. Europace 2007;9:516–522. [DOI] [PubMed] [Google Scholar]
- 18. Borne RT, Peterson PN, Greenlee R, Heidenreich PA, Wang Y, Curtis JP, Tzou WS, Varosy PD, Kremers MS, Masoudi FA.. Temporal trends in patient characteristics and outcomes among medicare beneficiaries undergoing primary prevention implantable cardioverter-defibrillator placement in the United States, 2006–2010. Circulation 2014;130:845–853. [DOI] [PubMed] [Google Scholar]
- 19. Hsu JC, Varosy PD, Bao H, Dewland TA, Curtis JP, Marcus GM.. Cardiac perforation from implantable cardioverter-defibrillator lead placement: insights from the national cardiovascular data registry. Circ Cardiovasc Qual Outcomes 2013;6:582–590. [DOI] [PubMed] [Google Scholar]
- 20. Dewland TA, Pellegrini CN, Wang Y, Marcus GM, Keung E, Varosy PD.. Dual-chamber implantable cardioverter-defibrillator selection is associated with increased complication rates and mortality among patients enrolled in the NCDR implantable cardioverter-defibrillator registry. J Am Coll Cardiol 2011;58:1007–1013. [DOI] [PubMed] [Google Scholar]
- 21. Kirkfeldt RE, Johansen JB, Nohr EA, Jørgensen OD, Nielsen JC.. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2013;eht511.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peterson PN, Daugherty SL, Wang Y, Vidaillet HJ, Heidenreich PA, Curtis JP, Masoudi FA.. National Cardiovascular Data Registry. Gender differences in procedure-related adverse events in patients receiving implantable cardioverter-defibrillator therapy. Circ Am Heart Assoc J 2009;119:1078–1084. [DOI] [PubMed] [Google Scholar]
- 23. Kovacs MJ, Kearon C, Rodger M, Anderson DR, Turpie AGG, Bates SM, Desjardins L, Douketis J, Kahn SR, Solymoss S, Wells PS.. Single-arm study of bridging therapy with low-molecular-weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin. Circulation 2004;110:1658–1663. [DOI] [PubMed] [Google Scholar]
- 24. Robinson M, Healey JS, Eikelboom J, Schulman S, Morillo CA, Nair GM, Baranchuk A, Ribas S, Evans G, Connolly SJ, Turpie AG.. Postoperative low-molecular-weight heparin bridging is associated with an increase in wound hematoma following surgery for pacemakers and implantable defibrillators. Pacing Clin Electrophysiol 2009;32:378–382. [DOI] [PubMed] [Google Scholar]
- 25. de Oliveira JC, Martinelli M, Nishioka SAD, Varejão T, Uipe D, Pedrosa AAA, Costa R, D'avila A, Danik SB.. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009;2:29–34. [DOI] [PubMed] [Google Scholar]
- 26. Raad D, Irani J, Akl EG, Choueiri S, Azar E, Abboud J, Afif C.. Implantable electrophysiologic cardiac device infections: a risk factor analysis. Eur J Clin Microbiol Infect Dis 2012;31:3015–3021. [DOI] [PubMed] [Google Scholar]
- 27. Pakarinen S, Oikarinen L, Toivonen L.. Short-term implantation-related complications of cardiac rhythm management device therapy: a retrospective single-centre 1-year survey. Europace 2010;12:103–108. [DOI] [PubMed] [Google Scholar]
- 28. Kirkfeldt RE, Johansen JB, Nohr EA, Moller M, Arnsbo P, Nielsen JC.. Pneumothorax in cardiac pacing: a population-based cohort study of 28 860 Danish patients. Europace 2012;14:1132–1138. [DOI] [PubMed] [Google Scholar]
- 29. van Rees JB, de Bie MK, Thijssen J, Borleffs CJW, Schalij MJ, van Erven L.. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices. A systematic review of randomized clinical trials. J Am Coll Cardiol 2011;58:995–1000. [DOI] [PubMed] [Google Scholar]
- 30. Dasgupta A, Montalvo J, Medendorp S, Lloyd-Jones DM, Ghossein C, Goldberger J, Passman R.. Increased complication rates of cardiac rhythm management devices in ESRD patients. Am J Kidney Dis 2007;49:656–663. [DOI] [PubMed] [Google Scholar]
- 31. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM.. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–1006. [DOI] [PubMed] [Google Scholar]
- 32. Kostis WJ, Demissie K, Marcella SW, Shao Y-H, Wilson AC, Moreyra AE, Weekend versus weekday admission and mortality from myocardial infarction NEJM 2007;356:1099–1109. [DOI] [PubMed]
- 33. Patel NJ, Deshmukh A, Pau D, Goyal V, Patel SV, Patel N, Agnihotri K, Asirvatham S, Noseworthy P, Di Biase L, Natale A, Viles-Gonzalez JF.. Contemporary utilization and safety outcomes of catheter ablation of atrial flutter in the United States: analysis of 89,638 procedures. Heart Rhythm 2016;13:1317–1325. [DOI] [PubMed] [Google Scholar]
- 34. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


