Abstract
Background
FG-4592 (roxadustat) is an oral hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor (HIF-PHI) promoting coordinated erythropoiesis through the transcription factor HIF. Two Phase 2 studies were conducted in China to explore the safety and efficacy of FG-4592 (USAN name: roxadustat, CDAN name:  ), a HIF-PHI, in patients with anemia of chronic kidney disease (CKD), both patients who were dialysis-dependent (DD) and patients who were not dialysis-dependent (NDD).
), a HIF-PHI, in patients with anemia of chronic kidney disease (CKD), both patients who were dialysis-dependent (DD) and patients who were not dialysis-dependent (NDD).
Methods
In the NDD study, 91 participants were randomized to low (1.1–1.75 mg/kg) or high (1.50–2.25 mg/kg) FG-4592 starting doses or to placebo. In the DD study, 87 were enrolled to low (1.1–1.8 mg/kg), medium (1.5–2.3 mg/kg) and high (1.7–2.3 mg/kg) starting FG-4592 doses or to continuation of epoetin alfa. In both studies, only oral iron supplementation was allowed.
Results
In the NDD study, hemoglobin (Hb) increase ≥1 g/dL from baseline was achieved in 80.0% of subjects in the low-dose cohort and 87.1% in the high-dose cohort, versus 23.3% in the placebo arm (P < 0.0001, both). In the DD study, 59.1%, 88.9% (P = 0.008) and 100% (P = 0.0003) of the low-, medium- and high-dose subjects maintained their Hb levels after 5- and 6-weeks versus 50% of the epoetin alfa-treated subjects.
In both studies, significant reductions in cholesterol were noted in FG-4592-treated subjects, with stability or increases in serum iron, total iron-binding capacity (TIBC) and transferrin (without intravenous iron administration). In the NDD study, hepcidin levels were significantly reduced across all FG-4592-treated arms as compared with no change in the placebo arm. In the DD study, hepcidin levels were also reduced in a statistically significant dose-dependent manner in the highest dose group as compared with the epoetin alfa-treated group. Adverse events were similar for FG-4592-treated and control subjects.
Conclusions
FG-4592 may prove an effective alternative for managing anemia of CKD. It is currently being investigated in a pivotal global Phase 3 program.
Keywords: anemia in chronic kidney disease, erythropoiesis, erythropoietin, hypoxia-inducible factor, FG-4592
INTRODUCTION
Chronic kidney disease (CKD) is a major global public health challenge affecting over 10% of the population in industrial countries [1], with an estimated 120 million affected people in China [1–3]. The prevalence and severity of anemia are higher at later stages of CKD, with >90% prevalence in Stage 5 CKD. Anemia is associated with significant morbidity and mortality [4, 5], progression of kidney disease in patients with diabetes [6] and higher transfusion rates [7].
In China, anemia in non-dialysis dependent CKD patients (NDD-CKD) generally remains untreated, reflected by the low hemoglobin (Hb) levels at dialysis initiation in both rural and large urban healthcare settings, e.g. mean Hb levels of 7.3 ±1.8 g/dL (Guangzhou), 8.2 ±1.8 g/dL (Beijing) and 7.7 ±1.9 g/dL (Shanghai) [8–11]. In a single-center study, rural subjects who initiated dialysis with mean Hb levels of 5.9 ±4.7 g/dL had twice the rate of heart failure (34.4% versus 16.2%) and twice the 1-year mortality rate (42.9% versus 21.9%) of urban subjects who began dialysis with mean Hb of 8.4 ±4.5 g/dL [8, 10, 11].
CKD anemia in dialysis-dependent (DD) patients also appears to be undertreated in China. According to the Chinese National Renal Data System (2011), the mean Hb in Chinese dialysis patients is 9.1 g/dL despite the use of recombinant erythropoiesis-stimulating agents (ESAs) in 82.6% of hemodialysis patients and the Chinese ESA label Hb target of 10–12 g/dL [12]. Although the USA has a more mature dialysis infrastructure, half of the US new dialysis patients (50.1%) have Hb levels <10 g/dL, and approximately 28% have Hb levels <9 g/dL [13].
Exogenous human recombinant erythropoietin (rhEPO) and derivatives thereof (ESAs) are approved for treating CKD anemia in most countries, including China. Although ESAs may improve quality of life [14], several studies in CKD patients (both NDD and DD) observed higher mortality in higher dose ESA-treated cohorts when the protocol objective was to treat to higher target Hb levels [15–18]. Meta-analyses support the association of adverse events with higher ESA dose administration [19–23]. Increased cardiovascular and/or cerebrovascular risk is associated with the dose of ESA used rather than with the Hb level achieved [21, 23]. In addition to these safety concerns, challenges of administering parenteral products like ESAs outside of dialysis centers have limited the use of such therapies in the NDD-CKD population.
While endogenous erythropoietin (EPO) levels are lower than expected for the degree of anemia in CKD [24, 25], the retained ability to increase production of endogenous EPO and the roles of inflammation and elevated hepcidin support the concept that anemia in CKD patients is not solely due to endogenous EPO deficiency but is rather the result of multiple factors [26–28].
Hypoxia-inducible factor (HIF) is a transcription factor that acts as the body's main sensor of oxygen tension [29]. HIF induces expression not only of EPO, but also of the EPO receptor and of proteins that promote iron absorption and recycling from the macrophage iron storage system [30]. HIF is regulated by a family of prolyl hydroxylases (HIF-PHs), which promote the degradation of HIF under normal oxygen conditions but are inhibited under hypoxic conditions. FG-4592 (USAN name: roxadustat, CDAN name: 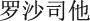 ) is a potent and reversible HIF-PH inhibitor (HIF-PHI) that transiently induces HIF stabilization and leads to a functional HIF transcriptional response that mimics the natural erythropoietic response associated with exposure of humans to intermittent hypoxia. Thus, FG-4592 pharmacologically stimulates erythropoiesis via the HIF pathway and in a manner consistent with the physiological response to hypoxia, but under normoxic conditions. The intermittent dosing strategy of FG-4592 for the treatment of CKD anemia [31] permits maintenance of the therapeutic effect over time without desensitization to treatment [32, 33]. Herein, we report the results of two randomized, controlled Phase 2 studies of FG-4592 for correction of anemia or Hb maintenance in Chinese NDD- and DD-CKD patients, respectively.
) is a potent and reversible HIF-PH inhibitor (HIF-PHI) that transiently induces HIF stabilization and leads to a functional HIF transcriptional response that mimics the natural erythropoietic response associated with exposure of humans to intermittent hypoxia. Thus, FG-4592 pharmacologically stimulates erythropoiesis via the HIF pathway and in a manner consistent with the physiological response to hypoxia, but under normoxic conditions. The intermittent dosing strategy of FG-4592 for the treatment of CKD anemia [31] permits maintenance of the therapeutic effect over time without desensitization to treatment [32, 33]. Herein, we report the results of two randomized, controlled Phase 2 studies of FG-4592 for correction of anemia or Hb maintenance in Chinese NDD- and DD-CKD patients, respectively.
MATERIALS AND METHODS
Ethical considerations
These studies were conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines, approved by appropriate Ethics Committees, and registered at Clinicaltrials.gov (NCT01599507 and NCT01596855). Subjects provided informed consent prior to participation.
Subjects and treatment
NDD study
The double-blinded NDD study included CKD subjects 18- to 80-years old with an estimated glomerular filtration rate (eGFR) [34] of 10 to <60 mL/min/1.73 m2, not requiring dialysis, with baseline Hb <10.0 g/dL. Key exclusions were thromboembolic events, ESA treatment or red blood cell (RBC) transfusion within 12 weeks or intravenous (IV) iron supplementation within 4 weeks of Day 1 of the study (see Supplementary data, Table S1). Qualified subjects were randomized 2:1 to FG-4592 or placebo orally thrice weekly (TIW) sequentially into first low- (1.1–1.75 mg/kg) and then high-dose (1.50–2.25 mg/kg) FG-4592 cohorts using weight-tiered dosing (40–60 kg, >60 to 80 kg or >80 to 100 kg), and were treated for 8 weeks. Dose increase could occur once (Week 5), while dose reductions for protocol-defined excessive erythropoiesis could occur at any time (see Supplementary data, Table S2).
DD study
The open-label DD study included subjects on hemodialysis whose mean Hb in three screening tests was between 9.0 and 12.0 g/dL, who received stable doses of epoetin alfa (administered IV or subcutaneously) during the 7 weeks prior to randomization, and who were without recent cardiovascular events (Supplementary data, Table S3). Subjects were stratified by baseline epoetin alfa dose (> or ≤6000 IU/week) and randomized 3:1 to FG-4592 or to epoetin alfa [FG-4592 sequentially to 1.1–1.8 mg/kg (low), 1.5–2.3 mg/kg (medium) and 1.7–2.3 mg/kg (high) per dose TIW on dialysis days], using weight-based dosing (Supplementary data, Table S4). Subjects randomized to epoetin alfa continued their pre-randomization dose, route and schedule with dose adjustments to maintain stable Hb level. Dose increase could occur once at Week 5 (see Supplementary data, Table S4). Dose decreases were allowed at any time during the dosing period for protocol-defined excessive erythropoiesis (see legend under Supplementary data, Table S4).
Three additional subjects at each dose level were assigned to FG-4592 and enrolled in a sub-study of FG-4592 pharmacokinetics and pharmacodynamics (PK/PD). These subjects are included in the safety population, but not in the efficacy-evaluable (EE) population (see below). The PK/PD data are expected to be reported in a subsequent manuscript.
Iron use and rescue
In both studies, the protocol allowed supplementation with oral iron at investigator discretion. The protocol restricted the use of ESAs, IV iron, androgens or RBC transfusions through end-of-treatment (EOT). In NDD subjects, rescue therapy with ESAs and/or IV iron was available if Hb fell below <8.0 g/dL and the investigator felt it was in the subject’s medical interest. In DD subjects, rescue was available if Hb decreased to <8.0 g/dL or to <9.0 g/dl with a ≥1.5 g/dL decrease from baseline.
Statistical analysis
Statistical methods used in both studies
Analyses were performed in SAS 9.1.3 (SAS Institute, Cary, NC, USA). A P-value of <0.05 two-sided was accepted as significant. No adjustments were made for multiple comparisons.
Biological correlates of iron utilization [e.g. total iron-binding capacity (TIBC), transferrin saturation (TSAT), ferritin and serum iron], soluble transferrin receptor (sTfR), hepcidin, C-reactive protein (CRP), red cell parameters [e.g. mean corpuscular volume (MCV)] and non-fasting cholesterol [total, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) associated] were evaluated in both studies. For Hb, baseline was defined as the mean of Day 1 Hb and the last two Hb prior to Day 1 values. For other parameters the laboratory value obtained immediately prior to the administration of the first dose of study drug was deemed baseline. The treatment group comparisons utilized mixed model repeated measure methodologies, taking into account repeated measurements over time. Laboratory and safety data were summarized using descriptive statistics based on data of all subjects. The number of subjects requiring rescue therapy was also assessed in both studies.
Analysis populations
The intent-to-treat (ITT) populations included all randomized subjects in each study. The safety populations in both studies included all subjects that had received at least one dose of study drug (FG-4592, placebo or active comparator). The pre-specified EE population in the DD study only consisted of all subjects who received study drug for at least 2 weeks and had corresponding Hb data. All baseline and safety analyses were done on the safety populations. All efficacy analyses were done on the ITT population in the NDD study, and on the EE population in the DD study.
Primary endpoint (NDD)
The primary efficacy endpoint for the NDD study was the maximum Hb change from baseline (ΔHbmax) at any time from baseline to 8 weeks of treatment. A sample size of 30 subjects per group provided 80% power to detect a difference between means of ΔHbmax of 1 g/dL in the FG-4592 arms and ΔHbmax of 0.5 g/dL in the placebo arm, assuming a common standard deviation of 0.6 g/dL and a 20% dropout rate, and using a two-group t-test with a 0.05 two-sided significance level. ΔHbmax for FG-4592-treated subjects was compared with that for placebo-treated subjects using the ANCOVA model with baseline Hb value as covariate. Time to response was estimated using the Kaplan–Meier method and compared using the log-rank test.
Other key efficacy endpoints were the proportion of subjects who were Hb responders (defined as an Hb increase of ≥1.0 g/dL from baseline at any time) and the proportion of subjects who achieved an Hb level of ≥11.0 g/dL. Fisher’s exact test was used to compare response rates.
Primary endpoint (DD)
The DD study’s primary endpoint was the percentage of subjects with successful dose conversion defined as a Hb level maintained at no <0.5 g/dL below mean baseline value during the last 2 weeks of the 6-week dosing period in the EE population. This endpoint was compared between treatment groups using the Cochran–Mantel–Haenszel test after adjusting for randomization stratification. The mean change from baseline (CFB) Hb (not due to an RBC transfusion, protocol-prohibited ESA treatment or IV iron supplementation during the dosing period) after 6 weeks of dosing was analyzed using the ANCOVA model including baseline Hb and randomization stratification as covariates. The sample size of the study was not determined by formal power calculations.
Hepcidin levels were measured at baseline and at EOT using a competitive ELISA kit from Intrinsic Life Sciences, Inc., La Jolla, CA, USA (Hepcidin IDxTM ELISA Kit, Cat. No. ICE-007). High-sensitivity C-reactive protein (hsCRP) assay was performed at baseline by LabCorp Central Laboratories (Beijing, China) using an immunoturbidimetric method. The assay’s lowest calibrator concentration (2.5 ng/mL) was used as the lower limit of quantitation.
RESULTS
Subject disposition
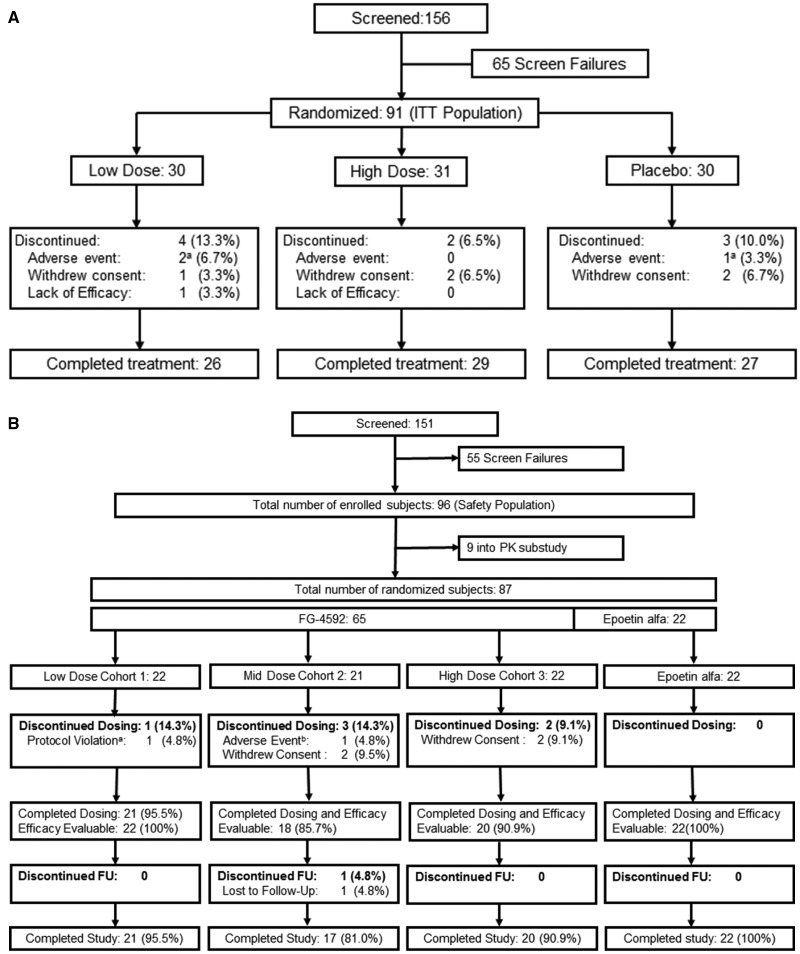
In the NDD study, 91 subjects were randomized from December 2011 to August 2012 to receive either FG-4592 (n = 61) or placebo (n = 30) at 11 study sites in China (Figure 1A), constituting both ITT and safety populations. In the DD study, 87 subjects were enrolled from September 2011 to June 2012 to one of the three FG-4592 dose cohorts or to the epoetin alfa cohort at nine sites in China (Figure 1B): 74 subjects were treated with FG-4592 (65 subjects randomized to FG-4592 and 9 PK subjects who were not randomized) and 22 subjects were treated with epoetin alfa. Of 65 subjects, 5 that were randomized to FG-4592 withdrew from the study within 2 weeks, including a subject who incorrectly received a single dose of epoetin-alfa and a subject who developed a Grade 1 rash. The other three early terminations withdrew consent. Therefore, the pre-specified EE population (see section ‘Materials and methods’) comprised 82 subjects, 60 of whom were randomized to FG-4592 and 22 of whom were randomized to epoetin alfa.
FIGURE 1.
Patient disposition. (A) NDD study: athe two adverse events in the low dose FG-4592 arm were urinary tract infection and worsening chronic renal failure. The one placebo subject was discontinued for adverse event of worsening anemia (and received rescue therapy). (B) DD study: awithdrawn due to having had one dose of epoetin alfa (prohibited medication) administered in error, brash (hypersensitivity). The latter subject was efficacy evaluable. FU, follow-up.
Demographics and baseline characteristics
Baseline characteristics were comparable across treatment groups, except in the NDD study for the proportion of subjects baseline eGFR <10 mL/min/1.73 m2 (18.0% of FG-4592-treated versus 3.3% of the placebo subjects; Table 1).
Table 1.
Demographics and baseline characteristics (ITT/safety population for NDD study, safety population for DD study)
| Parameter | NDD study |
DD study |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=30) | FG-4592 |
Total (n=91) | rhEPO (n=22) | FG-4592 |
Total (n=96) | ||||
| Low dose (n=30) | High dose (n=31) | Low dose (n=25) | Mid dose (n=24) | High dose (n=25) | |||||
| Gender (% male) | 26.7 | 26.7 | 32.3 | 28.6 | 59.1 | 64 | 58.3 | 60 | 60.4 |
| Race (% Chinese) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Age (years), mean (±SD) | 51.4 (±11.9) | 48.1 (±13.0) | 49.6 (±14.8) | 49.7 (±13.2) | 53.8 (±10.0) | 49.9 (±14.7) | 50.2 (±9.3) | 49.8 (±13.5) | 50.8 (±12.6) |
| Weighta (kg), mean (±SD) | 56.9 (±10.3) | 57.5 (±10.6) | 57.3 (±11.6) | 57.2 (±10.8) | 60.9 (±9.5) | 62.2 (±14.6) | 64.8 (±10.0) | 58.2 (±10.1) | 61.5 (±11.4) |
| GFR (mL/min/1.73 m2)b mean (±SD) | 23.0 (±13.4) | 21.1 (±10.2) | 17.7 (±8.6) | 20.5 (±11.0) | |||||
| BL eGFR <10 [n (%)] | 1 (3.3) | 5 (16.7) | 6 (19.4) | 12 (13.2) | |||||
| 10 ≤ BL eGFR < 15 [n (%)] | 8 (26.7) | 5 (16.7) | 7 (22.6) | 20 (22.0) | |||||
| 15 ≤ BL eGFR < 20 [n (%)] | 6 (20.0) | 5 (16.7) | 9 (29.0) | 20 (22.0) | |||||
| 20 ≤ BL eGFR < 25 [n (%)] | 4 (13.3) | 6 (20.0) | 4 (12.9) | 14 (15.4) | |||||
| 25 ≤ BL eGFR < 30 [n (%)] | 4 (13.3) | 4 (13.3) | 1 (3.2) | 9 (9.9) | |||||
| BL eGFR ≥ 30 [n (%)] | 7 (23.3) | 5 (16.7) | 4 (12.9) | 16 (17.6) | |||||
| hsCRP (mg/L), mean (±SD) | 1.48 (±2.19) | 4.00 (±12.75) | 1.87 (±3.80) | 2.44 (±7.75) | 3.00 (±4.70) | 4.04 (±5.3) | 6.65 (9.83) | 1.94 (±3.04) | 3.91 (±6.37) |
| Hematology, mean (±SD) | |||||||||
| Hemoglobin (g/dL) | 8.9 (±0.8) | 8.8 (±0.9) | 8.8 (±0.9) | 8.8 (±0.9) | 10.6 (±1.0) | 10.9 (±0.7) | 10.7 (±0.8) | 10.8 (±0.6) | 10.7 (±0.8) |
| Hematocrit (%) | 29.5 (±3.4) | 28.7 (±3.9) | 29.4 (±2.7) | 29.2 (±3.3) | 33.6 (±3.4) | 34.3 (±2.7) | 34.0 (±3.2) | 34.7 (±2.8) | 34.2 (±3.0) |
| RBC (×1012/L) | 3.01 (±0.32) | 3.09 (±0.54) | 2.98 (±0.31) | 3.03 (0.40) | 3.51 (±0.44) | 3.64 (±0.42) | 3.60 (±0.39) | 3.52 (±0.28) | 3.56 (±0.38) |
| MCV (fL) | 97.9 (±5.4) | 93.7 (±7.7) | 98.7 (±6.6) | 96.8 (±6.9) | 96.2 (±8.0) | 94.9 (±8.9) | 95.6 (±5.9) | 98.6 (±5.5) | 96.3 (±7.2) |
| MCHC (g/dL) | 30.7 (±1.0) | 30.7 (±1.1) | 30.0 (±1.0) | 30.5 (±1.1) | 31.3 (±1.1) | 31.5 (±0.9) | 31.4 (±0.7) | 30.8 (±1.2) | 31.3 (±1.0) |
| Reticulocyte counts (%) | 1.6 (±0.9) | 1.6 (±0.6) | 1.2 (±0.5) | 1.5 (±0.7) | 1.6 (±0.8) | 2.0 (±1.0) | 2.1 (±0.7) | 1.4 (±1.0) | 1.8 (±0.9) |
| CHr (pg) | 31.3 (±1.5) | 30.5 (±2.4) | 30.5 (±1.8) | 30.8 (±2.0) | 32.2 (±2.5) | 31.8 (±3.3) | 31.6 (±1.9) | 31.4 (±1.4) | 31.7 (±2.4) |
| Platelet count (×109/mL) | 178 (±79) | 183 (±67) | 149 (±49) | 170 (±67) | 170 (±55) | 208 (±91) | 213 (±76) | 160 (±58) | 188 (±75) |
| Neutrophils (×109/mL) | 3.16 (±1.12) | 3.70 (±1.20) | 3.41 (±1.62) | 3.42 (±1.34) | 3.33 (±1.19) | 4.10 (±1.40) | 3.30 (±1.46) | 3.37 (±1.07) | 3.53 (±1.32) |
| Lymphocytes (×109/mL) | 1.20 (±0.38) | 1.29 (±0.38) | 1.17 (±0.47) | 1.22 (±0.41) | 1.15 (±0.41) | 1.51 (±0.47) | 1.14 (±0.47) | 1.34 (±0.40) | 1.29 (±0.46) |
| Monocytes (×109/mL) | 0.27 (±0.11) | 0.30 (±0.11) | 0.26 (±0.14) | 0.28 (±0.12) | 0.26 (±0.13) | 0.36 (±0.18) | 0.33 (±0.18) | 0.29 (±0.10) | 0.31 (±0.15) |
| Eosinophils (×109/mL) | 0.14 (±0.08) | 0.16 (±0.12) | 0.09 (±0.07) | 0.13 (±0.10) | 0.33 (±0.38) | 0.25 (±0.21) | 0.25 (±0.19) | 0.26 (±0.20) | 0.27 (±0.25) |
| Basophils (×109/mL) | 0.03 (±0.02) | 0.03 (±0.03) | 0.04 (±0.03) | 0.03 (±0.02) | 0.04 (±0.03) | 0.04 (±0.04) | 0.04 (±0.03) | 0.05 (±0.03) | 0.04 (±0.03) |
| White blood cells (×109/L) | 4.80 (±1.46) | 5.49 (±1.37) | 4.97 (±1.84) | 5.09 (±1.58) | 5.10 (±1.65) | 6.20 (±1.88) | 5.06 (±1.94) | 5.31 (±1.28) | 5.43 (±1.75) |
| Iron utilization parameters | |||||||||
| Serum iron (μg/mL), mean (±SD) | 58.1 (±14.8) | 61.0 (±24.3) | 64.9 (±20.7) | 61.4 (±20.3) | 79.0 (±31.9) | 68.0 (±35.6) | 75.5 (±39.5) | 71.9 (±21.0) | 73.5 (±32.4) |
| TSAT (%), mean (±SD) | 21.9 (±6.3) | 22.1 (±11.4) | 24.2 (±8.8) | 22.7 (±9.0) | 34.1 (±14.6) | 29.8 (±16.7) | 32.1 (±18.2) | 32.8 (±15.8) | 32.2 (16.2) |
| TSAT ≥20%[n (%)] | 20 (66.7) | 16 (53.3) | 19 (61.3) | 55 (60.4) | 20 (90.9) | 18 (72.0) | 15 (62.5) | 24 (96.0) | 79 (82.3) |
| TSAT <20%[n (%)] | 10 (33.3) | 14 (46.7) | 12 (38.7) | 36 (39.6) | 2 (9.1) | 6 (24.0) | 9 (37.5) | 1 (4.0) | 18 (18.8) |
| Ferritin (ng/mL), mean (±SD) | 221 (±181) | 201 (±252) | 184 (±194) | 202 (±209) | 458 (±361) | 380 (±345) | 488 (±372) | 485 (±391) | 453 (±365) |
| Ferritin ≥100 ng/mL[n (%)] | 19 (63.3) | 14 (46.7) | 23 (74.2) | 56 (61.5) | 18 (81.8) | 20 (80.0) | 22 (91.7) | 19 (76.0) | 79 (82.3) |
| Ferritin <100 ng/mL[n (%)] | 11 (36.7) | 16 (53.3) | 8 (25.8) | 35 (38.5) | 4 (18.2) | 4 (16.0) | 2 (8.3) | 6 (24.0) | 16 (16.7) |
| TIBC (µg/dL), mean (±SD) | 240 (±49) | 263 (±52) | 242 (±37) | 248 (±47) | 214 (±38) | 218 (±46) | 221 (±41) | 213 (±61) | 217 (±47) |
| Transferrin (mg/dL), mean (±SD) | 216 (±45) | 233 (±49) | 219 (±35) | 223 (±43) | 187 (±35) | 188 (±39) | 194 (±36) | 186 (±58) | 189 (±43) |
| sTfR (mg/L), mean (±SD) | 3.5 (±1.2) | 3.7 (±1.9) | 3.5 (±1.4) | 3.5 (±1.5) | 2.9 (±1.2) | 3.9 (±1.8) | 3.4 (±1.2) | 3.4 (±1.1) | 3.4 (±1.4) |
| Hepcidin (ng/mL), mean (±SD) | 69.9 (±8.7) | 69.0 (±13.1) | 73.9 (±12.1) | 71.5 (±8.8) | 209.0 (±127.1) | 157.0 (±124.0) | 198.4 (±113.1) | 174.4 (±124.0) | 182.9 (±121.8) |
| Serum lipids, mean (±SD) | |||||||||
| Total cholesterol (mg/dL) | 183 (±52) | 164 (±33) | 169 (±45) | 172 (±44) | 158 (±28) | 172 (±38) | 169 (±32) | 172 (±36) | 168 (±34) |
| HDL-cholesterol (mg/dL) | 48 (±19) | 54 (±20) | 44 (±17) | 49 (±19) | 41 (±14) | 39 (±12) | 39 (±14) | 39 (±15) | 39 (±14) |
| LDL-cholesterol (mg/dL) | 115 (±40) | 96 (±24) | 110 (±36) | 107 (±34) | 91 (±24) | 103 (±31) | 100 (±30) | 103 (±24) | 99 (±27) |
| HDL/LDL ratio | 0.46 (±0.23) | 0.58 (±0.21) | 0.43 (±0.18) | 0.49 (±0.22) | 0.48 (±0.20) | 0.45 (±0.30) | 0.46 (±0.37) | 0.41 (±0.18) | 0.45 (±0.27) |
| Triglycerides (mg/dL) | 148 (±97) | 123 (±60) | 131 (±55) | 134 (±73) | 168 (±93) | 193 (±112) | 180 (±72) | 182 (±101) | 181 (±95) |
| VLDL-cholesterol (mg/dL) | 31 (±27) | 24 (±12) | 26 (±11) | 27 (±18) | 33 (±18) | 37 (±20) | 36 (±14) | 36 (±18) | 36 (±18) |
| Etiology of CKD [n (%)] | |||||||||
| Diabetic nephropathy | 5 (16.7) | 4 (13.3) | 2 (6.5) | 11 (12.1) | 1 (4.5) | 5 (20.0) | 1 (4.2) | 2 (8.0) | 8 (8.3) |
| Hypertensive nephropathy | 9 (30.0) | 4 (13.3) | 10 (32.3) | 23 (25.3) | 3 (13.6) | 1 (4.0) | 4 (16.7) | 5 (20.0) | 13 (13.5) |
| IgA nephropathy | 3 (10.0) | 5 (16.7) | 2 (6.5) | 10 (11.0) | 1 (4.5) | 1 (4.0) | 0 | 1 (4.0) | 3 (3.1) |
| Focal segmental glomerulosclerosis | 0 | 2 (6.7) | 0 | 2 (2.2) | 1 (4.5) | 1 (4.0) | 1 (4.2) | 2 (8.0) | 5 (5.2) |
| Glomerulonephritis unspecified | 12 (40.0) | 14 (46.7) | 20 (64.5) | 46 (50.5) | 12 (54.5) | 11 (44.0) | 15 (62.5) | 14 (56.0) | 52 (54.2) |
| Pyelonephritis | 1 (3.3) | 0 | 0 | 1 (1.1) | 0 | 0 | 0 | 0 | 0 |
| Polycystic kidney disease | 1 (3.2) | 0 | 1 (3.3) | 2 (2.2) | 0 | 0 | 0 | 0 | 0 |
| Urologic disorder(s) | 1 (3.3) | 0 | 3 (9.7) | 4 (4.4) | 0 | 0 | 0 | 0 | 0 |
| Toxic/drug induced | 0 | 0 | 0 | 0 | 0 | 2 (8.0) | 2 (8.3) | 0 | 4 (4.2) |
| Other | 3 (10.0) | 4 (13.3) | 2 (6.5) | 9 (9.9) | 4 (18.2) | 4 (16.0) | 2 (8.3) | 1 (4.0) | 11 (11.5) |
| Median [range] epoetin alfa dose at screening (IU/kg/week)c | 100.2 | 130.7 | 100.9 | 140.2 | 116.2 | ||||
| [37–190] | [28–210] | [35–231] | [32–245] | [28–245] | |||||
| Subjects prior on SC epoetin-alfa [n (%)] | 14 (63.6) | 16 (64.0) | 12 (50.0) | 18 (72.0) | 60 (62.5) | ||||
| Median [range] SC epoetin-alfa dose at screening (IU/kg/week) | 116.3 | 142.8 | 108.7 | 149.2 | 140.8 | ||||
| [43–190] | [71–210] | [41–231] | [61–245] | [41–245] | |||||
| Median [range] IV epoetin-alfa dose at screening (IU/kg/week) | 99.9 | 97.5 | 84.2 | 104.7 | 99.4 | ||||
| [37–136] | [28–200] | [35–146] | [32–140] | [28–201] | |||||
Post-dialysis weight in DD study.
Not applicable for DD study.
Not applicable for NDD study.
BL, baseline.
Efficacy
Hb CFB
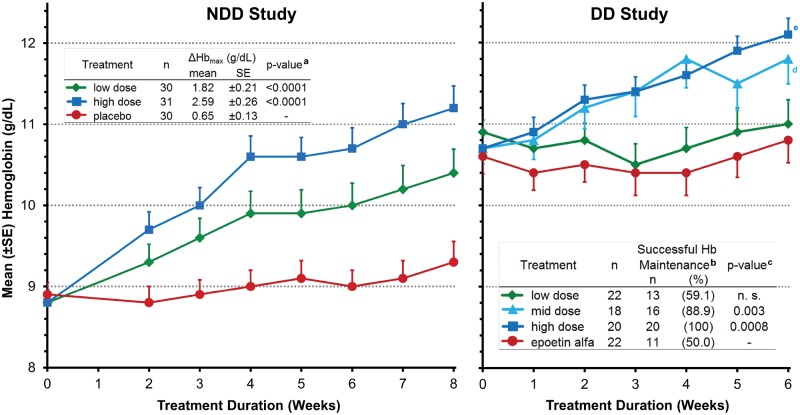
Mean [±standard error (SE)] Hb levels over time are shown for both studies (Figure 2). In the NDD study, the mean ΔHbmax at any time to EOT (primary NDD endpoint) was 1.82 g/dL and 2.59 g/dL in the low- and high-dose cohorts, respectively, versus 0.65 g/dL with placebo (P < 0.0001 for each cohort versus placebo, ITT; Table 2, and Figure left panel). At EOT, mean Hb increased by 1.55 g/dL and 2.38 g/dL in the low- and high-dose FG-4592 cohorts, respectively, as compared with 0.37 g/dL in the placebo group (P < 0.0001, for both versus placebo; Figure 2, left panel).
FIGURE 2.
Hb over time and Primary Efficacy Endpoints. NDD study: data are for the ITT population. aThe P-values for ΔHbmax are from the CFB lab values comparison of FG-4592 cohort to placebo group based on the ANCOVA model with baseline lab values as a covariate, and these were ≤0.0001 at all-time points after Week 2 for the high-dose cohort and after Week 6 for the low-dose cohort. In the low-dose cohort, the P-values for the difference between mean Hb CFB and placebo were 0.016 at Week 3, 0.003 at Week 4 and 0.0003 at Weeks 5 and 6 (these P-values are from mixed model for repeated measurements model with baseline as covariate, comparing FG-4592 CFB with placebo CFB). DD study: data are for the EE population using LOCF for missing data and are the mean (SE) Hb value at each time point. bDefined as a Hb level maintained at no less than 0.5 g/dL below mean baseline value during the last two weeks of the 6-week dosing period in the EE population. cCochran-Mantel-Haenszel test after adjusting for randomization stratification. Zero weeks (baseline) is the mean of three pre-dosing Hb values. dP = 0.005, eP = 0.0002, P-values are from the average CFB Hb comparison between FG-4592 Cohorts 1, 2 or 3 and epoetin alfa arm after 6 weeks of treatment, based on the ANCOVA model with baseline Hb and randomization stratification as covariates, and treatment groups as the classification variable.
Table 2.
Efficacy assessments (ITT population for NDD study, EE population for DD study)
| Parameter | NDD study |
DD study |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 30) | FG-4592 |
rhEPO (n = 22) | FG-4592 |
||||||
| Low dose (n = 30) | High dose (n = 31) | Total (n = 61) | Low dose (n = 22) | Mid dose (n = 18) | High dose (n = 20) | Total (n = 60) | |||
| Hemoglobin | |||||||||
| Hb CFB at EOT (g/dL), mean (±SD) (LOCF) | 0.37 (±0.87) | 1.55 (±1.23) | 2.38 (±1.46) | 1.97 (±1.40) | 0.17 (±0.96) | 0.11 (±1.0) | 1.10 (±1.00) | 1.42 (±1.12) | 0.84 (±1.18) |
| Maximum Hb CFB (g/dL), mean (±SD) (LOCF) | 0.65 (±0.73) | 1.82 (±1.15) | 2.59 (±1.45) | 2.21 (±1.35) | – | – | – | – | – |
| P-valuea | – | <0.0001 | <0.0001 | <0.0001 | – | 0.75 | 0.005 | 0.0002 | 0.02 |
| Hb CFB at EOS (g/dL), mean (±SD) | 0.40 (±0.67) | 0.92 (±1.16) | 1.39 (±1.30) | 1.16 (±1.24) | 0.52 (±1.77) | −0.49 (±0.93) | 0.89 (±1.07) | 0.45 (±1.29) | 0.21 (±1.23) |
| Hb responseb [n (%)] (LOCF) | 7 (23.3) | 24 (80.0) | 27 (87.1) | 51 (83.6) | 11 (50.0) | 13 (59.1) | 16 (88.9) | 20 (100) | 49 (81.7) |
| P-valuec | – | <0.0001 | <0.0001 | <0.0001 | – | 0.53 | 0.008 | 0.0003 | 0.004 |
| n (%) who achieved target Hb (≥11 g/dL) by EOT (LOCF) | 2 (6.7) | 15 (50.0) | 22 (71.0) | 37 (60.7) | |||||
| P-valuec comparison with control arm NDD study only | – | 0.0004 | <0.0001 | <0.0001 | |||||
| Hematocrit CFB by EOT (%), mean (±SD) | 0.5 (±2.7) | 6.9 (±4.1) | 8.5 (±4.4) | 7.7 (±4.3) | 1.85 (±3.4) | 1.05 (±3.474) | 3.90 (±3.47) | 6.8 (±4.87) | 3.88 (±4.63) |
| P-valued | – | <0.0001 | <0.0001 | <0.0001 | – | 0.62 | 0.009 | <0.0001 | 0.004 |
| Other hematology (CFB by EOT), mean (±SD) | |||||||||
| RBC (×1012/L) | 0.05 (±0.29) | 0.53 (±0.39) | 0.85 (±0.43) | 0.69 (±0.44) | 0.15 (±0.35) | 0.13 (±0.32) | 0.29 (±0.35) | 0.53 (±0.39) | 0.31 (±0.39) |
| P-valued | – | <0.0001 | <0.0001 | <0.0001 | – | 0.91 | 0.061 | <0.0001 | 0.013 |
| MCV (fL) | 0.30 (±3.38) | 4.70 (±3.92) | 0.29 (±3.51) | 2.45 (±4.30) | 1.12 (±2.73) | 1.26 (±3.25) | 2.79 (±2.26) | 5.42 (±3.67) | 3.14 (±3.56) |
| P-value d | – | <0.0001 | 0.80 | 0.01 | – | 0.81 | 0.04 | <0.0001 | 0.0045 |
| MCHC (g/dL) | 0.23 (±1.10) | −1.18 (±1.24) | 0.06 (±0.79) | −0.55 (±1.20) | −0.65 (±0.99) | −0.65 (±1.04) | −0.18 (±0.65) | −1.65 (±1.26) | −0.85 (±1.18) |
| P-valued | – | <0.0001 | 0.28 | 0.0003 | – | 0.92 | 0.14 | 0.0006 | 0.46 |
| Reticulocyte counts (%) (Week 3) | −0.14 (±0.43) | +0.93 (±0.91) | +1.54 (±1.16) | +1.21 (±1.07) | 0.05 (±0.87) | 0.15 (±1.25) | −0.74 (±0.90) | −0.56 (±0.94) | −0.36 (±1.10) |
| CHr (pg) | −0.13 (±1.35) | −1.13 (±1.75) | −0.87 (±1.90) | −1.00 (±1.81) | −1.30 (±1.07) | 0.62 (±1.47) | 0.84 (±1.50) | −0.90 (±1.88) | 0.17 (±1.78) |
| P-value d | – | 0.04 | 0.12 | 0.04 | – | 0.0006 | <0.0001 | 0.61 | 0.002 |
| Platelet count (×109/mL) | 4.5 (±39.5) | −15.5 (±44.1) | 35.1 (±49.7) | 10.3 (±53.1) | 11.3 (±45.18) | −0.8 (±33.06) | −14.4 (±56.18) | −26.4 (±38.50) | −13.6 (±43.63) |
| P-valued | – | 0.06 | 0.002 | 0.48 | – | 0.89 | 0.33 | 0.006 | 0.10 |
| Neutrophils (×109/mL) | 0.04 (±0.98) | −0.12 (±1.26) | 0.07 (±1.15) | −0.02 (±1.19) | 0.12 (±1.51) | −0.22 (±1.69) | 0.89 (±1.86) | 0.21 (±0.71) | 0.27 (±1.54) |
| Lymphocytes (×109/mL) | −0.03 (±0.32) | −0.09 (±0.25) | −0.04 (±0.32) | −0.06 (±0.29) | −0.05 (±0.36) | 0.07 (±1.00) | 0.09 (±0.41) | −0.18 (±0.41) | −0.01 (±0.67) |
| Monocytes CFB (×109/mL) | 0.00 (±0.11) | 0.04 (±0.24) | 0.06 (±0.16) | 0.05 (±0.20) | 0.06 (±0.15) | −0.02 (±0.18) | 0.02 (±0.15) | 0.01 (±0.11) | −0.00 (±0.15) |
| Eosinophils (×109/mL) | −0.022 (±0.070) | −0.003 (±0.106) | −0.004 (±0.042) | −0.004 (±0.079) | 0.026 (±0.249) | 0.037 (±0.134) | −0.033 (±0.166) | −0.083 (±0.120) | −0.026 (±0.146) |
| Basophils (×109/mL) | 0.003 (±0.013) | 0.011 (±0.035) | 0.002 (±0.023) | 0.006 (±0.029) | 0.000 (±0.036) | −0.002 (±0.042) | 0.003 (±0.018) | 0.018 (±0.033) | 0.007 (±0.033) |
| WBC (× 109/L) | −0.01 (±1.12) | −0.15 (±1.44) | 0.08 (±1.28) | −0.04 (±1.35) | 0.16 (±1.91) | −0.04 (±1.78) | 0.98 (±2.26) | −0.03 (±0.92) | 0.27 (±1.76) |
| Iron utilization parameters | |||||||||
| Serum iron CFB by EOT (μg/mL), mean (±SD) | 2.7 (±23.7) | 0.2 (±23.9) | −8.1 (±28.7) | −4.1 (±26.6) | −18.9 (±26.7) | 3.2 (±55.8) | −3.3 (±34.5) | 8.9 (±35.9)* | 3.1 (±43.0)f |
| TSAT CFB by EOT (%), mean (±SD) | 0.24 (±7.92) | −3.85 (±9.65) | −8.66 (±9.49) | −6.35 (±9.78) | −8.29 (±10.46) | −3.77 (±21.41) | −8.98 (±14.73) | −4.87 (±17.22) | −5.77 (±17.93) |
| P-valued comparison with control arm | – | 0.11 | <0.0001 | 0.001 | – | 0.80 | 0.98 | 0.57 | 0.74 |
| Ferritin CFB by EOT (ng/mL), mean (±SD) | −28 (±64) | −124 (±171) | −98 (±81) | −110 (±131) | −70 (±157) | 21 (±186) | −149 (±145) | −162 (±179) | −95 (±189) |
| P-valued comparison with control arm | – | <0.0001 | <0.0001 | <0.0001 | – | 0.06 | 0.13 | 0.04 | 0.52 |
| TIBC CFB by EOT (µg/dL), mean (±SD) | 1.2 (±22.1) | 65.1 (±47.9) | 102.0 (±56.2) | 84.3 (±55.1) | 0.5 (±17.4) | 41.5 (±37.5) | 50.6 (±46.0) | 59.1 (±40.5) | 50.5 (±41.3) |
| P-valued comparison with control arm | – | <0.0001 | <0.0001 | <0.0001 | – | 0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Transferrin CFB by EOT (mg/dL), mean (±SD) | 2.3 (±22.0) | 67.1 (±48.7) | 95.7 (±54.4) | 81.9 (±53.2) | 3.3 (±16.1) | 39.8 (±37.5) | 50.1 (±44.2) | 58.8 (±41.1) | 49.5 (±40.9) |
| P-valued comparison with control arm | – | <0.0001 | <0.0001 | <0.0001 | – | 0.0004 | <0.0001 | <0.0001 | <0.0001 |
| sTfR CFB by EOT (mg/L), mean (±SD) | 0.05 (±0.63) | 2.71 (±2.22) | 3.68 (±2.99) | 3.21 (±2.67) | 0.88 (±1.19) | 0.51 (±2.38) | 0.52 (±0.95) | 2.05 (±1.81) | 1.05 (±1.95) |
| P-valued comparison with control arm | – | <0.0001 | <0.0001 | <0.0001 | – | 0.59 | 0.48 | 0.011 | 0.52 |
| Hepcidin CFB by EOT (ng/mL), mean (±SD) | −4.8 (±8.17) | −37.8 (±9.91) | −37.2 (±9.31) | −37.5 (±6.73) | −77.9 (±75.18) | −25.7 (±108.68) | −86.0 (±109.41) | −102.7 (±80.40) | −70.2 (±104.19) |
| P-valuee comparison with control arm | 0.0004 | 0.0003 | <0.0001 | 0.13 | 0.65 | 0.005 | 0.71 | ||
| Serum lipids (CFB by EOT) mean (±SD) | |||||||||
| Total cholesterol (mg/dL) | 8.0 (±30.0) | −31.7 (±25.3) | −35.6 (±37.5) | −33.7 (±31.8) | 18.3 (±24.32) | −11.1 (±31.31) | −13.1 (±31.64) | −15.8 (±48.63) | −13.3 (±37.55) |
| P-valued | – | <0.0001 | <0.0001 | <0.0001 | – | 0.0045 | 0.0045 | 0.0012 | 0.0003 |
| Total cholesterol (%) | 5.5 | −19.3 | −17.5 | −18.4 | 11.2 | −6.9 | −6.9 | −7.6 | −7.1 |
| Total cholesterol (mg/dL) by EOS | 8.7 (±27.5) | 2.1 (±30.0) | 13.1 (±32.8) | 7.8 (±31.7) | 14.0 (±20.4) | 16.6 (±28.7) | 20.4 (±35.5) | 36.5 (±31.5) | 24.6 (±32.5) |
| HDL-cholesterol (mg/dL) | 1.7 (±10.6) | −7.7 (±10.5) | −6.9 (±7.0) | −7.3 (±8.9) | −1.9 (±7.4) | −8.2 (±7.8) | −6.6 (±8.4) | −6.6 (±12.5) | −7.2 (±9.6) |
| P-valued | – | 0.0001 | 0.0002 | <0.0001 | – | 0.005 | 0.034 | 0.014 | 0.002 |
| LDL-cholesterol (mg/dL) | 4 (±25.5) | −22.4 (±19.4) | −32.0 (±33.5) | −27.1 (±26.8) | −5.0 (±15.3) | −25.0 (±20.2) | −23.4 (±20.6) | −25.8 (±27.6) | −24.8 (±22.6) |
| P-valued | – | <0.0001 | <0.0001 | <0.0001 | – | 0.008 | 0.013 | 0.007 | 0.001 |
| HDL/LDL ratio | 0.01 (±0.12) | 0.08 (±0.17) | 0.10 (±0.15) | 0.09 (±0.16) | −0.01 (±0.10) | 0.06 (±0.22) | 0.09 (±0.14) | 0.06 (±0.19) | 0.07 (±0.18) |
| P-valued | – | 0.028 | 0.014 | 0.0072 | – | 0.081 | 0.026 | 0.074 | 0.019 |
| Triglycerides (mg/dL) | 6.5 (±50.0) | −26.3 (±39.9) | −21.9 (±42.5) | −24.1 (±40.8) | −4.1 (±86.6) | −16.5 (±53.0) | 30.8 (±131.6) | −21.3 (±93.0) | −3.4 (±97.0) |
| P-valuee | – | 0.0002 | 0.005 | 0.0001 | – | 0.96 | 0.56 | 0.59 | 0.99 |
| VLDL-cholesterol (mg/dL) | −0.8 (±18.8) | −5.2 (±7.9) | −4.5 (±8.5) | −4.9 (±8.1) | −0.5 (±18.2) | −1.0 (±14.0) | 5.7 (±25.4) | −3.1 (±17.5) | 0.4 (±19.3) |
| P-valued | – | <0.0001 | 0.005 | <0.0001 | – | 0.89 | 0.21 | 0.65 | 0.80 |
The P-values for ΔHbmax are from the change from baseline lab values comparison of FG-4592 cohort to placebo group based on the ANCOVA model with BL lab values as a covariate.
Hb response for the NDD study was the number (%) of subjects that by EOT experienced Hb increase from baseline (BL) of ≥1.0 g/dL. Hb response for the DD study was the number (%) of subjects with successful treatment after 5 and 6 weeks—Hb maintained at no more than 0.5 g/dL below mean baseline value.
P-value is from Fisher's exact test comparing proportion of FG-4592 group with comparator group for NDD study and is from Cochran–Mantel–Haenszel statistics for DD study. Where no P-values are provided, differences were not significant.
P-values were computed using mixed model repeated measure analysis with baseline as covariate and treatment, visit and treatment × visit as fixed effects.
P-values are computed based on rank ANCOVA; [47] comparison with control arm.
p<0.05.
At EOT in the DD study, Hb levels also increased in a dose-dependent manner. The low-dose FG-4592 cohort CFB was 0.11 g/dL compared with 0.17 g/dL in the epoetin alfa arm (P-value not significant) (Table 2). In both the mid- and high-dose cohorts, Hb increased by 1.10 and 1.42 g/dL, respectively (P = 0.005 for mid-dose and P = 0.0002 for high-dose cohort versus epoetin alfa; Figure 2, right panel; Table 2).
Hb response
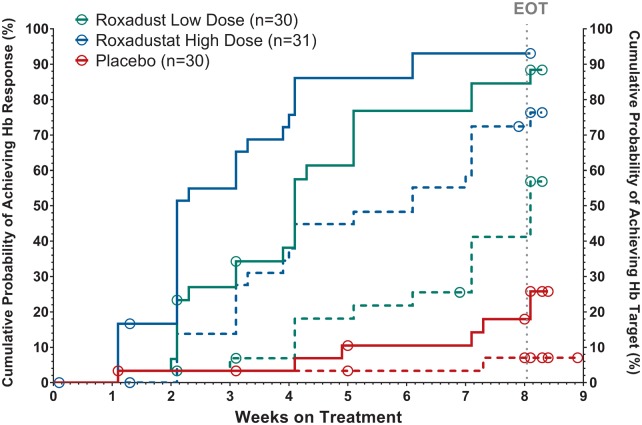
In the NDD study, Hb response, defined as a Hb rise of ≥1.0 g/dL from baseline at any time, was dose-dependent and significantly higher in the FG-4592 arms than in the placebo arm (Figure 3). In the completers (n = 82), Hb response was achieved in 88.5% and 93.1% of the low- and high-dose cohorts, respectively, versus in 25.9% of placebo subjects (P < 0.0001). The Hb response rates for the ITT population were 80.0% in the low-dose and 87.1% in the high-dose versus 23.3% in placebo cohorts (P < 0.0001 in comparison with placebo; Table 2). The median time to first Hb response was 29 days in the low-dose cohort and 16 days in the high-dose cohort but could not be estimated for the placebo group (P < 0.0001; Figure 3).
FIGURE 3.
Cumulative probability of Hb response and achievement of Hb target in NDD study (ITT population, Kaplan–Meier analysis). Response was defined as first instance of Hb rise from baseline ≥1.0 g/dL during the treatment period (full lines). Achievement was defined as first reaching of Hb level ≥11.0 g/dL during the treatment period (dashed lines). Empty circles represent censored subjects. P-values (log-rank test) for Hb response comparison against placebo were <0.0001 for both doses. P-value for comparison of high-dose with low-dose was 0.0665. P-values (log-rank test) for target achievement comparison against placebo were 0.0034 for the low FG-4592 dose and <0.0001 for the high dose. The P-value for comparison of high-dose to low-dose was 0.0259. EOT denotes protocol planned end of treatment with study drug.
The DD study’s primary endpoint was the number (%) of subjects in the EE population with successful dose conversion defined as subjects whose Hb levels were maintained at no less than 0.5 g/dL below their mean baseline value during the last 2 weeks of the 6-week dosing period. A total of 59.1%, 88.9% and 100% of subjects randomized to the low-, mid- and high-dose FG-4592 cohorts, respectively, met the primary endpoint as compared with only 50% of the subjects that continued on epoetin alfa (Figure 2, right panel; Table 2). The proportion of subjects who maintained Hb levels was significantly higher in the FG-4592 mid-dose (P = 0.008) and high-dose cohorts (P = 0.0003) than in the epoetin alfa arm. Analyses were also performed on the ITT (all randomized) subjects, and similar results were observed.
Fractions of subjects achieving Hb ≥11 g/dL
In the NDD study, the proportion of subjects achieving Hb ≥11 g/dL was dose-dependent (Figure 3) at 50% in the low-dose and 71% in the high-dose cohort compared with 6.7% in the placebo-treated cohort (P = 0.0004 and P < 0.0001 versus placebo, respectively; Table 2).
Leukocyte and platelet counts
Mean leukocyte and platelet counts did not change during treatment with FG-4592 in either the NDD or DD studies. However, subjects with the highest platelet counts may have experienced modest decreases in platelet numbers. In the DD study, the baseline mean platelet counts for the low, middle and high tertiles were 128 × 103/mm3, 185 × 103/mm3 and 280 × 103/mm3, respectively, compared with EOT mean platelet counts of 127 × 103/mm3, 190 × 103/mm3 and 241 × 103/mm3, respectively. The CFB in the high-dose tertile was significant (P = 0.02).
Rescue therapy
In the NDD study, one FG-4592 subject and one placebo subject required rescue therapy. A severely anemic FG-4592 subject (low-dose cohort) had a baseline Hb level of 5.8 g/dL at time of randomization, which fell to 5.1 g/dL by 2 weeks of treatment, after which IV iron and ESA therapy were provided. A placebo subject had a baseline Hb level of 7.0 g/dL, which fell to 6.3 g/dL; ESA therapy and transfusions were then provided. In the DD study, no subjects required rescue therapy.
Changes in erythrocyte and iron indices
For both the NDD and DD studies, the differences in CFB for TSAT, serum iron, TIBC and transferrin between FG-4592-treated subjects and the relevant comparator arm followed the same pattern and need to be interpreted in the context of a difference in red cell production in the NDD study and the absence of IV iron in the DD study (Table 2). In the FG-4592-treated arms, serum iron remained stable or increased, TIBC and transferrin rose significantly and TSAT was largely stable with some slight decreases. Specifically, in the NDD study, the CFBs for serum iron in these two groups were not significantly different (−4.1 versus 2.7 μg/mL), while TIBC (84.3 versus 1.2 μg/dL, P < 0.0001) and transferrin (81.9 versus 2.3 mg/dL, P < 0.0001) rose significantly in the FG-4592 arms. The CFB for TSAT in FG-4592-treated subjects was −6.35% as compared with 0.24% in placebo (P = 0.001). In the DD study, subjects receiving FG-4592 had stable serum iron levels as compared with a decline among those receiving epoetin alfa (CFB 3.1 versus −18.9 μg/mL, P = 0.03). The CFB for TIBC levels (50.5 versus 0.5 μg/dL, P < 0.0001) and transferrin (49.5 versus 3.3 mg/dL, P < 0.0001) rose significantly in the FG-4592 arms. There was no significant difference in TSAT between subjects receiving FG-4592 and those receiving epoetin alfa (−5.77% versus −8.29%, P = 0.74).
Concurrent with these changes in iron stores, the subjects receiving FG-4592 experienced a greater increase in MCV than in the comparator arms. In the NDD study, MCV increased by 2.45 fL as compared with 0.30 fL in the placebo arm (P = 0.01). In the DD study, MCV increased by 3.14 fL as compared with 1.12 fL in the epoetin alfa arm (P = 0.0045).
Changes in hepcidin
Treatment with FG-4592 resulted in a decline in serum hepcidin levels in both the NDD and DD studies. In the NDD trial, the CFB in hepcidin levels was significantly greater in subjects receiving FG-4592 than in subjects receiving placebo (−37.5 versus −4.8 ng/mL, respectively, P < 0.0001; Table 2). In the DD study, the CFB in hepcidin levels was dose-dependent in the subjects receiving FG-4592 in the low-, middle- and high-dose cohorts, at −25.7, −86.0 and −102.7 ng/mL, respectively. The difference between the groups receiving high-dose FG-4592 and epoetin alfa was statistically significant (−102.7 versus −77.9 ng/mL, P = 0.005). This difference was maintained when adjusted for CFB in Hb (data not shown).
Serum lipids
Compared with the applicable control, total cholesterol fell significantly in the FG-4592 group in both the NDD study (−33.7 versus 8.0 mg/dL, P <0.0001) and the DD study (−13.3 versus 18.3 mg/dL, P = 0.0003) (Table 2). LDL- and HDL-associated cholesterol levels also decreased with FG-4592 treatment (Table 2); the effect on LDL cholesterol was greater, resulting in an increase in the overall HDL/LDL ratio of 0.09 (P = 0.0072) in the NDD study and of 0.07 (P = 0.019) in the DD study. The magnitude of change in cholesterol level was related to baseline cholesterol levels. In the NDD trial, FG-4592 subjects in the highest tertile of baseline total cholesterol (mean 210.9 mg/dL) experienced the greatest absolute decrement of 48.1 mg/dL, while subjects in the middle (mean 165.6 mg/dL) and lower (mean 126.1 mg/dL) tertiles experienced lesser decrements of 33.8 and 16.4 mg/dL, respectively. In the DD trial, FG-4592 subjects in the highest tertile of baseline total cholesterol (mean 212.0 mg/dL) experienced the greatest absolute decrement of 21.9 mg/dL, while subjects in the middle (mean 168.0 mg/dL) and lower (mean 135.0 mg/dL) tertiles experienced lesser decrements of 14.3 and 7.0 mg/dL, respectively. Total cholesterol returned toward baseline after 4 weeks of follow-up (data not shown).
Among patients treated with FG-4592 in both NDD and DD studies, the CFB for total cholesterol did not differ between those receiving and not receiving concurrent statin therapy (data not shown).
Safety
Serious adverse events
Treatment-emergent serious adverse events (SAEs) were reported in four (13.3%) placebo-treated subjects and eight (13.1%) FG-4592-treated subjects in the blinded NDD study; no SAEs were deemed related to FG-4592. One cardiovascular SAE of unstable angina was reported in a placebo-treated subject but no such events were reported in FG-4592-treated subjects. No SAEs occurred during the DD study. No deaths or major adverse cardiac events occurred in FG-4592 subjects during either study.
Adverse events
Nineteen (63%) placebo-treated subjects and 36 (59%) FG-4592 subjects reported at least one treatment-emergent adverse event (TEAE) in the NDD study, while in the DD study, 32 subjects (43%) among the total of 74 FG-4592-treated subjects and 4 subjects (18%) among the total of 22 epoetin alfa-treated subjects reported having at least one TEAE. The majority of TEAEs in both studies were mild or moderate in severity. The most common TEAEs did not differ in frequency between the pooled FG-4592 and comparator arms (Table 3). In the NDD study, one placebo-treated but no FG-4592-treated subjects had elevations ≥3× upper limit of normal of either ALT or AST. Two adverse events of urinary tract infection and worsening chronic renal failure in the low-dose FG-4592 arm in the NDD study (a decline in eGFR from 11 mL/min at screening to 8 mL/min at EOT), and one adverse event of rash in the mid-dose FG-4592 arm in the DD study led to treatment discontinuation (Figure 1).
Table 3.
TEAEs occurring in ≥5% of subjects in a treatment group (safety populations for both studies)
| NDD study: FG-4592 |
DD study: FG-4592 |
NDD study | DD study | All FG-4592 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low dose | High dose | All | Low dose | Mid dose | High dose | All | Placebo | Epoetin alfa | ||
| Preferred terma | (n = 30) | (n = 31) | (n = 61) | (n = 25) | (n = 24) | (n = 25) | (n = 74) | (n = 30) | (n = 22) | (n = 135) |
| Subjects with TEAE(s) [n (%)] | 17 (57) | 19 (61) | 36 (59) | 10 (40) | 13 (54) | 9 (36) | 32 (43) | 19 (63) | 4 (18) | 68 (50) |
| Muscle spasms | 0 | 1 (3) | 1 (3) | 2 (8) | 2 (8) | 0 | 4 (5) | 0 | 3 (14) | 5 (4) |
| Diarrhoea | 2 (6.7) | 0 | 2 (3.3) | 0 | 1 (4.2) | 0 | 1 (1.4) | 1 (3.3) | 0 | 3 (2) |
| Vomiting | 2 (6.7) | 0 | 2 (3.3) | 0 | 0 | 0 | 0 | 0 | 1 (5) | 2 (1) |
| Abdominal discomfort | 0 | 0 | 0 | 0 | 2 (8) | 1 (4) | 3 (4) | 1 (3) | 0 | 3 (2) |
| Nausea | 1 (3) | 3 (10) | 4 (7) | 0 | 0 | 0 | 0 | 1 (3) | 1 (5) | 4 (3) |
| Dizziness | 3 (10) | 0 | 3 (5) | 0 | 1 (4) | 0 | 1 (1) | 3 (10) | 0 | 4 (3) |
| Headache | 0 | 2 (6.5) | 2 (3.3) | 0 | 0 | 0 | 0 | 0 | 1 (5) | |
| Hypertension | 1 (3) | 3 (10) | 4 (7) | 0 | 2 (8) | 1 (4) | 3 (4) | 0 | 1 (5) | 7 (5) |
| Hyperkalemia | 3 (10) | 3 (10) | 6 (10) | 0 | 0 | 0 | 0 | 2 (7) | 0 | 6 (4) |
| Liver injuryb | 0 | 0 | 0 | 0 | 0 | 2 (8) | 2 (3) | 1 (3) | 0 | 2 (1) |
| Decreased appetite | 0 | 0 | 0 | 1 (4) | 2 (8) | 2 (8) | 5 (7) | 0 | 1 (5) | 5 (4) |
| TSAT decreased | 5 (17) | 3 (10) | 8 (13) | 0 | 0 | 0 | 0 | 1 (3) | 0 | 8 (6) |
| Renal failure chronic | 1 (3) | 3 (10) | 4 (7) | 0 | 0 | 0 | 0 | 0 | NA | 4 (3) |
| Nasopharyngitis | 2 (6.7) | 0 | 2 (3.3) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (1) |
| Upper respiratory tract infections | 2 (7) | 1 (3) | 3 (5) | 2 (8) | 1 (4) | 0 | 3 (4) | 3 (10) | 0 | 6 (4) |
MedDRA version 14.1.
Two subjects had transient elevations in ALT and/or AST levels. One-subject had elevations of AST and ALT that were between 1 × and 1.7 × ULN; both values returned to normal in 33 days. The other patient had ALT elevation up to 2.7 × ULN and AST elevation up to l.5 × ULN; both values returned to normal in 35 days. Neither subject exhibited any symptoms associated with the observed levels. Both cases of transaminase elevations resolved without change in study drug administration as per protocol.
NA, not applicable.
DISCUSSION
The two Phase 2 clinical trials shown here demonstrate the potential of FG-4592 to effectively treat CKD-related anemia in Chinese CKD patients on and not on dialysis. The trial of patients not on dialysis is the first prospective, double-blind, placebo-controlled trial for the assessment of anemia agents reported in a registrational study in China. The overall median time to Hb response was 16 and 29 days in the high- and low-dose NDD-CKD cohorts, respectively. Despite moderately severe anemia at baseline [mean (±SE) Hb level of 8.8 ±0.9 g/dL], 67% of the FG-4592-treated NDD-CKD subjects reached 11.0 g/dL within the short period of 8 weeks of dosing. In both the NDD study and the DD study, FG-4592 showed a dose–response effect on Hb levels. In the DD study, all doses of FG-4592 were at least comparable to ESAs in maintaining Hb following conversion, as was seen in a Phase 2 trial with FG-4592 in US hemodialysis patients [28], with the lowest dose arm maintaining the closest comparability.
FG-4592 appears to affect Hb selectively. Treatment with FG-4592 was not associated with an increase in platelet levels in the NDD study. Similarly, in the DD study, there was no increase in platelet levels in the low-dose and mid-dose FG-4592-treated cohorts. A small decrease in platelets levels was observed among FG-4592-treated subjects in the highest baseline tertile in the dialysis study. As ESAs are known to increase platelet levels [35], withdrawal of ESA and improved iron utilization with FG-4592 in the DD study may account for that particular change.
The effect of FG-4592 treatment on iron biomarkers is particularly noteworthy given that IV iron supplementation was prohibited in each of the studies. Serum iron levels were clinically stable in FG-4592 subjects despite robust erythropoiesis in the setting of hepcidin decrements. The comparison between this stability and the decline in serum iron seen in the group receiving epoetin alfa should be underscored in view of the fact that patients on dialysis have an almost universal reliance on IV iron [36, 37]. In the absence of IV iron, serum iron levels fell in epoetin alfa subjects and were significantly lower by EOT than serum iron levels in FG-4592 subjects. The ability of FG-4592 to also reduce hepcidin should be noted. Hepcidin is increased in inflammation and in CKD, blocking export from macrophages of iron that is required for RBC production [28, 38]. In fact, while optimal erythropoietic response to epoetin alfa requires IV iron, with oral iron found ineffective [39], in another Phase 2 study with FG-4592 for correction of anemia in incident dialysis patients, oral iron supplementation has been shown to be as effective with FG-4592 as IV iron [40]. Accordingly, FG-4592 may reduce the need for IV iron typically required to supplement ESA treatment, potentially limiting patient exposure to the documented safety liabilities [41] and costs of IV iron associated with ESA use.
FG-4592 lowered plasma cholesterol in subjects receiving FG-4592 in both the NDD and the DD trials. Cholesterol-lowering has been reported with high altitude exposure [42] and this result is consistent with results demonstrated in previously conducted roxadustat studies in healthy volunteers as well as NDD-CKD and DD-CKD patients [43]. The potential cholesterol-lowering effect of FG-4592 is independent of statin-use, and may be mediated, in part, by the effects of HIF on the degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase, a rate-limiting enzyme in cholesterol biosynthesis [44]. This effect is apparently greater in subjects with the highest baseline cholesterol levels. While cholesterol reduction with statins reduces major atherosclerotic events among patients with CKD [45], further study is required to determine if the cholesterol reduction mediated by FG-4592 also leads to cardiovascular benefits in CKD patients.
Taken together, these data suggest that roxadustat addresses multiple etiologic contributors to anemia in CKD patients with central common initiators such as inflammation and/or the failure to respond to hypoxia to the same degree as people with normal kidney function [26, 46]. It is of some interest that, in the two China Phase 2 studies presented here, the relative doses of FG-4592 used to correct anemia in advanced NDD-CKD patients (84% with eGFR <30 mL/min) were similar to those used to maintain a desired Hb level in DD patients. This observation is perhaps predictive of an easier dose transition between the two CKD states and FG-4592’s ability to overcome inflammation.
FG-4592 was well tolerated in these populations. No cardiovascular safety signals were noted in the FG-4592-treated patients. The adverse event experience was similar for FG-4592- and placebo-treated subjects in the blinded NDD trial. In the DD study, there were no cardiovascular SAEs, deaths or study drug-related SAEs in FG-4592-treated patients. It should be underscored that as with all Phase 2 studies, the safety data are limited in their ability to examine less common potential risks due to the small sample size and short-term follow-up [48]. A more conclusive safety assessment is being undertaken in the much larger Phase 3 trials currently underway.
In summary, 8 weeks of treatment with FG-4592 at a dose of 1.1–2.2 mg/kg TIW stimulated erythropoiesis and increased Hb without IV iron in Chinese NDD-CKD patients. Additionally, FG-4592 successfully maintained Hb levels over 6-weeks in Chinese DD patients, while epoetin alfa did not. Further, FG-4592 improved the functional delivery of iron to the erythron in NDD and in DD patients as reflected by stable iron levels, improved transferrin levels and increased MCV values. A potential role for hepcidin is suggested in the concurrent decline of levels of this master iron regulator in both NDD and DD subjects receiving FG-4592. The demonstrated potential safety and efficacy profile of FG-4592 and the advantages associated with use of an oral agent (rather than injectable ESAs) in improving patient access to treatment support further development in this large, underserved patient population.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Jessica Charpentier, Jenny Li and Patrick Jiang for managing study operations.
AUTHORS' CONTRIBUTIONS
FibroGen Inc. was the study sponsor that designed the study in consultation with the Principal Investigators (N.C. and J.Q.). All authors except those employed by the sponsor contributed patients to the study. FibroGen was responsible for data collection and analysis. All authors had full access to the study data and the analyses. S.H. served as lead medical writer and illustrator of this manuscript. All authors reviewed the manuscript and signed off on its accuracy.
CONFLICT OF INTEREST STATEMENT
D.N., C.L., S.H., L.S., A.B., T.B.N., K.P.Y. and F.H.V. are employees of FibroGen and hold stock and/or stock options in FibroGen. T.B.N. and K.P.Y. have patents regarding FG-4592 and HIF-PHIs. N.C., J.Q., C.M., C.H. and L.Z. received honoraria and nonfinancial support from FibroGen for speaking engagements and advisory board participation related to physician education. Institutions of N.C., J.Q., J.C., X.Y., C.M., H.L., C.H., G.J., L.Z., X.Z. and X.L. received funding from FibroGen to conduct the clinical study covered by the work herein. There are no other disclosures.
REFERENCES
- 1. US Center for Disease Control. National Chronic Kidney Disease Fact Sheet2014. http://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf (9 July 2016, date last accessed)
- 2. Zhang L, Wang F, Wang L. et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379: 815–822 [DOI] [PubMed] [Google Scholar]
- 3. Chen N, Wang W, Huang Y. et al. Community-based study on CKD subjects and the associated risk factors. Nephrol Dial Transplant 2009; 24: 2117–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins AJ, Ma JZ, Xia A. et al. Trends in anemia treatment with erythropoietin usage and patient outcomes. Am J Kidney Dis 1998; 32: S133–S141 [DOI] [PubMed] [Google Scholar]
- 5. Levin A. The treatment of anemia in chronic kidney disease: understandings in 2006. Curr Opin Nephrol Hypertens 2007; 16: 267–271 [DOI] [PubMed] [Google Scholar]
- 6. Mohanram A, Zhang Z, Shahinfar S. et al. The effect of losartan on hemoglobin concentration and renal outcome in diabetic nephropathy of type 2 diabetes. Kidney Int 2008; 73: 630–636 [DOI] [PubMed] [Google Scholar]
- 7. Lawler EV, Bradbury BD, Fonda JR. et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010; 5: 667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu L, Yang L, Wang M.. The clinical stage at which dialysis began in patients with non-diabetic end stage renal disease: a retrospective study. Chin J Blood Purif 2007; 6: 242–246 [Google Scholar]
- 9. Liu RQ, Chen LFJ, Chen H-X.. Retrospective study of baseline rebal function in hemodialysis patients before treatment at hemodialysis centers. Chinese Journal of Integrated Traditional and Western Nephrology 2013; 14: 326–328 [Google Scholar]
- 10. Zhang W, Gong Z, Peng X. et al. Clinical characteristics and outcomes of rural patients with ESRD in Guangxi, China: one dialysis center experience. Int Urol Nephrol 2010; 42: 195–204 [DOI] [PubMed] [Google Scholar]
- 11. Zhu L-N, Lv WI, Teng J. et al. Association of residual renal function at initiation of dialysis with prognosis in maintenance. Chin J Nephrol 2012; 28: 757–764 [Google Scholar]
- 12. Li Y, Shi H, Wang WM. et al. Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease: first multicenter, cross-sectional study. Medicine (Baltimore) 2016; 95: e3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. United States Renal Data System. Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013
- 14. Beusterien KM, Nissenson AR, Port FK. et al. The effects of recombinant human erythropoietin on functional health and well-being in chronic dialysis patients. J Am Soc Nephrol 1996; 7: 763–773 [DOI] [PubMed] [Google Scholar]
- 15. Besarab A, Bolton WK, Browne JK. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339: 584–590 [DOI] [PubMed] [Google Scholar]
- 16. Drueke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 17. Pfeffer MA, Burdmann EA, Chen CY. et al. Baseline characteristics in the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT). Am J Kidney Dis 2009; 54: 59–69 [DOI] [PubMed] [Google Scholar]
- 18. Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 19. Besarab A, Frinak S, Yee J.. What is so bad about a hemoglobin level of 12 to 13 g/dL for chronic kidney disease patients anyway? Adv Chronic Kidney Dis 2009; 16: 131–142 [DOI] [PubMed] [Google Scholar]
- 20. Fishbane S. Anemia and cardiovascular risk in the patient with kidney disease. Heart Fail Clin 2008; 4: 401–410 [DOI] [PubMed] [Google Scholar]
- 21. Szczech LA, Barnhart HX, Inrig JK. et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008; 74: 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unger EF. FDA Perspectives on ESAs for Anemia of Chronic Renal Failure: Hemoglobin Target and Dose Optimization Joint Meeting of the Cardiovascular and Renal Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee (11 September 2007). http://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4315s1-10-FDA-Unger_files/frame.htm (23 September 2016, date last accessed)
- 23. Zhang Y, Thamer M, Stefanik K. et al. Epoetin requirements predict mortality in hemodialysis patients. Am J Kidney Dis 2004; 44: 866–876 [PubMed] [Google Scholar]
- 24. Radtke HW, Claussner A, Erbes PM. et al. Serum erythropoietin concentration in chronic renal failure: relationship to degree of anemia and excretory renal function. Blood 1979; 54: 877–884 [PubMed] [Google Scholar]
- 25. Erslev AJ. Erythropoietin. N Engl J Med 1991; 324: 1339–1344 [DOI] [PubMed] [Google Scholar]
- 26. Babitt JL, Lin HY.. Mechanisms of anemia in CKD. J Am Soc Nephrol 2012; 23: 1631–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bernhardt WM, Wiesener MS, Scigalla P. et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol 2010; 21: 2151–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Provenzano R, Besarab A, Wright S. et al. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis 2016; 67: 912–924 [DOI] [PubMed] [Google Scholar]
- 29. Semenza GL, Agani F, Booth G. et al. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int 1997; 51: 553–555 [DOI] [PubMed] [Google Scholar]
- 30. Peyssonnaux C, Nizet V, Johnson RS.. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle 2008; 7: 28–32 [DOI] [PubMed] [Google Scholar]
- 31. Nangaku M, Kojima I, Tanaka T. et al. Novel drugs and the response to hypoxia: HIF stabilizers and prolyl hydroxylase. Recent Patents Cardiovasc Drug Discov 2006; 1: 129–139 [DOI] [PubMed] [Google Scholar]
- 32. Besarab A, Provenzano R, Hertel J. et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 2015; 30: 1665–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Provenzano R, Besarab A, Sun CH. et al. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor Roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol 2016; 11: 982–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levey AS, Bosch JP, Lewis JB. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 35. Eschbach JW, Abdulhadi MH, Browne JK. et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 1989; 111: 992–1000 [DOI] [PubMed] [Google Scholar]
- 36. Karaboyas A, Zee J, Morgenstern H. et al. Understanding the recent increase in ferritin levels in United States dialysis patients: potential impact of changes in intravenous iron and erythropoiesis-stimulating agent dosing. Clin J Am Soc Nephrol 2015; 10: 1814–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. KDIGO Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
- 38. Provenzano R, Goodkin D, Klaus S. et al. Evaluation of FG-4592, a novel oral hypoxia-inducible factor prolyl hydroxylase inhibitor, to treat anemia in hemodialysis patients. Am J Kidney Dis 2011; 57: B80 [Google Scholar]
- 39. Macdougall IC, Tucker B, Thompson J. et al. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int 1996; 50: 1694–1699 [DOI] [PubMed] [Google Scholar]
- 40. Besarab A, Chernyavskaya E, Motylev I. et al. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol 2016; 27: 1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bailie GR, Larkina M, Goodkin DA. et al. Data from the dialysis outcomes and practice patterns study validate an association between high intravenous iron doses and mortality. Kidney Int 2015; 87: 162–168 [DOI] [PubMed] [Google Scholar]
- 42. Ferezou J, Richalet JP, Coste T. et al. Changes in plasma lipids and lipoprotein cholesterol during a high altitude mountaineering expedition (4800 m). Eur J Appl Physiol Occup Physiol 1988; 57: 740–745 [DOI] [PubMed] [Google Scholar]
- 43. Besarab A, Leong R, Franco M. et al. FG-4592, a novel oral hypoxia-nducible factor stabilizer, raises hemoglobin in diabetic subjects with anemia of chronic kidney disease. Abstract # 5847 from the Scientific Sessions of the American Diabetes Association (21–25 June 2013), Chicago, IL
- 44. Nguyen AD, McDonald JG, Bruick RK. et al. Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase through accumulation of lanosterol and hypoxia-inducible factor-mediated induction of insigs. J Biol Chem 2007; 282: 27436–27446 [DOI] [PubMed] [Google Scholar]
- 45. Baigent C, Landray MJ, Reith C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377: 2181–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wenger RH, Hoogewijs D.. Regulated oxygen sensing by protein hydroxylation in renal erythropoietin-producing cells. Am J Physiol Renal Physiol 2010; 298: F1287–F1296 [DOI] [PubMed] [Google Scholar]
- 47. Koch GG, Amara IA, Stokes ME. et al. Categorical data analysis In: Berry DA. (ed).Statistical Methodology in Pharmaceutical Sciences. New York, NY: Marcel Dekker, 1990, 291–475 [Google Scholar]
- 48. Wenger RH, Hoogewijs D., Koury MJ. et al. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol 2015; 11: 394–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.