Abstract
Aims
Previous studies have identified sex disparities in the use of cardiac resynchronization therapy (CRT) and implantable cardioverter defibrillators (ICD), although the basis of underutilization in women remains poorly understood. The aim of this study was to assess sex differences in patterns of CRT use with our without ICD.
Methods and results
In this cross-sectional study using the National Inpatient Sample database we identified 311 009 patients undergoing CRT implantation in the United States between 2006 and 2012. Demographic and clinical characteristics were compared between men and women undergoing CRT implantation, with special attention to clinical predictors of left ventricular reverse remodelling (CRT response, score range: 0–4) and reduced ICD efficacy (score range: 0–7). When compared to men, women undergoing CRT implantation were significantly more likely to have ≥ 3 predictors of CRT response (47.3 vs. 33.2%, P < 0.001) and less likely to have ≥3 predictors of reduced ICD efficacy (27.0 vs. 37.3%, P < 0.001). Despite this, men were significantly more likely to undergo CRT with ICD (CRT-D) as the type of CRT (88.6 vs. 80.1% of all CRT implants). Compared to those with the greatest likelihood of CRT response (score ≥ 3), those with the least likelihood of CRT response had a significant decreased odds of CRT-D implant (adj odds ratio 0.27 [0.24–0.31], P < 0.001), with a greater decreased odds in women compared to men (P, for sex interaction <0.001). The difference in the % of CRT-D implant in men vs. women increased over the study period (P, sex Δ time trend = 0.012).
Conclusion
In this large, contemporary cohort, sex differences in CRT-D implantation were inversely related to predicted CRT efficacy and have increased over time. Future efforts to narrow the gap in CRT-D implantation in men and women may help better align device selection with those most likely to benefit.
Keywords: Cardiac resynchronization therapy , Implantable cardioverter-defibrillator , Sex disparities
Introduction
Cardiac resynchronization therapy (CRT) and implantable cardioverter-defibrillator (ICD) therapy each improve survival in appropriately selected patients with heart failure.1 Implantable cardioverter-defibrillator therapy reduces the risk of sudden arrhythmic death, whereas the mortality benefit of CRT is related, in part, to favourable left ventricular reverse remodelling with related attenuation of both heart failure death as well as ventricular arrhythmias.2,3 As guideline indications for each device therapy frequently overlap,4 ICD implantation is often performed in conjunction with CRT (CRT-D).5
Despite the overall efficacy of CRT and ICD therapy, there is an expanding recognition that their benefit may not be uniform. For example, the survival benefit of ICD therapy may be nullified in the context of increasing comorbidity burden and competing modes of non-arrhythmic death.6 Likewise, the efficacy of CRT may be attenuated in patients with comorbidities which mitigate effective pacing (e.g. atrial fibrillation, AF) or blunt remodelling (e.g. ischaemic cardiomyopathy).7 Of the myriad predictors of CRT efficacy, female sex has been consistently associated with greater reverse remodelling and improved survival.8 Despite this, women are significantly less likely than men to undergo CRT and ICD implantation,9 although the basis for this underutilization remains poorly understood.
In this study, we utilized the National Inpatient Sample to evaluate sex differences in 311 009 individuals undergoing inpatient CRT implant in the United States between 2006 and 2012. Our aims were to (1) assess sex differences in CRT implant type (CRT-D, CRT-P) stratifying by predictors of device efficacy and (2) identify and compare predictors of CRT implant type in men and women. (3) Finally, we examine temporal trends in CRT device selection stratified by sex, with comparison to sex-specific trends in 423 507 individuals undergoing ICD-only implantation.
Methods
Data source
The National Inpatient Sample (NIS) is the largest publicly available all-payer inpatient health care database in the United States, yielding national estimates of diagnoses, procedure utilization, and outcomes for hospital inpatient stays.10 The database contains a nationally representative sample from more than 7 million hospitalizations annually. Applying sample weights provided by NIS, the database projects estimates for more than 36 million hospitalizations annually. The NIS provides data on patient demographics, in-hospital clinical outcomes, hospital characteristics, and hospital charges. Federal hospitals are not included in the NIS. Quality control and validation of the NIS are performed by the Agency for Healthcare Research and Quality (AHRQ; Rockville, MD). The database was provided with de-identified patient information and thus was deemed exempt from institutional review by the Human Research Committee at Massachusetts General Hospital.
Study sample
We identified all adults ≥18 years old who received a CRT device during a hospitalization between 1 January 2006 and 31 December 2012. CRT was defined by the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes for CRT-P (00.50) and CRT-D (00.51). These two ICD-9-CM codes identify procedures where a total CRT system is implanted and do not identify CRT generator replacement. Sex was documented on all except 77 patients (99.98%). In sensitivity analysis, we additionally examined all adults ≥ 18 years old who underwent ICD implantation (excluding CRT-D) during the same time period. ICD was defined by ICD-9-CM code 37.94.
Covariates and device efficacy scores
Demographic covariates of interest included age, sex, race/ethnicity, primary insurance type (Medicare, Medicaid, Private, and other), and income quartile. Race/ethnicity was reclassified as non-Hispanic white, non-Hispanic black, other (Hispanic, Asian, and Native American), and missing. Other covariates included hospital type (rural, urban non-teaching, and urban teaching), type of admission (elective vs. acute), geographic region, and year of hospital discharge. Cardiovascular comorbidities were identified by ICD-9-CM codes and included coronary artery disease, non-ischaemic cardiomyopathy, history of ventricular arrhythmia or cardiac arrest, AF, left bundle branch block (LBBB), and complete atrio-ventricular (AV) block. Non-cardiovascular comorbidity burden was assessed using the Elixhauser comorbidity measure, which was originally developed using administrative data relying on the ICD-9-CM coding manual for 30 comorbidities.11 In this study, Elixhauser comorbidities were generated from ICD-9-CM diagnosis codes using the AHRQ Comorbidity Software.12 Of note, cardiomyopathy, chronic kidney disease, and history of ventricular arrhythmia were removed from the comorbidity score in order to be analysed independently, and hence, the total number of Elixhauser comorbidities analysed was 27. ICD-9-CM codes used in the data analysis are listed in (see Supplementary material online, Table S1).
To assess the impact of predicted device efficacy on type of CRT implantation, we generated two morbidity-based scores previously shown to be associated with CRT and ICD efficacy. An ICD efficacy score was comprised of seven comorbidities (ischaemic heart disease, chronic kidney disease, diabetes, chronic pulmonary disease, AF, peripheral vascular disease, tobacco use) previously shown to be associated with reduced ICD efficacy in patients with heart failure.6,13,14 For this study, the ICD efficacy score (range 0–7) was reclassified as 0, 1–2, ≥3 given previous studies suggesting that the survival benefit of ICD implant was nullified in the presence of ≥ 3 of the identified comorbidities.6 A CRT response score was comprised of four clinical conditions known to be associated with left ventricular reverse remodelling after CRT implant: non-ischaemic heart disease, LBBB, absence of chronic kidney disease, and absence of AF.8,15,16 The sum of the CRT response variables (range 0–4) was classified as 0, 1–2, or ≥3. While female sex is an established predictor of reverse remodelling following CRT,8 we excluded sex from the CRT response score to allow for direct comparability of CRT response scores between men and women. Exclusion of sex from the CRT response score would underestimate the likelihood of reverse remodelling in women, therefore yielding a conservative estimate by biasing any identified sex differences towards the null.
Statistical analyses
Frequencies, proportions and 95% confidence intervals (CI) were calculated and weighted to reflect national estimates using inverse sampling weights provided by NIS. χ2 tests were used to compare the demographic and clinical characteristics between men and women. Additionally, χ2 tests were used to compare the unadjusted percentage of CRT-D use between female and male patients stratified by different clinical factors. Multivariable logistic regression was used to identify predictors of CRT-D use vs. CRT-P and included demographic, geographic, and clinical covariates (including the CRT response score) detailed above, as well as calendar year (treated as a continuous variable). In addition to the multivariable logistic regression model for the total cohort, separate logistic regression models were fit for each sex in order to show differences in the magnitude of association between covariates and the odds of CRT-D implant in men vs. women. To assess for sex differences in the covariates predictive of CRT-D implantation, covariate-sex interactions were included in the multivariable logistic model. All analyses were performed using survey procedures in SAS version 9.4 (SAS Institute, Cary, NC). A 2-tailed P < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
The baseline characteristics of the 311 009 inpatient CRT implants between 2006 and 2012 in the United States are shown in Table 1. In keeping with a contemporary cohort of patients undergoing CRT, the majority of the study cohort was ≥ 65 years of age (N = 222 696; 71.6%). The most prevalent non-heart failure comorbidities were hypertension, diabetes mellitus, and chronic kidney disease. Approximately one-third of patients had ≥ 3 comorbidities associated with reduced ICD efficacy and a similar proportion had ≥ 3 comorbidities associated with CRT response.
Table 1.
Characteristics of patients undergoing CRT implantation, stratified by sex
| All | Female | Male | P-value | |
|---|---|---|---|---|
| (n = 311 009) | (n = 92 126) | (n = 218 883) | ||
| Age category | 0 .002 | |||
| 18–54 | 32 953 (10 .6) | 10 016 (10 .9) | 22 937 (10 .5) | |
| 55–64 | 55 360 (17.8) | 16 166 (17.5) | 39 194 (17.9) | |
| 65–74 | 95 763 (30 .8) | 27 786 (30 .2) | 67 977 (31.1) | |
| 75–84 | 102 047 (32.8) | 30 254 (32.8) | 71 792 (32.8) | |
| >85 | 24 886 (8.0) | 7903 (8.6) | 16 982 (7.8) | |
| Race | <0 .001 | |||
| White | 196 786 (63.3) | 54 702 (59.4) | 142 084 (64.9) | |
| Black | 27 584 (8.9) | 10 776 (11.7) | 16 808 (7.7) | |
| Othera | 27 600 (8.9) | 8795 (9.5) | 18 805 (8.6) | |
| Unknown | 59 039 (19.0) | 17 852 (19.4) | 41 187 (18.8) | |
| Primary payer | <0 .001 | |||
| Medicare | 225 428 (72.5) | 67 457 (73.2) | 157 971 (72.2) | |
| Medicaid | 14 861 (4.8) | 5374 (5.8) | 9487 (4.3) | |
| Private | 59 977 (19.3) | 16 524 (17.9) | 43 453 (19.9) | |
| Otherb | 10 338 (3.3) | 2652 (2.9) | 7686 (3.5) | |
| Low income quartile | 80 855 (26.0) | 26 183 (28.4) | 54 671 (25.0) | <0 .001 |
| Region | <0 .001 | |||
| Northeast | 60 734 (19.5) | 16 833 (18.3) | 43 901 (20 .1) | |
| Midwest | 81 012 (26.0) | 24 653 (26.8) | 56 359 (25.7) | |
| South | 117 156 (37.7) | 35 909 (39.0) | 81 246 (37.1) | |
| West | 52 107 (16.8) | 14 730 (16.0) | 37 377 (17.1) | |
| Hospital location/teaching | 0 .076 | |||
| Rural | 12 334 (4.0) | 3904 (4.2) | 8430 (3.9) | |
| Urban, non-teaching | 111 393 (35.8) | 32 466 (35.2) | 78 927 (36.1) | |
| Urban, teaching | 185 034 (59.5) | 55 117 (59.8) | 129 917 (59.4) | |
| Elective admission | 157 275 (50 .6) | 46 533 (50 .5) | 110 742 (50 .6) | 0 .804 |
| CHD and Arrhythmic History | ||||
| Coronary disease | 204 248 (65.7) | 47 311 (51.4) | 156 937 (71.7) | <0 .001 |
| Nonischaemic cardiomyopathy | 133 107 (42.8) | 49 417 (53.6) | 83 691 (38.2) | <0 .001 |
| Left bundle branch block | 103 625 (33.3) | 36 142 (39.2) | 67 483 (30 .8) | <0 .001 |
| Ventricular arrhythmia | 77 926 (25.1) | 18 473 (20 .1) | 59 453 (27.2) | <0 .001 |
| Atrial fibrillation | 116 400 (37.5) | 32 257 (35.0) | 84 243 (38.5) | <0 .001 |
| Complete AV block | 28 083 (9.0) | 8455 (9.2) | 19 628 (9.0) | 0 .382 |
| Comorbidities | ||||
| Diabetes | 102 483 (33.0) | 29 820 (32.4) | 72 663 (33.2) | 0 .038 |
| Chronic kidney disease | 64 714 (20 .8) | 16 110 (17.5) | 48 604 (15.6) | <0 .001 |
| Chronic lung disease | 65 309 (21.0) | 20 033 (21.7) | 45 276 (20 .7) | 0 .007 |
| Anemia | 33 404 (10 .7) | 11 332 (12.3) | 22 072 (10 .1) | <0 .001 |
| Hypertension | 180 764 (58.1) | 54 028 (58.6) | 126 736 (57.9) | 0 .082 |
| Peripheral vascular disease | 27 789 (8.9) | 6364 (6.9) | 21 425 (9.8) | <0 .001 |
| Tobacco use | 60 481 (19.4) | 13 807 (15.0) | 46 673 (21.3) | <0 .001 |
| Reduced ICD efficacy score ≥3c | 106 558 (34.3) | 24 919 (27.0) | 81 639 (37.3) | <0 .001 |
| CRT response score ≥3d | 116 223 (37.4) | 43 560 (47.3) | 72 663 (33.2) | <0 .001 |
Percentages may not sum to 100 given missing data.
AV, atrio-ventricular; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator.
Includes Hispanic, Asian or Pacific Islander, Native American, and other.
Includes self-pay, no charge, and other.
Comprised of seven comorbidities associated with increased mortality and reduced ICD efficacy in patients with heart failure: ischaemic heart disease, atrial fibrillation, diabetes mellitus, chronic lung disease, chronic kidney disease, peripheral vascular disease, and smoking. Presence of ≥ 3 comorbidities previously shown to negate survival benefit of primary prevention ICD 6.
Comprised of four clinical covariates associated with left ventricular reverse remodeling and improved clinical outcomes following CRT: non-ischaemic cardiomyopathy, left bundle branch block, absence of chronic kidney disease, and absence of atrial fibrillation.
When stratified by sex, there were significant differences in demographic and clinical characteristics (Table 1). Men undergoing CRT were more likely to be white (64.9 vs. 59.4%, P < 0.001) and were more likely to be undergoing ICD for secondary prevention (i.e. history of ventricular arrhythmia and/or cardiac arrest) (27.2 vs. 20.1%, P < 0.001) compared to women. In contrast, women were more likely to have ≥ 3 predictors of CRT response (47.3 vs. 33.2%, P < P < 0.001) including non-ischaemic cardiomyopathy (53.6 vs. 38.2%, P < 0.001) and were less likely to have ≥ 3 comorbidities associated with reduced ICD efficacy (27.0 vs. 37.3%, P < 0.001).
Sex differences in use of CRT-D
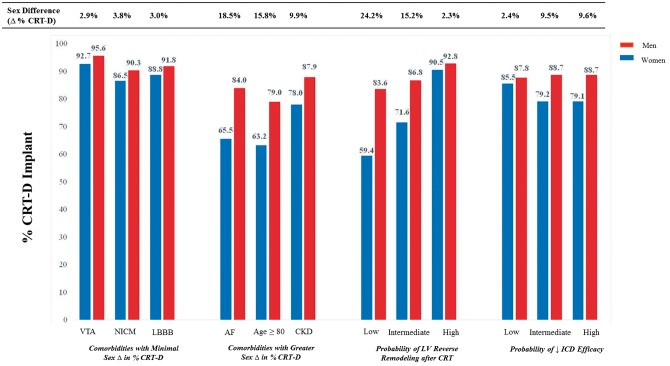
In the total cohort, there was a higher percentage of CRT-D use in men compared to women (88.6 vs. 80.1%, P < 0.001) and this excess proportion was consistently present in multiple subgroups (Table 2). The majority of both men and women with a secondary prevention indication for ICD (i.e. ventricular arrhythmia or cardiac arrest) underwent CRT-D implant (95.6 vs. 92.7%) and the sex difference in % CRT-D implant was similarly small in those with non-ischaemic cardiomyopathy and LBBB. In contrast, the difference in percentage of CRT-D use in men vs. women was greatest in those with AF (absolute Δ: +18.6 [17.0 to 20.3]%) and was also increased in those with chronic kidney disease and those ≥ 80 years of age (Table 2; Figure 1).
Table 2.
Percentage of CRT implants with ICD (CRT-D), stratified by sex
| % CRT-D |
|||
|---|---|---|---|
| Female | Male | Difference, % (95% CI)a | |
| (n = 73 822) | (n = 194 016) | ||
| Overall | 80.1 | 88.6 | 8.5 (7.6–9.4) |
| Age | |||
| < 80 years | 85.3 | 91.4 | 6.2 (5.3–7.0) |
| ≥ 80 years | 63.2 | 79.0 | 15.8 (14.0–17.6) |
| Type of admission | |||
| Elective | 82.3 | 89.7 | 7.4 (6.3–8.6) |
| Acute | 77.9 | 87.6 | 9.7 (8.4–10.9) |
| Cardiac history | |||
| Ventricular arrhythmia | 92.7 | 95.6 | 2.9 (2.0–3.9) |
| Non-ischaemic cardiomyopathy | 86.5 | 90.3 | 3.8 (3.0–4.7) |
| Left bundle branch block | 88.8 | 91.8 | 3.0 (2.2–3.9) |
| Atrial fibrillation | 65.5 | 84.0 | 18.6 (17.0–20.3) |
| Complete AV block | 59.1 | 73.8 | 14.6 (11.9–17.4) |
| Chronic kidney disease | 78.0 | 87.9 | 10.0 (8.4–11.5) |
| Reduced ICD Efficacyb | |||
| 0 | 85.5 | 87.8 | 2.4 (0.6–4.1) |
| 1–2 | 79.2 | 88.7 | 9.5 (8.3–10.7) |
| ≥3 | 79.1 | 88.7 | 9.6 (8.3–10.7) |
| CRT response variablesc | |||
| 0 | 59.4 | 83.6 | 24.2 (19.9–28.6) |
| 1–2 | 71.6 | 86.8 | 15.2 (13.8–16.7) |
| ≥3 | 90.5 | 92.8 | 2.3 (1.6–3.1) |
All comparisons between female and age male had P < 0.05.
Comprised of seven comorbidities associated with increased mortality and reduced ICD efficacy in patients with heart failure: ischaemic heart disease, atrial fibrillation, diabetes mellitus, chronic lung disease, chronic kidney disease, peripheral vascular disease, and smoking.
Comprised of four conditions associated with an increased likelihood of left ventricular reverse remodeling following CRT: non-ischaemic cardiomyopathy, left bundle block, absence of chronic kidney disease, and absence of atrial fibrillation.
Figure 1.
Sex differences in CRT-D implantation: impact of demographics, comorbidities, and predicted device efficacy. Shown is the percentage of CRT implantation with concurrent ICD (CRT-D) in men compared to women within demographic and clinical subgroups of interest. Highlighted is the sex difference in % CRT-D in those with LBBB, NICM, or prior VTA compared to the sex differences amongst those with AF, age ≥ 80 years, and CKD. As comorbidities are known to influence device efficacy, sex differences in CRT-D implantation are shown as stratified by predicted probability of LV reverse remodelling following CRT and probability of diminished ICD efficacy. CRT, cardiac resynchronization therapy; CRT-D, CRT with implantable cardioverter defibrillator; CRT-P, CRT with pacemaker; AF, atrial fibrillation; LBBB, left bundle branch block; NICM, non-ischaemic cardiomyopathy; VTA, ventricular tachyarrhythmia; CKD, chronic kidney disease; LV, left ventricular.
We next assessed unadjusted sex differences in CRT-D implant within strata of predicted device efficacy (Table 2). Amongst those with the greatest predicted CRT efficacy (i.e. CRT response score ≥3), more than 90% of men and women underwent CRT-D implant (absolute Δ: +2.3 [1.6–3.1]%). Likewise, in those with the greatest predicted benefit from ICD implant (i.e. reduced ICD efficacy score of 0), more than 85% underwent CRT-D implant with a similarly minimal difference between sexes (Δ, men vs. women: +2.4 [0.6–4.1]%). In contrast, the sex difference in % CRT-D increased significantly in those with decreased predicted device efficacy. For example, the absolute difference in percentage of CRT-D use in men vs. women was +15.2 [13.8 to 16.7]% in those with 1–2 CRT response predictors and +24.2 [19.9–28.6]% in those with 0 CRT response predictors (Figure 1). Likewise, the sex difference increased in those predicted to have reduced ICD benefit (e.g. Δ, men vs. women with reduced ICD efficacy score ≥3: +9.6 [8.3–10.7]%).
Predictors of CRT-D in men compared to women
We next assessed multivariable predictors of CRT implant type in men compared to women. As shown in Table 3, demographic (younger age, black race), geographic (Northeast region), temporal (calendar year), and clinical predictors (history of ventricular arrhythmia, coronary artery disease) of CRT-D were similar in men compared women in multivariable-adjusted models. In contrast, the impact of predicted CRT response on the odds of CRT-D implant was significantly different in men compared to women, even after multivariable-adjustment. When compared to those with ≥ 3 CRT response predictors, the presence of 0 response predictors was associated with a 60% decreased odds of CRT-D in men (odds ratio (OR) 0.40 [0.34 to 0.46]) and an 84% decreased odds of CRT-D in women (OR 0.16 [0.13 to 0.19]) (P, for sex interaction <0.001).
Table 3.
Multivariable predictors of CRT-D implantation
| CRT-D All | CRT-D Female | CRT-D Male OR (95% CI) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Male | 1.80 (1.71–1.90) | - | - |
| Calendar Yeara | 0.90 (0.88–0.93) | 0.88 (0.86–0.91) | 0.92 (0.89–0.94) |
| Age | |||
| 18–54 | ref | Ref | ref |
| 55–64 | 0.92 (0.81–1.04) | 0.99 (0.81–1.21) | 0.87 (0.75–1.01) |
| 65–74 | 0.65 (0.57–0.74) | 0.70 (0.57–0.86) | 0.61 (0.53–0.71) |
| 75–84 | 0.39 (0.34–0.44) | 0.38 (0.31–0.47) | 0.38 (0.33–0.44) |
| ≥85 | 0.12 (0.11–0.14) | 0.12 (0.09–0.15) | 0.12 (0.10–0.15) |
| Race | |||
| White | ref | ref | ref |
| Black | 1.61 (1.40–1.86) | 1.79 (1.48–2.16) | 1.48 (1.26–1.75) |
| Otherb | 1.10 (0.97–1.25) | 1.20 (1.00–1.44) | 1.05 (0.91–1.21) |
| Primary payer | |||
| Private | Ref | Ref | Ref |
| Medicare | 1.16 (1.07–1.26) | 1.09 (0.94 -1.26) | 1.20 (1.09 -1.31) |
| Medicaid | 1.25 (1.06–1.47) | 1.17 (0.93–1.47) | 1.30 (1.05–1.61) |
| Otherc | 0.95 (0.79–1.13) | 0.91 (0.69–1.20) | 0.97 (0.79–1.20) |
| Elective admission | 1.30 (1.20–1.40) | 1.24 (1.11–1.38) | 1.32 (1.22–1.43) |
| Region | |||
| Midwest | ref | ref | ref |
| Northeast | 1.65 (1.33–2.07) | 1.76 (1.39–2.28) | 1.59 (1.25–2.03) |
| South | 1.06 (0.87–1.30) | 1.09 (0.88–1.35) | 1.04 (0.84–1.29) |
| West | 1.06 (0.88–1.27) | 1.00 (0.82–1.23) | 1.09 (0.89–1.33) |
| Hospital location/teaching | |||
| Urban, teaching | ref | ref | ref |
| Urban, non-teaching | 1.14 (0.98–1.32) | 1.11 (0.94–1.32) | 1.16 (0.99–1.35) |
| Rural | 0.60 (0.33–1.08) | 0.52 (0.29–0.96) | 0.65 (0.36–1.18) |
| Coronary artery disease | 2.60 (2.43–2.77) | 2.60 (2.37–2.85) | 2.58 (2.37–2.81) |
| Ventricular arrhythmia | 3.71 (3.36–4.10) | 3.93 (3.38–4.56) | 3.62 (3.22–4.06) |
| Elixhauser indexd | 0.96 (0.94–0.99) | 0.96 (0.93–0.99) | 0.97 (0.94–1.00) |
| CRT response variablese | |||
| 0 | 0.27 (0.24–0.31) | 0.16 (0.13–0.19) | 0.40 (0.35–0.47) |
| 1–2 | 0.35 (0.32–0.37) | 0.24 (0.21–0.27) | 0.47 (0.43–0.52) |
| ≥3 | ref | ref | ref |
2006–12.
Includes Hispanic, Asian or Pacific Islander, Native American, and other.
Includes self-pay, no charge, and other.
Measures 27 comorbidities of the Elixhauser index.
Comprised of four conditions associated with an increased likelihood of left ventricular reverse remodeling following CRT: non-ischaemic cardiomyopathy, left bundle block, absence of chronic kidney disease, and absence of atrial fibrillation.
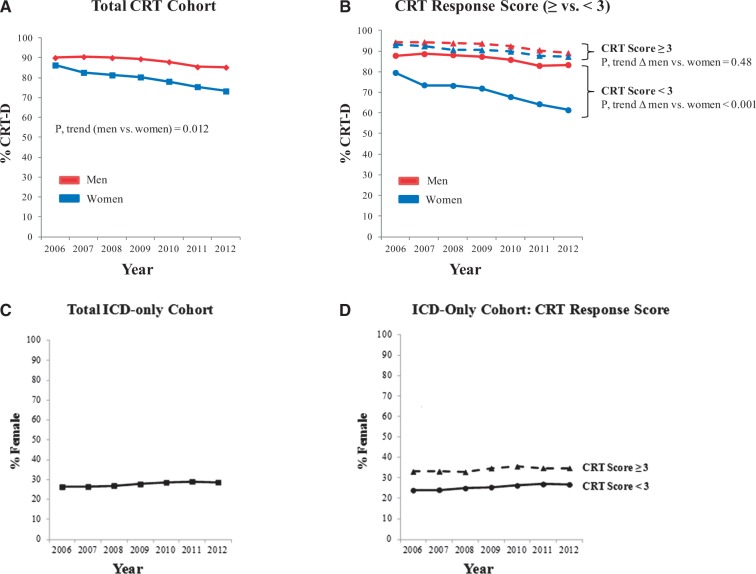
Temporal trends in CRT-D implantation by sex
Finally, we assessed temporal trends in CRT implant type in men compared to women, with parallel assessment of sex trends in patients undergoing ICD-only (Figure 2). Baseline characteristics of patients undergoing CRT-D, CRT-P, and ICD-only are shown in (see Supplementary material online, Table S2). As expected, there was a greater prevalence of LBBB in CRT recipients compared to ICD-only, and a greater proportion of ICD-only recipients had a secondary prevention indication (N= 204 701 [48.3%]) compared to CRT-D recipients (N= 73 945 [27.6%]). Importantly, the prevalence of comorbidities associated with reduced ICD efficacy was similar across all groups (Reduced ICD Efficacy Score ≥ 3—ICD-only: 32.1%, CRT-D: 34.4%, CRT-P: 33.4%).
Figure 2.
Secular trend in the percentage of CRT devices implanted with an ICD in men vs. women between 2006 and 2012.(A) Panel A shows the percentage of CRT-D implant for the total CRT cohort over the study period, stratified by sex. The p-value shown is for the comparison of time trend in women vs. men. (B) Panel B shows % CRT-D implant in men and women, further stratified by likelihood left ventricular reverse remodelling following CRT as defined by the presence of the following predictors: non-ischaemic cardiomyopathy, left bundle branch block, absence of atrial fibrillation, absence of chronic kidney disease (CRT response score, rage: 0–4). Sex differences are shown within strata of CRT response score (≥ vs. < 3). Within each subgroup of CRT response score (≥ vs. < 3), the p-value shown is for the comparison of time trend in women vs. men. Of recipients undergoing ICD-only during the same time period, Panels C and D show the % women in the total ICD-only cohort (C) and stratified by CRT response score (D).
Over the study period, between 2006 and 2012, there was a greater decrease in the percentage of CRT-D implants for women (2006 vs. 2012: 86.3% vs. 73.2%) compared to men (90.0 vs. 85.2%) (P, for sex Δ in time trend = 0.012) (Figure 2A). When stratified by the likelihood of CRT response, the percentage of CRT-D implant decreased significantly over the study period for women with a CRT response score < 3 (2006 vs. 2012: 79.5% vs. 61.4%) but not for men (2006 vs. 2012: 87.8% vs. 83.3%) (P, for sex Δ in time trend <0.001) (Figure 2B). In contrast, the percentage of CRT-D implants in those with ≥ 3 CRT response predictors remained high for both women and men over the study period (P, for sex Δ in time trend = 0.48). Over the same time period, the proportion of women amongst ICD-only recipients was unchanged (Figure 2C), including when stratified by CRT response score (Figure 2D).
Discussion
In this study of greater than 300 000 CRT implants in the US between 2006 and 2012, men were significantly more likely to undergo CRT-D implant compared to women. Sex differences in CRT-D implantation were sensitive to comorbidities known to impact device efficacy. We find that sex differences increased significantly over the study period, despite greater predicted CRT and ICD efficacy in women compared to men. Taken together, these data highlight an expanding sex disparity in CRT implant type in the USA, and further identify potential opportunities to narrow this gap in order to better align device selection with those most likely to benefit.
There is an expanding recognition that CRT and ICD efficacy may not be uniform in men compared to women. First, left ventricular reverse remodelling is postulated to be an important mechanism of survival benefit in CRT.3 In our study, women were significantly more likely to have clinical characteristics associated with a greater likelihood of reverse remodelling, which is consistent with previous findings that women have improved survival following CRT implant compared to men.17 Second, ICD benefit may be attenuated in the presence of competing modes of death and comorbidities associated with these competing risks may differ in men and women.13,14 In our study, women undergoing CRT were more likely to have a comorbidity profile associated with CRT and ICD benefit, which is in keeping with the sex distribution of clinical characteristics described in other contemporary CRT cohorts, including the European CRT Survey.18 Consistent with these findings, others have identified similar and potentially superior survival following ICD implant in women compared to men.19
Our group5 has previously identified the presence of a sex differences in CRT implant patterns in the US and others have highlighted similar findings in Europe.20 Our findings extend these previous observations in several ways. First, we assessed sex differences in several clinically relevant subgroups. In keeping with contemporary guidelines,4 we observed consistently high and similar percentage of CRT-D device use among men and women with a secondary prevention indication for ICD (i.e. ventricular arrhythmia or cardiac arrest). Likewise, there was similarly minimal sex differences in patients with non-ischaemic cardiomyopathy. In contrast, we identified significant sex differences in CRT-D implant use in the presence of other relevant subgroups including AF, chronic kidney disease, and the elderly. We would further highlight that sex remained a significant predictor of CRT device type even after adjusting for ICD indication and clinical risk factors, including non-ischaemic cardiomyopathy. Second, we explore the clinical implications of the identified sex differences by leveraging previous reports linking comorbidities with device efficacy.6,8 While >90% of both men and women predicted to have high likelihood CRT response underwent CRT-D implantation, the difference in CRT-D use in men vs. women increased significantly in those less likely to respond to CRT. Similarly, there were minimal sex differences in CRT-D implantation amongst those with the greatest predicted ICD efficacy, but a widening disparity in those with lower predicted ICD efficacy. By coupling comorbidity-specific sex differences in CRT-D implant with the established relationship between comorbidities and device efficacy, our findings highlight the clinical implications of these sex disparities.
We also show that the sex disparity in CRT implant type increased over the study period, and specifically in those predicted to have a lower likelihood of CRT response. Our temporal trend findings extend findings from a recent report which demonstrated static sex differences in ICD implantation between 2005 and 2009 in the US.9 In contrast to previous ICD-only studies,9 we leverage the simultaneous presence of patients with and without ICD implantation (i.e. CRT-D vs. CRT-P) available in the National Inpatient Sample database. As an internal control, we show that there was no evidence that our findings of a widening disparity were related to a secular decrease in ICD-only implantation amongst women. The similar distribution of comorbidities in ICD-only and CRT-D recipients suggests that our findings may be applicable to ICD-only recipients, generally, although additional investigation in ICD-eligible HF patients appears warranted.
Maximizing the benefit of CRT and ICD therapy in the ever-expanding population of patients with heart failure will require improved alignment of device implantation with those most likely to benefit. The complexity of decision-making regarding ICD implantation at the time of CRT is further increased given that CRT associated improvements in LV function may attenuate both sudden arrhythmic death as well as non-sudden cardiac death.2 Indeed, despite the established efficacy of ICD therapy in appropriately selected patients, there remains no direct comparison establishing the incremental efficacy of CRT-D compared to CRT-P alone.21 While CRT-related improvements in left ventricular function may attenuate risk of ventricular arrhythmias, we would note that anticipated ventricular response to CRT is not represented in guidelines regarding ICD implantation,4 and that patients with reverse remodelling remain at risk for ventricular arrhythmias.22 In addition, CRT-related reverse remodelling may also modify the risk of other ‘competing modes of death’ (e.g. heart failure death)2 which, in turn, could influence the potential lifetime survival benefit of ICD therapy.23 Taken together, our findings highlight the ongoing need to refine our understanding of the dynamic influence of CRT on modes of death after implant and furthermore, how such anticipated responses may influence sex-specific decision making at the time of CRT implant.
Contemporary implant practices regarding CRT device selection differ by region, with a greater proportion of CRT-D implant in the US as compared to Europe.5,24 As suggested by the recent report from the CeRtiTuDe registry which identified an excess of non-arrhythmic death in those undergoing CRT-P,25 ICD device selection should consider the competing risk of non-arrhythmic deaths. Indeed, contemporary implant practices suggest significant opportunities for improvement. For example, Kramer and colleagues recently showed that approximately half of US Medicare beneficiaries undergoing ICD implantation enrol in hospice or die within 5 years of implant.26 Likewise, the recent Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischaemic Systolic Heart Failure (DANISH) failed to find ICD benefit in the elderly (age > 59 years).27 Finally, the inclusion of an ICD in those undergoing CRT has significant clinical implications including the risk of device complications (∼15% at 3 years), increased healthcare utilization (battery depletion/need for generator change), and the deleterious clinical impact of inappropriate ICD therapy.28
Viewed in this context, our findings highlight potentially divergent opportunities to ‘narrow the gap’ for more effective deployment of CRT-D. For example, in those with a low likelihood of ICD efficacy, the sex gap may be appropriately addressed by decreasing CRT-D implants in men unlikely to benefit (e.g. ≥ age 80 with ischaemic heart disease and chronic kidney disease). Conversely, the sex disparity in CRT-D implantation for those with an intermediate probability of CRT response (e.g. 1-2 CRT response predictors) may be best addressed by targeted augmentation of CRT-D implant in women. Our data also highlight the need to better understand the influence of comorbidities and predicted device efficacy on patient-physician decision making at the time of device selection. Emerging literature supports the possibility of sex-specific differences in physician attitudes and patient preferences regarding trading quality of life and survival time.29 Whether specific comorbidities influence such quality of life preferences differentially may warrant additional exploration.
Limitations
The findings of this study should be interpreted within the limitations of its study design. First, these data represent serial cross-sectional assessments and availability of longitudinal clinical and echocardiographic outcomes would offer a more robust understanding of the potential clinical consequences of the identified differences. Second, while there remains no consensus definition for CRT response, left ventricular reverse remodelling is a robust and specific metric of biventricular pacing therapy.1 The selected measures of CRT response in our study have been consistently identified in systematic reviews and post-hoc analyses of randomized controlled trials in CRT.1,7 However, some proposed predictors of echocardiographic response after CRT implantation were not available (e.g. QRS duration, left ventricular lead location) and would have enhanced our ability to stratify the likelihood of reverse remodelling. To the extent that specific measures highlighted in recent consensus guidelines may identify greater device benefit in women as compared to men (e.g. QRS duration < 150 ms),30 our findings would only be underestimating the sex disparities present within strata of predicted CRT efficacy. Third, our analyses relied on using ICD-9-CM diagnosis codes with known high specificity and lower sensitivity.31 This likely explains the lower than expected prevalence of certain morbidities (e.g. 33% prevalence of LBBB). Other lifestyle factors considered in our study (e.g. tobacco smoking) have shown significant correlation with direct survey methods.32 In addition, certain baseline characteristics (e.g. race) were unknown in a significant minority of patients, although we anticipate that missingness was random with respect to sex. Both non-differential misclassification and underestimation of comorbidity prevalence would have biased our findings towards the null. We acknowledge that there may be heterogeneity in CRT indication in men as compared to women (e.g. bundle branch block morphology, cardiomyopathy etiology). For this reason, we assessed sex differences within strata of potential phenotypic heterogeneity. Finally, guidelines regarding CRT implantation were updated after the latest date considered in our study4 and our findings do not reflect any potential impact of these changes on sex disparity in CRT implant type.
Conclusions
In this contemporary cohort reflecting more than 300 000 CRT implants between 2006 and 2012, women were significantly less likely to undergo CRT-D implant compared to men despite greater predicted device efficacy. Amongst those with a lower predicted benefit from ICD, there was an excess of CRT-D implant in men. An improved understanding of the determinants of CRT device selection in men compared to women would help maximize the benefit of these devices for our ever-expanding population of patients with heart failure.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors would like to thank the Institute for Quantitative Social Science at Harvard University for providing access to cluster computing.
Funding
National Heart Lung and Blood Institute (T-32 HL-007575 to N.A.C.), Junior Faculty Career Development Award from the National Palliative Care Research Center (C.L.), Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Institutes of Health Award UL1 TR001102), and financial contributions from Harvard University and its affiliated academic medical centres.
Conflict of interest: J.P.S. receives consulting fees from St. Jude Medical, Sorin, Boston Scientific, Medtronic, CardioInsight, Respicardia, Backbeat, and Medscape and receives research funding from Sorin, St. Jude Medical, Medtronic, and Boston Scientific. N.A.C., R.B., Y.C., J.L., V.A.J., J.P.M., C.L. report no conflicts.
References
- 1. Leyva F, Nisam S, Auricchio A.. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol 2014;64:1047–1058. [DOI] [PubMed] [Google Scholar]
- 2. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L.. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J 2006;27:1928–1932. [DOI] [PubMed] [Google Scholar]
- 3. Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, Brown MW, Hall WJ, Pfeffer MA, Moss AJ.. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation 2010;122:985–992. [DOI] [PubMed] [Google Scholar]
- 4. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ.. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 5. Lindvall C, Chatterjee NA, Chang Y, Chernack B, Jackson VA, Singh JP, Metlay JP.. National trends in the use of cardiac resynchronization therapy with or without implantable cardioverter-defibrillator. Circulation 2016;133:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steinberg BA, Al-Khatib SM, Edwards R, Han J, Bardy GH, Bigger JT, Buxton AE, Moss AJ, Lee KL, Steinman R, Dorian P, Hallstrom A, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Inoue LY, Sanders GD.. Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities: results from a combined analysis of 4 randomized clinical trials. JACC Heart Fail 2014;2:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chatterjee NA, Singh JP.. Cardiac resynchronization therapy: past, present, and future. Heart Fail Clin 2015;11:287–303. [DOI] [PubMed] [Google Scholar]
- 8. Hsu JC, Solomon SD, Bourgoun M, McNitt S, Goldenberg I, Klein H, Moss AJ, Foster E.. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: the MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy) study. J Am Coll Cardiol 2012;59:2366–2373. [DOI] [PubMed] [Google Scholar]
- 9. Al-Khatib SM, Hellkamp AS, Hernandez AF, Fonarow GC, Thomas KL, Al-Khalidi HR, Heidenreich PA, Hammill S, Yancy C, Peterson ED.. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation 2012;125:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2006–2012. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nisoverview.jsp (20 October 2015).
- 11. Elixhauser A, Steiner C, Harris DR, Coffey RM.. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 12.HCUP Comorbidity Software. Healthcare Cost and Utilization Project (HCUP). 2008. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp (20 October 2015).
- 13. Bilchick KC, Stukenborg GJ, Kamath S, Cheng A.. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol 2012;60:1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Rees JB, Borleffs CJ, van Welsenes GH, van der Velde ET, Bax JJ, van Erven L, Putter H, van der Bom JG, Schalij MJ.. Clinical prediction model for death prior to appropriate therapy in primary prevention implantable cardioverter defibrillator patients with ischaemic heart disease: the FADES risk score. Heart 2012;98:872–877. [DOI] [PubMed] [Google Scholar]
- 15. Healey JS, Hohnloser SH, Exner DV, Birnie DH, Parkash R, Connolly SJ, Krahn AD, Simpson CS, Thibault B, Basta M, Philippon F, Dorian P, Nair GM, Sivakumaran S, Yetisir E, Wells GA, Tang AS.. Cardiac resynchronization therapy in patients with permanent atrial fibrillation: results from the Resynchronization for Ambulatory Heart Failure Trial (RAFT). Circ Heart Fail 2012;5:566–570. [DOI] [PubMed] [Google Scholar]
- 16. Mathew J, Katz R, St John Sutton M, Dixit S, Gerstenfeld EP, Ghio S, Gold MR, Linde C, Shlipak MG, Deo R.. Chronic kidney disease and cardiac remodelling in patients with mild heart failure: results from the REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction (REVERSE) study. Eur J Heart Fail 2012;14:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zusterzeel R, Spatz ES, Curtis JP, Sanders WE, Selzman KA, Pina IL, Bao H, Ponirakis A, Varosy PD, Masoudi FA, Canos DA, Strauss DG.. Cardiac resynchronization therapy in women versus men: observational comparative effectiveness study from the National Cardiovascular Data Registry. Circ Cardiovasc Qual Outcomes 2015;8(2 Suppl 1):S4–11. [DOI] [PubMed] [Google Scholar]
- 18. Bogale N, Priori S, Gitt A, Alings M, Linde C, Dickstein K.. The European cardiac resynchronization therapy survey: patient selection and implantation practice vary according to centre volume. Europace 2011;13:1445–1453. [DOI] [PubMed] [Google Scholar]
- 19. Zeitler EP, Hellkamp AS, Schulte PJ, Fonarow GC, Hernandez AF, Peterson ED, Sanders GD, Yancy CW, Al-Khatib SM.. Comparative effectiveness of implantable cardioverter defibrillators for primary prevention in women. Circ Heart Fail 2016;9:e002630.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marijon E, Leclercq C, Narayanan K, Boveda S, Klug D, Lacaze-Gadonneix J, Defaye P, Jacob S, Piot O, Deharo JC, Perier MC, Mulak G, Hermida JS, Milliez P, Gras D, Cesari O, Hidden-Lucet F, Anselme F, Chevalier P, Maury P, Sadoul N, Bordachar P, Cazeau S, Chauvin M, Empana JP, Jouven X, Daubert JC, Le Heuzey JY.. Causes-of-death analysis of patients with cardiac resynchronization therapy: an analysis of the CeRtiTuDe cohort study. Eur Heart J 2015;36: 2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lam SK, Owen A.. Combined resynchronisation and implantable defibrillator therapy in left ventricular dysfunction: Bayesian network meta-analysis of randomised controlled trials. BMJ 2007;335:925.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chatterjee NA, Roka A, Lubitz SA, Gold MR, Daubert C, Linde C, Steffel J, Singh JP, Mela T.. Reduced appropriate implantable cardioverter-defibrillator therapy after cardiac resynchronization therapy-induced left ventricular function recovery: a meta-analysis and systematic review. Eur Heart J 2015;36:2780–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raphael CE, Finegold JA, Barron AJ, Whinnett ZI, Mayet J, Linde C, Cleland JG, Levy WC, Francis DP.. The effect of duration of follow-up and presence of competing risk on lifespan-gain from implantable cardioverter defibrillator therapy: who benefits the most? Eur Heart J 2015;36:1676–1688. [DOI] [PubMed] [Google Scholar]
- 24. Dickstein K, Bogale N, Priori S, Auricchio A, Cleland JG, Gitt A, Limbourg T, Linde C, van Veldhuisen DJ, Brugada J.. The European cardiac resynchronization therapy survey. Eur Heart J 2009;30:2450–2460. [DOI] [PubMed] [Google Scholar]
- 25. Marijon E, Leclercq C, Narayanan K, Boveda S, Klug D, Lacaze-Gadonneix J, Defaye P, Jacob S, Piot O, Deharo JC, Perier MC, Mulak G, Hermida JS, Milliez P, Gras D, Cesari O, Hidden-Lucet F, Anselme F, Chevalier P, Maury P, Sadoul N, Bordachar P, Cazeau S, Chauvin M, Empana JP, Jouven X, Daubert JC,L, Heuzey JY.. Causes-of-death analysis of patients with cardiac resynchronization therapy: an analysis of the CeRtiTuDe cohort study. Eur Heart J 2015;36:2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kramer DB, Reynolds MR, Normand SL, Parzynski CS, Spertus JA, Mor V, Mitchell SL.. Hospice use following implantable cardioverter-defibrillator implantation in older patients: results from the NCDR(R). Circulation 2016;133:2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp-Pedersen C, Pehrson S.. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221. [DOI] [PubMed] [Google Scholar]
- 28. Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Bardy GH.. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008;359:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brunner-La Rocca HP, Rickenbacher P, Muzzarelli S, Schindler R, Maeder MT, Jeker U, Kiowski W, Leventhal ME, Pfister O, Osswald S, Pfisterer ME, Rickli H.. End-of-life preferences of elderly patients with chronic heart failure. Eur Heart J 2012;33:752–759. [DOI] [PubMed] [Google Scholar]
- 30. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE. Guidelines ESCCfP Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bansch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, L, Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM.. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in Collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 31. Quan H, Parsons GA, Ghali WA.. Validity of information on comorbidity derived rom ICD-9-CCM administrative data. Med Care 2002;40:675–685. [DOI] [PubMed] [Google Scholar]
- 32. Al Kazzi ES, Lau B, Li T, Schneider EB, Makary MA, Hutfless S.. Differences in the prevalence of obesity, smoking and alcohol in the United States nationwide inpatient sample and the behavioral risk factor surveillance system. PLoS One 2015;10:e0140165.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.