Abstract
Background: Sodium disarrays are common in peritoneal dialysis (PD) patients, and may be associated with adverse outcomes in this population. However, few studies of limited sample size have examined the association of serum sodium with mortality in PD patients, with inconsistent results. We hypothesized that both hypo- and hypernatremia are associated with higher death risk in a nationally representative cohort of US PD patients.
Methods: We sought to examine the association of serum sodium over time and mortality among 4687 adult incident PD patients from a large US dialysis organization who underwent one or more serum sodium measurements within the first 3 months of dialysis over January 2007 to December 2011. We examined the association of time-dependent and baseline sodium with all-cause mortality as a proxy of short- and long-term sodium–mortality associations, respectively. Hazard ratios were estimated using Cox models with three adjustment levels: minimally adjusted, case-mix adjusted, and case-mix + laboratory adjusted.
Results: In time-dependent analyses, sodium levels <140 mEq/L were associated with incrementally higher death risk in case-mix models (ref: 140 to <142 mEq/L); following laboratory covariate adjustment, associations between lower sodium and higher mortality remained significant for levels <136 mEq/L. In analyses using baseline values, sodium levels <140 mEq/L were associated with higher mortality risk across all models (ref: 140 to <142 mEq/L).
Conclusions: In PD patients, lower time-dependent and baseline sodium levels were independently associated with higher death risk. Further studies are needed to determine whether correction of dysnatremia improves longevity in this population.
Keywords: hypernatremia, hyponatremia, mortality, peritoneal dialysis, sodium
INTRODUCTION
Sodium derangements are the most frequently encountered electrolyte disorder among advanced chronic kidney disease (CKD) patients, including those undergoing maintenance dialysis [1]. In comparison with the general population, observational data show that pre-dialysis CKD and hemodialysis patients have a 2-fold higher prevalence of hyponatremia compared with the general population, likely resulting from volume overload, excess free water intake, impaired free water clearance, protein-energy wasting and co-existing illnesses (e.g. congestive heart failure, cirrhosis) [1–4].
Peritoneal dialysis (PD) patients, too, may have unique predisposition to hypo- and hypernatremia ensuing from various causes. While water is removed via both aquaporins and ‘small pores’ with PD, sodium is removed across the ‘small pores’. Movement of water through the aquaporins occurs primarily during early phases of the dwell resulting in greater removal of water than sodium, long recognized as sodium sieving with PD [2, 5]. Hence, the length of the dwell has an important influence on the relative removal of sodium and water, which in turn is likely to have a significant effect on serum sodium levels. The PD prescription for any given peritoneal solute transfer rate is, thus, possibly an important determinant of the prevalence of hypo- or hypernatremia. As importantly, glucose absorption from dextrose-based dialysate or icodextrin metabolites can result in lower serum sodium levels [2, 5, 6]. That the effects of PD on serum electrolytes are different is exemplified by a significantly higher prevalence of hypokalemia and lower prevalence of metabolic acidosis compared with patients treated with maintenance hemodialysis [7, 8].
While several studies have shown that dysnatremia is associated with higher death risk in advanced CKD and hemodialysis patients, given these considerations, separate examination of the association of serum sodium with mortality is warranted in the PD population [3, 9–13]. However, there have been few studies of the association between serum sodium and mortality in PD patients; they provide limited evidence owing to small sample size precluding the possibility of granular examination of serum sodium levels, pooling data from incident and prevalent PD patients with distinct residual kidney function profiles, and inability to consider short- and long-term effects of serum sodium on mortality [14–16]. Thus, to address these limitations, we sought to examine the association of repeated and baseline measures of serum sodium level over time with all-cause mortality among incident PD patients from a large US dialysis organization with comprehensive availability of comorbidity and laboratory data. We also examined clinical characteristics associated with low baseline sodium level in these patients. We hypothesized that both hypo- and hypernatremia were independently associated with higher death risk in this nationally representative population of patients undergoing PD.
MATERIALS AND METHODS
Source cohort
We conducted an observational study using national data from a large dialysis organization in the USA with detailed patient-level information on socio-demographics, comorbidities, laboratory tests, dialysis treatment characteristics, clinical events and vital status [17, 18]. Our source population was an incident cohort of 6651 adult dialysis patients who solely received PD from one of the facilities operated by the dialysis provider over a 5-year period (1 January 2007 to 31 December 2011). Patients were included provided that at the time of baseline sodium measurement they had a dialysis vintage of >60 days, and had one or more sodium measure(s) during their baseline quarter (first 91 days) of dialysis. Patients were excluded from the study if they underwent treatment with a dialysis modality other than PD at any time during follow-up or had an outlier sodium value below the 0.5th percentile of observed values (<125 mEq/L; Supplementary data, Figure S1). The study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA, University of California Irvine Medical Center and University of Washington.
Exposure ascertainment
The exposure of interest was serum sodium level, and we sought to examine the association between sodium level and all-cause mortality using two approaches: (i) time-dependent sodium–mortality associations were examined in order to ascertain short-term exposure–mortality associations, and (ii) baseline sodium–mortality associations were assessed in order to ascertain long-term exposure–mortality associations [19]. For the time-dependent analyses, the median [interquartile range (IQR)] number of quarterly-averaged sodium measurements contributed by each patient was 4 (2, 8).
In primary analyses, serum sodium level was divided into seven a priori selected categories: <134, 134 to <136, 136 to <138, 138 to <140, 140 to <142 (reference group), 142 to <144, and ≥144 mEq/L. In sensitivity analyses, we examined serum sodium as a continuous predictor using restricted cubic spline analyses with knots defined at the 25th, 50th and 75th percentiles of observed sodium values (sodium levels 138, 140 and 141 mEq/L, respectively, for both time-dependent and baseline sodium analyses).
Laboratory data collection and peritoneal equilibrium test characteristics
Serum samples for laboratory testing, including serum sodium, were drawn within the outpatient dialysis clinics of the large dialysis organization using uniform techniques, and were transported to a single central laboratory in Deland, Florida typically within 24 h of collection, for sodium measurement. All laboratory testing was conducted using automated and standardized methods. All laboratory results, including serum sodium, were ascertained as the mean of all values over successive 91-day periods from the date of first dialysis.
We also considered peritoneal equilibrium test (PET) data in a subcohort of 3035 patients among whom testing was conducted [18]. The PET is a standardized test used to assess peritoneal solute transfer rate [20, 21]. Following infusion of PD dextrose-based dialysis solution, the ratio of the dialysate to plasma creatinine (D/PCr ratio) at the end of a 4-h dwell was calculated and used to classify peritoneal membrane transport capability, defined as low (<0.52), average (0.52 to <0.78) or high (≥0.78) in this study. In a subcohort of 2948 patients, we also examined data examining the ultrafiltration volume at the end of the 4-h dwell, defined as ‘4-h PET ultrafiltration volume = 4-h drain volume − 4-h infusion volume’ [18].
Outcome ascertainment
The primary outcome of interest was all-cause mortality. At-risk time began the day after the baseline quarter of sodium measurement. Patients were censored for kidney transplantation, transfer to a dialysis facility operated by another provider or at the end of the study (31 December 2011).
Statistical analyses
We first sought to determine clinical characteristics associated with low versus high serum sodium, in which sodium levels were dichotomized as <136 mEq/L versus ≥136 mEq/L, respectively. We conducted logistic regression analyses examining the association between baseline socio-demographic, comorbidity, PD treatment and laboratory test characteristics with baseline serum sodium level.
We then estimated the association between sodium level and mortality using Cox proportional hazard models with three hierarchical levels of covariate adjustment. In time-dependent analyses, laboratory data were examined as time-dependent covariates summarized over 91-day periods (i.e. mean or median values over the quarter for each patient).
Minimally adjusted model: adjusted for patient's calendar quarter of entry into the cohort;
Case-mix adjusted model: adjusted for covariates in the minimally adjusted model, as well as age, sex, race/ethnicity, primary insurance and baseline comorbidities including diabetes, hypertension, congestive heart failure, atherosclerotic heart disease, cerebrovascular disease, other cardiovascular disease, chronic obstructive pulmonary disease, human immunodeficiency virus, history of cancer and alcohol abuse;
Case-mix + laboratory test adjusted model: adjusted for covariates in the case-mix model, as well as serum albumin, serum creatinine, total iron binding capacity, calcium, phosphorus, parathyroid hormone, ferritin, iron saturation, hemoglobin, white blood cell count, peritoneal urea clearance (peritoneal Kt/v), renal urea clearance (renal Kt/V), use of automated PD during the baseline quarter and use of automated PD anytime during the follow-up period.
We a priori defined the case-mix adjusted model as our preferred model, which forced into the model core socio-demographic measures and other confounders of the association between sodium and mortality. Based on prior data highlighting hyponatremia as a mortality predictor in patients with acute-on-chronic liver failure, which in and of itself is associated with higher death risk, we also conducted sensitivity analyses that incrementally adjusted for liver disease as a potential confounder of the sodium–mortality association [22]. In order to explore potential causal pathways that might mediate the association between serum sodium level and mortality, we designated the case-mix + laboratory adjusted model as an exploratory model in which we added covariate terms for potential pathway intermediates to the case-mix adjusted model and observed for attenuation of effect estimates. To determine the impact of key confounders highlighted in prior literature upon estimates of the sodium–mortality association, namely serum potassium, serum glucose and serum bicarbonate, we examined three additional models that separately adjusted for these covariates in addition to case-mix + laboratory test covariates: (iv) case-mix + laboratory + potassium, (v) case-mix + laboratory + glucose and (vi) case-mix + laboratory + bicarbonate adjusted models [2, 14–16]. Proportional hazards assumptions were checked by graphical and formal testing.
In addition to conducting glucose-adjusted analyses (case-mix + laboratory test + glucose adjusted model), we also separately conducted analyses in which sodium was corrected for serum glucose using the following formula: glucose-corrected sodium = measured sodium + 0.016 × (serum glucose − 100) using unadjusted, case-mix and case-mix + laboratory test adjusted models [12]. The mean ± standard deviation (SD), median (IQR) and minimum-maximum values of observed baseline glucose values were 158 ± 67, 140 (108, 193) and 27–742 mEq/L, respectively.
Missing data were handled using methods that included multiple imputation. There were no missing values for age, sex, diabetes, primary insurance, baseline comorbidities or baseline serum potassium. The remaining covariates ascertained at baseline had ≤1% missing values, except for ferritin (4%), peritoneal Kt/V (15%), renal Kt/V (15%), parathyroid hormone (2%), white blood cell count (25%) and serum glucose (37%).
Analyses and figures were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), Stata version 13.1 (Stata Corporation, College Station, TX, USA) and SigmaPlot Version 12.5 (Systat Software, San Jose, CA, USA).
RESULTS
Study population
Among 4687 patients who met the inclusion and exclusion criteria (Supplementary data, Figure S1), we observed that 9% (n = 399) had hyponatremia (defined as sodium <136 mEq/L) and 4% (n = 170) had hypernatremia (defined as sodium ≥144 mEq/L) at study entry according to baseline serum sodium levels. In the overall cohort, the mean ± SD, median (IQR) and minimum-maximum values of observed sodium levels were 140 ± 3, 140 (138, 141) and 125–149 mEq/L, respectively. Comparison of characteristics among incident PD patients who did (n = 4689) versus did not undergo baseline serum sodium measurement (n = 1962) showed that those who underwent serum sodium measurement were more likely to be Caucasian, less likely to be Hispanic and less likely to be treated with automated PD at baseline or anytime during follow-up, while other socio-demographic, comorbidity and laboratory test characteristics were largely similar (Supplementary data, Table S1).
Characteristics of patients according to baseline serum sodium level are shown in Table 1. Compared with patients in the highest sodium category (≥144 mEq/L), patients in the lowest sodium category (<134 mEq/L) tended to be female, were more likely to be Hispanic and less likely to be African-American, were more likely to have a history of diabetes and thyroid disease, were less likely to be receiving automated PD at study entry and had higher net 4-h ultrafiltration volumes with PET, had higher serum glucose and ferritin levels, and had lower residual kidney function (defined by renal urea clearance [renal Kt/V]), serum albumin and parathyroid hormone levels.
Table 1.
Baseline characteristics among incident peritoneal dialysis patients according to baseline sodium level
| Serum sodium level (mEq/L) |
P* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | <134 | 134 to <136 | 136 to <138 | 138 to <140 | 140 to <142 | 142 to <144 | ≥144 | ||
| N | 4687 | 148 | 251 | 654 | 1368 | 1424 | 672 | 170 | N/A |
| Age, years (±SD) | 58 ± 15 | 59 ± 14 | 58 ± 16 | 56 ± 15 | 56 ± 16 | 58 ± 15 | 60 ± 15 | 59 ± 16 | <0.001 |
| Female (%) | 45 | 61 | 47 | 48 | 46 | 44 | 40 | 32 | <0.001 |
| Race/ethnicity (%) | |||||||||
| Caucasian | 60 | 67 | 58 | 58 | 58 | 61 | 64 | 63 | 0.16 |
| African-American | 20 | 10 | 20 | 18 | 21 | 21 | 20 | 24 | 0.009 |
| Asian | 5 | 5 | 4 | 6 | 5 | 4 | 4 | 1 | 0.04 |
| Hispanic | 11 | 14 | 14 | 15 | 12 | 10 | 9 | 7 | <0.001 |
| Other | 4 | 4 | 4 | 3 | 4 | 4 | 3 | 5 | 0.58 |
| Primary insurance (%) | |||||||||
| Medicare | 44 | 51 | 44 | 46 | 44 | 45 | 44 | 42 | 0.20 |
| Medicaid | 4 | 5 | 6 | 5 | 4 | 4 | 2 | 4 | 0.03 |
| Other | 52 | 45 | 50 | 50 | 53 | 51 | 54 | 54 | <0.001 |
| Comorbidities (%) | |||||||||
| Diabetes | 58 | 67 | 69 | 71 | 60 | 53 | 52 | 48 | <0.001 |
| Hypertension | 48 | 39 | 49 | 48 | 45 | 49 | 51 | 50 | 0.06 |
| Atherosclerotic heart disease | 13 | 12 | 14 | 13 | 13 | 12 | 14 | 13 | 0.98 |
| Other cardiovascular disease | 9 | 7 | 10 | 12 | 9 | 9 | 10 | 7 | 0.37 |
| Congestive heart failure | 6 | 5 | 9 | 8 | 5 | 5 | 6 | 4 | 0.03 |
| Cerebrovascular disease | <1 | <1 | 1 | <1 | 1 | 0 | <1 | <1 | 0.48 |
| Thyroid disorders | 15 | 18 | 17 | 16 | 14 | 14 | 16 | 12 | 0.41 |
| COPD | 2 | 1 | 3 | 3 | 1 | 2 | 2 | 3 | 0.77 |
| Malignancy | 1 | <1 | <1 | 1 | 1 | 2 | 1 | 1 | 0.92 |
| HIV | <1 | 0 | 0 | <1 | 0 | 0 | 0 | 0 | 0.76 |
| Alcohol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.07 |
| Liver disease | <1 | <1 | 1 | <1 | 1 | 0 | <1 | 0 | 0.09 |
| Use of APD (%) | |||||||||
| At study entry | 45 | 35 | 37 | 42 | 45 | 46 | 47 | 51 | <0.001 |
| Any time during study follow-up | 83 | 80 | 76 | 81 | 85 | 84 | 85 | 87 | 0.001 |
| Laboratory tests [mean ± SD or median (IQR)] | |||||||||
| 4-h PET D/P creatinine | 0.64 ± 0.12 | 0.64 ± 0.12 | 0.65 ± 0.12 | 0.65 ± 0.12 | 0.63 ± 0.12 | 0.64 ± 0.12 | 0.64 ± 0.12 | 0.64 ± 0.13 | 0.18 |
| 4-h PET ultrafiltration volume (mL) | 300 (100, 400) | 400 (200, 500) | 300 (175, 400) | 300 (100, 450) | 300 (200, 450) | 300 (100, 400) | 275 (100, 400) | 225 (0, 400) | <0.001 |
| Renal urea clearance (renal Kt/V) | 1.2 ± 0.8 | 1.0 ± 0.7 | 1.0 ± 0.8 | 1.1 ± 0.8 | 1.3 ± 0.8 | 1.3 ± 0.8 | 1.3 ± 0.8 | 1.3 ± 0.8 | <0.001 |
| Peritoneal clearance (peritoneal Kt/V) | 1.4 ± 0.4 | 1.6 ± 0.5 | 1.5 ± 0.4 | 1.5 ± 0.5 | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 | <0.001 |
| Serum albumin (g/dL) | 3.7 ± 0.5 | 3.4 ± 0.5 | 3.5 ± 0.6 | 3.6 ± 0.5 | 3.7 ± 0.5 | 3.8 ± 0.4 | 3.7 ± 0.4 | 3.7 ± 0.5 | <0.001 |
| Serum creatinine (mg/dL) | 5.9 ± 2.4 | 5.4 ± 2.3 | 5.7 ± 2.4 | 6.0 ± 2.6 | 5.9 ± 2.4 | 5.9 ± 2.4 | 5.8 ± 2.4 | 5.8 ± 2.4 | 0.86 |
| Serum potassium (mEq/L) | 4.2 ± 0.5 | 4.2 ± 0.5 | 4.2 ± 0.5 | 4.2 ± 0.5 | 4.2 ± 0.5 | 4.2 ± 0.5 | 4.1 ± 0.5 | 4.1 ± 0.6 | <0.001 |
| Glucose (mg/dL) | 158 ± 67 | 207 ± 144 | 200 ± 89 | 185 ± 79 | 160 ± 63 | 143 ± 51 | 133 ± 40 | 124 ± 36 | <0.001 |
| TIBC (mg/dL) | 257 ± 47 | 248 ± 25 | 249 ± 54 | 256 ± 47 | 260 ± 48 | 259 ± 45 | 256 ± 45 | 255 ± 43 | 0.05 |
| Bicarbonate (mEq/L) | 25 ± 3 | 25 ± 3 | 25 ± 3 | 24 ± 3 | 24 ± 3 | 25 ± 3 | 25 ± 3 | 26 ± 3 | <0.001 |
| Calcium (mg/dL) | 9.1 ± 0.7 | 9.2 ± 0.5 | 9.2 ± 0.8 | 9.2 ± 0.6 | 9.1 ± 0.7 | 9.1 ± 0.7 | 9.1 ± 0.7 | 9.2 ± 0.7 | <0.001 |
| Phosphorus (mg/dL) | 4.7 ± 1.1 | 4.8 ± 1.1 | 4.7 ± 1.0 | 4.9 ± 1.2 | 4.8 ± 1.0 | 4.7 ± 1.0 | 4.6 ± 1.1 | 4.7 ± 1.2 | <0.001 |
| Intact PTH (pg/mL) | 266 (159, 429) | 206 (127, 368) | 238 (144, 400) | 249 (154, 420) | 268 (159, 426) | 281 (166, 445) | 271 (167, 458) | 246 (147, 379) | 0.03 |
| Ferritin (ng/mL) | 199 (110, 348) | 222 (124, 348) | 241 (122, 478) | 209 (106, 375) | 200 (108, 335) | 192 (107, 340) | 200 (115, 344) | 180 (94, 319) | 0.006 |
| Iron saturation (%) | 26 ± 9 | 26 ± 10 | 28 ± 10 | 27 ± 11 | 26 ± 9 | 26 ± 9 | 26 ± 9 | 26 ± 10 | 0.25 |
| Hemoglobin (g/dL) | 11.4 ± 1.3 | 11.3 ± 1.4 | 11.4 ± 1.2 | 11.3 ± 1.3 | 11.4 ± 1.3 | 11.4 ± 1.3 | 11.5 ± 1.3 | 11.4 ± 1.4 | 0.06 |
| WBC (×103/µL) | 7.4 ± 2.4 | 7.9 ± 3.4 | 7.6 ± 2.4 | 7.4 ± 2.3 | 7.4 ± 2.5 | 7.4 ± 2.3 | 7.2 ± 2.0 | 7.2 ± 2.3 | 0.007 |
*P-for-trend values.
COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; PET, peritoneal equilibrium test; D/P, dialysate to plasma; APD, automated peritoneal dialysis; TIBC, total iron binding capacity; PTH, parathyroid hormone; WBC, white blood cell count.
Predictors of serum sodium level
In case-mix adjusted analyses, patients who were female, with underlying diabetes, and higher peritoneal clearance (defined by peritoneal Kt/V), ferritin, white blood cell count and serum glucose levels were more likely to have low baseline serum sodium levels (<136 mEq/L; Supplementary data, Table S2). In contrast, patients with higher residual kidney function (defined by renal Kt/V), serum albumin, total iron binding capacity and parathyroid hormone levels were less likely to have low baseline sodium levels.
Time-dependent serum sodium and mortality
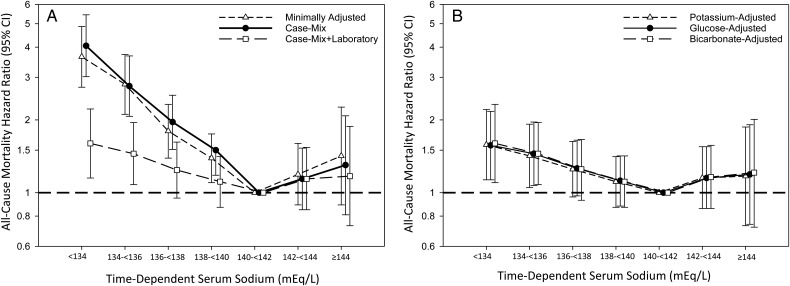
Patients contributed a total of 7478 years of follow-up during which time 649 deaths occurred. Median (IQR) at-risk time was 11.9 (6.0, 20.9) months. In time-dependent analyses, we observed a strong, graded association between lower sodium level and higher mortality risk for sodium levels <140 mEq/L (ref: sodium 140 to <142 mEq/L) in minimally adjusted, case-mix and case-mix + liver disease adjusted analyses (Figure 1A and Supplementary data, Table S3). In case-mix + laboratory adjusted analyses, we observed an attenuation in the estimates of sodium and mortality for sodium levels <140 mEq/L, although associations remained statistically significant for sodium levels <136 mEq/L (Figure 1A and Supplementary data, Table S3). A similar pattern of findings was observed in analyses additionally adjusted for serum potassium, glucose and bicarbonate (Figure 1B and Supplementary data, Table S4). In analyses using a correction factor for glucose, we found that glucose-corrected sodium levels <134 mEq/L were associated with higher mortality risk in case-mix + laboratory adjusted models (Supplementary data, Table S5 and Figure S3).
FIGURE 1.
Associations between time-dependent sodium level and all-cause mortality in minimally adjusted, case-mix and case-mix + laboratory adjusted models (A) and models additionally adjusted for serum potassium, glucose and bicarbonate (B) among incident peritoneal dialysis patients. Minimally adjusted analyses adjusted for entry calendar quarter. Case-mix analyses adjusted for covariates in the minimally adjusted model, as well as age, sex, race/ethnicity, primary insurance, diabetes, alcohol use, congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, human immunodeficiency virus, malignancy, hypertension, atherosclerotic heart disease and other cardiovascular disease. Case-mix + laboratory adjusted analyses adjusted for covariates in the case-mix model, as well as serum albumin, serum creatinine, total iron binding capacity, calcium, ferritin, hemoglobin, iron saturation, renal urea clearance (renal Kt/V), peritoneal urea clearance (peritoneal Kt/V), phosphorus, parathyroid hormone, white blood cell count, automated peritoneal dialysis status at baseline and automated peritoneal dialysis status during follow-up. Case-mix + laboratory + potassium adjusted analyses adjusted for covariates in the case-mix + laboratory adjusted model, as well as serum potassium. Case-mix + laboratory + glucose adjusted analyses adjusted for covariates in the case-mix + laboratory adjusted model, as well as serum glucose. Case-mix + laboratory + bicarbonate adjusted analyses adjusted for covariates in the case-mix + laboratory adjusted model, as well as serum bicarbonate.
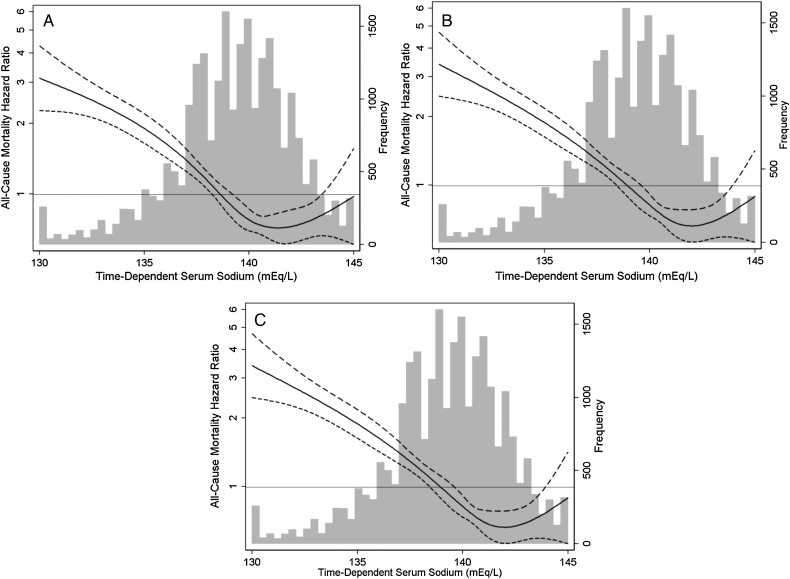
In analyses of time-dependent serum sodium and mortality as restricted cubic splines, we observed that sodium levels <138 mEq/L were associated with a higher mortality risk, whereas levels 138–143 mEq/L were associated with a lower mortality risk, above which the survival benefit plateaued in case-mix + laboratory adjusted analyses (Figure 2C).
FIGURE 2.
Association between time-dependent sodium level gradations and all-cause mortality in incident peritoneal dialysis patients. Figures present hazard ratios (short-dashed lines indicate 95% confidence intervals) for sodium analyzed as a spline with knots at the 25th, 50th and 75th percentiles of observed values (sodium levels 138, 140 and 141 mEq/L, respectively). A histogram of observed baseline sodium values and a hazard reference ratio of 1 (long-horizontal line) is overlaid. Sodium level <130 and >145 mEq/L replaced with 130 and 145 mEq/L, respectively, for the purposes of the spline analyses. Minimally adjusted analyses adjusted for entry calendar quarter (A). Case-mix analyses adjusted for covariates in the minimally adjusted model, as well as age, sex, race/ethnicity, primary insurance, diabetes, alcohol use, congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, human immunodeficiency virus, malignancy, hypertension, atherosclerotic heart disease and other cardiovascular disease (B). Case-mix + laboratory adjusted analyses adjusted for covariates in the case-mix model, as well as serum albumin, serum creatinine, total iron binding capacity, calcium, ferritin, hemoglobin, iron saturation, renal urea clearance (renal Kt/V), peritoneal urea clearance (peritoneal Kt/V), phosphorus, parathyroid hormone, white blood cell count, automated peritoneal dialysis status at baseline and automated peritoneal dialysis status during follow-up (C).
Baseline serum sodium and mortality
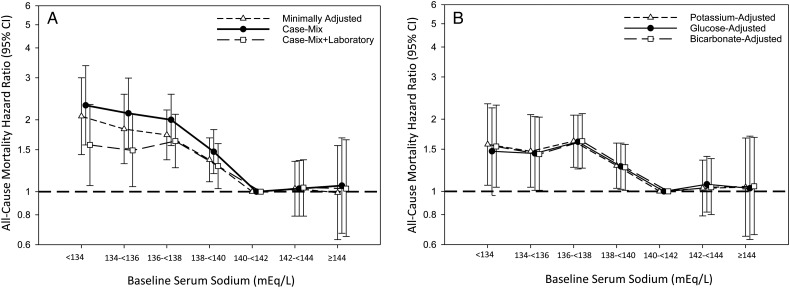
We observed a graded association between lower sodium level (<140 mEq/L) at baseline and higher mortality risk (ref: sodium 140 to <142 mEq/L) in minimally adjusted, case-mix and case-mix + liver disease adjusted models (Figure 3A and Supplementary data, Table S3). In case-mix + laboratory adjusted analyses, there was an attenuation in the estimates of sodium and mortality for sodium levels <140 mEq/L, although associations remained statistically significant (Figure 3A and Supplementary data, Table S3). Analyses additionally adjusted for serum potassium, glucose and bicarbonate showed similar findings, although the association between the lowest serum sodium category (<134 mEq/L) and mortality was no longer statistically significant in the model adjusted for blood glucose (Figure 3B and Supplementary data, Table S4). In analyses using a correction factor for glucose, we also found that glucose-corrected sodium categories <140 mEq/L were associated with higher mortality risk in case-mix + laboratory adjusted models, although the lowest category (<134 mEq/L) did not achieve statistical significance (Supplementary data, Table S5 and Figure S3).
FIGURE 3.
Associations between baseline sodium level and all-cause mortality in minimally adjusted, case-mix and case-mix + laboratory adjusted models (A) and models additionally adjusted for serum potassium, glucose and bicarbonate (B) among incident peritoneal dialysis patients. Minimally adjusted analyses adjusted for entry calendar quarter. Case-mix analyses adjusted for covariates in the minimally adjusted model, as well as age, sex, race/ethnicity, primary insurance, diabetes, alcohol use, congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, human immunodeficiency virus, malignancy, hypertension, atherosclerotic heart disease and other cardiovascular disease. Case-mix + laboratory adjusted analyses adjusted for covariates in the case-mix model, as well as serum albumin, serum creatinine, total iron binding capacity, calcium, ferritin, hemoglobin, iron saturation, renal urea clearance (renal Kt/V), peritoneal urea clearance (peritoneal Kt/V), phosphorus, parathyroid hormone, white blood cell count, automated peritoneal dialysis status at baseline and automated peritoneal dialysis status during follow-up. Case-mix + laboratory + potassium adjusted analyses adjusted for covariates in the case-mix + laboratory adjusted model, as well as serum potassium. Case-mix + laboratory + glucose adjusted analyses adjusted for covariates in the case-mix + laboratory adjusted model, as well as serum glucose. Case-mix + laboratory + bicarbonate adjusted analyses adjusted for covariates in the case-mix + laboratory adjusted model, as well as serum bicarbonate.
We then examined baseline serum sodium as a continuous variable in restricted cubic spline analyses. Sodium levels <140 mEq/L were associated with a higher mortality risk, whereas levels 140–143 mEq/L were associated with a lower mortality risk, above which the survival benefit plateaued in minimally adjusted, case-mix and case-mix + laboratory adjusted analyses (Supplementary data, Figure S2).
Subgroup analyses
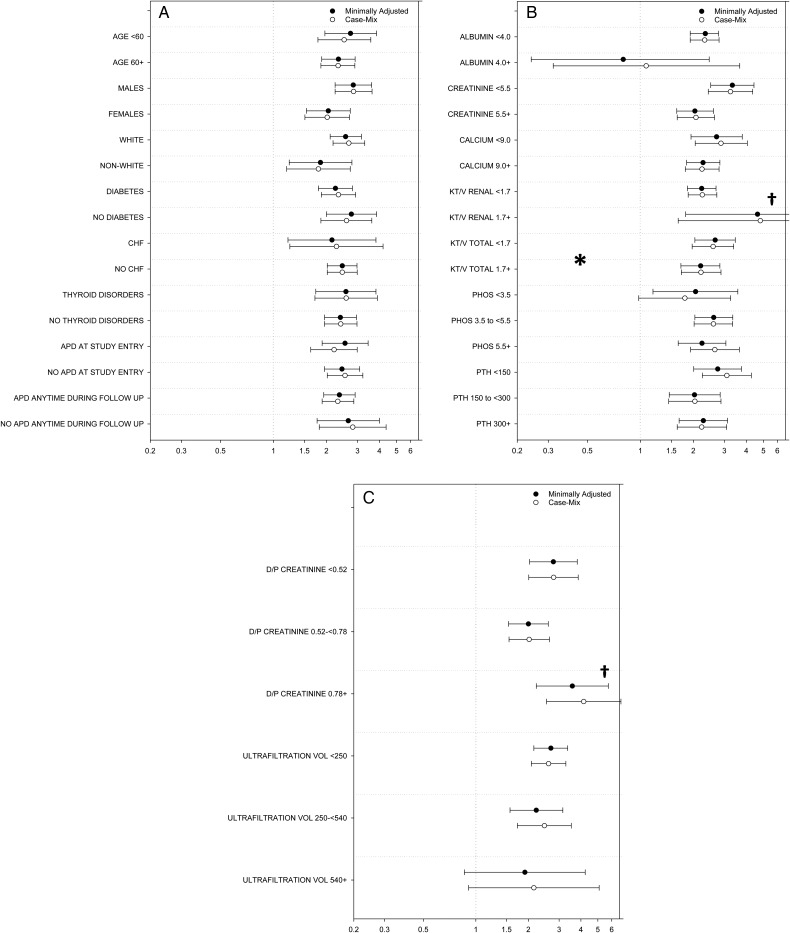
We then examined the association between sodium level dichotomized as <136 versus ≥136 mEq/L (based upon the time-dependent serum sodium threshold below which higher mortality risk was observed) and mortality across clinically relevant subgroups (ref: ≥136 mEq/L). In time-dependent analyses adjusted for case-mix covariates, point estimates of the association of serum sodium with mortality were incrementally higher for subgroups with lower values of net 4-h PET ultrafiltration (i.e. those with tendency toward net volume retention) (Figure 4 and Supplementary data, Table S6). However, interaction tests demonstrated that differences in estimates of the sodium–mortality association across subcategories were statistically significant only for subgroups of total urea clearance: P-interaction 0.03 (Figure 4 and Supplementary data, Table S6). In analyses using baseline serum sodium, interaction tests demonstrated that differences in estimates of the association across subcategories were statistically significant for subgroups of serum creatinine: P-interaction 0.003 (Supplementary data, Figure S4 and Table S6).
FIGURE 4.
Associations between time-dependent sodium level (dichotomized as sodium <136 mEq/L versus ≥136 mEq/L; ref: sodium ≥136 mEq/L) and all-cause mortality across clinically relevant subgroups of incident peritoneal dialysis patients. (A) Stratified by subgroups of socio-demographics and comorbidities, (B) stratified by subgroups of laboratory results, (C) stratified by subgroups of peritoneal equilibrium test characteristics. Minimally adjusted analyses adjusted for entry calendar quarter. Case-mix analyses adjusted for covariates in the minimally adjusted model, as well as age, sex, race/ethnicity, primary insurance, diabetes, alcohol use, congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, human immunodeficiency virus, malignancy, hypertension, atherosclerotic heart disease and other cardiovascular disease. *Denotes statistically significant interaction P-value (P-value <0.05). †Upper bound of 95% confidence interval exceeds figure limits. CHF, congestive heart failure; APD, automated peritoneal dialysis; PHOS, phosphorus; PTH, parathyroid hormone; D/P, dialysate to plasma.
DISCUSSION
In the largest study of serum sodium in PD patients conducted to date, we found that time-dependent sodium levels <140 mEq/L (as a proxy of short-term sodium–mortality associations) were associated with higher death risk independent of socio-demographics and comorbidities. Associations were somewhat attenuated but remained significant for sodium levels <136 mEq/L after accounting for PD modality and laboratory characteristics such as serum potassium, residual kidney function, and markers of nutritional and inflammatory status (e.g. serum albumin). Similarly, we observed that lower baseline sodium levels <140 mEq/L reflecting long-term sodium–mortality associations were associated with higher death risk, independent of socio-demographics, comorbidities, PD modality and laboratory tests.
To date, there has been sparse examination of the association between serum sodium level and mortality in the PD population, which has shown inconsistent findings. In a study of 387 PD patients with baseline normal sodium levels from a single center in South Korea, those who developed hyponatremia (defined as sodium <135 mEq/L) did not have higher mortality risk compared with those who remained normonatremic in multivariable-adjusted analyses [16]. Similarly, in a study of 318 Taiwanese PD patients, baseline serum sodium divided into quartiles (124–135, 136–139, 140–141 and 142–148 mEq/L) was not associated with 2-year mortality [15]. However, in a recent study of 441 incident Korean PD patients in whom time-averaged sodium levels were examined as tertiles (sodium <137, 137 to <139 and ≥139 mEq/L), those in the lowest tertile had higher all-cause and infection-related mortality risk; when examined in continuous increments, a 1 mEq/L higher serum sodium was associated with a 21 and 23% lower all-cause and infection-related mortality risk, respectively [14].
Our study adds new knowledge to the field given its granular examination of both repeated and baseline sodium measurements in a multi-center cohort of US patients undergoing PD for the first time. We found a strikingly consistent association between lower sodium level and higher mortality risk across a number of secondary and subgroup analyses, although the exact sodium threshold below which higher mortality risk was observed varied across analyses (134–140 mEq/L). There are a number of potential mechanisms by which low serum sodium may lead to higher death risk in the PD population. For example, it is well known that severe hyponatremia is directly toxic to the brain, leading to cerebral edema and herniation, encephalopathy, seizure and coma [23–25]. However, even mild declines in serum sodium level can have adverse consequence upon the central nervous system, including disequilibrium, gait abnormalities, fall and fracture risk [1, 26–29]. Indeed, a recent cross-sectional study of 472 PD patients showed that hyponatremia was associated with cognitive impairment ascertained by various formal testing methods [4]. Emerging data also suggest that hyponatremia leads to derangements in cardiac conduction and function, owing to inhibition of calcium channel circuits [30]. Hyponatremia was associated with higher risk of cardiovascular mortality in two large cohorts of maintenance hemodialysis patients from the Dialysis Outcomes and Practice Patterns and Accelerated Mortality on Renal Replacement studies, and lower serum sodium was associated with higher incidence of fatal and non-fatal cardiovascular events in a recent analysis of 441 incident PD patients [9, 12, 14]. A growing body of evidence also shows that hyponatremia may be a risk factor for infection due to (i) impaired interleukin-17 producing helper T cells that function in host immunity, and (ii) mucosal membrane and cellular edema leading to breakdown of microbial barrier function [31–35]. This bears particular relevance to PD patients, given that infection is the second most common cause of death following cardiovascular disease in this population, as well as a major risk factor for patients undergoing PD to transfer to hemodialysis [36, 37]. Furthermore, hyponatremia has been shown to be a risk factor for worse outcomes in patients with PD-related peritonitis as well as infection-related mortality risk in PD patients [14, 38].
Our study also provides important insights into clinical characteristics linked with lower serum sodium level among incident PD patients. Corroborating findings from another study of risk factors for hyponatremia among 166 PD patients, we observed that lower residual kidney function (defined by renal Kt/V) was associated with lower serum sodium independent of case-mix covariates [2]. As the transition from normo- to hyponatremia may be a hallmark of loss of residual kidney function in PD patients, further study is needed to determine whether dysnatremia underlies one potential link between loss of residual kidney function and higher mortality in dialysis patients [39–43].
The strengths of our study include: its examination of a large, nationally representative cohort of PD patients; focus upon an incident PD cohort whose survival is distinct from that of prevalent patients; availability of repeated measures of serum sodium that were obtained in the ambulatory setting and analyzed in a single laboratory; and granular data on comorbidities, dialysis treatment characteristics and laboratory tests. However, several limitations of our study should be acknowledged. First, included patients were required to have at least one serum sodium value, and while the indications for which sodium measurement within the study population cannot be ascertained, this was likely at the discretion of medical providers. Comparison of characteristics among patients who did versus did not undergo sodium measurement showed that there were clinically meaningful differences in race/ethnicity, as well as in utilization of automated peritoneal dialysis. Second, while we were able to adjust for a large number of confounders of the association of serum sodium with all-cause mortality, we were unable to account for certain dietary factors (sodium, potassium, fluid intake) and PD treatment characteristics (use of icodextrin versus glucose-based PD solutions), which may have resulted in residual confounding. However, it should be noted that the use of icodextrin within the large dialysis organization was infrequent during the study period, and hence is unlikely to be a confounder [18]. Third, while it is possible that some of the lower sodium values may have been observed in the context of hyperglycemia, we accounted for serum glucose derangements in multivariable models. Fourth, we did not have information on cause-specific death (e.g. cardiovascular, infection). Lastly, given the observational cohort study design, our findings do not confirm a causal association between dysnatremia and mortality risk in PD patients.
In conclusion, our study shows that lower serum sodium levels measured over time and at baseline are associated with higher death risk in incident PD patients. Further studies are needed to determine (i) the mechanistic pathways by which lower serum sodium levels are linked with higher mortality in PD patients, (ii) whether hyponatremia may contribute to the heightened mortality associated with loss of residual kidney function and (iii) whether correction of sodium derangements improves outcomes in the PD population.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
J.C.A., A.N.A. and K.K.-Z. have received honoraria from Otsuka. J.J.S. has received an investigator initiated grant from Otsuka. None of the other authors declares any relevant conflicts of interest. We certify that the submission is original work and is not being considered for publication elsewhere, in whole or in part, except in abstract form.
Supplementary Material
ACKNOWLEDGEMENTS
Portions of these data were presented as an oral abstract at the 2015 American Society of Nephrology Kidney Week Meeting in San Diego, CA (3–8 November 2015). The authors are supported by the research grants from the NIH/NIDDK including K23-DK102903 (C.M.R.), K24-DK091419 (K.K.-Z.), R01-DK09568 (R.M. and K.K.-Z.), R01-DK078106 (K.K.-Z.), R01-DK096920 (C.P.K. and K.K.-Z.) and U01-DK102163 (K.K.-Z. and C.P.K.), and philanthropist grants from Mr Harold Simmons, Mr Louis Chang and Dr Joseph Lee.
REFERENCES
- 1. Hoorn EJ, Zietse R. Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis 2013; 62: 139–149 [DOI] [PubMed] [Google Scholar]
- 2. Dimitriadis C, Sekercioglu N, Pipili C. et al. Hyponatremia in peritoneal dialysis: epidemiology in a single center and correlation with clinical and biochemical parameters. Perit Dial Int 2014; 34: 260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kovesdy CP, Lott EH, Lu JL. et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation 2012; 125: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu R, Pi HC, Xiong ZY. et al. Hyponatremia and cognitive impairment in patients treated with peritoneal dialysis. Clin J Am Soc Nephrol 2015; 10: 1806–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diaz-Buxo JA. Prescription writing: CAPD and PD plus. Clin Nephrol 2007; 68: 349–353 [DOI] [PubMed] [Google Scholar]
- 6. Fourtounas C, Hardalias A, Dousdampanis P. et al. Sodium removal in peritoneal dialysis: the role of icodextrin and peritoneal dialysis modalities. Adv Perit Dial 2008; 24: 27–31 [PubMed] [Google Scholar]
- 7. Torlen K, Kalantar-Zadeh K, Molnar MZ. et al. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol 2012; 7: 1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vashistha T, Kalantar-Zadeh K, Molnar MZ. et al. Dialysis modality and correction of uremic metabolic acidosis: relationship with all-cause and cause-specific mortality. Clin J Am Soc Nephrol 2013; 8: 254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hecking M, Karaboyas A, Saran R. et al. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2012; 59: 238–248 [DOI] [PubMed] [Google Scholar]
- 10. Hecking M, Karaboyas A, Saran R. et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol 2012; 7: 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mc Causland FR, Brunelli SM, Waikar SS. Dialysate sodium, serum sodium and mortality in maintenance hemodialysis. Nephrol Dial Transplant 2012; 27: 1613–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nigwekar SU, Wenger J, Thadhani R. et al. Hyponatremia, mineral metabolism, and mortality in incident maintenance hemodialysis patients: a cohort study. Am J Kidney Dis 2013; 62: 755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waikar SS, Curhan GC, Brunelli SM. Mortality associated with low serum sodium concentration in maintenance hemodialysis. Am J Med 2011; 124: 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang TI, Kim YL, Kim H. et al. Hyponatremia as a predictor of mortality in peritoneal dialysis patients. PLoS One 2014; 9: e111373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen KH, Chen CY, Lee CC. et al. Baseline hyponatremia does not predict two-year mortality in patients with chronic peritoneal dialysis. Ren Fail 2014; 36: 1371–1375 [DOI] [PubMed] [Google Scholar]
- 16. Kang SH, Cho KH, Park JW. et al. Characteristics and clinical outcomes of hyponatraemia in peritoneal dialysis patients. Nephrology (Carlton) 2013; 18: 132–137 [DOI] [PubMed] [Google Scholar]
- 17. Kuttykrishnan S, Kalantar-Zadeh K, Arah OA. et al. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant 2015; 30: 1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehrotra R, Ravel V, Streja E. et al. Peritoneal equilibration test and patient outcomes. Clin J Am Soc Nephrol 2015; 10: 1990–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dekker FW, de Mutsert R, van Dijk PC. et al. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int 2008; 74: 994–997 [DOI] [PubMed] [Google Scholar]
- 20. Pannekeet MM, Imholz AL, Struijk DG. et al. The standard peritoneal permeability analysis: a tool for the assessment of peritoneal permeability characteristics in CAPD patients. Kidney Int 1995; 48: 866–875 [DOI] [PubMed] [Google Scholar]
- 21. Teitelbaum I, Burkart J. Peritoneal dialysis. Am J Kidney Dis 2003; 42: 1082–1096 [DOI] [PubMed] [Google Scholar]
- 22. Cordoba J, Ventura-Cots M, Simon-Talero M. et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol 2014; 60: 275–281 [DOI] [PubMed] [Google Scholar]
- 23. Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med 1986; 314: 1529–1535 [DOI] [PubMed] [Google Scholar]
- 24. Ayus JC, Achinger SG, Arieff A. Brain cell volume regulation in hyponatremia: role of sex, age, vasopressin, and hypoxia. Am J Physiol Renal Physiol 2008; 295: F619–F624 [DOI] [PubMed] [Google Scholar]
- 25. Riggs JE. Neurologic manifestations of fluid and electrolyte disturbances. Neurol Clin 1989; 7: 509–523 [PubMed] [Google Scholar]
- 26. Ayus JC, Moritz ML. Bone disease as a new complication of hyponatremia: moving beyond brain injury. Clin J Am Soc Nephrol 2010; 5: 167–168 [DOI] [PubMed] [Google Scholar]
- 27. Ayus JC, Negri AL, Kalantar-Zadeh K. et al. Is chronic hyponatremia a novel risk factor for hip fracture in the elderly? Nephrol Dial Transplant 2012; 27: 3725–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kinsella S, Moran S, Sullivan MO. et al. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol 2010; 5: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Renneboog B, Musch W, Vandemergel X. et al. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 2006; 119: 71.e1–71.e8 [DOI] [PubMed] [Google Scholar]
- 30. Movafagh S, Cleemann L, Morad M. Regulation of cardiac Ca(2+) channel by extracellular Na(+). Cell Calcium 2011; 49: 162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ayus JC, Caputo D, Bazerque F. et al. Treatment of hyponatremic encephalopathy with a 3% sodium chloride protocol: a case series. Am J Kidney Dis 2015; 65: 435–442 [DOI] [PubMed] [Google Scholar]
- 32. Kleinewietfeld M, Manzel A, Titze J. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013; 496: 518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mandai S, Kuwahara M, Kasagi Y. et al. Lower serum sodium level predicts higher risk of infection-related hospitalization in maintenance hemodialysis patients: an observational cohort study. BMC Nephrol 2013; 14: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Meer JW, Netea MG. A salty taste to autoimmunity. N Engl J Med 2013; 368: 2520–2521 [DOI] [PubMed] [Google Scholar]
- 35. Wu C, Yosef N, Thalhamer T. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013; 496: 513–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li PK, Szeto CC, Piraino B. et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30: 393–423 [DOI] [PubMed] [Google Scholar]
- 37. US Renal Data System. USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: NIH, 2014 [Google Scholar]
- 38. Tseng MH, Cheng CJ, Sung CC. et al. Hyponatremia is a surrogate marker of poor outcome in peritoneal dialysis-related peritonitis. BMC Nephrol 2014; 15: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ates K, Nergizoglu G, Keven K. et al. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int 2001; 60: 767–776 [DOI] [PubMed] [Google Scholar]
- 40. Bargman JM. The CANUSA study and the importance of residual kidney function in dialysis patients. Kidney Int 2010; 77: 931; author reply 931–932 [DOI] [PubMed] [Google Scholar]
- 41. Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12: 2158–2162 [DOI] [PubMed] [Google Scholar]
- 42. Chaudhary K, Sangha H, Khanna R. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol 2011; 6: 447–456 [DOI] [PubMed] [Google Scholar]
- 43. Lukowsky LR, Mehrotra R, Kheifets L. et al. Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: a marginal structural model analysis. Clin J Am Soc Nephrol 2013; 8: 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.