Abstract
Aims
Fractional flow reserve by computerized tomography (FFR-CT) provides non-invasive functional assessment of the hemodynamic significance of coronary artery stenosis. We determined the FFR-CT values, receiver operator characteristic (ROC) curves, and predictive ability of FFR-CT for actual standard of care guided coronary revascularization.
Methods and results
Consecutive outpatients who underwent coronary CT angiography (coronary CTA) followed by invasive angiography over a 24-month period from 2012 to 2014 were identified. Studies that fit inclusion criteria (n = 75 patients, mean age 66, 75% males) were sent for FFR-CT analysis, and results stratified by coronary artery calcium (CAC) scores. Coronary CTA studies were re-interpreted in a blinded manner, and baseline FFR-CT values were obtained retrospectively. Therefore, results did not interfere with clinical decision-making. Median FFR-CT values were 0.70 in revascularized (n = 69) and 0.86 in not revascularized (n = 138) coronary arteries (P < 0.001). Using clinically established significance cut-offs of FFR-CT ≤0.80 and coronary CTA ≥70% stenosis for the prediction of clinical decision-making and subsequent coronary revascularization, the positive predictive values were 74 and 88% and negative predictive values were 96 and 84%, respectively. The area under the curve (AUC) for all studied territories was 0.904 for coronary CTA, 0.920 for FFR-CT, and 0.941 for coronary CTA combined with FFR-CT (P = 0.001). With increasing CAC scores, the AUC decreased for coronary CTA but remained higher for FFR-CT (P < 0.05).
Conclusion
The addition of FFR-CT provides a complementary role to coronary CTA and increases the ability of a CT-based approach to identify subsequent standard of care guided coronary revascularization.
Keywords: coronary computerized tomography angiography, fractional flow reserve by computerized tomography, coronary revascularization
Introduction
Previous observations demonstrated the need for improved risk stratification and increased yield of patients who undergo invasive coronary angiography and subsequent revascularization.1 Mounting evidence over recent years supports the use of coronary computerized tomography angiography (coronary CTA) as an attractive non-invasive approach that may fulfil this gatekeeper role.2,3 However, coronary artery calcium (CAC) significantly reduces the diagnostic specificity4,5 and overall accuracy6 of coronary CTA.
Coronary CTA provides non-invasive assessment of CAD with a high correlation to invasive coronary angiography.4,5 Recent large clinical trials7,8 further support increased diagnostic certainty and improved efficiency of triage to invasive coronary angiography when using coronary CTA. Despite these findings however, coronary CTA has not been widely adopted—in part due to its initial inability to determine the physiologic importance of CAD. Fractional flow reserve derived from coronary CTA (FFR-CT) enhances the accuracy of coronary CTA and has emerged as a potential tool to provide functional characterization of the hemodynamic significance of coronary artery stenosis with correlation to invasive FFR.9 Furthermore, the accuracy of FFR-CT is superior to coronary CTA stenosis in the presence of calcification,10,11 thereby providing enhanced interpretation ability.
An ideal non-invasive strategy should go beyond the prediction of the anatomical narrowing of an artery and aggregate risk factor profiles, intrinsically reflect clinical parameters, and predict the physiological need for coronary revascularization on a per vessel basis. Multiple studies have compared FFR-CT to coronary artery stenosis and invasive FFR12–14 and determined its ability to identify obstructive CAD during invasive angiography15 with associated cost reduction.16 Despite these studies analysing CT functional imaging of coronary obstructions,17 none have compared FFR-CT to actual decision-making at the time of invasive coronary angiography while integrating all available parameters including risk factor profile, clinical presentation, and anatomical findings.
Accordingly, in this study, we retrospectively determined whether FFR-CT predicts standard of care guided coronary revascularization in ‘real world’ clinical practice, above and beyond percent stenosis determination. We calculated the performance of coronary CTA alone, FFR-CT alone, or coronary CTA combined with FFR-CT to predict actual standard of care guided clinical decision-making leading to medical management vs. coronary revascularization—integrating clinical presentation as well as clinical and diagnostic parameters with findings during invasive coronary angiography. In addition, we further established the range of FFR-CT values in patients who underwent coronary revascularization and analysed the performance of FFR-CT and coronary CTA in studies with significant calcium burden.
Methods
Patient population
Consecutive outpatients who underwent computerized tomography (CT) followed by invasive angiography (mean delay 45.8 days) over a 24-month period from 2012 to 2014 at the Cedars Sinai Heart Institute—Cardiovascular Medical Group of Southern California (Los Angeles) were identified using a retrospective approach. Patient characteristics (Table 1) at the time of coronary CTA were obtained through review of electronic medical records. The decision to obtain CT studies (Table 1) and to proceed with invasive coronary angiography was at the discretion of the care providers. The decision to advance to coronary revascularization was based on standard of care. This study was approved by the Institutional Review Board.
Table 1.
Patient characteristics and coronary CTA indication
| Patient characteristics | |
| Age, years | 66 ± 10 |
| Gender, male | 56 (75) |
| Medical history | |
| LVEF, % | 60 ± 8 |
| Hypertension | 41 (55) |
| Dyslipidaemia | 59 (79) |
| Diabetes mellitus | 8 (11) |
| Tobacco use | |
| Current | 10 (14) |
| Former | 25 (33) |
| Never | 40 (53) |
| Family history of CAD | 40 (53) |
| Medications | |
| Aspirin | 45 (60) |
| Second antiplatelet agent | 6 (8) |
| β-Blocker | 22 (29) |
| ACE-I/ARB | 32 (43) |
| Statin | 42 (56) |
| Fish oil | 9 (12) |
| Ezetimibe | 8 (11) |
| Laboratory results | |
| Creatinine, µmol/L | 87 (77–99) |
| GFR, mL/min/1.73 m2 | 75 (63–86) |
| Glucose, mmol/L | 5.4 (4.9–5.9) |
| HbA1C, % | 5.6 (5.4–5.9) |
| Total cholesterol, mmol/L | 4.6 (4.0–5.5) |
| LDL cholesterol, mmol/L | 2.8 (2.2–3.7) |
| HDL cholesterol, mmol/L | 1.3 (1.1–1.5) |
| Triglycerides, mmol/L | 1.3 (0.8–1.7) |
| Coronary CTA indication | |
| Symptomatic with typical angina | 14 (19) |
| Symptomatic with atypical angina | 34 (45) |
| Asymptomatic with known CADa | 6 (8) |
| Asymptomatic with CAD risk factors | 21 (28) |
Values are mean ± standard deviation, n (%), or median (interquartile range).
aKnown CAD defined as previous CAD identification by invasive angiography or coronary CT angiography.
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; GFR, glomerular filtration rate; HbA1C, haemoglobin A1C; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction.
Coronary CT angiography studies
Coronary CTA images were acquired using a 64-slice multi-detector row Lightspeed VCT scanner (General Electric Healthcare). Patients received β-blockers to achieve a heart rate of <60 bpm. Following a scout X-ray of the chest, a timing bolus [10–20 mL iodixanol (Visipaque), General Electric Healthcare] was performed to detect time to reach optimal contrast opacification in the axial image at a level immediately superior to the ostium of the left main coronary artery. Nitroglycerin was given prior to contrast administration. A triple phase contrast protocol was used during image acquisition: iodixanol (60 mL), followed by a ½–½ mixture of iodixanol and normosaline (40 mL), followed by a normosaline flush (50 mL). The scan parameters were 64 × 0.625 mm collimation, tube voltage 100 or 120 kV, effective mA 350–780 and 512 × 512 matrix size. All coronary CTA studies were transferred to a workstation (Vitrea–Vital Images, Toshiba).
Coronary CT angiography interpretation
The coronary CTA studies were re-interpreted separately by two readers (R.R.S.P. and R.P.K.) blinded to all patient characteristics, FFR-CT and invasive coronary angiography results. The coronary CTA readers were permitted to use any or all of the available post-processing image reconstruction algorithms, including 2D axial and 3D maximal intensity projection, multi-planar reformat, cross-sectional analysis, and volume-rendered technique. A semi-quantitative scale was used by the coronary CTA readers to grade the extent of luminal stenosis as a percentage of the vessel diameter. Stenosis severity was recorded in the following manner: 0, 1–24, 25–49, 50–69, 70–99, and 100%. There was 95% overall inter-observer agreement of the two blinded readers. In the four cases with discrepant reads, consensus was reached by joint interpretation.
FFR-CT studies
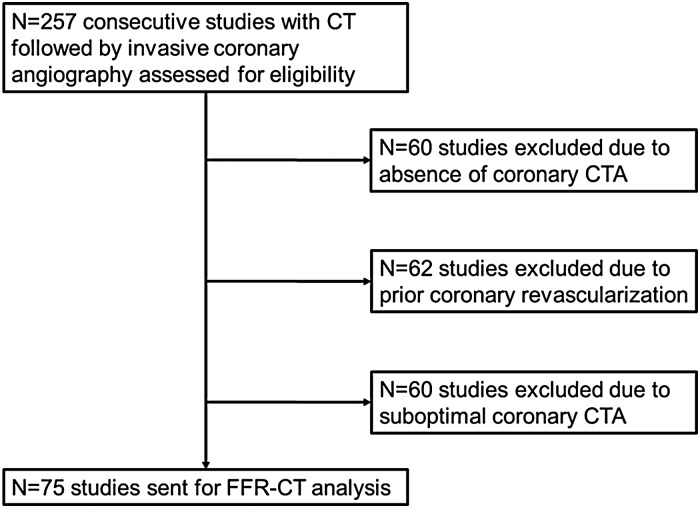
CT studies that did not fit inclusion criteria (absence of coronary CT angiography, prior coronary revascularization, incomplete or suboptimal datasets) were excluded (Figure 1). The remaining studies (n = 75) were sent for FFR-CT analysis (HeartFlow), which was performed as previously described.15,18 Three-dimensional blood flow simulations in the coronary vasculature were performed using proprietary software, with qualitative and quantitative image analysis, image segmentation, and physiological modelling using principles of computational fluid dynamics.9 Coronary blood flow was simulated under conditions that modelled coronary hyperemia to mirror pressure and flow data and the FFR values that would have been obtained during an invasive evaluation. Data provided to the investigators included the lowest FFR-CT value in each coronary distribution and a colour-scale representation showing FFR-CT values in all vessels >1.8 mm in diameter. FFR-CT values from left main coronary arteries were excluded because of inconsistent reporting when vascular territories were short, and FFR-CT values from chronic total occlusions (CTOs) were excluded to avoid skewing of the data and given this additional measure is of limited clinical utility. Baseline FFR-CT values were obtained retrospectively and therefore did not interfere with clinical decision-making. FFR-CT values were compared in revascularized (n = 50 patients, n = 69 arteries) and not revascularized arteries (n = 138) (Figures 2 and 3).
Figure 1.
Study flowchart.
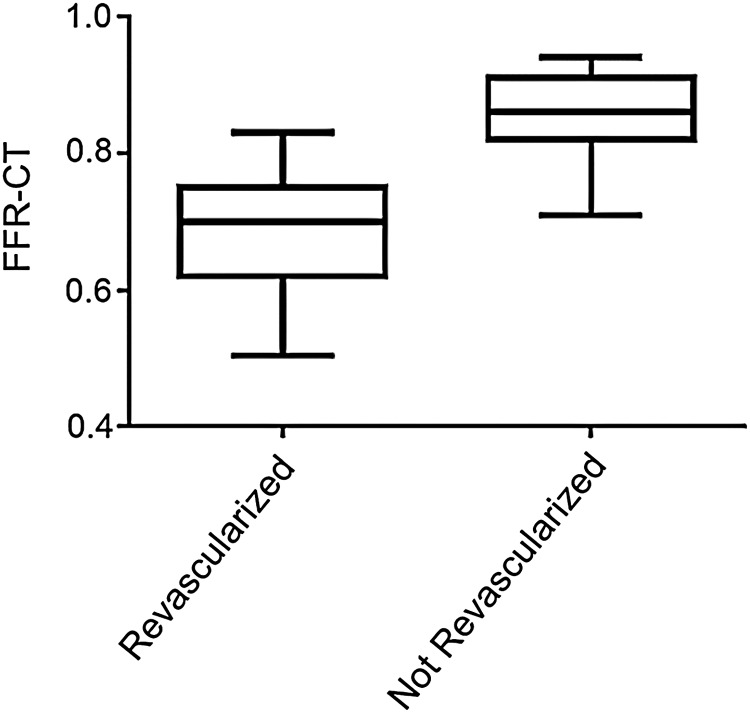
Figure 2.
FFR-CT distributions in revascularized vs. not revascularized coronary arteries. Bow and whisker plots with the boxes depicting the 25th–75th percentile and the whiskers the 5th–95th percentile of data distribution.
Figure 3.
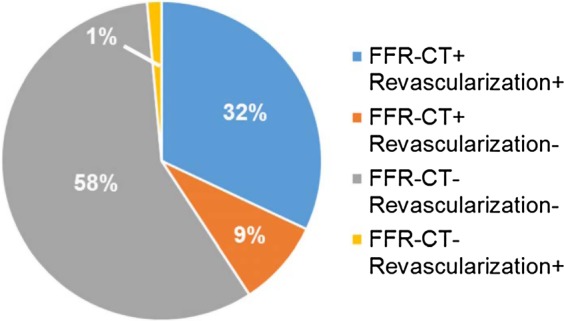
FFR-CT and clinical decision of CAD management. Concordance between FFR-CT findings and clinical management was observed in 90% of the coronary territories (n = 186), whereas 10% were discordant (n = 21).
Comparative performance of FFR-CT and coronary CTA
The positive and negative predictive abilities for coronary revascularization of previously established and clinically used significance cut-offs of FFR-CT ≤0.80 and coronary CTA ≥70% stenosis were compared (Table 2). Receiver operator characteristic (ROC) curves for the prediction of coronary revascularization were determined for coronary CTA alone, FFR-CT alone, and coronary CTA combined with FFR-CT (Figure 4). For the ROC curves, FFR-CT values were used as continuous variables ranging from 0.50 to 1.0, whereas the following discrimination thresholds were used for coronary CTA stenosis: 0, 1–24, 25–49, 50–69, and 70–99%. ROC curves were analysed in the entire population to determine global test performance and in the subgroups with a CAC score of >0, >200, >400, >600, >800, and >1000 (Figure 5).
Table 2.
FFR-CT vs. coronary CTA analyses using routine clinical significance cut-offs and subsequent coronary revascularization
| FFR-CT ≤ 0.80 |
Coronary CTA ≥ 70% |
|||
|---|---|---|---|---|
| P-value | P-value | |||
| Accuracy | 86% | <0.001 | 85% | <0.001 |
| Positive predictive value | 74% (63–83%) | <0.001 | 88% (75–95%) | <0.001 |
| Positive likelihood ratio | 5.6 (3.8–8.1) | <0.001 | 14.3 (6.4–32.0) | <0.001 |
| Negative predictive value | 96% (91–99%) | <0.001 | 84% (77–89%) | <0.001 |
| Negative likelihood ratio | 0.09 (0.04–0.2) | <0.001 | 0.39 (0.29–0.53) | <0.001 |
Values in parentheses depict the 95% confidence intervals.
Figure 4.
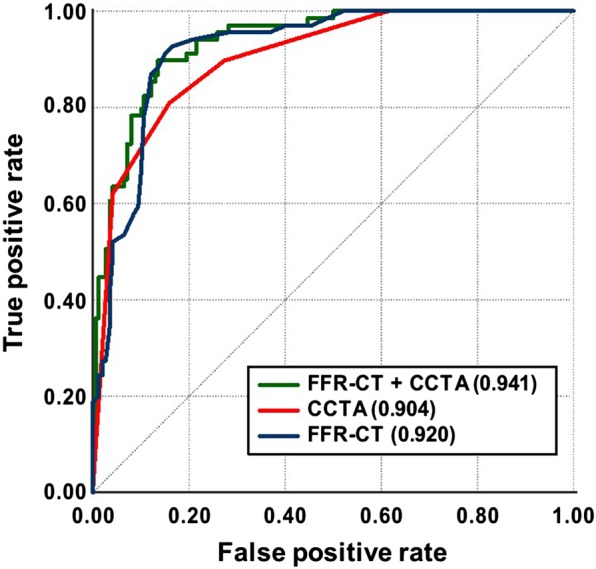
Receiver operator characteristic curves of coronary CTA, FFR-CT, or coronary CTA + FFR-CT to predict subsequent coronary revascularization. Coronary CTA alone was the reference group and had an AUC of 0.904, whereas FFR-CT alone had an AUC of 0.92 and combining coronary CTA with FFR-CT had the highest AUC of 0.941.
Figure 5.
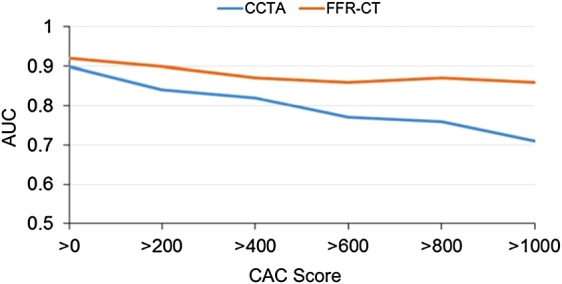
AUC plots of coronary CTA vs. FFR-CT according to increasing CAC scores for the prediction of subsequent revascularization. AUC plots depicting relative performance of coronary CTA vs. FFR-CT in territories with increasing calcification burden ranging from CAC score >0 to >1000 demonstrate a superior performance of FFR-CT at all levels of coronary artery calcification compared with coronary CTA. AUC, area under the curve; CAC, coronary artery calcium.
Statistical analyses
Statistics were performed using the Mann–Whitney test for differences in median FFR-CT values, the Kolmogorov–Smirnov test for FFR-CT value distributions, and the c-statistic to compare the relative performance of FFR-CT and coronary CTA as well as to analyse significance of the ROC curves. To test the significance of differences in areas under two independent ROC curves, Cochran's c-statistic was applied based on multiple comparisons, as previously described.19 To this end, we first calculated the areas under the ROC curve (AUC) for coronary CTA, FFR-CT, and the combination of coronary CTA and FFR-CT. Next, we used predictors with the SAS ROCcomp command to apply both nested and non-nested models.20 IBM SPSS version 20 and GraphPad version 6 were used to perform the statistical analyses. A P-value <0.05 was considered statistically significant.
Results
Over a 24-month period (2012–14), 257 consecutive outpatients who had a CT followed by invasive coronary angiography were identified (Figure 1). Of the excluded studies, 60 did not have a coronary CTA with either a non-contrast CAC score only, an aortogram, a pre-electrophysiological study, a TAVR (trans-catheter aortic valve replacement) or PE (pulmonary embolism) protocol; 62 had prior revascularization (given FFR-CT is not approved in patients with prior surgical or interventional revascularization) with 47 who had prior PCI (percutaneous coronary intervention) and 15 prior CABG (coronary artery bypass grafting); and 60 studies were excluded or rejected because of misregistrations such as a ‘skip’ in the axial data, motion artefact, or missing left ventricular segments. No studies were rejected due to blooming artefact.
All the remaining coronary CTA studies underwent successful FFR-CT analysis (n = 75 patients, n = 207 territories). Patient characteristics, medical history, medications, and laboratory results are presented in Table 1. The 10-year ACC/AHA atherosclerotic cardiovascular disease (ASCVD) risk,21 defined as coronary death or non-fatal myocardial infarction, or fatal or non-fatal stroke, of our study population was as follows: 27% had an ASCVD <7.5%, placing them in the low-risk category, whereas 73% had an ASCVD ≥7.5%, categorizing them as high risk. None of the patients had a previous myocardial infarction. Indications for the coronary CTA studies are detailed in Table 1. Of the patients who underwent a coronary CTA study, 64% were symptomatic with 19% experiencing typical angina and 45% reporting atypical anginal symptoms. In the remaining 36% of patients who were asymptomatic, coronary CTA was used as a screening tool for the determination of underlying CAD burden and severity. Eight percent of patients had previously identified CAD by coronary CT or invasive angiography, and 28% had significant CAD risk factors. The mean CAC score was 705.
Baseline FFR-CT distributions (median, 25th–75th percentile) (Figure 2) in revascularized 0.70 (0.62–0.75) compared with not revascularized territories 0.86 (0.82–0.91) displayed significantly different medians (P < 0.001) and value distributions (P < 0.001). We retrospectively analysed the proportion of cases in which FFR-CT recapitulated the clinical decision-making process to medically manage or revascularize coronary territories (Figure 3). Concordance was observed in 90% of the territories whereas 10% were discordant. Fifty-eight percent of territories (n = 120) had a FFR-CT of >0.80 and were managed medically, whereas 32% of territories (n = 66) had a FFR-CT ≤0.80 and were revascularized. One percent of the territories (n = 3) had a FFR-CT >0.80 but were revascularized, with review of cases illustrating coronary stenoses ≥50% and presence of typical anginal symptoms. Invasive FFR was not obtained in these cases. Of the 9% territories (n = 18) in which an FFR-CT ≤0.80 was reported but were medically managed, the following contributing factors were observed: invasive coronary stenosis <25% (n = 8), invasive coronary stenosis 25–50% (n = 3), discrepancy with an invasive FFR >0.80 during cardiac catheterization (n = 5), or significant stenosis deemed too distal and not amenable to intervention (n = 2). In addition, the above patients with invasive coronary stenoses <25 or 25–50% underwent invasive coronary angiography following coronary CTA falsely concerning for significant stenoses which were obtained in the setting of known CAD, CAD screening, or atypical symptoms (Table 1).
Next, we sought to compare the discriminative ability of FFR-CT with coronary CTA in the prediction of standard of care guided coronary revascularization (Table 2). To this end, clinically accepted and routinely used significance cut-offs were studied, i.e. FFR-CT ≤0.80 and coronary CTA stenosis ≥70%. The overall accuracies for the prediction of revascularization were similar—86% for FFR-CT and 85% for coronary CTA. Importantly, a significant complementary role for these two strategies was observed, with coronary CTA having a superior positive predictive value of 88% and a positive likelihood ratio of 14.3, whereas FFR-CT had a superior negative predictive value of 96% and a negative likelihood ratio of 0.09.
We further determined ROC curves for the comparison of three strategies: coronary CTA alone, FFR-CT alone, and coronary CTA combined with FFR-CT for the prediction of subsequent coronary revascularization in the entire study population (Figure 4). Using continuous variables portrayed in the ROC curves, the global statistical accuracy was not used to test the study properties of coronary CTA vs. invasive coronary angiography, but the overall ability of coronary CTA vs. FFR-CT to predict actual clinical decision-making, i.e. medical management vs. coronary revascularization. The reference group was coronary CTA alone (AUC, 95% CI): 0.904 (0.863–0.944), with the ROC curve demonstrating the strong performance of this test by itself. FFR-CT alone (AUC, 95% CI): 0.92 (0.883–0.957) had a greater AUC than coronary CTA which did not achieve significance (P = 0.4). However, combining coronary CTA with FFR-CT (AUC, 95% CI): 0.941 (0.911–0.971) had the highest AUC for the prediction of subsequent revascularization and was significant (P = 0.001). In addition, we identified that from the ROC curve the optimal FFR-CT cut-off value to predict downstream revascularization may be a lower FFR-CT value of 0.76, and not 0.80.22 In order to test the performance of this FFR-CT cut-off value, a comparison of ROC curves (on a per-patient basis) was applied, yielding an AUC of 0.842 for an FFR-CT cut-off of 0.76 and an AUC of 0.829 for an FFR-CT cut-off of 0.80 (P = 0.043).
AUCs were also stratified according to increasing CAC burden categories and demonstrated the increasing contribution of FFR-CT to overall accuracy with worsening calcification (Figure 5). Although coronary CTA AUC progressively decreased with worsening calcification burden, ranging from 0.90 (CAC > 0) to 0.71 (CAC > 1000), this phenomenon was less observed in the FFR-CT AUC which remained more stable in its discriminative ability, ranging from 0.92 (CAC > 0) to 0.86 (CAC > 1000) (P < 0.05, FFR-CT combined with coronary CTA vs. coronary CTA alone for all CAC scores).
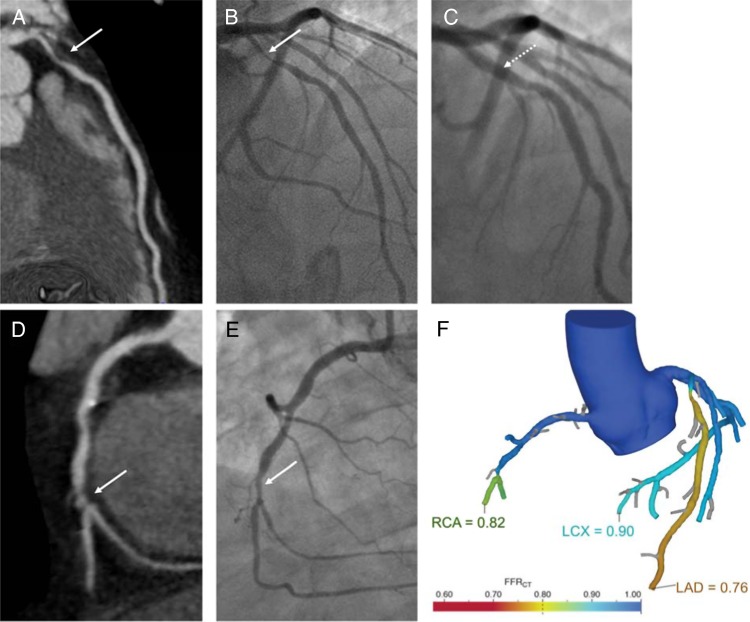
A case example of a patient with short clinical vignette comparing coronary CTA, FFR-CT and invasive coronary angiography results is illustrated (Figure 6).
Figure 6.
Case example comparing coronary CTA, FFR-CT, and invasive angiography results. A 71-year-old male presented with exertional chest pain. He exercised for 10 min on a Bruce protocol, experienced reproducible chest pain but had no significant ECG changes. He underwent coronary CTA, which showed a 70–95% ostial left anterior descending (LAD) coronary artery narrowing (A). Invasive angiography confirmed the 70–95% ostial LAD narrowing (B) and the artery was stented (C). The coronary CTA also showed a 25–49% mid right coronary artery (RCA) narrowing followed by a 70–95% distal narrowing (D). The mid RCA narrowing was judged minimal on invasive angiography and the distal RCA a 50% narrowing that did not undergo stenting (E). FFR-CT was 0.76 in the LAD that underwent stenting and 0.82 in the RCA that did not undergo stenting (F), predicting decision-making in the cardiac catheterization laboratory. The patient was asymptomatic at 2-year follow-up. The solid white arrows represent the stenotic segments identified on coronary CTA (A and D) or invasive angiography (B and E). The dashed white arrow represents the revascularized LAD stenotic segment (C). The FFR-CT values in each coronary territory are depicted in colour scale, and the numerical values of the worst FFR-CT determined in distal segments provided (F). A colour-scale graph of FFR-CT values is also presented (F). LCX, left circumflex coronary artery.
Discussion
The management of patients with stable coronary artery disease (CAD) has evolved significantly23–25 with efforts to determine which coronary stenoses cause myocardial ischaemia. A strategy of artery-specific flow-guided revascularization was explored in the FAME trials.26,27 In stable CAD patients, routine measurement of invasive FFR during coronary angiography significantly reduces death and non-fatal myocardial infarction compared with an angiographically driven approach when stenting of indicated lesions only if the FFR is ≤0.80.26 Conversely, similar patients with an FFR >0.80 should receive medical therapy alone to improve outcomes.27 Thus, the routine measurement of FFR is supported by large clinical trials and integrated into American28 and European guidelines29 recommending the use of FFR with a cut-off of 0.80 to guide the decision to medically manage or revascularize patients with stable CAD.30
FFR-CT may offer a non-invasive equivalent to invasive FFR.17 FFR-CT provides functional characterization of the hemodynamic significance of coronary artery stenosis without changes in acquisition, medication, contrast, or radiation.9,18 FFR-CT computation is based on calculations of coronary flow and pressure fields from anatomic data, in particular, construction of an anatomic model of the coronary arteries, a mathematical model of coronary physiology to derive boundary conditions representing cardiac output, aortic pressure, microcirculatory resistance, and their combination with fluid dynamics principles that relate to conservation of mass and balance of momentum.18
This study sought to take findings beyond previous reports12–14 by predicting not just the significance of coronary artery narrowing, but also establishing the distribution of FFR-CT values, inclusive of studies with high calcium scores, in an integrated manner to predict coronary revascularization behaviour in patients commonly treated with standard of care in a large urban cardiology practice. We applied this measure to consecutive outpatients over a 24-month period who had previously undergone invasive coronary angiography following a coronary CTA study. Given baseline FFR-CT values were obtained retrospectively, results did not interfere with clinical decision-making. We determined baseline FFR-CT values in patients undergoing coronary revascularization and those treated with optimal medical therapy, establishing significant differences in median values (0.70 vs. 0.86, respectively). A comparison of accepted and routinely used clinical significance cut-offs of FFR-CT ≤0.80 and coronary CTA stenosis ≥70%26–29 highlighted important different behaviours of these two tests. Indeed, a coronary CTA approach had a significant positive predictive value and positive likelihood ratio, indicating that in the presence of an angiographically significant stenosis, the odds of having a downstream revascularization were high, reflecting the ability of coronary CTA to identify high-risk patients. In contrast, an FFR-CT approach had a significant negative predictive value and negative likelihood ratio, supporting the hypothesis that in the presence of a hemodynamically insignificant FFR-CT value >0.80, the odds of having downstream revascularization were low, thereby reflecting the ability of FFR-CT to exclude high-risk patients. With the use of FFR-CT values as continuous variables and incremental discrimination thresholds for coronary CTA stenoses, we further established global performance measures of these tests in the prediction of coronary revascularization. Accordingly, our data demonstrate that even though coronary CTA alone had a high predictive ability of subsequent coronary revascularization, FFR-CT alone performed better, and the combination of coronary CTA and FFR-CT performed best, particularly in patients with advanced disease and elevated CAC scores. In addition, our findings suggest that the best cut-off value for the prediction of downstream revascularization may be a lower FFR-CT value of 0.76, and not 0.80 as reported with invasive FFR.
Previous CT trials determined the diagnostic characteristics of coronary CTA compared with invasive coronary angiography—i.e. % angiographic stenosis by CT vs. % angiographic stenosis by cardiac catheterization,4,5,31 a correlation dependent on target lesion, coronary calcium score, and vessel diameter. In these coronary CTA validation studies, coronary artery segments <2 mm in diameter were not evaluated.32 In this study, we examined the ability of coronary CTA alone, FFR-CT alone, or coronary CTA combined with FFR-CT to integrate all available parameters including clinical presentation, laboratory findings, non-invasive imaging workup, and findings on invasive coronary angiography with actual clinical decision-making regarding medical management vs. coronary revascularization on a per-vessel basis. The difference in approach we adopted with an end point consisting of standard of care guided clinical decision-making to manage with optimal medical therapy or revascularize coronary territories, as opposed to previous studies where the end point consisted of anatomic findings during cardiac catheterization,4,5,31 explain differences in the observed predictive abilities of coronary CTA and FFR-CT we illustrate.
Our study has several limitations. This was a single centre retrospective study. We analysed FFR-CT only in patients who had a coronary CTA followed by invasive angiography. The decision to proceed with revascularization was at the discretion of the primary cardiologist, based on medical history, clinical presentation, non-invasive imaging tests, and findings during angiography. We believe however these limitations also constitute a strength of our study population which is representative of ‘real world’ patient care and may avoid the patient selection bias of clinical trials.
Conclusions
FFR-CT predicts standard of care guided coronary revascularization and provides additive predictive value to coronary CTA, improving overall accuracy, particularly in territories with significant calcification. Future studies are needed to validate these observations prospectively and to determine the optimal FFR-CT cut-off for the prediction of coronary revascularization. Our results suggest that coronary CTA combined with FFR-CT allows individual patient-level, artery-specific decision-making, thereby enhancing the gatekeeping function of coronary CTA and increasing the diagnostic and therapeutic yield of invasive coronary angiography.
Funding
The authors are supported by NIH grant T32HL007895 (R.R.S.P.), the UCLA STAR program (R.R.S.P.), and an unrestricted grant from the Cardiovascular Research Foundation of Southern California (R.P.K.).
Acknowledgements
The authors thank James Sayre, PhD, and Boback Ziaeian, MD, for insightful discussions.
Conflict of interest: None declared.
References
- 1. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV et al. . Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karlsberg RP, Budoff MJ, Thomson LE, Friedman JD, Berman DS. Integrated coronary computed tomographic angiography in an office-based cardiology practice. Rev Cardiovasc Med 2009;10:194–201. [DOI] [PubMed] [Google Scholar]
- 3. Shaw LJ, Hausleiter J, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ et al. . Coronary computed tomographic angiography as a gatekeeper to invasive diagnostic and surgical procedures: results from the multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter) registry. J Am Coll Cardiol 2012;60:2103–14. [DOI] [PubMed] [Google Scholar]
- 4. Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E et al. . Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–32. [DOI] [PubMed] [Google Scholar]
- 5. Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA et al. . Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–44. [DOI] [PubMed] [Google Scholar]
- 6. Kruk M, Noll D, Achenbach S, Mintz GS, Pregowski J, Kaczmarska E et al. . Impact of coronary artery calcium characteristics on accuracy of CT angiography. JACC Cardiovasc Imaging 2014;7:49–58. [DOI] [PubMed] [Google Scholar]
- 7. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B et al. . Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. [DOI] [PubMed] [Google Scholar]
- 9. Min JK, Taylor CA, Achenbach S, Koo BK, Leipsic J, Nørgaard BL et al. . Noninvasive fractional flow reserve derived from coronary CT angiography: clinical data and scientific principles. JACC Cardiovasc Imaging 2015;8:1209–22. [DOI] [PubMed] [Google Scholar]
- 10. Min JK, Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S et al. . Effect of image quality on diagnostic accuracy of noninvasive fractional flow reserve: results from the prospective multicenter international DISCOVER-FLOW study. J Cardiovasc Comput Tomogr 2012;6:191–9. [DOI] [PubMed] [Google Scholar]
- 11. Nørgaard BL, Gaur S, Leipsic J, Ito H, Miyoshi T, Park SJ et al. . Influence of coronary calcification on the diagnostic performance of CT angiography derived FFR in coronary artery disease: a substudy of the NXT trial. JACC Cardiovasc Imaging 2015;8:1045–55. [DOI] [PubMed] [Google Scholar]
- 12. Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS et al. . Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (diagnosis of ischemia-causing stenoses obtained via noninvasive fractional flow reserve) study. J Am Coll Cardiol 2011;58:1989–97. [DOI] [PubMed] [Google Scholar]
- 13. Nakazato R, Park HB, Berman DS, Gransar H, Koo BK, Erglis A et al. . Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: results from the DeFACTO study. Circ Cardiovasc Imaging 2013;6:881–9. [DOI] [PubMed] [Google Scholar]
- 14. Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H et al. . Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol 2014;63:1145–55. [DOI] [PubMed] [Google Scholar]
- 15. Douglas PS, Pontone G, Hlatky MA, Patel MR, Nørgaard BL, Byrne RA et al. . Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFRct: outcome and resource impacts study. Eur Heart J 2015;47:3359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hlatky MA, De Bruyne B, Pontone G, Patel MR, Nørgaard BL, Byrne RA et al. . Quality of life and economic outcomes of assessing fractional flow reserve with computed tomography angiography: the PLATFORM study. J Am Coll Cardiol 2015;66:2315–23. [DOI] [PubMed] [Google Scholar]
- 17. de Feyter PJ. CT functional imaging using intracoronary gradient analysis: an indispensable boost for CT coronary angiography. Eur Heart J Cardiovasc Imaging 2012;13:971–2. [DOI] [PubMed] [Google Scholar]
- 18. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61:2233–41. [DOI] [PubMed] [Google Scholar]
- 19. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839–43. [DOI] [PubMed] [Google Scholar]
- 20. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 21. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH et al. . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
- 22. Faraggi D, Reiser B. Estimation of the area under the ROC curve. Stat Med 2002;21:3093–106. [DOI] [PubMed] [Google Scholar]
- 23. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ et al. . Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16. [DOI] [PubMed] [Google Scholar]
- 24. Group BDS, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF et al. . A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A et al. . Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011;364:1607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M et al. . Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24. [DOI] [PubMed] [Google Scholar]
- 27. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z et al. . Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014;371:1208–17. [DOI] [PubMed] [Google Scholar]
- 28. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B et al. . 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44–122. [DOI] [PubMed] [Google Scholar]
- 29. Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C et al. . 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 30. Pijls NH, Tanaka N, Fearon WF. Functional assessment of coronary stenoses: can we live without it? Eur Heart J 2013;34:1335–44. [DOI] [PubMed] [Google Scholar]
- 31. Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I et al. . Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324–36. [DOI] [PubMed] [Google Scholar]
- 32. Sandfort V, Lima JA, Bluemke DA. Noninvasive imaging of atherosclerotic plaque progression: status of coronary computed tomography angiography. Circ Cardiovasc Imaging 2015;8:e003316. [DOI] [PMC free article] [PubMed] [Google Scholar]